Corneal Sensory Receptors and Pharmacological Therapies to Modulate Ocular Pain
Abstract
1. Introduction
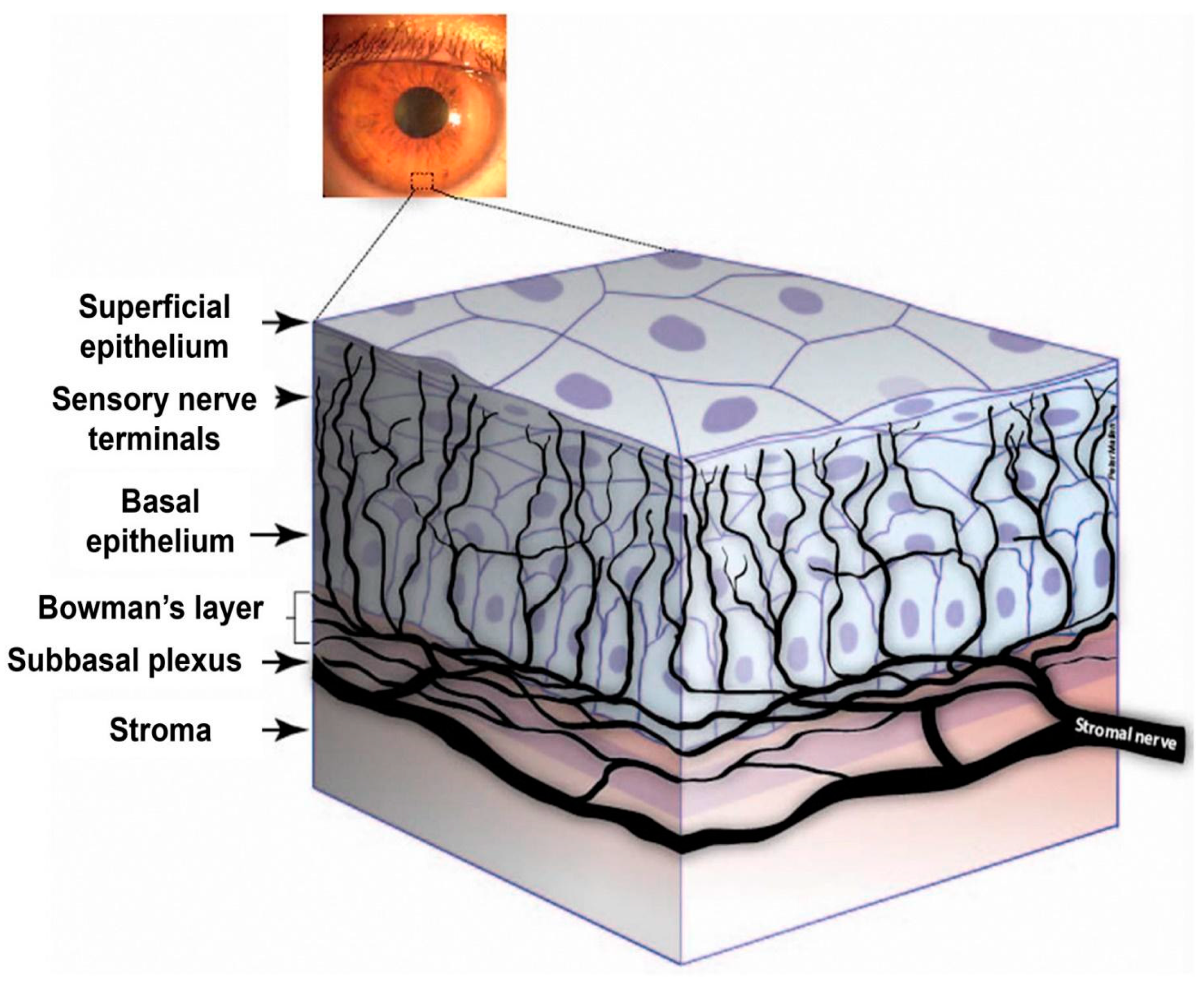
2. Cornea Nerve Anatomy, Physiology, and Development
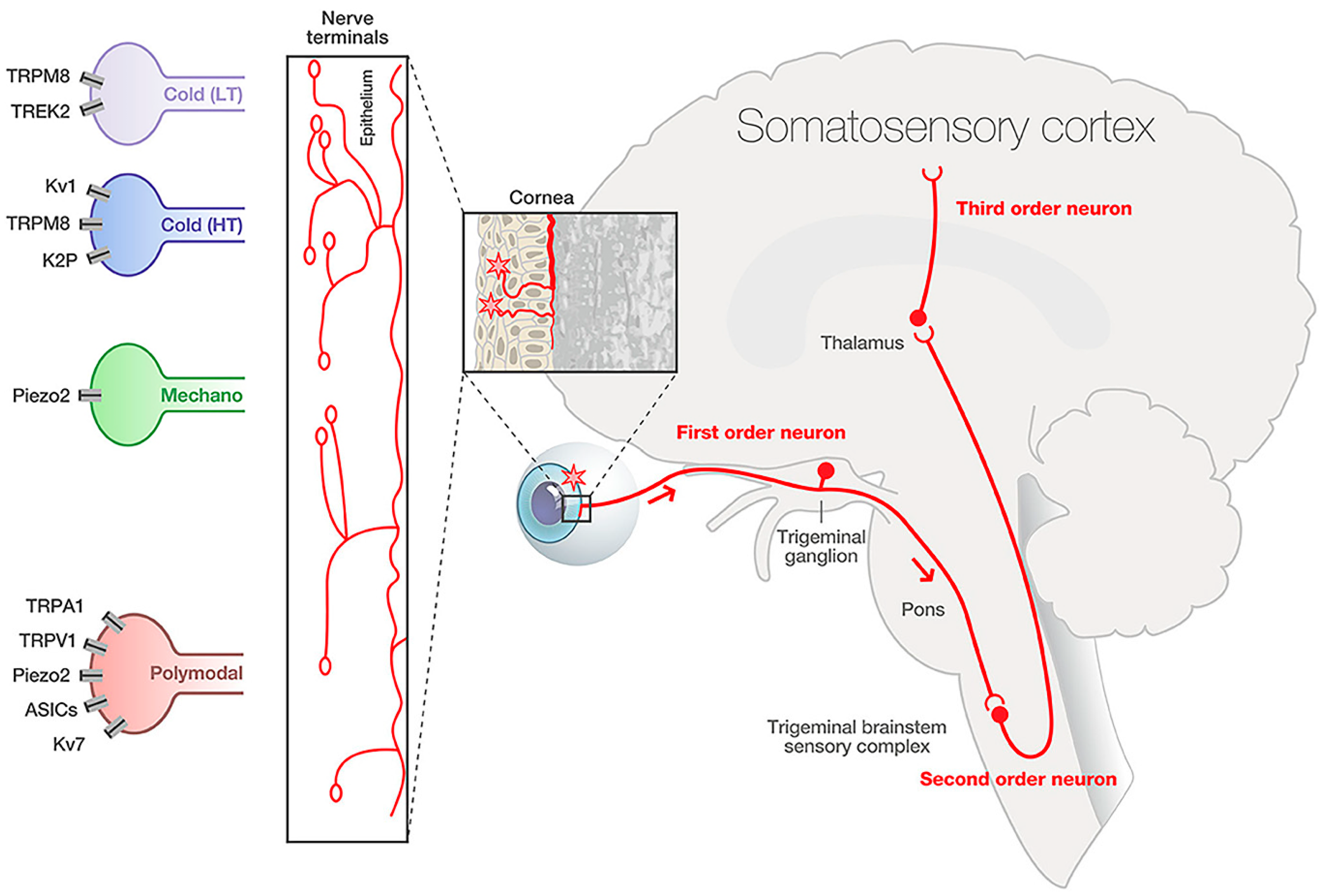
3. Cornea Nociceptor Receptors, Channels, and Neurotransmitters
3.1. Nociceptors and the Cornea
3.2. Cornea Nociceptor Receptors and Channels
3.2.1. TRP Channel Superfamily
3.2.2. Acid-Sensing Ion Channels (ASICs)
3.2.3. Mechanosensing Ion Channels
3.2.4. Coding Channels
3.2.5. Interactions and Distinctions Between Nociceptive Channels
3.2.6. Age- and Sex-Related Variation in Ion Channel Expression and Function
3.3. Corneal Neurotransmitters
4. Corneal Nerve Pathology
4.1. Corneal Nerve Lesions and Inflammation
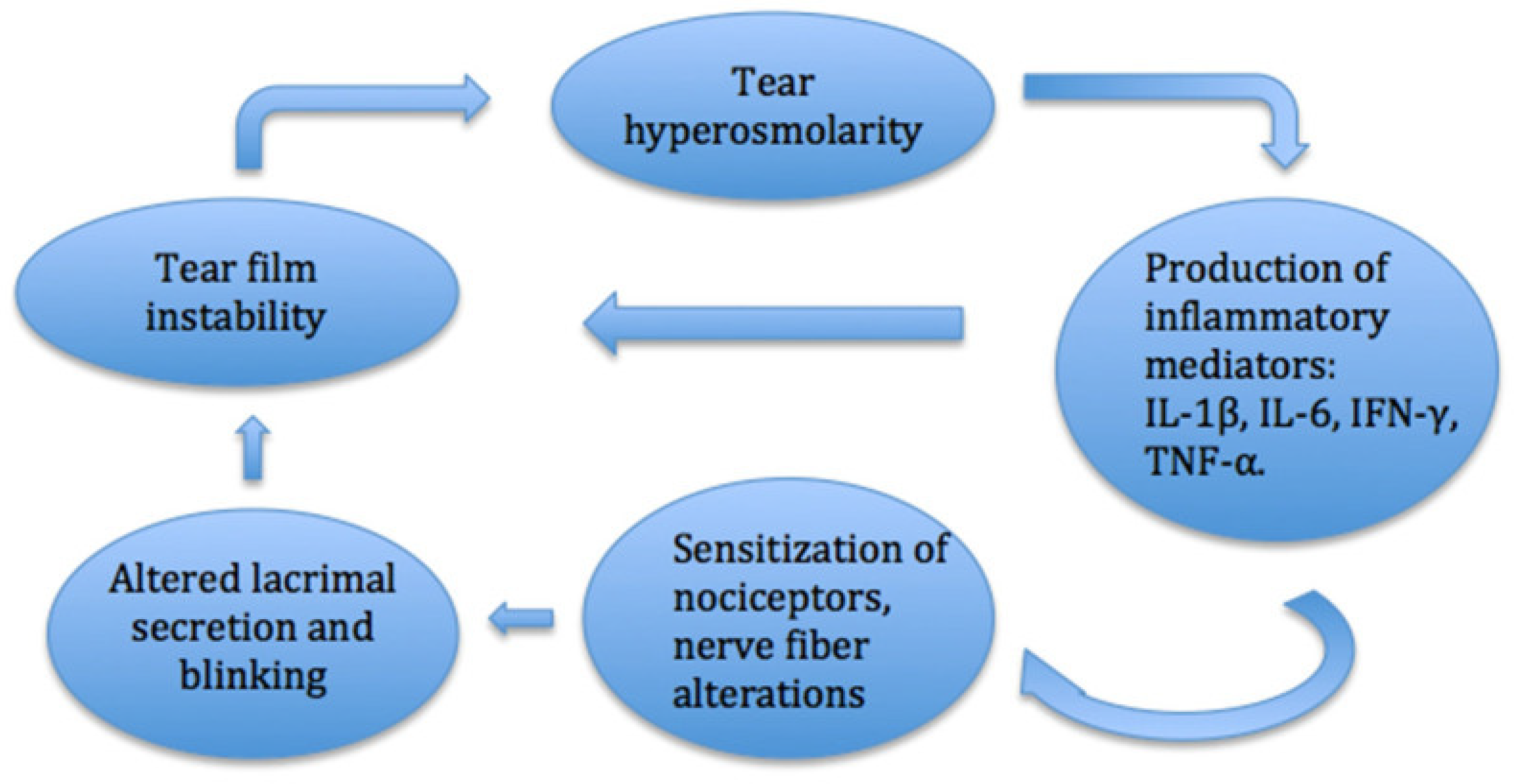
4.2. Dry Eye Disease
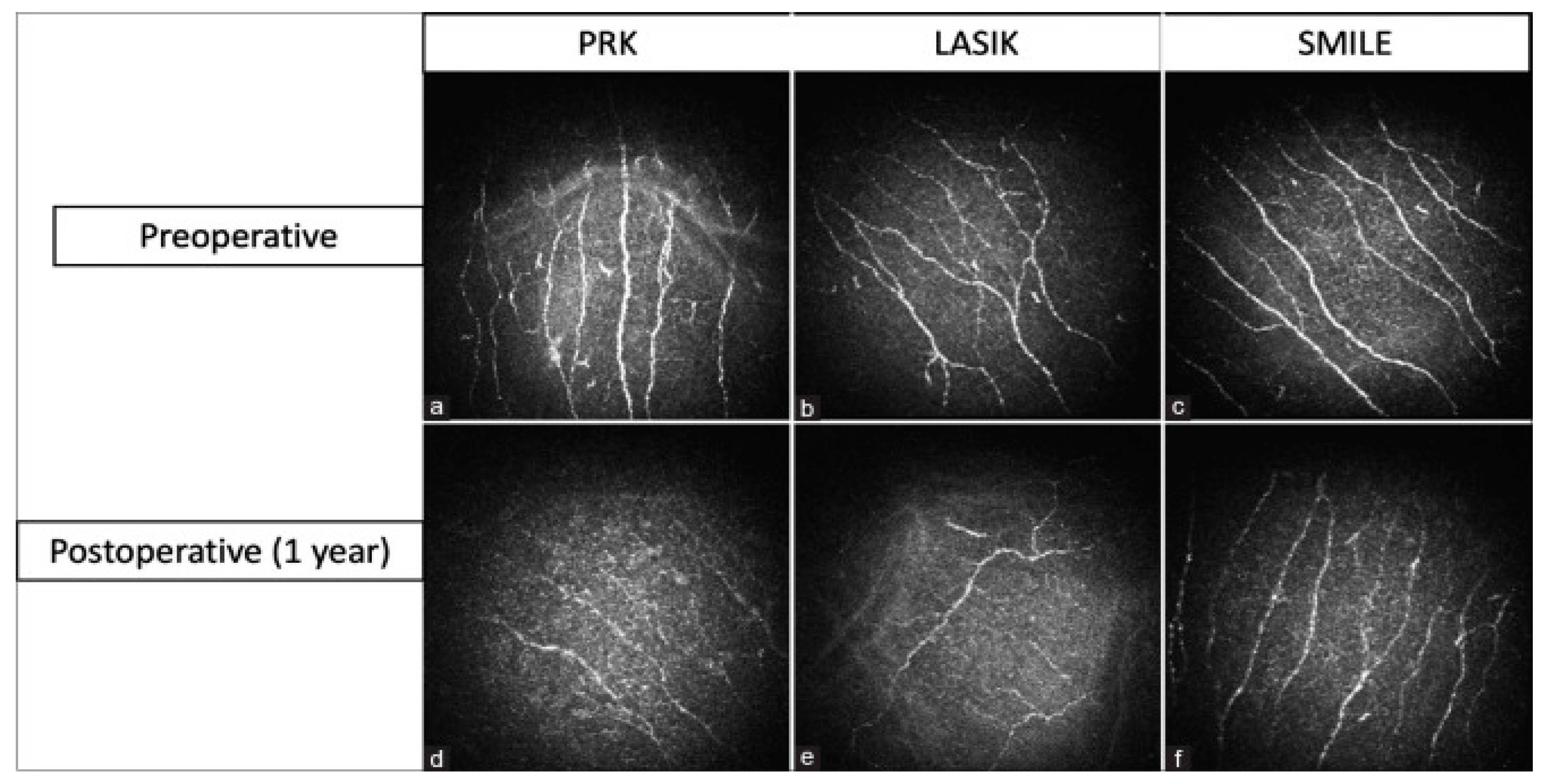
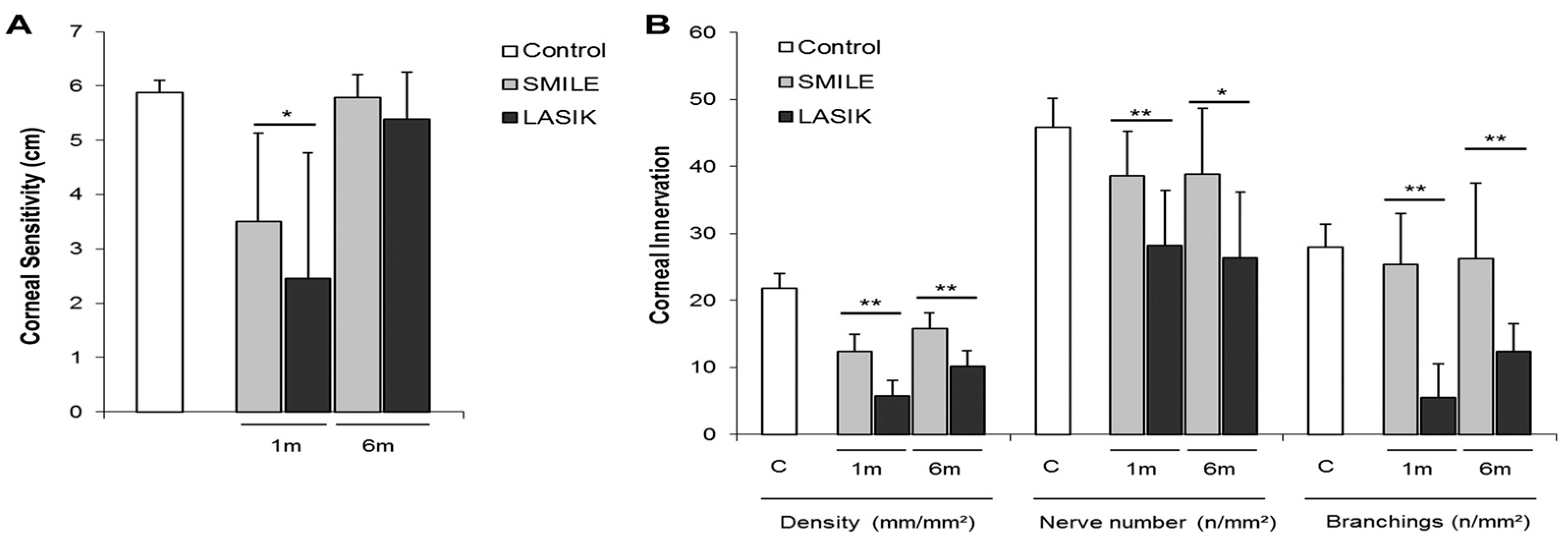
4.3. Refractive Surgery
4.4. Keratitis
4.5. Corneal Nerve Growth and Regeneration
5. Nociceptors, Inflammation, and Sensitization
6. Current FDA-Approved Nociceptor-Related Therapies for Ocular Pain
7. Future Therapies and Targets
7.1. Therapies in Development
7.1.1. TRP-Based Therapeutics
7.1.2. NGF-Based Therapeutics
7.1.3. TrkA-Based Therapeutics
7.1.4. Novel Mu-Receptor Therapeutics
7.1.5. Neurokinin 1 Receptor Antagonist Therapeutics
7.1.6. Dual Enkephalinase Inhibitors (DENKIs)
7.1.7. Nav Channel Inhibitors
7.2. Potential Future Targets
7.2.1. PIRT
7.2.2. ASICs
7.2.3. TRPA1
7.2.4. TRPV4
7.2.5. PIEZO2
7.2.6. K2p K+ Channels: TRESK and TREK1
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASIC | Acid-Sensing Ion Channel |
| ATP | Adenosine Triphosphate |
| BCL | Bandage Contact Lens |
| BDNF | Brain-derived Neurotrophic Factor |
| CCL2 | Chemokine Ligand 2 |
| CCL3 | Chemokine Ligand 3 |
| CGRP | Calcitonin Gene-related Peptide |
| CN | Cranial Nerve |
| CNS | Central Nervous System |
| COX | Cyclooxygenase |
| CX3CR1 | CX3C Motif Chemokine Receptor 1 |
| DAG | Diacylglycerol |
| DAMGO | [D-Ala2, N-MePhe4, Gly-ol]-enkephalin |
| DENKI | Dual Enkephalinase Inhibitor |
| DRG | Dorsal Root Ganglion |
| EGF | Epidermal Growth Factor |
| F6H8 | Perfluorohexyloctane |
| FDA | Food and Drug Administration |
| FM | Fluorescence Microscopy |
| GABA | Gamma-aminobutyric Acid |
| GDNF | Glial Cell-derived Neurotrophic Factor |
| GsMTx4 | Grammostola Mechanotoxin 4 |
| IL | Interleukin |
| IVCM | In Vivo Confocal Microscopy |
| K2P | Two-pore Domain Potassium Channels |
| LASIK | Laser-Assisted In Situ Keratomileusis |
| LC | Locus Coeruleus |
| LIF | Leukemia Inhibitory Factor |
| LINE | Laser In Situ Keratomileusis-induced Neurotrophic Epitheliopathy |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated Protein Kinase |
| MDSC | Myeloid-derived Suppressor Cell |
| MOR | Mu Opioid Receptor |
| mTORC1 | Mammalian Target of Rapamycin Complex 1 |
| NGF | Nerve Growth Factor |
| NK1R | Neurokinin-1 Receptor |
| NSAID | Non-steroidal Anti-inflammatory Drug |
| NT | Neurotrophin |
| OCT | Optical Coherence Tomography |
| OGF | Opioid Growth Factor |
| P2 | ATP Receptors |
| PACAP | Pituitary Adenylate Cyclase-activating Peptide |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-Kinase |
| PIEZO2 | Piezo Type Mechanosensitive Ion Channel Component 2 |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIRT | Phosphoinositide Interacting Regulator |
| PNS | Peripheral Nervous System |
| PRK | Photorefractive Keratectomy |
| PROSE | Prosthetic Replacement of the Ocular Surface Ecosystem |
| RNA | Ribonucleic Acid |
| ROBO | Transmembrane Roundabout |
| RVM | Rostral Ventromedial Medulla |
| SAF312 | Libvatrep |
| SMILE | Small Incision Lenticule Extraction |
| SP | Substance P |
| SPRY2 | Sprouty RTK Signaling Antagonist 2 |
| T3 | Triiodothyronine |
| TASK | TWIK-related Acid-sensitive Potassium Channel 1 |
| TGF | Transforming Growth Factor |
| TMEM120A | Transmembrane Protein 120A |
| TNF | Tumor Necrosis Factor |
| TREK | TWIK-related K+ Channel |
| TRESK | TWIK-related Spinal Cord K+ Channel |
| TrkA | Tropomyosin Receptor Kinase A |
| TRP | Transient Receptor Potential |
| TRPA | Transient Receptor Potential Ankyrin |
| TRPC | Transient Receptor Potential Canonical |
| TRPM | Transient Receptor Potential Subfamily M |
| TRPP | Transient Receptor Potential Polycystic |
| TRPV | Transient Receptor Potential Vanilloid |
| TWIK | Tandem of P Domains in a Weak Inward Rectifying K+ Channel |
| VEGF | Vascular Endothelial Growth Factor |
| YFP | Yellow Fluorescent Protein |
References
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Moreno, A.; Baudouin, C.; Parsadaniantz, S.M.; Goazigo, A.R.L. Morphological and Functional Changes of Corneal Nerves and Their Contribution to Peripheral and Central Sensory Abnormalities. Front. Cell. Neurosci. 2020, 14, 610342. [Google Scholar] [CrossRef] [PubMed]
- Koaik, M.; Baig, K. Corneal neurotization. Curr. Opin. Ophthalmol. 2019, 30, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, A.; Qazi, Y.; Hamrah, P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul. Surf. 2017, 15, 15–47. [Google Scholar] [CrossRef]
- Belmonte, C.; Tervo, T.; Gallar, J. Sensory Innervation of the Eye; US Elsevier Health: New York, NY, USA, 2024. [Google Scholar]
- Eguchi, H.; Hiura, A.; Nakagawa, H.; Kusaka, S.; Shimomura, Y. Corneal Nerve Fiber Structure, Its Role in Corneal Function, and Its Changes in Corneal Diseases. BioMed Res. Int. 2017, 2017, 3242649. [Google Scholar] [CrossRef]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef]
- Fakih, D.; Migeon, T.; Moreau, N.; Baudouin, C.; Réaux-Le Goazigo, A.; Mélik Parsadaniantz, S. Transient Receptor Potential Channels: Important Players in Ocular Pain and Dry Eye Disease. Pharmaceutics 2022, 14, 1859. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Hegarty, D.M.; Hermes, S.M.; Largent-Milnes, T.M.; Aicher, S.A. Capsaicin-responsive corneal afferents do not contain TRPV1 at their central terminals in trigeminal nucleus caudalis in rats. J. Chem. Neuroanat. 2014, 61–62, 1–12. [Google Scholar] [CrossRef][Green Version]
- Schecterson, L.C.; Pazevic, A.A.; Yang, R.; Matulef, K.; Gordon, S.E. TRPV1, TRPA1, and TRPM8 are expressed in axon terminals in the cornea: TRPV1 axons contain CGRP and secretogranin II; TRPA1 axons contain secretogranin 3. Mol. Vis. 2020, 26, 392–404. [Google Scholar]
- Benitez-Angeles, M.; Morales-Lazaro, S.L.; Juarez-Gonzalez, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef] [PubMed]
- Leffler, A.; Monter, B.; Koltzenburg, M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience 2006, 139, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Callejo, G.; Castellanos, A.; Castany, M.; Gual, A.; Luna, C.; Acosta, M.C.; Gallar, J.; Giblin, J.P.; Gasull, X. Acid-sensing ion channels detect moderate acidifications to induce ocular pain. Pain 2015, 156, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Takayama, Y.; Ohno, N.; Kanda, H.; Dai, Y.; Sokabe, T.; Tominaga, M. Increased TRPV4 expression in non-myelinating Schwann cells is associated with demyelination after sciatic nerve injury. Commun. Biol. 2020, 3, 716. [Google Scholar] [CrossRef]
- Guarino, B.D.; Paruchuri, S.; Thodeti, C.K. The role of TRPV4 channels in ocular function and pathologies. Exp. Eye Res. 2020, 201, 108257. [Google Scholar] [CrossRef]
- Yamada, Y.; Terada, Y.; Yamanaka, R.; Enoyoshi, M.; Ito, K. TRPV4 activation in human corneal epithelial cells promotes membrane mucin production. Biochem. Biophys. Res. Commun. 2024, 731, 150402. [Google Scholar] [CrossRef]
- Lapajne, L.; Lakk, M.; Rudzitis, C.N.; Vemaraju, S.; Lang, R.A.; Hawlina, M.; Krizaj, D. Neuropsin, TRPV4 and intracellular calcium mediate intrinsic photosensitivity in corneal epithelial cells. Ocul. Surf. 2025, 36, 1–9. [Google Scholar] [CrossRef]
- Moore, C.; Cevikbas, F.; Pasolli, H.A.; Chen, Y.; Kong, W.; Kempkes, C.; Parekh, P.; Lee, S.H.; Kontchou, N.A.; Yeh, I.W.; et al. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling (vol 110, pg E3225, 2013). Proc. Natl. Acad. Sci. USA 2013, 110, 15502. [Google Scholar] [CrossRef]
- Alamri, A.S.; Wood, R.J.; Ivanusic, J.J.; Brock, J.A. The neurochemistry and morphology of functionally identified corneal polymodal nociceptors and cold thermoreceptors. PLoS ONE 2018, 13, e0195108. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Chen, O.; Donnelly, C.R.; Ji, R.-R. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr. Opin. Neurobiol. 2020, 62, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.X. Multiple roles of calmodulin and other Ca2+-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 2005, 451, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Staaf, S.; Maxvall, I.; Lind, U.; Husmark, J.; Mattsson, J.P.; Ernfors, P.; Pierrou, S. Down regulation of TRPC1 by shRNA reduces mechanosensitivity in mouse dorsal root ganglion neurons. Neurosci. Lett. 2009, 457, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Garrison, S.R.; Dietrich, A.; Stucky, C.L. TRPC1 contributes to light-touch sensation and mechanical responses in low-threshold cutaneous sensory neurons. J. Neurophysiol. 2012, 107, 913–922. [Google Scholar] [CrossRef]
- Giblin, J.P.; Comes, N.; Strauss, O.; Gasull, X. Chapter Five—Ion Channels in the Eye: Involvement in Ocular Pathologies. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 104, pp. 157–231. [Google Scholar]
- Comes, N.; Gasull, X.; Callejo, G. Proton Sensing on the Ocular Surface: Implications in Eye Pain. Front. Pharmacol. 2021, 12, 773871. [Google Scholar] [CrossRef]
- Murthy, S.E.; Loud, M.C.; Daou, I.; Marshall, K.L.; Schwaller, F.; Kühnemund, J.; Francisco, A.G.; Keenan, W.T.; Dubin, A.E.; Lewin, G.R.; et al. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med. 2018, 10, eaat9897. [Google Scholar] [CrossRef]
- Szczot, M.; Liljencrantz, J.; Ghitani, N.; Barik, A.; Lam, R.; Thompson, J.H.; Bharucha-Goebel, D.; Saade, D.; Necaise, A.; Donkervoort, S.; et al. PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci. Transl. Med. 2018, 10, eaat9892. [Google Scholar] [CrossRef]
- Fernández-Trillo, J.; Florez-Paz, D.; Iñigo-Portugués, A.; González-González, O.; del Campo, A.G.; González, A.; Viana, F.; Belmonte, C.; Gomis, A. Piezo2 Mediates Low-Threshold Mechanically Evoked Pain in the Cornea. J. Neurosci. 2020, 40, 8976–8993. [Google Scholar] [CrossRef]
- Ghovanloo, M.R.; Tyagi, S.; Zhao, P.; Waxman, S.G. Nav1.8, an analgesic target for nonpsychotomimetic phytocannabinoids. Proc. Natl. Acad. Sci. USA 2025, 122, e2416886122. [Google Scholar] [CrossRef]
- Renganathan, M.; Cummins, T.R.; Waxman, S.G. Contribution of Na 1.8 sodium channels to action potential electrogenesis in DRG neurons. J. Neurophysiol. 2001, 86, 629–640. [Google Scholar] [CrossRef]
- Rasband, M.N.; Park, E.W.; Vanderah, T.W.; Lai, J.; Porreca, F.; Trimmer, J.S. Distinct potassium channels on pain-sensing neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 13373–13378. [Google Scholar] [CrossRef] [PubMed]
- Sabbadini, M.; Yost, C.S. Molecular Biology of Background K Channels: Insights from K2P Knockout Mice. J. Mol. Biol. 2009, 385, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Alessandri-Haber, N.; Dina, O.A.; Chen, X.J.; Levine, J.D. TRPC1 and TRPC6 Channels Cooperate with TRPV4 to Mediate Mechanical Hyperalgesia and Nociceptor Sensitization. J. Neurosci. 2009, 29, 6217–6228. [Google Scholar] [CrossRef]
- Staruschenko, A.; Jeske, N.A.; Akopian, A.N. Contribution of TRPV1-TRPA1 Interaction to the Single Channel Properties of the TRPA1 Channel. J. Biol. Chem. 2010, 285, 15167–15177. [Google Scholar] [CrossRef]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Memon, T.; Chase, K.; Leavitt, L.S.; Olivera, B.M.; Teichert, R.W. Trpa1 Expression Levels and Excitability Brake by K Channels Influence Cold Sensitivity of Trpa1-Expressing Neurons. Neuroscience 2017, 353, 76–86. [Google Scholar] [CrossRef]
- Karashima, Y.; Talavera, K.; Everaerts, W.; Janssens, A.; Kwan, K.Y.; Vennekens, R.; Nilius, B.; Voets, T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 1273–1278. [Google Scholar] [CrossRef]
- del Camino, D.; Murphy, S.; Heiry, M.; Barrett, L.B.; Earley, T.J.; Cook, C.A.; Petrus, M.J.; Zhao, M.; D’Amours, M.; Deering, N.; et al. TRPA1 Contributes to Cold Hypersensitivity. J. Neurosci. 2010, 30, 15165–15174. [Google Scholar] [CrossRef]
- Munns, C.; AlQatari, M.; Koltzenburg, M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium 2007, 41, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Oto, T.; Urata, K.; Hayashi, Y.; Hitomi, S.; Shibuta, I.; Iwata, K.; Iinuma, T.; Shinoda, M. Age-Related Differences in Transient Receptor Potential Vanilloid 1 and 2 Expression Patterns in the Trigeminal Ganglion Neurons Contribute to Changes in the Palatal Mucosal Heat Pain Sensitivity. Tohoku J. Exp. Med. 2022, 256, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Frutos-Rincón, L.; Luna, C.; Aleixandre-Carrera, F.; Velasco, E.; Diaz-Tahoces, A.; Meseguer, V.; Gallar, J.; Acosta, M.C. The Contribution of TRPA1 to Corneal Thermosensitivity and Blink Regulation in Young and Aged Mice. Int. J. Mol. Sci. 2023, 24, 12620. [Google Scholar] [CrossRef] [PubMed]
- Alcalde, I.; Iñigo-Portugués, A.; González-González, O.; Almaraz, L.; Artime, E.; Morenilla-Palao, C.; Gallar, J.; Viana, F.; Merayo-Lloves, J.; Belmonte, C. Morphological and functional changes in TRPM8-expressing corneal cold thermoreceptor neurons during aging and their impact on tearing in mice. J. Comp. Neurol. 2018, 526, 1859–1874. [Google Scholar] [CrossRef]
- Méndez-Reséndiz, K.A.; Enciso-Pablo, O.; González-Ramírez, R.; Juárez-Contreras, R.; Rosenbaum, T.; Morales-Lázaro, S.L. Steroids and TRP Channels: A Close Relationship. Int. J. Mol. Sci. 2020, 21, 3819. [Google Scholar] [CrossRef]
- Goswami, C.; Kuhn, J.; Dina, O.A.; Fernández-Ballester, G.; Levine, J.D.; Ferrer-Montiel, A.; Hucho, T. Estrogen destabilizes microtubules through an ion-conductivity-independent TRPV1 pathway. J. Neurochem. 2011, 117, 995–1008. [Google Scholar] [CrossRef]
- Seol, S.H.; Chung, G. Review article Estrogen-dependent regulation of transient receptor potential vanilloid 1 (TRPV1) and P2X purinoceptor 3 (P2X3): Implication in burning mouth syndrome. J. Dent. Sci. 2022, 17, 8–13. [Google Scholar] [CrossRef]
- Alarcón-Alarcón, D.; Cabañero, D.; De Andrés-López, J.; Nikolaeva-Koleva, M.; Giorgi, S.; Fernández-Ballester, G.; Fernández-Carvajal, A.; Ferrer-Montiel, A. TRPM8 contributes to sex dimorphism by promoting recovery of normal sensitivity in a mouse model of chronic migraine. Nat. Commun. 2022, 13, 6304. [Google Scholar] [CrossRef]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M.T. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- Yang, J.M.; Wei, E.T.; Kim, S.J.; Yoon, K.C. TRPM8 Channels and Dry Eye. Pharmaceuticals 2018, 11, 125. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Santhiago, M.R. Corneal nerves anatomy, function, injury and regeneration. Exp. Eye Res. 2020, 200, 108243. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Hamrah, P. Understanding Neuropathic Corneal Pain—Gaps and Current Therapeutic Approaches. Semin. Ophthalmol. 2016, 31, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Acosta, M.C.; Gallar, J. Neural basis of sensation in intact and injured corneas. Exp. Eye Res. 2004, 78, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, M.H.; Dana, R. Corneal Nerve Alterations in Dry Eye-associated Ocular Surface Disease. Int. Ophthalmol. Clin. 2009, 49, 11. [Google Scholar] [CrossRef]
- Fang, W.; Lin, Z.-X.; Yang, H.-Q.; Zhao, L.; Liu, D.-C.; Pan, Z.-Q. Changes in corneal nerve morphology and function in patients with dry eyes having type 2 diabetes. World J. Clin. Cases 2022, 10, 3014–3026. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Dohlman, T.H.; Amparo, F.; Arnoldner, M.A.; Jamali, A.; Hamrah, P.; Dana, R. Effects of Corneal Nerve Density on the Response to Treatment in Dry Eye Disease. Ophthalmology 2015, 122, 662–668. [Google Scholar] [CrossRef]
- Dieckmann, G.; Borsook, D.; Moulton, E. Neuropathic corneal pain and dry eye: A continuum of nociception. Br. J. Ophthalmol. 2021, 106, 1039–1043. [Google Scholar] [CrossRef]
- Li, F.; Yang, W.; Jiang, H.; Guo, C.; Huang, A.J.W.; Hu, H.; Liu, Q. TRPV1 activity and substance P release are required for corneal cold nociception. Nat. Commun. 2019, 10, 5678. [Google Scholar] [CrossRef]
- Pizzano, M.; Vereertbrugghen, A.; Cernutto, A.; Sabbione, F.; Keitelman, I.A.; Shiromizu, C.M.; Aguilar, D.V.; Fuentes, F.; Giordano, M.N.; Trevani, A.S.; et al. Transient Receptor Potential Vanilloid-1 Channels Facilitate Axonal Degeneration of Corneal Sensory Nerves in Dry Eye. Am. J. Pathol. 2024, 194, 810–827. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Mikalauskiene, L.; Grzybowski, A.; Zemaitiene, R. Ocular Surface Changes Associated with Ophthalmic Surgery. J. Clin. Med. 2021, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Kaur, M.; Sharma, N.; Titiyal, J.S. Refractive surgery and dry eye-An update. Indian J. Ophthalmol. 2023, 71, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Linna, T.U.; Pérez-santonja, J.J.; Tervo, K.M.; Sakla, H.F.; AliÓ y Sanz, J.L.; Tervo, T.M.T. Recovery of Corneal Nerve Morphology Following Laser in situ Keratomileusis. Exp. Eye Res. 1998, 66, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, J.A.O.; Holopainen, J.M.; Vesaluoma, M.H.; Tervo, T.M.T. Corneal recovery after lasik for high myopia: A 2-year prospective confocal microscopic study. Br. J. Ophthalmol. 2008, 92, 1397–1402. [Google Scholar] [CrossRef]
- Erie, J.C.; McLaren, J.W.; Hodge, D.O.; Bourne, W.M. Recovery of Corneal Subbasal Nerve Density After PRK and LASIK. Am. J. Ophthalmol. 2005, 140, 1059–1064.e1. [Google Scholar] [CrossRef]
- Stachs, O.; Zhivov, A.; Kraak, R.; Hovakimyan, M.; Wree, A.; Guthoff, R. Structural-functional Correlations of Corneal Innervation After LASIK and Penetrating Keratoplasty. J. Refract. Surg. 2010, 26, 159–167. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, M.; Cañadas, P.; Gros-Otero, J.; Rodriguez-Perez, I.; Cañones-Zafra, R.; Kozobolis, V.; Teus, M.A. Long-term corneal subbasal nerve plexus regeneration after laser in situ keratomileusis. J. Cataract Refract. Surg. 2019, 45, 966. [Google Scholar] [CrossRef]
- Bragheeth, M.A.; Dua, H.S. Corneal sensation after myopic and hyperopic LASIK: Clinical and confocal microscopic study. Br. J. Ophthalmol. 2005, 89, 580–585. [Google Scholar] [CrossRef]
- Chao, C.; Lum, E.; Golebiowski, B.; Stapleton, F. Alteration of the pattern of regenerative corneal subbasal nerves after laser in-situ keratomileusis surgery. Ophthalmic Physiol. Opt. 2020, 40, 577–583. [Google Scholar] [CrossRef]
- Moshirfar, M.; Bhavsar, U.M.; Durnford, K.M.; McCabe, S.E.; Ronquillo, Y.C.; Lewis, A.L.; Hoopes, P.C. Neuropathic Corneal Pain Following LASIK Surgery: A Retrospective Case Series. Ophthalmol. Ther. 2021, 10, 677–689. [Google Scholar] [CrossRef]
- Denoyer, A.; Landman, E.; Trinh, L.; Faure, J.-F.; Auclin, F.; Baudouin, C. Dry Eye Disease after Refractive Surgery: Comparative Outcomes of Small Incision Lenticule Extraction versus LASIK. Ophthalmology 2015, 122, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.; Hertzog, L.; Garbus, J.J.; McDonnell, P.J. Corneal Sensitivity After Photorefractive Keratectomy. Am. J. Ophthalmol. 1992, 114, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Nejima, R.; Miyata, K.; Tanabe, T.; Okamoto, F.; Hiraoka, T.; Kiuchi, T.; Oshika, T. Corneal barrier function, tear film stability, and corneal sensation after photorefractive keratectomy and laser in situ keratomileusis. Am. J. Ophthalmol. 2005, 139, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, J.A.O.; Vesaluoma, M.H.; Müller, L.J.; Tervo, T.M.T. Long-Term Corneal Morphology after PRK by In Vivo Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1064–1069. [Google Scholar] [CrossRef][Green Version]
- Woreta, F.A.; Gupta, A.; Hochstetler, B.; Bower, K.S. Management of post-photorefractive keratectomy pain. Surv. Ophthalmol. 2013, 58, 529–535. [Google Scholar] [CrossRef]
- Slade, S.G.; Durrie, D.S.; Binder, P.S. A Prospective, Contralateral Eye Study Comparing Thin-Flap LASIK (Sub-Bowman Keratomileusis) with Photorefractive Keratectomy. Ophthalmology 2009, 116, 1075–1082. [Google Scholar] [CrossRef]
- Garcia, R.; de Andrade, D.C.; Teixeira, M.J.; Nozaki, S.S.; Bechara, S.J. Mechanisms of Corneal Pain and Implications for Postoperative Pain After Laser Correction of Refractive Errors. Clin. J. Pain 2016, 32, 450–458. [Google Scholar] [CrossRef]
- Gallar, J.; Acosta, M.C.; Gutiérrez, A.R.; Belmonte, C. Impulse activity in corneal sensory nerve fibers after photorefractive keratectomy. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4033–4037. [Google Scholar] [CrossRef]
- Recchioni, A.; Sisó-Fuertes, I.; Hartwig, A.; Hamid, A.; Shortt, A.J.; Morris, R.; Vaswani, S.; Dermott, J.; Cerviño, A.; Wolffsohn, J.S.; et al. Short-Term Impact of FS-LASIK and SMILE on Dry Eye Metrics and Corneal Nerve Morphology. Cornea 2020, 39, 851–857. [Google Scholar] [CrossRef]
- Demirok, A.; Ozgurhan, E.B.; Agca, A.; Kara, N.; Bozkurt, E.; Cankaya, K.I.; Yilmaz, O.F. Corneal Sensation After Corneal Refractive Surgery with Small Incision Lenticule Extraction. Optom. Vis. Sci. 2013, 90, 1040. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, J.; Wu, J.; Yu, K. Comparison of Corneal Biological Healing After Femtosecond LASIK and Small Incision Lenticule Extraction Procedure. Curr. Eye Res. 2016, 41, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Golebiowski, B.; Stapleton, F. The Role of Corneal Innervation in LASIK-Induced Neuropathic Dry Eye. Ocul. Surf. 2014, 12, 32–45. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Seyed-Razavi, Y.; Ghezzi, C.E.; Dieckmann, G.; Nieland, T.J.F.; Cairns, D.M.; Pollard, R.E.; Hamrah, P.; Kaplan, D.L. Corneal pain and experimental model development. Prog. Retin. Eye Res. 2019, 71, 88–113. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.T.; Abedi, F.; Cruzat, A.; Witkin, D.; Baniasadi, N.; Cavalcanti, B.M.; Jamali, A.; Chodosh, J.; Dana, R.; Pavan-Langston, D.; et al. Degeneration and Regeneration of Subbasal Corneal Nerves after Infectious Keratitis: A Longitudinal In Vivo Confocal Microscopy Study. Ophthalmology 2015, 122, 2200–2209. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Massaro-Giordano, G.; Nubile, M.; Sacchetti, M. Understanding the Pathogenesis of Neurotrophic Keratitis: The Role of Corneal Nerves. J. Cell. Physiol. 2017, 232, 717–724. [Google Scholar] [CrossRef]
- Lambiase, A.; Sacchetti, M.; Mastropasqua, A.; Bonini, S. Corneal Changes in Neurosurgically Induced Neurotrophic Keratitis. JAMA Ophthalmol. 2013, 131, 1547–1553. [Google Scholar] [CrossRef]
- Acosta, M.C.; Luna, C.; Quirce, S.; Belmonte, C.; Gallar, J. Corneal Sensory Nerve Activity in an Experimental Model of UV Keratitis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3403–3412. [Google Scholar] [CrossRef]
- Sacchetti, M.; Lambiase, A. Neurotrophic factors and corneal nerve regeneration. Neural Regen. Res. 2017, 12, 1220–1224. [Google Scholar]
- Kowtharapu, B.S.; Stachs, O. Corneal Cells: Fine-tuning Nerve Regeneration. Curr. Eye Res. 2020, 45, 291–302. [Google Scholar] [CrossRef]
- Yam, G.H.F.; Williams, G.P.; Fuest, M.; Lee, X.W.; Zhou, L.; Mehta, J. Nerve regeneration by human corneal stromal keratocytes (CSKs) and stromal fibroblasts (SFs). Investig. Ophthalmol. Vis. Sci. 2017, 58, 1173. [Google Scholar]
- Pham, T.L.; Bazan, H.E.P. Docosanoid signaling modulates corneal nerve regeneration: Effect on tear secretion, wound healing, and neuropathic pain. J. Lipid Res. 2021, 62, 100033. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Ivanusic, J.J.; McMenamin, P.G.; Chinnery, H.R. Distribution of Corneal TRPV1 and Its Association With Immune Cells During Homeostasis and Injury. Investig. Ophthalmol. Vis. Sci. 2021, 62, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, S.Y.; Yu, R.X.; Chen, X.W.; Li, F.Y.; Sun, X.; Xu, P.Y.; Huang, Y.J.; Xue, Y.X.; Fu, T.; et al. TRPV1+ sensory nerves modulate corneal inflammation after epithelial abrasion via RAMP1 and SSTR5 signaling. Mucosal Immunol. 2022, 15, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Yuan, X.-b. TRP Channels and Axon Pathfinding. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Li, Y.; Jia, Y.-C.; Cui, K.; Li, N.; Zheng, Z.-Y.; Wang, Y.-Z.; Yuan, X.-B. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 2005, 434, 894–898. [Google Scholar] [CrossRef]
- Wang, G.X.; Poo, M.-M. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature 2005, 434, 898–904. [Google Scholar] [CrossRef]
- Shim, S.; Goh, E.L.; Ge, S.; Sailor, K.; Yuan, J.P.; Roderick, H.L.; Bootman, M.D.; Worley, P.F.; Song, H.; Ming, G.-l. XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat. Neurosci. 2005, 8, 730–735. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Verri, W.A.; Chiu, I.M. Nociceptor Sensory Neuron–Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef]
- Shinoda, M.; Kubo, A.; Hayashi, Y.; Iwata, K. Peripheral and Central Mechanisms of Persistent Orofacial Pain. Front. Neurosci. 2019, 13, 1227. [Google Scholar] [CrossRef]
- Ye, Y.; Salvo, E.; Romero-Reyes, M.; Akerman, S.; Shimizu, E.; Kobayashi, Y.; Michot, B.; Gibbs, J. Glia and Orofacial Pain: Progress and Future Directions. Int. J. Mol. Sci. 2021, 22, 5345. [Google Scholar] [CrossRef]
- Constantin, C.E.; Mair, N.; Sailer, C.A.; Andratsch, M.; Xu, Z.Z.; Blumer, M.J.; Scherbakov, N.; Davis, J.B.; Bluethmann, H.; Ji, R.R.; et al. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J. Neurosci. 2008, 28, 5072–5081. [Google Scholar] [CrossRef]
- Puri, S.; Kenyon, B.M.; Hamrah, P. Immunomodulatory Role of Neuropeptides in the Cornea. Biomedicines 2022, 10, 1985. [Google Scholar] [CrossRef] [PubMed]
- Ro, L.-S.; Chen, S.-T.; Tang, L.-M.; Jacobs, J.M. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain 1999, 79, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Eskander, M.A.; Ruparel, S.; Green, D.P.; Chen, P.B.; Por, E.D.; Jeske, N.A.; Gao, X.; Flores, E.R.; Hargreaves, K.M. Persistent Nociception Triggered by Nerve Growth Factor (NGF) Is Mediated by TRPV1 and Oxidative Mechanisms. J. Neurosci. 2015, 35, 8593–8603. [Google Scholar] [CrossRef]
- Ji, R.R.; Samad, T.A.; Jin, S.X.; Schmoll, R.; Woolf, C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Allchorne, A.; Safieh-Garabedian, B.; Poole, S. Cytokines, nerve growth factor and inflammatory hyperalgesia: The contribution of tumour necrosis factor α. Br. J. Pharmacol. 1997, 121, 417–424. [Google Scholar] [CrossRef]
- Fortingo, N.; Melnyk, S.; Sutton, S.H.; Watsky, M.A.; Bollag, W.B. Innate Immune System Activation, Inflammation and Corneal Wound Healing. Int. J. Mol. Sci. 2022, 23, 14933. [Google Scholar] [CrossRef]
- Simeoli, R.; Montague, K.; Jones, H.R.; Castaldi, L.; Chambers, D.; Kelleher, J.H.; Vacca, V.; Pitcher, T.; Grist, J.; Al-Ahdal, H.; et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017, 8, 1778. [Google Scholar] [CrossRef]
- Strickland, I.T.; Richards, L.; Holmes, F.E.; Wynick, D.; Uney, J.B.; Wong, L.-F. Axotomy-Induced miR-21 Promotes Axon Growth in Adult Dorsal Root Ganglion Neurons. PLoS ONE 2011, 6, e23423. [Google Scholar] [CrossRef]
- Malcangio, M. Role of the immune system in neuropathic pain. Scand. J. Pain 2019, 20, 33–37. [Google Scholar] [CrossRef]
- Dong, C.L.; Ubogu, E.E. Pro-inflammatory cytokines and leukocyte integrins associated with chronic neuropathic pain in traumatic and inflammatory neuropathies: Initial observations and hypotheses. Front. Immunol. 2022, 13, 935306. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.L.; Hill, L.J.; Downie, L.E.; Chinnery, H.R. Neuroimmune crosstalk in the cornea: The role of immune cells in corneal nerve maintenance during homeostasis and inflammation. Prog. Retin. Eye Res. 2022, 91, 101105. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Burns, A.R.; Han, L.; Rumbaut, R.E.; Smith, C.W. IL-17 and VEGF are necessary for efficient corneal nerveregeneration. Am. J. Pathol. 2011, 178, 1106–1116. [Google Scholar] [CrossRef]
- Murata, Y.; Masuko, S. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res. 2006, 1085, 87–94. [Google Scholar] [CrossRef]
- Milligan, E.D.; Sloane, E.M.; Watkins, L.R. Glia in pathological pain: A role for fractalkine. J. Neuroimmunol. 2008, 198, 113–120. [Google Scholar] [CrossRef]
- Maruyama, K. Senso-immunology: Crosstalk between nociceptive and immune systems. Febs J. 2022, 289, 4132–4145. [Google Scholar] [CrossRef]
- Bonelli, F.; Demirsoy, I.; Lasagni Vitar, R.M.; Fonteyne, P.; Ferrari, G. Topical formulations of Aprepitant are safe and effective in relieving pain and inflammation, and drive neural regeneration. Ocul. Surf. 2023, 30, 92–103. [Google Scholar] [CrossRef]
- D’Arcy, Y.; Mantyh, P.; Yaksh, T.; Donevan, S.; Hall, J.; Sadrarhami, M.; Viktrup, L. Treating osteoarthritis pain: Mechanisms of action of acetaminophen, nonsteroidal anti-inflammatory drugs, opioids, and nerve growth factor antibodies. Postgrad. Med. 2021, 133, 879–894. [Google Scholar] [CrossRef]
- Jayakar, S.; Shim, J.; Jo, S.; Bean, B.P.; Singeç, I.; Woolf, C.J. Developing nociceptor-selective treatments for acute and chronic pain. Sci. Transl. Med. 2021, 13, eabj9837. [Google Scholar] [CrossRef]
- Chaudhary, S.; Ghimire, D.; Basu, S.; Agrawal, V.; Jacobs, D.S.; Shanbhag, S.S. Contact lenses in dry eye disease and associated ocular surface disorders. Indian J. Ophthalmol. 2023, 71, 1142–1153. [Google Scholar] [CrossRef]
- Jacobs, D.S. Diagnosis and Treatment of Ocular Pain: The Ophthalmologist’s Perspective. Curr. Ophthalmol. Rep. 2017, 5, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Roucaute, E.; Huertas-Bello, M.; Sabater, A.L. Novel treatments for dry eye syndrome. Curr. Opin. Pharmacol. 2024, 75, 102431. [Google Scholar] [CrossRef] [PubMed]
- Delicado-Miralles, M.; Velasco, E.; Díaz-Tahoces, A.; Gallar, J.; Acosta, M.C.; Aracil-Marco, A. Deciphering the Action of Perfluorohexyloctane Eye Drops to Reduce Ocular Discomfort and Pain. Front. Med. 2021, 8, 709712. [Google Scholar] [CrossRef] [PubMed]
- Protzko, E.E.; Segal, B.A.; Korenfeld, M.S.; Krösser, S.; Vittitow, J.L. Long-Term Safety and Efficacy of Perfluorohexyloctane Ophthalmic Solution for the Treatment of Patients With Dry Eye Disease: The KALAHARI Study. Cornea 2023, 43, 1100–1107. [Google Scholar] [CrossRef]
- Hua, X.; Deng, R.; Li, J.; Chi, W.; Su, Z.; Lin, J.; Pflugfelder, S.C.; Li, D.-Q. Protective Effects of L-Carnitine Against Oxidative Injury by Hyperosmolarity in Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5503. [Google Scholar] [CrossRef]
- Kaercher, T.; Thelen, U.; Brief, G.; Morgan-Warren, R.; Leaback, R. A prospective, multicenter, noninterventional study of Optive Plus® in the treatment of patients with dry eye: The prolipid study. Clin. Ophthalmol. 2014, 8, 1147–1155. [Google Scholar]
- Mogi, M.; Mendonza, A.E.; Chastain, J.; Demirs, J.T.; Medley, Q.G.; Zhang, Q.; Papillon, J.P.N.; Yang, J.; Gao, Y.; Xu, Y.; et al. Ocular Pharmacology and Toxicology of TRPV1 Antagonist SAF312 (Libvatrep). Trans. Vis. Sci. Technol. 2023, 12, 5. [Google Scholar] [CrossRef]
- Bai, J.; Liu, F.; Wu, L.-F.; Wang, Y.-F.; Li, X.-Q. Attenuation of TRPV1 by AMG-517 after nerve injury promotes peripheral axonal regeneration in rats. Mol. Pain 2018, 14, 1744806918777614. [Google Scholar] [CrossRef]
- Fakih, D.; Guerrero-Moreno, A.; Baudouin, C.; Réaux-Le Goazigo, A.; Parsadaniantz, S.M. Capsazepine decreases corneal pain syndrome in severe dry eye disease. J. Neuroinflamm. 2021, 18, 111. [Google Scholar] [CrossRef]
- Yang, M.; Jung, S.; Sethi, G.; Ahn, K. Pleiotropic Pharmacological Actions of Capsazepine, a Synthetic Analogue of Capsaicin, against Various Cancers and Inflammatory Diseases. Molecules 2019, 24, 995. [Google Scholar] [CrossRef]
- Ang, S.-F.; Moochhala, S.M.; MacAry, P.A.; Bhatia, M. Hydrogen Sulfide and Neurogenic Inflammation in Polymicrobial Sepsis: Involvement of Substance P and ERK-NF-κB Signaling. PLoS ONE 2011, 6, e24535. [Google Scholar] [CrossRef] [PubMed]
- Lucius, A.; Chhatwal, S.; Valtink, M.; Reinach, P.S.; Li, A.; Pleyer, U.; Mergler, S. L-Carnitine Suppresses Transient Receptor Potential Vanilloid Type 1 Activation in Human Corneal Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 11815. [Google Scholar] [CrossRef]
- Fakih, D.; Baudouin, C.; Réaux-Le Goazigo, A.; Mélik Parsadaniantz, S. TRPM8: A Therapeutic Target for Neuroinflammatory Symptoms Induced by Severe Dry Eye Disease. Int. J. Mol. Sci. 2020, 21, 8756. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-F.; Zhang, Y.; Rittenhouse, K.D.; Pickering, E.H.; McDowell, M.T. Evaluations of Tear Protein Markers in Dry Eye Disease: Repeatability of Measurement and Correlation with Disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4556. [Google Scholar] [CrossRef] [PubMed]
- De Caro, C.; Russo, R.; Avagliano, C.; Cristiano, C.; Calignano, A.; Aramini, A.; Bianchini, G.; Allegretti, M.; Brandolini, L. Antinociceptive effect of two novel transient receptor potential melastatin 8 antagonists in acute and chronic pain models in rat. Br. J. Pharmacol. 2018, 175, 1691–1706. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, J.; Yang, J.M.; Wei, E.T.; Kim, S.J.; Yoon, K.C. Topical TRPM8 Agonist for Relieving Neuropathic Ocular Pain in Patients with Dry Eye: A Pilot Study. J. Clin. Med. 2021, 10, 250. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Q.; Deng, P.; Yang, J.; Yang, L.; Lin, M.; Yu, Z.; Zhou, Z. Critical role of TRPC1 in thyroid hormone-dependent dopaminergic neuron development. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 1900–1912. [Google Scholar] [CrossRef]
- Bonini, S.; Lambiase, A.; Rama, P.; Caprioglio, G.; Aloe, L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology 2000, 107, 1347–1351. [Google Scholar] [CrossRef]
- Sarkar, J.; Chaudhary, S.; Jassim, S.H.; Ozturk, O.; Chamon, W.; Ganesh, B.; Tibrewal, S.; Gandhi, S.; Byun, Y.S.; Hallak, J.; et al. CD11b+GR1+Myeloid Cells Secrete NGF and Promote Trigeminal Ganglion Neurite Growth: Implications for Corneal Nerve Regeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5920–5936. [Google Scholar] [CrossRef]
- Marlin, M.C.; Li, G.P. Biogenesis and Function of the NGF/TrkA Signaling Endosome. Int. Rev. Cell Mol. Biol. 2015, 314, 239–257. [Google Scholar]
- Liu, D.; Li, X.; Zhang, L.; Hu, B.; Hu, S.; Zhang, X.; Hu, J. Small molecule inhibitors of osteoarthritis: Current development and future perspective. Front. Physiol. 2023, 14, 1156913. [Google Scholar] [CrossRef] [PubMed]
- Clewes, O.; Fahey, M.S.; Tyler, S.J.; Watson, J.J.; Seok, H.; Catania, C.; Cho, K.; Dawbarn, D.; Allen, S.J. Human ProNGF: Biological effects and binding profiles at TrkA, P75 and sortilin. J. Neurochem. 2008, 107, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Nykjaer, A.; Lee, R.; Teng, K.K.; Jansen, P.; Madsen, P.; Nielsen, M.S.; Jacobsen, C.; Kliemannel, M.; Schwarz, E.; Willnow, T.E.; et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004, 427, 843–848. [Google Scholar] [CrossRef]
- Krupka, E.; Jiang, G.L.; Jan, C. Efficacy and safety of intra-articular injection of tropomyosin receptor kinase A inhibitor in painful knee osteoarthritis: A randomized, double-blind and placebo-controlled study. Osteoarthr. Cartil. 2019, 27, 1599–1607. [Google Scholar] [CrossRef]
- Barker, P.A.; Mantyh, P.; Arendt-Nielsen, L.; Viktrup, L.; Tive, L. Nerve Growth Factor Signaling and Its Contribution to Pain. J. Pain Res. 2020, 13, 1223–1241. [Google Scholar] [CrossRef]
- Watt, F.E.; Blauwet, M.B.; Fakhoury, A.; Jacobs, H.; Smulders, R.; Lane, N.E. Tropomyosin-related kinase A (TrkA) inhibition for the treatment of painful knee osteoarthritis: Results from a randomized controlled phase 2a trial. Osteoarthr. Cartil. 2019, 27, 1590–1598. [Google Scholar] [CrossRef]
- Asiedu, K. Neurophysiology of corneal neuropathic pain and emerging pharmacotherapeutics. J. Neurosci. Res. 2024, 102, e25285. [Google Scholar] [CrossRef]
- Joubert, F.; Guerrero-Moreno, A.; Fakih, D.; Reboussin, E.; Gaveriaux-Ruff, C.; Acosta, M.C.; Gallar, J.; Sahel, J.A.; Bodineau, L.; Baudouin, C.; et al. Topical treatment with a mu opioid receptor agonist alleviates corneal allodynia and corneal nerve sensitization in mice. Biomed. Pharmacother. 2020, 132, 110794. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome REPLY. N. Engl. J. Med. 2017, 377, 699–700. [Google Scholar]
- Wong, H.; Cairns, B.E. Cannabidiol, cannabinol and their combinations act as peripheral analgesics in a rat model of myofascial pain. Arch. Oral Biol. 2019, 104, 33–39. [Google Scholar] [CrossRef]
- Huang, J.; Fan, X.; Jin, X.Q.; Jo, S.; Zhang, H.B.; Fujita, A.; Bean, B.P.; Yan, N. Cannabidiol inhibits Nav channels through two distinct binding sites. Nat. Commun. 2023, 14, 3613. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.R.; Dib-Hajj, S.D.; Goodchild, S.J.; Ruben, P.C.; Waxman, S.G. Non-psychotropic phytocannabinoid interactions with voltage-gated sodium channels: An update on cannabidiol and cannabigerol. Front. Physiol. 2022, 13, 1066455. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lopez, C.; Gomez-Huertas, C.; Sanchez-Gonzalez, J.M.; Borroni, D.; Rodriguez-Calvo-de-Mora, M.; Romano, V.; Rachwani-Anil, R.; Ramos-Lopez, J.F.; Ortiz-Perez, S.; Rocha-de-Lossada, C. Opioids and Ocular Surface Pathology: A Literature Review of New Treatments Horizons. J. Clin. Med. 2022, 11, 1424. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Gu, L.Y.; Ruan, Y.L.; Gegen, T.; Yu, L.; Zhu, C.; Yang, Y.; Zhou, Y.; Yu, G.; Tang, Z.X. Pirt Together with TRPV1 Is Involved in the Regulation of Neuropathic Pain. Neural Plast. 2018, 2018, 4861491. [Google Scholar] [CrossRef]
- Carlin, D.; Halevi, A.E.; Ewan, E.E.; Moore, A.M.; Cavalli, V. Nociceptor Deletion of Tsc2 Enhances Axon Regeneration by Inducing a Conditioning Injury Response in Dorsal Root Ganglia. Eneuro 2019, 6, ENEURO.0168-19.2019. [Google Scholar] [CrossRef]
- Gong, Q.; Zhang, S.; Jiang, L.; Lin, M.; Xu, Z.; Yu, Y.; Wang, Q.; Lu, F.; Hu, L. The effect of nerve growth factor on corneal nerve regeneration and dry eye after LASIK. Exp. Eye Res. 2021, 203, 108428. [Google Scholar] [CrossRef]
- Pedrotti, E.; Bonacci, E.; Chierego, C.; De Gregorio, A.; Cozzini, T.; Brighenti, T.; Caldarella, G.; Pastore, G.; Fasolo, A.; Marchini, G. Eight months follow-up of corneal nerves and sensitivity after treatment with cenegermin for neurotrophic keratopathy. Orphanet J. Rare Dis. 2022, 17, 63. [Google Scholar] [CrossRef]
- Verkest, C.; Salinas, M.; Diochot, S.; Deval, E.; Lingueglia, E.; Baron, A. Mechanisms of Action of the Peptide Toxins Targeting Human and Rodent Acid-Sensing Ion Channels and Relevance to Their In Vivo Analgesic Effects. Toxins 2022, 14, 709. [Google Scholar] [CrossRef]
- Kung, C.-C.; Huang, Y.-C.; Hung, T.-Y.; Teng, C.-Y.; Lee, T.-Y.; Sun, W.-H. Deletion of Acid-Sensing Ion Channel 3 Relieves the Late Phase of Neuropathic Pain by Preventing Neuron Degeneration and Promoting Neuron Repair. Cells 2020, 9, 2355. [Google Scholar] [CrossRef]
- Dong, X.; Dong, X. Peripheral and Central Mechanisms of Itch. Neuron 2018, 98, 482–494. [Google Scholar] [CrossRef]
- Nativi, C.; Gualdani, R.; Dragoni, E.; Di Cesare Mannelli, L.; Sostegni, S.; Norcini, M.; Gabrielli, G.; la Marca, G.; Richichi, B.; Francesconi, O.; et al. A TRPA1 antagonist reverts oxaliplatin-induced neuropathic pain. Sci. Rep. 2013, 3, 2005. [Google Scholar] [CrossRef] [PubMed]
- Marcotti, A.; Fernández-Trillo, J.; González, A.; Vizcaíno-Escoto, M.; Ros-Arlanzón, P.; Romero, L.; Vela, J.M.; Gomis, A.; Viana, F.; de la Peña, E. TRPA1 modulation by Sigma-1 receptor prevents oxaliplatin-induced painful peripheral neuropathy. Brain 2023, 146, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Gabrielle, M.; Yudin, Y.; Wang, Y.J.; Su, X.Y.; Rohacs, T. Phosphatidic acid is an endogenous negative regulator of PIEZO2 channels and mechanical sensitivity. Nat. Commun. 2024, 15, 7020. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-T.; Siregar, A.S.; Kim, E.-J.; Lee, E.-S.; Nyiramana, M.M.; Woo, M.S.; Hah, Y.-S.; Han, J.; Kang, D. Upregulation of TRESK Channels Contributes to Motor and Sensory Recovery after Spinal Cord Injury. Int. J. Mol. Sci. 2020, 21, 8997. [Google Scholar] [CrossRef]



| Associated Corneal Nerve Type | Neurotransmitter |
|---|---|
| Sensory nerves | Substance P (SP) |
| Calcitonin gene-related peptide (CGRP) | |
| Pituitary adenylate cyclase-activating peptide (PACAP) | |
| Galanin | |
| Excitatory amino acids (glutamate, aspartate) | |
| Sympathetic nerves | Norepinephrine |
| Neuropeptide Y | |
| Serotonin (5-HT) | |
| Parasympathetic nerves | Vasoactive inhibitory peptide |
| Met-enkephalin | |
| Neuropeptide Y | |
| Galanin | |
| Acetylcholine | |
| Undetermined | Cholecystokinin |
| Brain natriuretic peptide | |
| Vasopressin | |
| Neurotensin | |
| B-endorphin |
| Type | Therapeutic | Mechanism | Effect |
|---|---|---|---|
| Broad-acting | NSAID | COX inhibitor reduces PGE2 release | Decreases nerve sensitization in PNS and CNS |
| Acetaminophen | CNS | Decreases nerve sensitization | |
| Specific | Bandage contact lenses | Direct physical barrier against mechanical irritants | Increases corneal healing and prevents chronic nociceptive pain |
| Perfluorohexyl-octane (F6H8) | TRPM8 activator | Decreases nociceptive pain by improving dry eye symptoms; increases tear production and blinking rate | |
| Optive Plus artificial tears | TRPV1 antagonist containing L-carnitine | Decreases pain after 4 weeks |
| Stage | Therapeutic | Mechanism | Effect |
|---|---|---|---|
| Clinical trial—phase 2 | Anti-TrkA monoclonal antibodies | TrkA inhibitor | Decreases nociceptive pain and inflammation |
| Dual enkephalinase inhibitors | Inhibit enkephalin degradation, increasing opioid receptor binding | Reduces pain and inflammation | |
| Pre-clinical | TrkA inhibitor | Monoclonal antibodies binding TrkA | Decreases nociceptive pain and sensitization in PNS and CNS |
| SAF312, TRPV1 antagonist | TRPV1 selective, non-competitive antagonist, Ca2+ influx inhibitor | Inhibits Ca2+ influx into nociceptor cells, decreases inflammation | |
| Joint treatment with L-carnitine and capsazepine | TRPV1 antagonist, Ca2+ influx inhibitor | Reduces pain and discomfort | |
| Capsazepine, TRPV1, TRPV4, TRPM8 antagonist | Inhibits SP expression; Ca2+ influx inhibitor. | Inhibits Ca2+ influx into nociceptor cells, decreases corneal sensitization and inflammation | |
| TRPM8 ion channel antagonist | TRPM8 antagonist | Reduces inflammation | |
| DAMGO | Mu opioid receptor (MOR) ligand | Reduces responsiveness of nociceptors | |
| Aprepitant | Neurokinin 1 receptor (NK1R) antagonist | Decreases pain sensitivity and BDNF upregulation | |
| PIRT | Positive regulator of TRPV1 activity in nociceptive neurons | Influences pain perception, inflammation, and immune response; enhances nerve regeneration | |
| NGF | Promotes epithelial migration and proliferation | Improves would healing and nociceptor sensitivity | |
| Thy-1 YFP-positive myeloid-derived suppressor cells (MDSCs) | Producers of nerve growth factor (NGF) | Induces nociceptor growth | |
| Opioid growth factors | Analgesic | Supports wound healing and nociceptive sensitization |
| Channel | Role in Nerve Recovery | Model | Source |
|---|---|---|---|
| TRPV4 | Ablation of TRPV4 after nerve injury is related to the delay of nerve functional recovery | Mouse cell culture | [1] |
| TRPA1 | Blocking TRPA1 results in decreased neuropathic pain in rat models | Rat | [3] |
| ASIC3 | ASIC3 might improve tissue repair via a change in the M1: M2 macrophage ratio | Mouse | [4] |
| TRESK/TREK1 | Overexpression of TRESK leads to faster mice paralysis recovery and lower TNF-α in blood | Mouse | [5] |
| PIEZO2 | PIEZO2 is associated with mechanical allodynia after nerve injury | Mouse, human | [2,6] |
| PIRT | PIRT regulates TRPV1 with potential for nerve regeneration | Mouse | [7,8] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, R.; Spritz, S.; Zeng, A.Y.; Erukulla, R.; Zavala, D.; Merchant, T.; Gascon, A.; Jung, R.; Bigit, B.; Azar, D.T.; et al. Corneal Sensory Receptors and Pharmacological Therapies to Modulate Ocular Pain. Int. J. Mol. Sci. 2025, 26, 4663. https://doi.org/10.3390/ijms26104663
Park R, Spritz S, Zeng AY, Erukulla R, Zavala D, Merchant T, Gascon A, Jung R, Bigit B, Azar DT, et al. Corneal Sensory Receptors and Pharmacological Therapies to Modulate Ocular Pain. International Journal of Molecular Sciences. 2025; 26(10):4663. https://doi.org/10.3390/ijms26104663
Chicago/Turabian StylePark, Ryan, Samantha Spritz, Anne Y. Zeng, Rohith Erukulla, Deneb Zavala, Tasha Merchant, Andres Gascon, Rebecca Jung, Bianca Bigit, Dimitri T. Azar, and et al. 2025. "Corneal Sensory Receptors and Pharmacological Therapies to Modulate Ocular Pain" International Journal of Molecular Sciences 26, no. 10: 4663. https://doi.org/10.3390/ijms26104663
APA StylePark, R., Spritz, S., Zeng, A. Y., Erukulla, R., Zavala, D., Merchant, T., Gascon, A., Jung, R., Bigit, B., Azar, D. T., Chang, J.-H., Jalilian, E., Djalilian, A. R., Guaiquil, V. H., & Rosenblatt, M. I. (2025). Corneal Sensory Receptors and Pharmacological Therapies to Modulate Ocular Pain. International Journal of Molecular Sciences, 26(10), 4663. https://doi.org/10.3390/ijms26104663







