The Current Understanding of the Molecular Pathogenesis of Papillary Thyroid Cancer
Abstract
1. The Thyroid Gland: Anatomy and Function
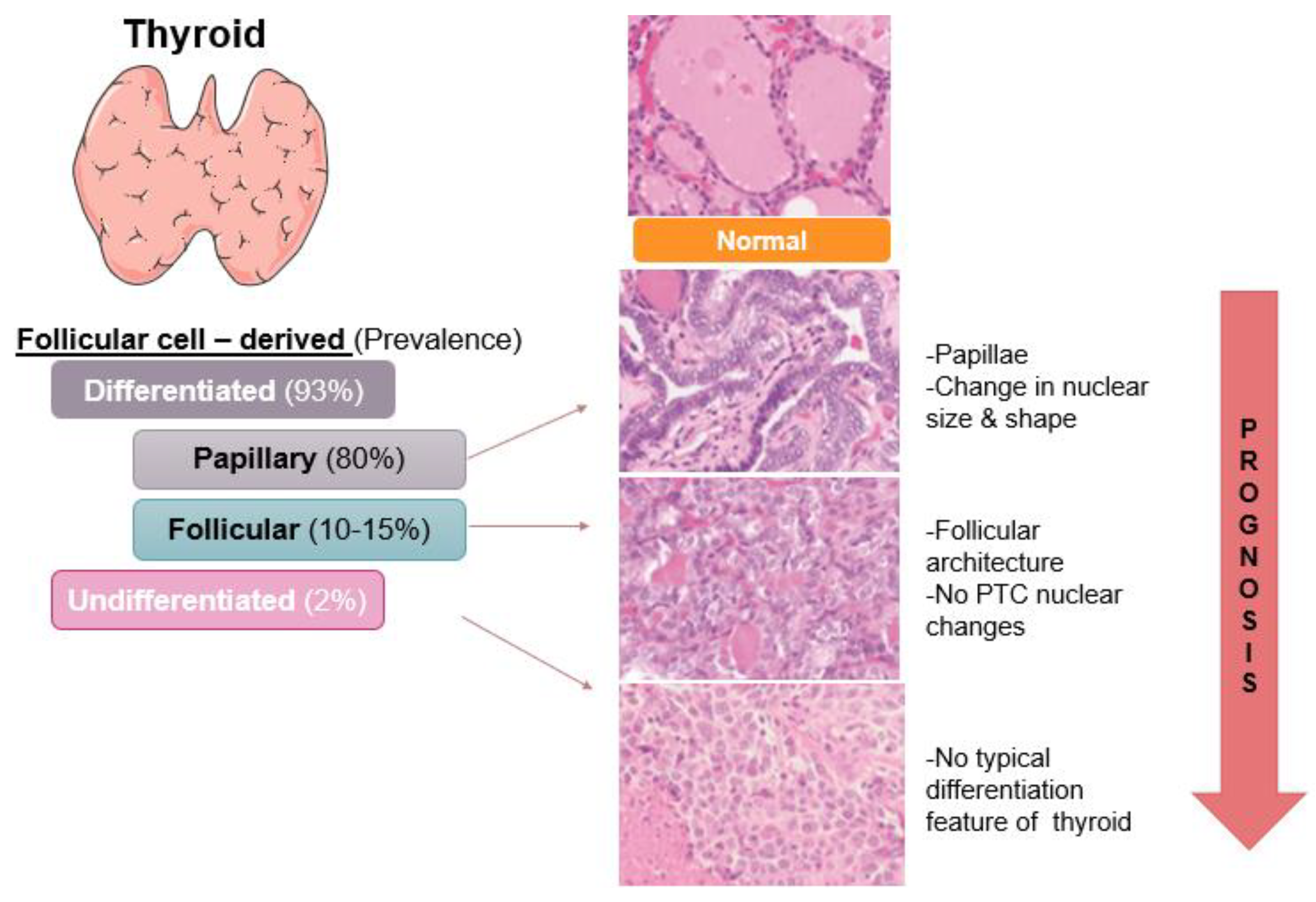
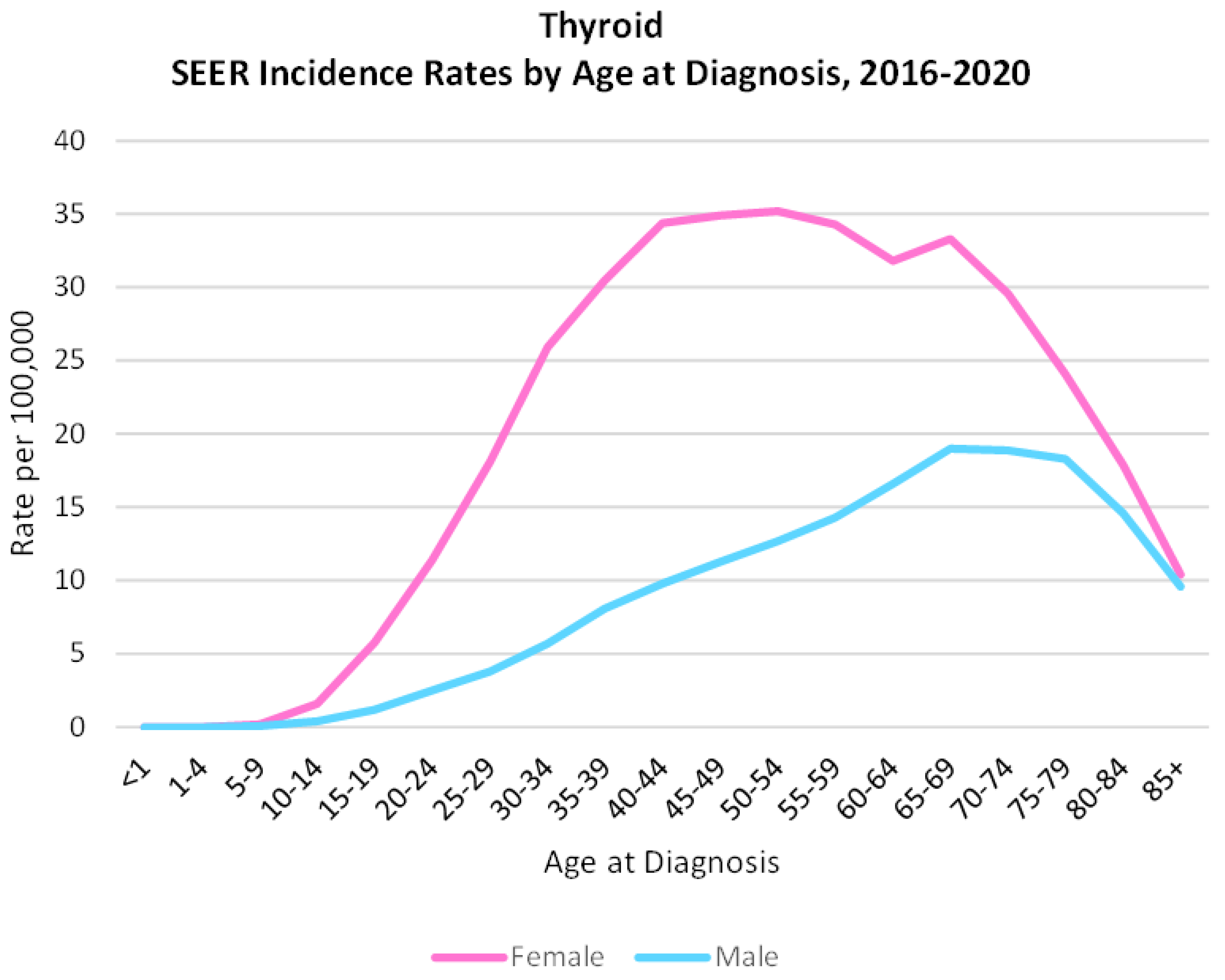
2. Thyroid Cancer: Incidence and Subtypes
3. Papillary Thyroid Cancer
4. Diagnosis, Prognosis, Treatment, and Recurrence
4.1. Tumor
4.2. Node
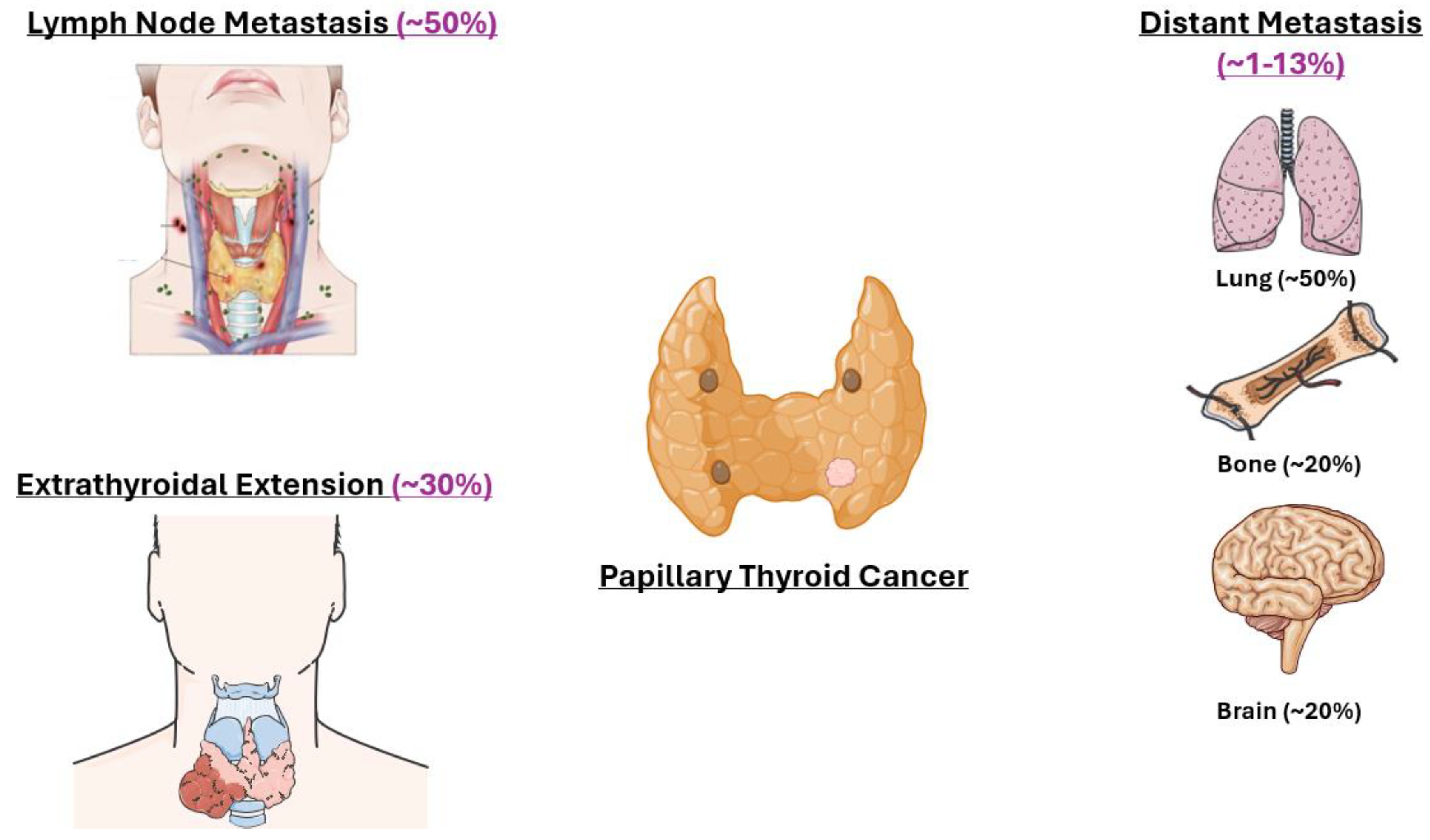
4.3. Metastasis
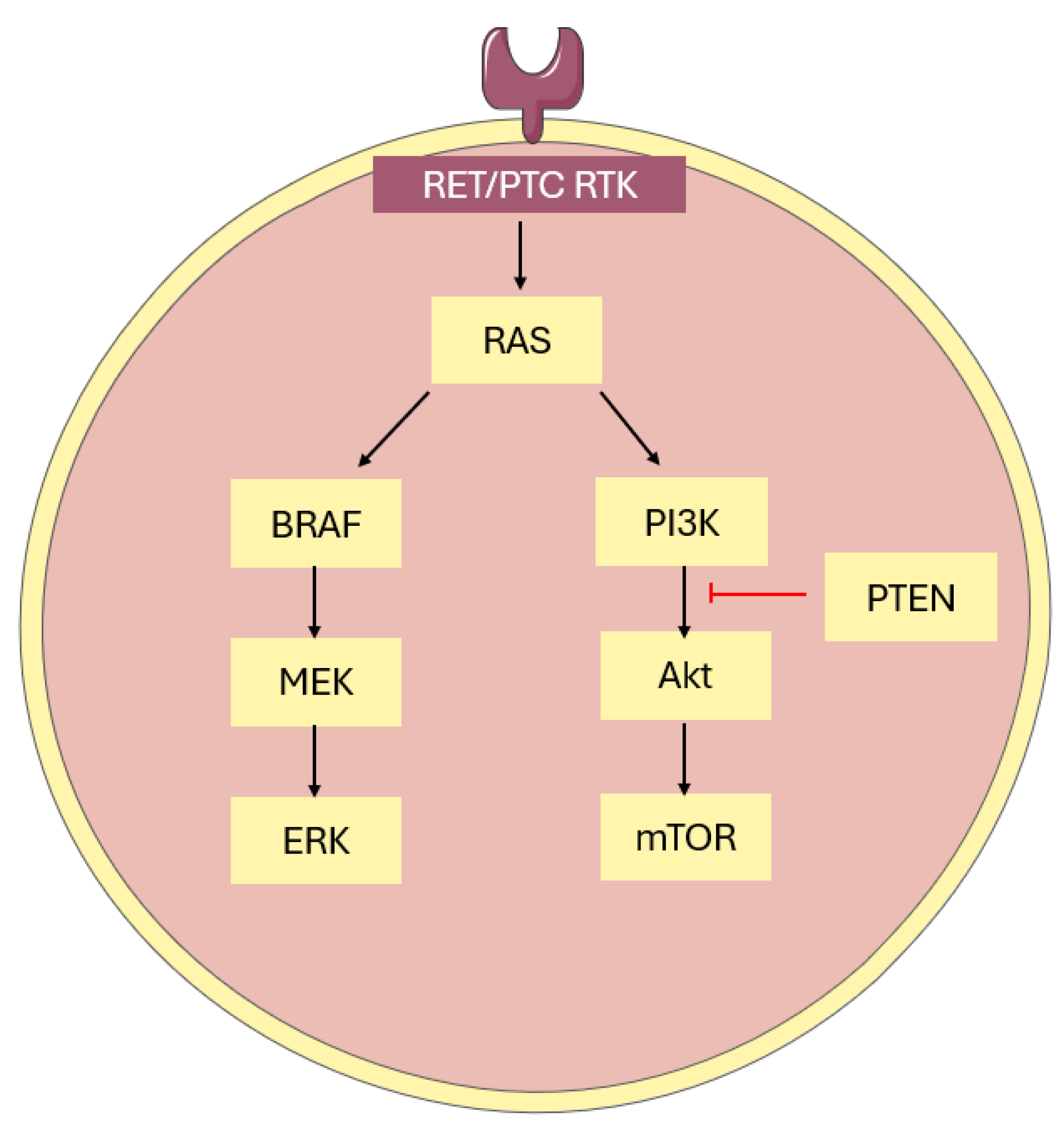
5. Molecular Pathways and Characteristics of Papillary Thyroid Cancer
6. Biomarkers in Papillary Thyroid Cancer
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TC | Thyroid cancer |
| PTC | Papillary thyroid cancer |
| DIO | Deiodinase |
| NIS | Sodium–iodide symporter |
| Tg | Thyroglobulin |
| TPO | Thyroid peroxidase |
| DUOX | Dual oxidase |
| TRH | Thyroid-releasing hormone |
| TSH | Thyroid-stimulating hormone |
| TSHR | Thyroid-stimulating hormone receptor |
| RAI | Radioactive iodine ablation |
| HRT | Hormone replacement therapy |
| DTC | Differentiated thyroid cancer |
| FTC | Follicular thyroid cancer |
| ATC | Anaplastic thyroid cancer |
| AR | Androgen receptor |
| ER | Estrogen receptor |
| PD-L1 | Programmed Death Receptor Ligand 1 |
| LNM | Lymph node metastasis |
| FNAB | Fine needle aspiration biopsy |
| ENE | Extranodal extension |
| ETE | Extrathyroidal extension |
| ERBT | External beam radiation |
| RR-PTC | Radioiodine-refractory papillary thyroid cancer |
| TKI | Tyrosine kinase inhibitor |
| AJCC | American Joint Committee on Cancer |
| TNM | Tumor-Node-Metastasis |
| ATA | American Thyroid Association |
| MACIS | Metastasis-Age-Complete resection-Invasion-Size |
| MAPK | Mitogen-activated protein kinase |
| PI3K/Akt | Phosphatidylinositol 3-kinase/protein kinase C |
| BRAF | B- rapidly accelerated fibrosarcoma |
| DNA | Deoxyribonucleic acid |
| RL | Rat sarcoma virus-like |
| TDS | Thyroid differentiation score |
References
- Kaplan, E.; Angelos, P.; Applewhite, M.; Mercier, F.; Grogan, R.H. Chapter 21 surgery of the thyroid. In Endotext [Internet]; Feingold, K., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Nilsson, M.; Fagmanm, H. Development of the thyroid gland. Development 2017, 144, 2123–2140. [Google Scholar] [CrossRef] [PubMed]
- van der Spek, A.H.; Fliers, E.; Boelen, A. The classic pathways of thyroid hormone metabolism. Mol. Cell. Endocrinol. 2017, 458, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Thienpont, L.M.; van Uytfanghe, K.; Poppe, K.; Velkeniers, B. Determination of free thyroid hormones. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 689–700. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Hu, H.; Qu, H.; Xu, Y.; Li, Q. Prevalence and trends of thyroid disease among adults, 1999–2018. Endocr. Pract. 2023, 29, 875–880. [Google Scholar] [CrossRef]
- Alarcon, G.; Figueredo, V.; Tarkoff, J. Thyroid Disorders. Pediatr. Rev. 2021, 42, 604–618. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Di Domenicantonio, A.; Fallahi, P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015, 14, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Kiess, W.; Kirstein, A.S.; Kratzsch, J.; Gesing, J.; Pfaffle, R. Thyroid—What is a healthy thyroid function test? J. Pediatr. Endocrinol. Metab. 2023, 36, 223–224. [Google Scholar] [CrossRef]
- Hanley, P.; Lord, K.; Bauer, A.J. Thyroid disorders in children and adoelscents: A review. JAMA Pediatr. 2016, 170, 1008–1019. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Elia, G.; Ragusa, F.; Ruffilli, I.; Paparao, S.R.; Antonelli, A. Thyroid autoimmune disorders and cancer. Semin. Cancer Biol. 2020, 64, 135–146. [Google Scholar] [CrossRef]
- Prete, A.; de Souza, P.B.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 1, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Schneider, D.F.; Chen, H. New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J. Clin. 2013, 63, 373–394. [Google Scholar] [CrossRef]
- Shah, J. Thyroid Carcinoma: Epidemiology, Histology, and Diagnosis. Clin. Adv. Hematol. Oncol. 2015, 13, 3–6. [Google Scholar]
- Giani, F.; Vella, V.; Tumino, D.; Maladrino, P.; Frasca, F. The Possible Role of Cancer Stem Cells in the Resistance to Kinase Inhibitors of Advanced Thyroid Cancer. Cancers 2020, 12, 2249. [Google Scholar] [CrossRef]
- Koh, B.; Tan, D.J.H.; Ng, C.H.; Fu, C.E.; Lim, W.H.; Zeng, R.W.; Yong, J.N.; Koh, J.H.; Syn, N.; Meng, W.; et al. Patterns in cancer incidence among people younger than 50 years in the US, 2010–2019. JAMA Netw. Open 2023, 6, e2328171. [Google Scholar] [CrossRef]
- Parad, M.T.; Fararouei, M.; Mirahmadizadeh, A.R.; Afrashteh, S. Thyroid cancer and its associated factors: A population-based case-control study. Int. J. Cancer 2021, 149, 514–521. [Google Scholar] [CrossRef]
- N. C. I. Surveillance Research Program. SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 10 December 2023).
- Tran, O.; Davies, L. Thyroid cancer incidence differences between men and women. Curr. Opin. Endocr. Metab. Res. 2023, 31, e100472. [Google Scholar] [CrossRef]
- Derwahl, M.; Nicula, D. Estrogen and its role in thyroid cancer. Endocr.-Relat. Cancer 2014, 21, 273–283. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, T.J.; Dadafarin, S.; Jones, M.; Rodriguez, T.; Gupta, A.; Shin, E.; Moscatello, A.; Iacob, C.; Islam, H.; Tiwari, R.K.; et al. Androgen Activity Is Associated With PD-L1 Downregulation in Thyroid Cancer. Front. Cell Dev. Biol. 2021, 9, 663130. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; O’Connell, T.J.; Zhao, H.; Darzynkiewicz, Z.; Gupta, A.; Buchsbaum, J.; Shin, E.; Iacob, C.; Suslina, N.; Moscatello, A.; et al. Andorgen receptor activation decreases proliferation in thyroid cancer cells. J. Cell. Biochem. 2020, 122, 1113–1125. [Google Scholar] [CrossRef]
- Gupta, A.; Carnazza, M.; Jones, M.; Darzynkiewicz, Z.; Halicka, D.; O’Connell, T.; Zhao, H.; Dadafarin, S.; Shin, E.; Schwarcz, M.D.; et al. Androgen receptor activation induces senescence in thyroid cancer cells. Cancers 2023, 15, 2198. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.; Jo, J.Q.; Zanganeh, S. Sex-specific outcomes in cancer therapy: The central role of hormones. Front. Med. Technol. 2024, 6, 1320690. [Google Scholar] [CrossRef]
- Aurilio, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. Androgen receptor signaling pathway in prostate cancer: From genetics to clinical applications. Cells 2020, 9, 2653. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, A.H.; Hankinson, S.E. Endogenous hormone levels and risk of breast, endometrial, and ovarian cancers: Prospective studies. Adv. Exp. Med. Biol. 2008, 630, 148–165. [Google Scholar]
- Li, P.; Ding, Y.; Liu, M.; Wang, W.; Li, X. Sex disparities in thyroid cancer: A SEER population study. Gland Surg. 2021, 10, 3200–3210. [Google Scholar] [CrossRef]
- LeClair, K.; Bell, K.J.L.; Furuya-Kanamori, L.; Doi, S.A.; Francis, D.O.; Davies, L. Evaluation of gender inequity in thyroid cancer diagnosis. JAMA Intern. Med. 2021, 181, 1351–1358. [Google Scholar] [CrossRef]
- McGary, C.T.; Shaw, A. Educational Case: Cytology for Staging Neoplasia and Thyroid Neoplasms. Acad. Pathol. 2019, 6, 2374289519851218. [Google Scholar] [CrossRef]
- Xu, B.; Ghossein, R. Evolutin of histologic classification of thyroid neoplasms and its impact on clinical management. Eur. J. Surg. Oncol. 2018, 44, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Network, C.G.A.R. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Poller, D.N.; Glaysher, S. Molecular pathology and thyroid FNA. Cytopathology 2017, 28, 475–481. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Yazici, P.; Mihmanli, M.; Bozkurt, E.; Ozturk, F.; Uludag, M. Which is the best predictor of thyroid cancer: Thyroptropin, thyroglobulin, or their ratio? Hormones 2016, 15, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Ming, X.; Tian, Y.; Yao, M.; Ni, Y.; Liu, Y.; Li, Z. Mapping global epidemiology of thyroid nodules among general population: A systemic review and meta-analysis. Front. Oncol. 2022, 12, 1029926. [Google Scholar] [CrossRef]
- Ali, S.; Baloch, Z.W.; Cochland-Priollet, B.; Schmitt, F.C.; Vielh, P.; VanderLaan, P.A. The 2023 Bethesda system for reporting thyroid cytopathology. Thyroid 2023, 33, 1039–1044. [Google Scholar] [CrossRef]
- Filetti, S.; Duranted, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment, and follow-up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- Xu, B.; Ghossein, R. Crucial parameteres in thyroid carcinoma reporting- challenges, controversies, and clinical implications. Histopathology 2018, 72, 32–39. [Google Scholar] [CrossRef]
- Xu, B.; Ghossein, R. Critical prognostic parameters in the anatomic pathology reporting of differentiated follicular cell-derived thyroid carcinoma. Cancers 2019, 11, 1100. [Google Scholar] [CrossRef]
- Glover, A.R.; Gundara, J.S.; Norlén, O.; Lee, J.C.; Sidhu, S.B. The pros and cons of prophylactic central neck dissection in papillary thyroid carcinoma. Gland Surg. 2013, 2, 196. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, J.; Kukulska, A.; Oczko-Wojciechowska, M.; Kotecka-Blicharz, A.; Drosik-Rutowicz, K.; Haras-Gil, M.; Jarzab, B.; Handkiewicz-Junak, D. Early diagnosis of low-risk papillary thyroid cancer results rather in overtreatment than a better survival. Front. Endocrinol. 2020, 11, 571421. [Google Scholar] [CrossRef]
- Sugitani, I.; Fujimoto, Y.; Yamamoto, N. Papillary thyroid carcinoma with distant metastases: Survival predictors and the importance of local control. Surgery 2008, 143, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Toraih, E.A.; Hussein, M.H.; Zerfaoui, M.; Attia, A.S.; Ellythy, A.M.; Mostafa, A.; Ruiz, E.; Sharma, M.; Russel, J.O.; Randolph, G.W.; et al. Site-specific metastasis and survival in papillary thyroid cancer: The importance of brain and multi-organ disease. Cancers 2021, 13, 1625. [Google Scholar] [CrossRef]
- Metere, A.; Aceti, V.; Giacomelli, L. The surgical management of locally advanced well-differentiated thyroid carcinoma: Changes over the years according to the AJCC 8th edition Cancer Staging Manual. Thyroid Res. 2019, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Nilubol, N.; Boufraqech, M. New therapies for advanced thyroid cancer. Front. Endocrinol. 2020, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, F.; Dedhia, P.H.; Ringel, M.D. Thyroid cancer, recent advances in diagnosis and therapy. Int. J. Cancer 2021, 149, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Ryder, M.; Jimenez, C. Targeted therapy for advance thyroid cancer: Kinase inhibitors and beyond. Endocr. Rev. 2019, 40, 1573–1605. [Google Scholar] [CrossRef]
- Macerola, E.; Poma, A.M.; Vignali, P.; Proietti, A.; Ugolini, C.; Torregrossa, L.; Basolo, A.; Elisei, R.; Santini, F.; Basolo, F. Predictive biomarkers in thyroid cancer. Front. Oncol. 2022, 12, 901004. [Google Scholar] [CrossRef] [PubMed]
- Hyogo, Y.; Kiyota, N.; Goto, S.; Imamura, Y.; Chayahara, N.; Toyoda, M.; Nibu, K.; Hyodo, T.; Hara, S.; Masuoka, H.; et al. Thrombotic microangiopathy with severe proteinuria induced by Lenvatinib for radioactive iodine-refractory papillary thyroid carcinoma. Case Rep. Oncol. 2018, 11, 735–741. [Google Scholar] [CrossRef]
- Kluijfhout, W.P.; Rotstein, L.; Pasternak, J.D. Well-differentiated thyroid cancer: Thyroidectomy or lobectomy? CMAJ 2016, 188, 517–520. [Google Scholar] [CrossRef]
- Medas, F.; Canu, G.L.; Cappellacci, F.; Anedda, G.; Conzo, G.; Erdas, E.; Calò, P.G. Prophylactic Central Lymph Node Dissection Improves Disease-Free Survival in Patients with Intermediate and High Risk Differentiated Thyroid Carcinoma: A Retrospective Analysis on 399 Patients. Cancers 2020, 12, 1658. [Google Scholar] [CrossRef] [PubMed]
- Vassaux, G.; Zwarthoed, C.; Signetti, L.; Guglielmi, J.; Compin, C.; Guigonis, J.; Juhel, T.; Humbert, O.; Benisvy, D.; Pourcher, T.; et al. Iodinated contrast agents perturb iodine uptake by thyroid independent of free iodide. J. Nucl. Med. 2018, 59, 121–126. [Google Scholar] [CrossRef] [PubMed]
- De la Vieja, A.; Riesco-Eizaguirre, G. Radio-iodide treatment from molecular aspects to clinical view. Cancers 2021, 13, 995. [Google Scholar] [CrossRef]
- Sparano, C.; Moog, S.; Hadoux, J.; Dupuy, C.; Ghuzlan, A.A.; Breuskin, I.; Guerlain, J.; Hartl, D.; Baudin, E.; Lamartina, L. Strategies for radioiodine treatment: What’s new. Cancers 2022, 14, 3800. [Google Scholar] [CrossRef]
- Aashiq, M.; Silverman, D.A.; Na’ara, S.; Takahashi, H.; Amit, M. Radioiodine-refractory thyroid cancer: Molecular basis of redifferentiation therapies, management, and novel therapies. Cancers 2019, 11, 1382. [Google Scholar] [CrossRef] [PubMed]
- Van Houten, P.; Neta-Maier, R.T.; Smit, J.W. Differentiated thyroid carcinoma: An update. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101687. [Google Scholar] [CrossRef]
- Ulisse, S.; Baldini, E.; Lauro, A.; Pironi, D.; Tripodi, D.; Lori, E.; Ferent, I.C.; Amabile, M.I.; Catania, A.; Matteo, F.M.D.; et al. Papillary Thyroid Cancer Prognosis: An Evolving Field. Cancers 2021, 13, 5567. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, C.; Cui, K.; Ma, X. Papillary thyroid carcinoma with tracheal invasion. Medicine 2019, 98, e17033. [Google Scholar] [CrossRef]
- Jiang, K.; Zhou, D.; Luo, D. Microscopic extrathyroidal extension does not affect the prognosis of patients with papillary thyroid carcinoma: A propensity score matching analysis. Heliyon 2024, 10, e25280. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Rodrigo, J.P.; Shah, J.P.; Nixon, L.J.; Hartl, D.M.; Robbins, K.T.; Kowalski, L.P.; Makitie, A.A.; Hamoir, M.; Lopez, F.; et al. Recurrent differentiated thyroid cancer: The current treatment options. Cancers 2023, 15, 2692. [Google Scholar] [CrossRef] [PubMed]
- Grogan, R.H.; Kaplan, S.P.; Cao, H.; Weiss, R.E.; DeGroot, L.J.; Simon, C.A.; Embia, O.M.A.; Angelos, P.; Kaplan, E.L.; Schechter, R.B. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery 2013, 154, 1436–1447. [Google Scholar] [CrossRef]
- CS, G. Recurrence of papillary thyroid cancer after optimized surgery. Gland Surg. 2014, 4, 52–62. [Google Scholar] [CrossRef]
- Yarchoan, M.; LiVolsi, V.A.; Brose, M.S. BRAF Mutation and Thyroid Cancer Recurrence. J. Clin. Oncol. 2015, 33, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Chatchomchuan, W.; Thewjitcharoen, Y.; Karndumri, K.; Porramatikul, S.; Krittiyawong, S.; Wanothayaroj, E.; Vongterapak, S.; Butadej, S.; Veerasomboonsin, V.; Kanchanapitak, A.; et al. Recurrence Factors and Characteristic Trends of Papillary Thyroid Cancer over Three Decades. Int. J. Endocrinol. 2021, 2021, 9989757. [Google Scholar] [CrossRef]
- Sun, J.H.; Li, Y.R.; Chang, K.H.; Liou, M.J.; Lin, S.F.; Tsai, S.S.; Yu, M.C.; Hsueh, C.; Chen, S.T. Evaluation of recurrence risk in patients with papillary thyroid cancer through tumor-node-metastasis staging: A single center observational study in Taiwan. Biomed. J. 2022, 45, 923–930. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Tala, H.; Shah, J.; Leboeuf, R.; Ghossein, R.; Gonen, M.; Brokhin, M.; Omry, G.; Fagin, J.A.; Shaha, A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: Using response to therapy variables to modify initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010, 20, 1341–1349. [Google Scholar] [CrossRef]
- Dwamena, S.; Patel, N.; Egan, R.; Stechman, M.; Scott-Coombes, D. Impact of the change from seventh to eighth edition of the AJCC TNM classification of malignant tumors and comparison with the MACIS prognostic scoring system in non-medullary thyroid cancer. BJS Open 2019, 3, 623–628. [Google Scholar] [CrossRef]
- Younis, E. Oncogenesis of thyroid cancer. Asian Pac. J. Cancer Prev. 2017, 18, 1191–1199. [Google Scholar] [CrossRef]
- Fakhruddin, N.; Jabbour, M.; Novy, M.; Tamim, H.; Bahmad, H.; Farhat, F.; Zaatari, G.; Aridi, T.; Kriegshauser, G.; Oberkanins, C.; et al. BRAF and NRAS mutations in papillary thyroid carcinoma and concordance in BRAF mutations between primary and corresponding lymph node metastases. Sci. Rep. 2017, 7, 4666. [Google Scholar] [CrossRef]
- Cho, Y.; Park, S.; Shin, J.; Oh, Y.; Choe, J.; Kim, J.; Yim, H.; Kim, Y.; Ki, C.; Kim, T.; et al. Highly sensitive and specific molecular test for mutations in the diagnosis of thyroid nodules: A prospective study of BRAF-prevalent population. Int. J. Mol. Sci. 2020, 21, 5629. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Bychkov, A.; Jung, C. Emerging biomarkers in thyroid practic and research. Cancers 2022, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Cheng, L.; Sa, R.; Jin, Y.; Chen, L. Combined tazemetostat and MAPKi enhances differentiation of thyroid cancer cells harboring BRAFV600E by synergistically decreasing global trimethylation of H3K27. J. Cell. Mol. Med. 2020, 24, 3336–3345. [Google Scholar] [CrossRef]
- Santoro, M.; Moccia, M.; Federico, G.; Carlomagno, F. RET gene fusions in malignancies of the thyroid and other tissues. Genes 2020, 11, 424. [Google Scholar] [CrossRef]
- Khonrak, T.; Watcharadetwittaya, S.; Chamgramol, Y.; Intarawichian, P.; Deenonpoe, R. RET rearrangements are relevant to histopathologic subtypes and clinicopathological features of Thai papillary thyroid carcinoma patients. Pathol. Oncol. Res. 2023, 29, 1611138. [Google Scholar] [CrossRef]
- Xing, M. Clinical utility of RAS mutations in thyroid cancer: A blurred picture now emerging clear. BMC Med. 2016, 14, 12. [Google Scholar] [CrossRef]
- Marotta, V.; Bifulco, M.; Vitale, M. Significance of RAS mutations in thyroid benign nodules and non-medullary thyroid cancer. Cancers 2021, 13, 3785. [Google Scholar] [CrossRef]
- Howell, G.; Hodak, S.P.; Yip, L. RAS mutations in thyroid cancer. Oncologist 2013, 18, 926–932. [Google Scholar] [CrossRef]
- Yoo, S.; Song, Y.; Lee, E.; Hwang, J.; Kim, H.; Jung, G.; Kim, Y.; Cho, S.; Won, J.; Chung, E.; et al. Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer. Nat. Commun. 2019, 10, 2764. [Google Scholar] [CrossRef]
- Wong, K.; Lang, B.H. New molecular targeted therapy and redifferentiation therapy for radioiodine-refractory advanced papillary thyroid carcinoma: Literature review. J. Thyroid Res. 2012, 2012, 818204. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, V.K.; Armengol, G. Molecular biomarkers in cancer. Biomolecules 2022, 12, 1021. [Google Scholar] [CrossRef] [PubMed]
- DeSouza, N.R.; Quaranto, D.; Carnazza, M.; Jarboe, T.; Tiwari, R.K.; Geliebter, J. Interactome of long-noncoding RNAs: Transcriptomic expression patterns and shaping cancer cell phenotypes. Int. J. Mol. Sci. 2023, 24, 9914. [Google Scholar] [CrossRef]
- Entezari, M.; Taheriazam, A.; Orouei, S.; Fallah, S.; Sanaei, A.; Hejazi, E.S.; Kakavand, A.; Rezaei, S.; Heidari, H.; Behroozaghdam, M.; et al. LncRNA-miRNA axis in tumor progression and therapy response: An emphasis on molecular interactions and therapeutic interventions. Biomed. Pharmacother. 2022, 154, 113609. [Google Scholar] [CrossRef]
- Nixon, A.M.; Provatopoulou, X.; Kalogera, E.; Zografos, G.N.; Gournaris, A. Circulating thyroid cancer biomarkers: Current limitations and future prospects. Clin. Endocrinol. 2017, 87, 117–126. [Google Scholar] [CrossRef]
- Macvanin, M.T.; Gluvic, Z.M.; Zaric, B.L.; Essack, M.; Gao, X.; Isenovic, E.R. New biomarkers: Prospect for diagnosis and monitoring of thyroid disease. Front. Endocrinol. 2023, 14, 1218320. [Google Scholar] [CrossRef]
- Dadafarin, S.; Rodriguez, T.; Carnazza, M.; Tiwari, R.K.; Moscatello, A.; Geliebter, J. MEG3 Expression Indicates Lymph Node Metastasis and Presence of Cancer-Associated Fibroblasts in Papillary Thyroid Cancer. Cells 2022, 11, 3181. [Google Scholar] [CrossRef]
- Rutter, M.M.; Jha, P.; Schultz, K.A.P.; Sheil, A.; Harris, A.K.; Bauer, A.J.; Field, A.L.; Geller, J.; Hill, A.D. DICER1 Mutations and Differentiated Thyroid Carcinoma: Evidence of a Direct Association. J. Clin. Endocrinol. Metab. 2016, 101, 1–5. [Google Scholar] [CrossRef]
- Chai, L.; Li, J.; Lv, Z. An integrated analysis of cancer genes in thyroid cancer. Oncol. Rep. 2016, 35, 962–970. [Google Scholar] [CrossRef]
- Geisler, D.L.; French, E.K.; Yip, L.; Nikiforova, M.N.; Nikiforov, Y.E.; Schoedel, K.E.; Seethala, R.R.; Ohori, N.P. A thyroid EIF1AX story: How clinical, cytologic, and molecular surveillance led to appropriate management. J. Am. Soc. Cytopathol. 2023, 12, 105–111. [Google Scholar] [CrossRef]
- Htwe, T.T.; Karim, N.; Wong, J.; Jahanfar, S.; Mansur, M.A. Differential expression of galectin-3 in advancing thyroid cancer cells: A clue toward understanding tumour progression and metastasis. Singapore Med. J. 2010, 51, 856–859. [Google Scholar] [PubMed]
- Banerjee, S.; Nahar, U.; Dahiya, D.; Mukherjee, S.; Dey, P.; Gupta, R.; Radotra, B.; Sachdeva, N.; Sood, A.; Bhadada, S.K.; et al. Role of cytotoxic T cells and PD-1 immune checkpoint pathway in papillary thyroid carcinoma. Front. Endocrinol. 2022, 13, 931647. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kim, T.H.; Kim, S.W.; Ki, C.S.; Jang, H.W.; Kim, J.S.; Kim, J.H.; Choe, J.; Shin, J.H.; Hahn, S.Y.; et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr. Relat. Cancer 2017, 24, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Chintakuntlawar, S.V.; Rumilla, K.M.; Smith, C.Y.; Jenkins, S.M.; Foote, R.L.; Kasperbauer, J.L.; Morris, J.C.; Ryder, M.; Alsidwai, S.; Hilger, C.; et al. Expression of PD-1 and PD-L1 in Anaplastic Thyroid Cancer Patients Treated With Multimodal Therapy: Results From a Retrospective Study. J. Clin. Endocrinol. Metab. 2017, 102, 1943–1950. [Google Scholar] [CrossRef]
- Boruah, M.; Gaddam, P.; Agarwal, S.; Mir, Y.A.; Gupta, R.; Sharma, M.C.; Deo, S.; Nilima, N. PD-L1 expression in rare and aggressive thyroid cancers: A preliminary investigation for a role of immunotherapy. J. Cancer Res. Ther. 2023, 19, 312–320. [Google Scholar] [CrossRef]
- Shin, E.; Koo, J.S. Cell component and function of tumor microenvironment in thyroid cancer. Int. J. Mol. Sci. 2022, 23, 12578. [Google Scholar] [CrossRef]


| AJCC Stage | Age | Description |
|---|---|---|
| Stage I | <55 (A1) | Any T, Any N, M0 |
| ≥55 (A2) | T1, N0 or NX, M0 | |
| ≥55 (A2) | T2, N0 or NX, M0 | |
| Stage II | <55 (A1) | Any T, Any N, M1 |
| ≥55 (A2) | T1, N1, M0 | |
| ≥55 (A2) | T2, N1, M0 | |
| ≥55 (A2) | T3a or T3b, Any N, M0 | |
| Stage III | ≥55 (A2) | T4a, Any N, M0 |
| Stage IVA | ≥55 (A2) | T4b, Any N, M0 |
| Stage IVB | ≥55 (A2) | Any T, Any N, M1 |
| Recurrence Risk | Description |
|---|---|
| Low | No local or distant metastasis, the tumor is completely resected, no vascular invasion or invasion of locoregional tissues was observed, and the tumor does not have an aggressive histology |
| Medium | Microscopic invasion in the perithyroidal soft tissue, cervical lymph node metastasis is present, or the tumor has an aggressive histology or vascular invasion |
| High | Evidence of macroscopic tumor invasion, tumor resection was incomplete with gross residual disease, or distant metastases are present |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnazza, M.; Quaranto, D.; DeSouza, N.; Moscatello, A.L.; Garber, D.; Hemmerdinger, S.; Islam, H.K.; Tiwari, R.K.; Li, X.-M.; Geliebter, J. The Current Understanding of the Molecular Pathogenesis of Papillary Thyroid Cancer. Int. J. Mol. Sci. 2025, 26, 4646. https://doi.org/10.3390/ijms26104646
Carnazza M, Quaranto D, DeSouza N, Moscatello AL, Garber D, Hemmerdinger S, Islam HK, Tiwari RK, Li X-M, Geliebter J. The Current Understanding of the Molecular Pathogenesis of Papillary Thyroid Cancer. International Journal of Molecular Sciences. 2025; 26(10):4646. https://doi.org/10.3390/ijms26104646
Chicago/Turabian StyleCarnazza, Michelle, Danielle Quaranto, Nicole DeSouza, Augustine L. Moscatello, David Garber, Steven Hemmerdinger, Humayun K. Islam, Raj K. Tiwari, Xiu-Min Li, and Jan Geliebter. 2025. "The Current Understanding of the Molecular Pathogenesis of Papillary Thyroid Cancer" International Journal of Molecular Sciences 26, no. 10: 4646. https://doi.org/10.3390/ijms26104646
APA StyleCarnazza, M., Quaranto, D., DeSouza, N., Moscatello, A. L., Garber, D., Hemmerdinger, S., Islam, H. K., Tiwari, R. K., Li, X.-M., & Geliebter, J. (2025). The Current Understanding of the Molecular Pathogenesis of Papillary Thyroid Cancer. International Journal of Molecular Sciences, 26(10), 4646. https://doi.org/10.3390/ijms26104646







