The Anisotropic Osteoinductive Capacity of a Nickel–Titanium Alloy Fabricated Through Laser Powder Bed Fusion
Abstract
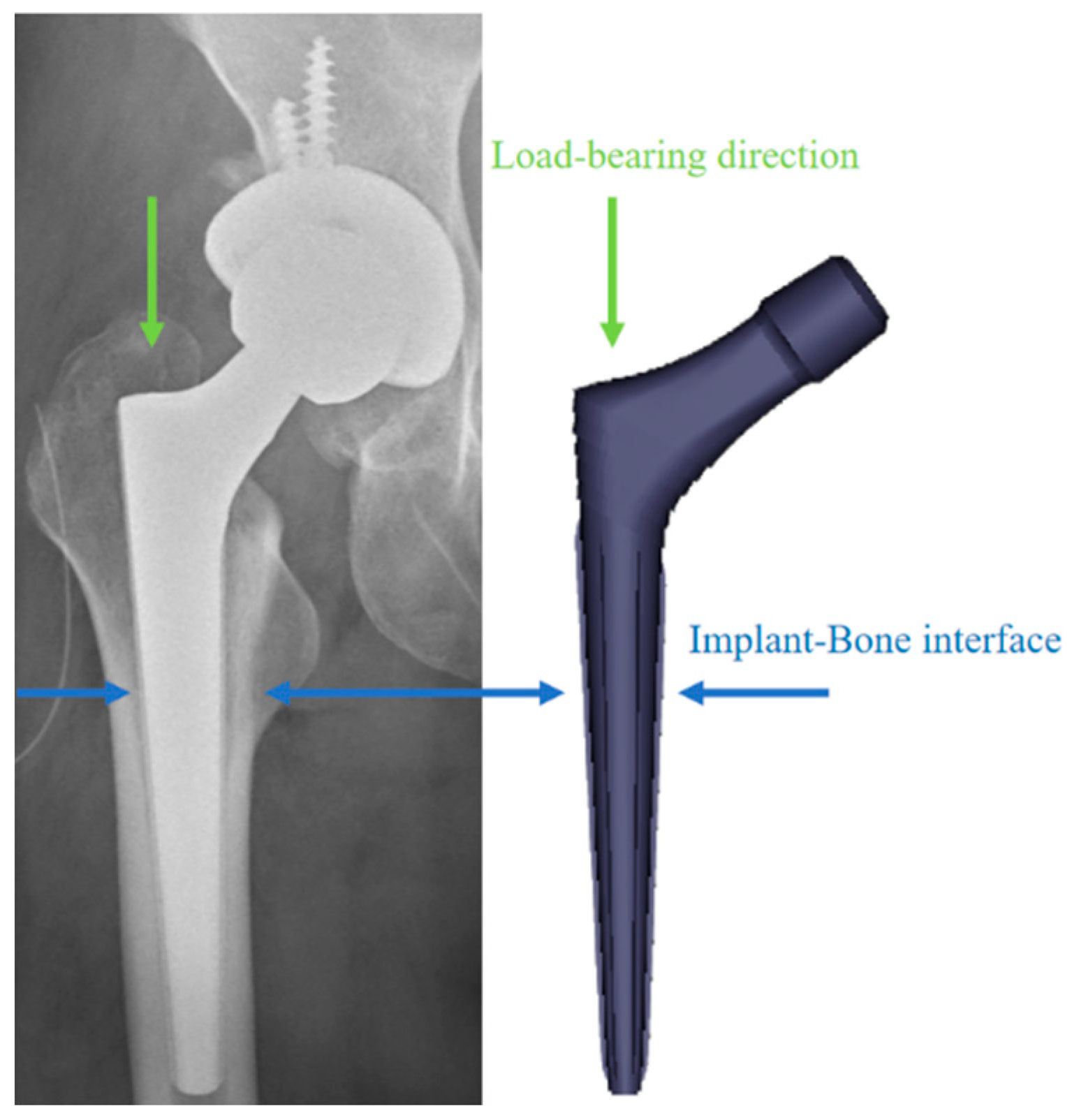
1. Introduction
2. Results
2.1. Cytotoxicity
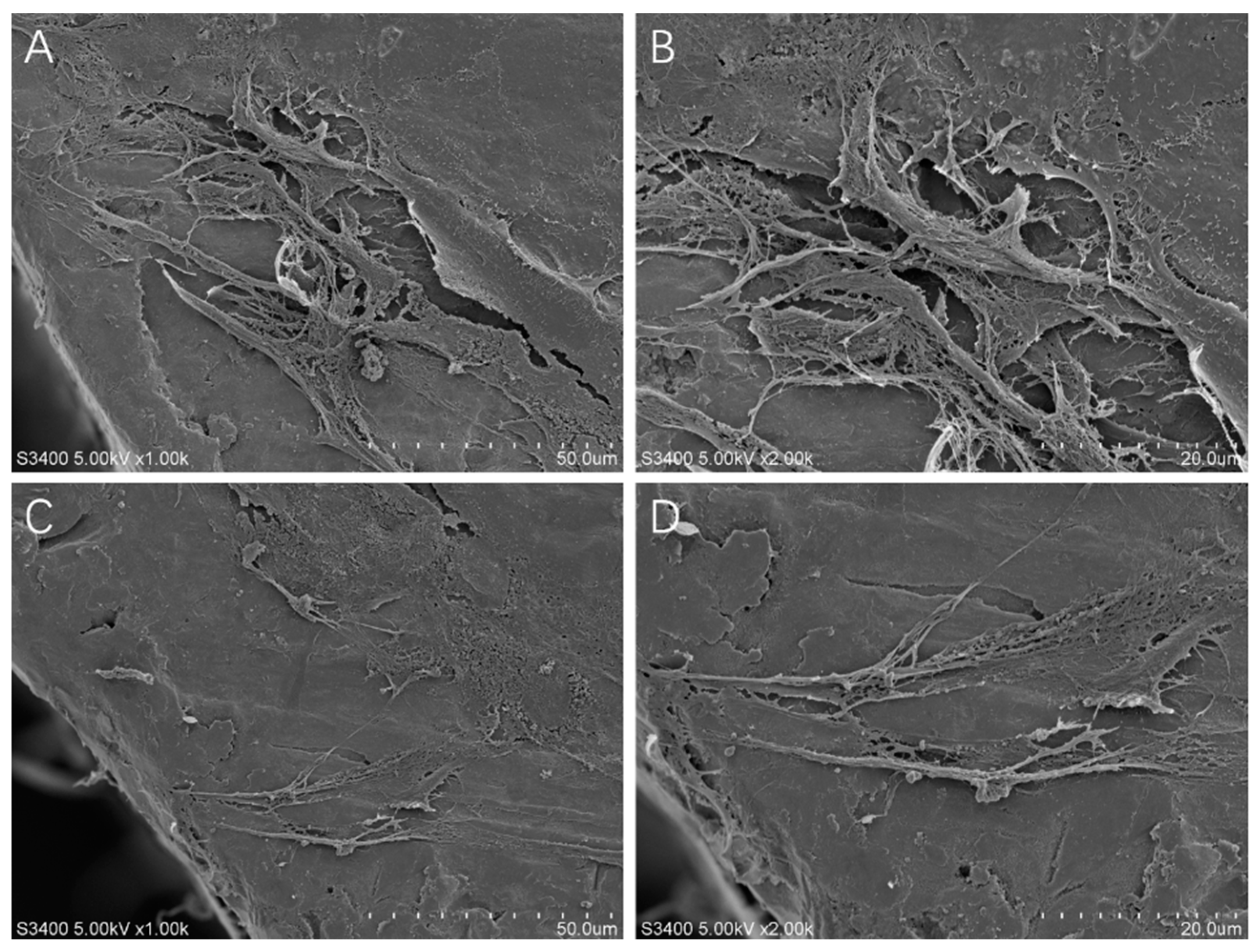
2.2. Cell Adhesion Morphology
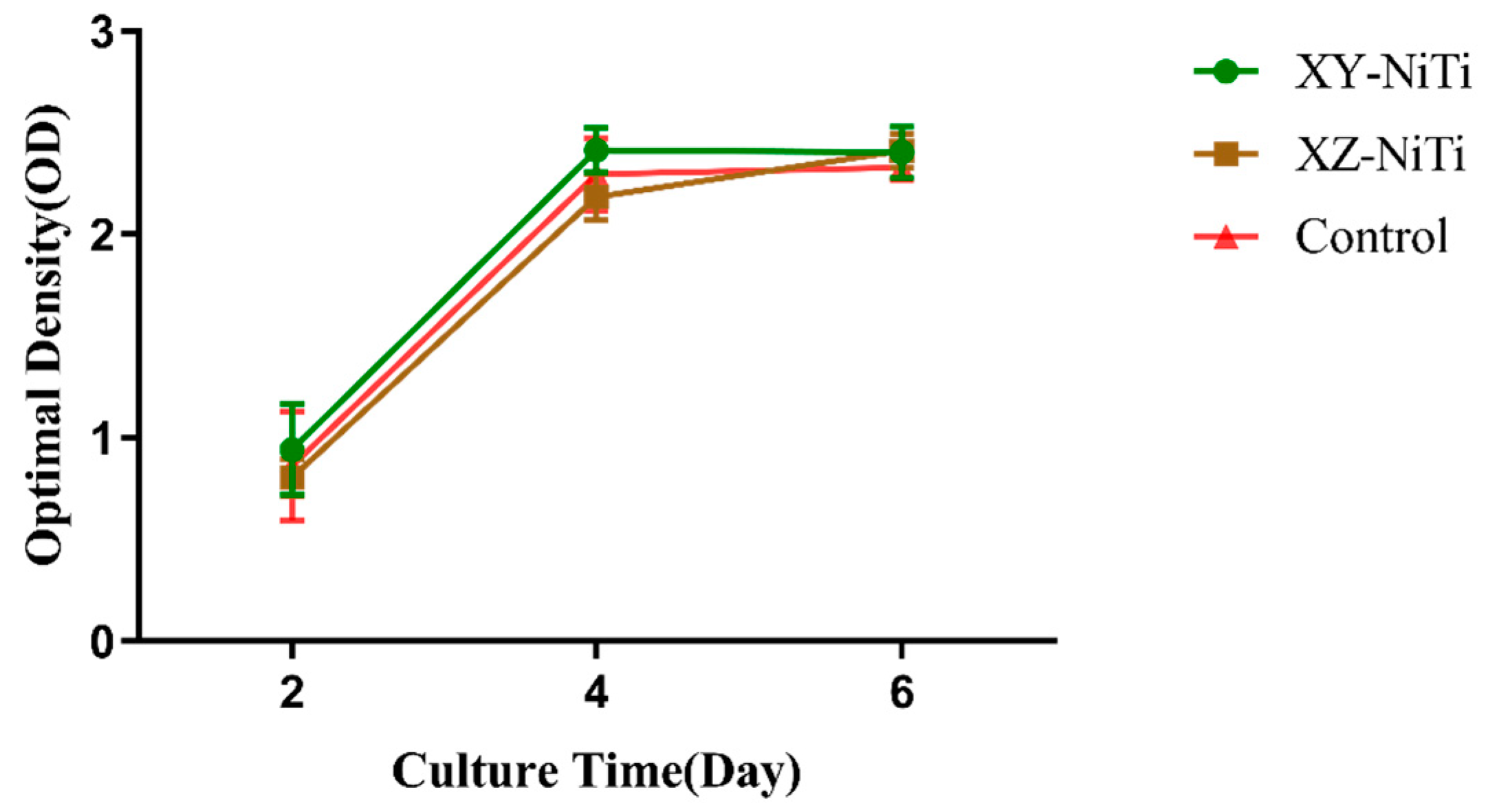
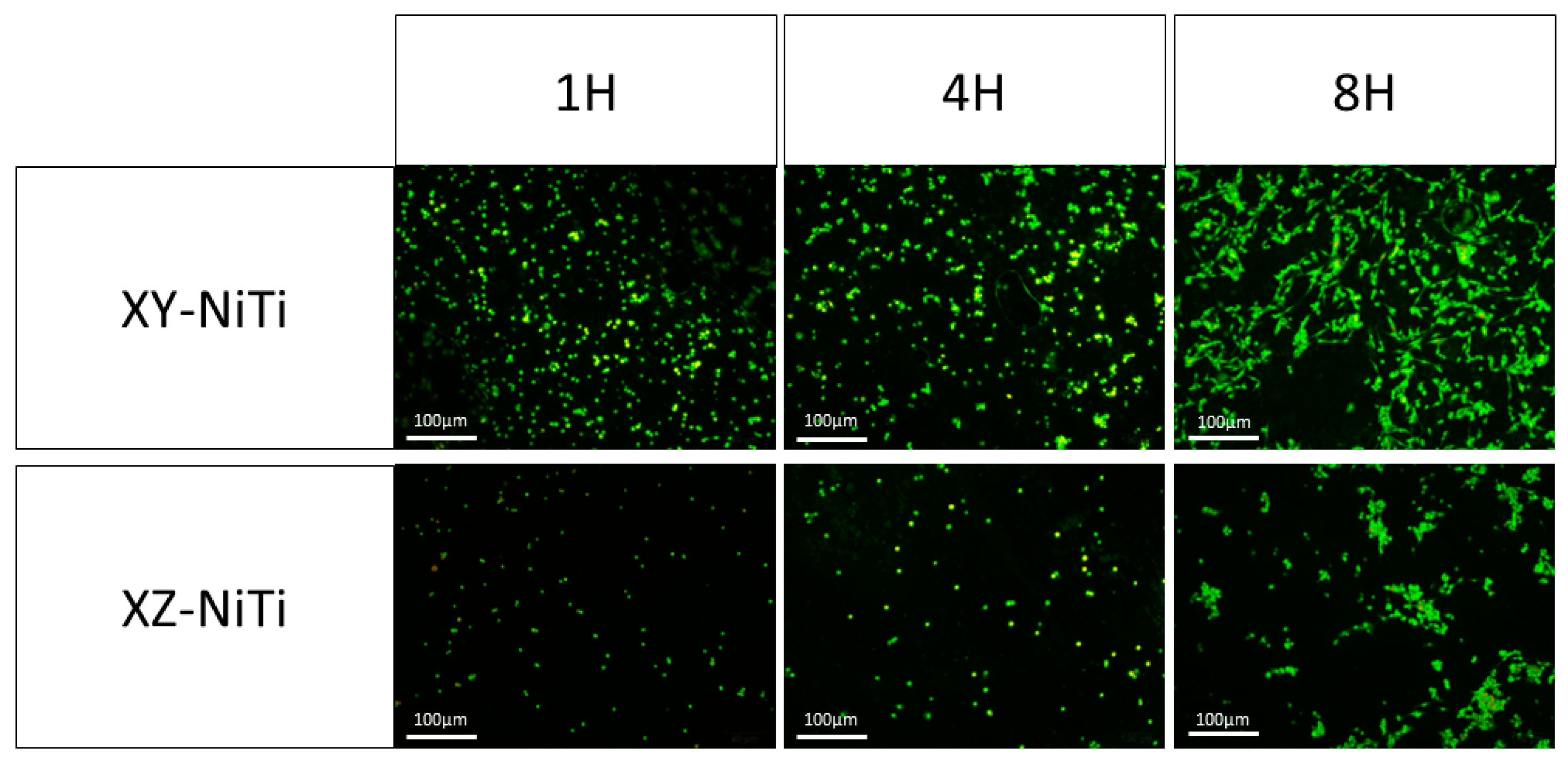
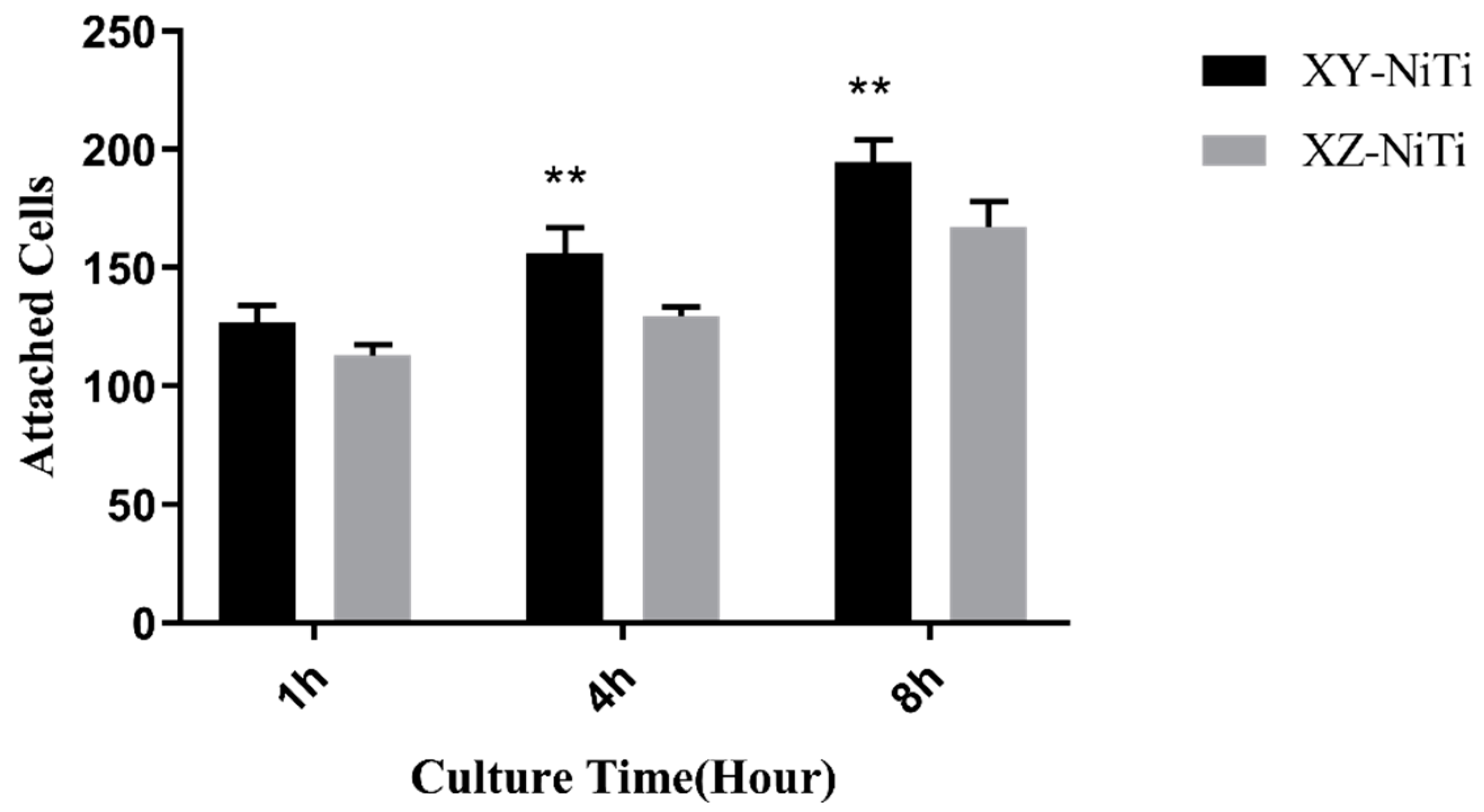
2.3. Adherent Cell Counting
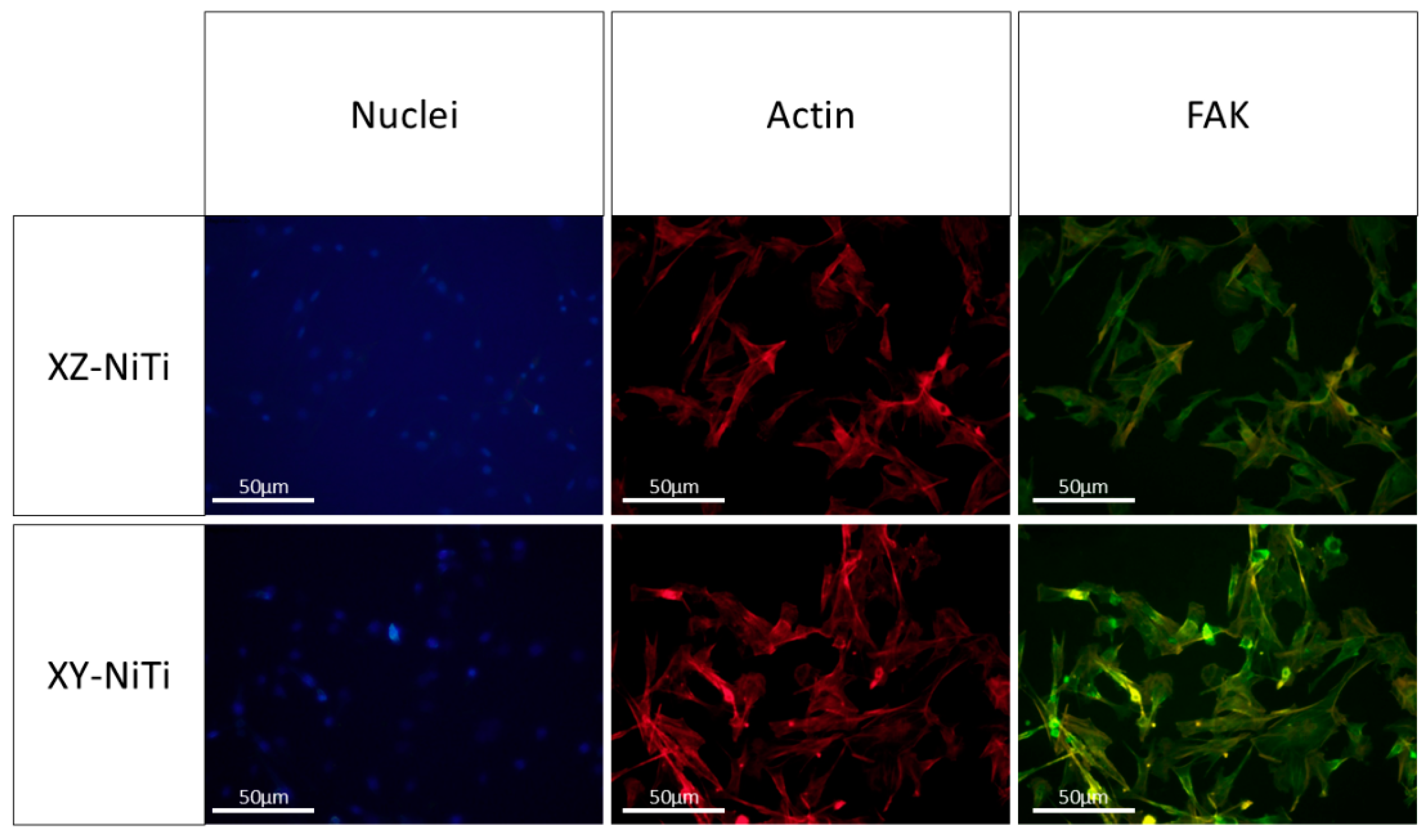
2.4. Nuclei–Actin–FAK Staining
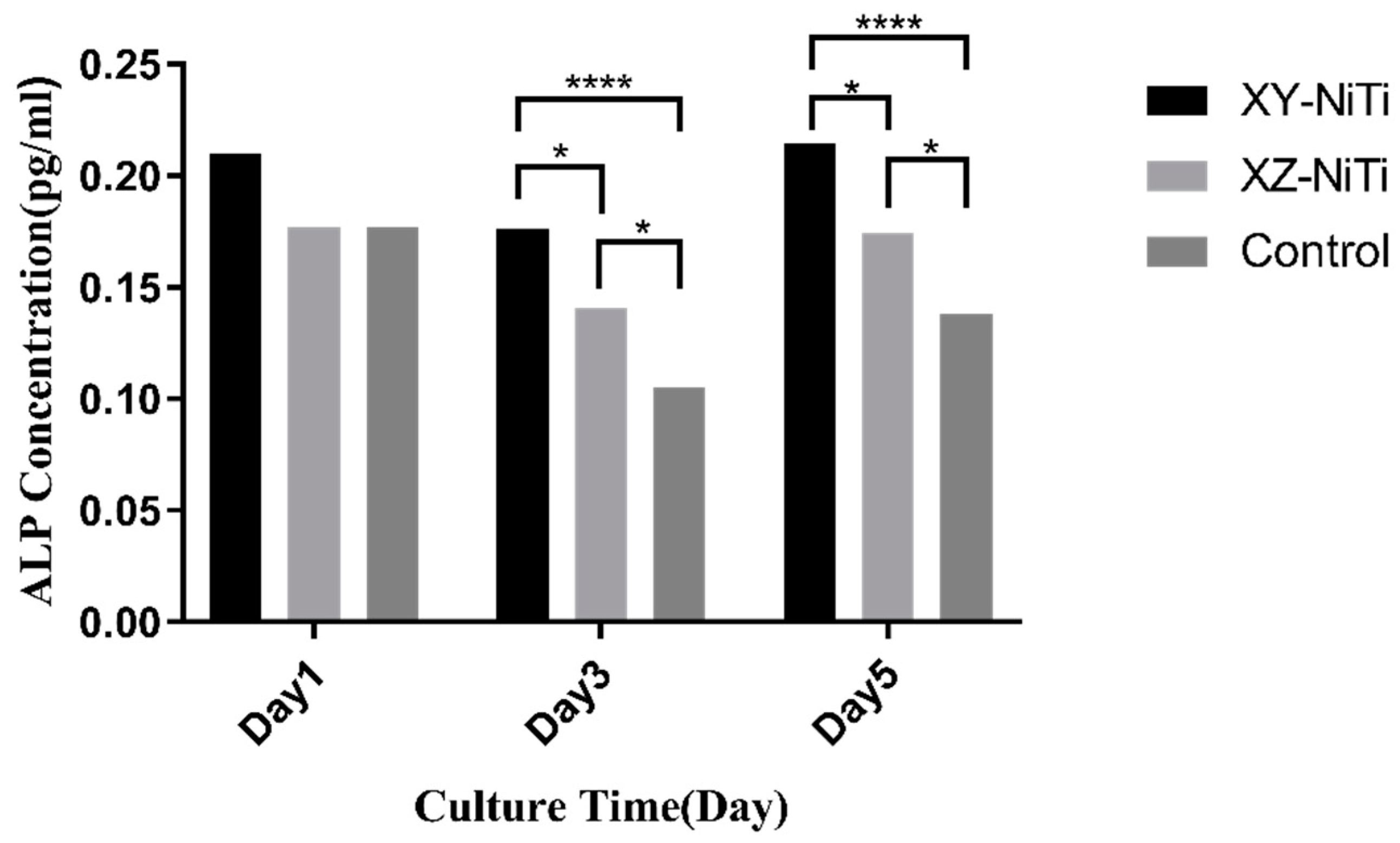
2.5. Early Osteogenic Differentiation
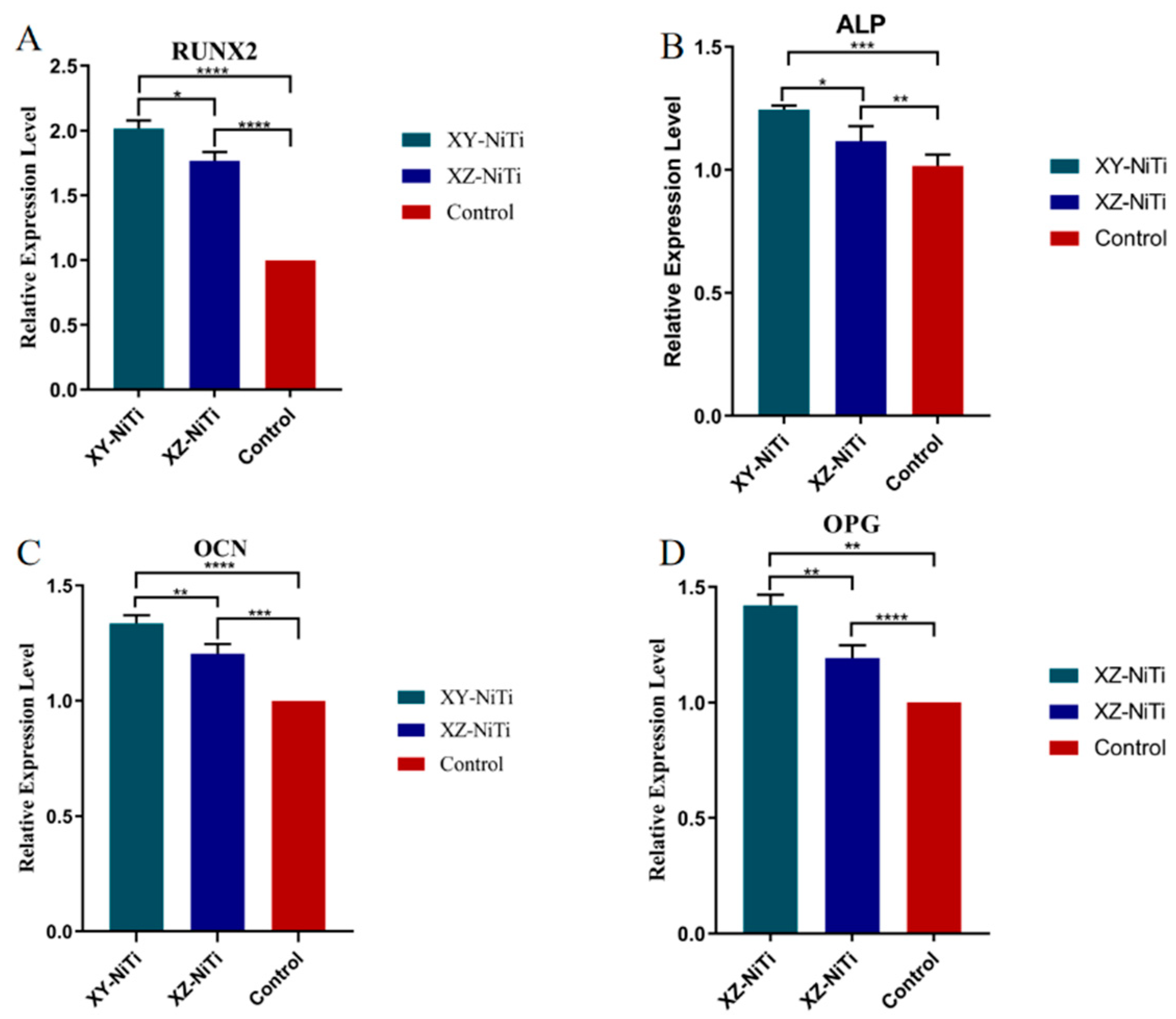
2.6. Osteogenic-Related Gene Expression
3. Discussion
4. Materials and Methods
4.1. Production of LPBF-NiTi Specimens
4.2. Isolation of Mouse Bone Marrow Mesenchymal Cells
4.3. Cytotoxicity and Viability
4.4. Cell Adhesion
4.5. Adherent Cell Morphology
4.6. Cytoskeletal Staining
4.7. ALP Secretion
4.8. RT-PCR of Osteogenic-Related Gene
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elahinia, M.H.; Hashem, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing and processing of NiTi implants: A review. Prog. Mater. Sci. 2012, 57, 911–946. [Google Scholar] [CrossRef]
- Elahinia, M.H. Shape Memory Alloy Actuators (Design, Fabrication, and Experimental Evaluation); Experimental Characterization of Shape Memory Alloys; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 239–277. [Google Scholar]
- Jani, J.M.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Oh, I.-H.; Nomura, N.; Masahashi, N.; Hanada, S. Mechanical properties of porous titanium compacts prepared by powder sintering. Scr. Mater. 2003, 9, 1197–1202. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Xiong, J.; Hodgson, P.D.; Wen, C.E. Porous TiNbZr alloy scaffolds for biomedical applications. Acta Biomater. 2009, 5, 3616–3624. [Google Scholar] [CrossRef]
- Ibrahim, H.; Jahadakbar, A.; Dehghan, A.; Moghaddam, N.S.; Elahinia, M. In Vitro Corrosion Assessment of Additively Manufactured Porous NiTi Structures for Bone Fixation Applications. Met.-Open Access Metall. J. 2018, 8, 164. [Google Scholar] [CrossRef]
- Kityk, A.; Protsenko, V.; Danilov, F.; Pavlik, V.; Hnatko, M.; Soltys, J. Enhancement of the surface characteristics of Ti-based biomedical alloy by electropolishing in environmentally friendly deep eutectic solvent (Ethaline). Colloids Surf. A-Physicochem. Eng. Asp. 2021, 613, 126125. [Google Scholar] [CrossRef]
- Angadi, S.V.; Nayak, S.H.; Kumar, G.S.R.; Buradi, A.; Yadav, S.P.S. Recent advancements in the manufacture of nitinol including its characterization and properties. In Proceedings of the International Conference on Advances in Materials and Mechanical Engineering (ICAMME), Ghaziabad, India, 18–19 February 2022; pp. 9–17. [Google Scholar]
- Gatto, E.; Matarese, G.; Di Bella, G.; Nucera, R.; Borsellino, C.; Cordasco, G. Load-deflection characteristics of superelastic and thermal nickel-titanium wires. Eur. J. Orthod. 2013, 35, 115–123. [Google Scholar] [CrossRef]
- Javid, F.; Angeles, J.; Pasini, D.; Cecere, R. Shape Optimization of a Self-deployable Anchor Designed for Percutaneous Mitral Valve Repair. J. Med. Devices-Trans. Asme 2012, 6, 011003. [Google Scholar] [CrossRef]
- Thompson, S.A. An overview of nickel-titanium alloys used in dentistry. Int. Endod. J. 2000, 33, 297–310. [Google Scholar] [CrossRef]
- Thierry, B.; Merhi, Y.; Bilodeau, L.; Trépanier, C.; Tabrizian, M. Nitinol versus stainless steel stents:: Acute thrombogenicity study in an ex vivo porcine model. Biomaterials 2002, 23, 2997–3005. [Google Scholar] [CrossRef] [PubMed]
- Ryhanen, J.; Kallioinen, M.; Tuukkanen, J.; Junila, J.; Niemela, E.; Sandvik, P.; Serlo, W. In vivo biocompatibility evaluation of nickel-titanium shape memory metal alloy: Muscle and perineural tissue responses and encapsule membrane thickness. J. Biomed. Mater. Res. 1998, 41, 481–488. [Google Scholar] [CrossRef]
- Wang, X.; Kustov, S.; Van Humbeeck, J. A Short Review on the Microstructure, Transformation Behavior and Functional Properties of NiTi Shape Memory Alloys Fabricated by Selective Laser Melting. Materials 2018, 11, 1683. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.V.; Rometsch, P.; Jarvis, T.; Rao, J.; Cao, S.; Davies, C.; Wu, X. Porosity formation mechanisms and fatigue response in Al-Si-Mg alloys made by selective laser melting. Mater. Sci. Eng. A 2018, 712, 166–174. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, Z.; Sun, Z.; Hao, S.; Yang, Y.; Li, M.; Song, C.; Qiu, P.; Cui, L. Selective laser melting of NiTi alloy with superior tensile property and shape memory effect. J. Mater. Sci. Technol. 2019, 35, 2238–2242. [Google Scholar] [CrossRef]
- Carter, L.N.; Martin, C.; Withers, P.J.; Attallah, M.M. The influence of the laser scan strategy on grain structure and cracking behaviour in SLM powder-bed fabricated nickel superalloy. J. Alloys Compd. 2014, 615, 338–347. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Scudino, S.; Eckert, J. Defining the tensile properties of Al-12Si parts produced by selective laser melting. Acta Mater. 2017, 126, 25–35. [Google Scholar] [CrossRef]
- Riemer, A.; Leuders, S.; Thoene, M.; Richard, H.A.; Troester, T.; Niendorf, T. On the fatigue crack growth behavior in 316L stainless steel manufactured by selective laser melting. Eng. Fract. Mech. 2014, 120, 15–25. [Google Scholar] [CrossRef]
- Zhang, Q.; Hao, S.; Liu, Y.; Xiong, Z.; Zhang, Z. The microstructure of a selective laser melting (SLM)-fabricated NiTi shape memory alloy with superior tensile property and shape memory recoverability. Appl. Mater. Today 2020, 19, 100547. [Google Scholar] [CrossRef]
- Bimber, B.A.; Hamilton, R.F.; Palmer, T.A.; Keist, J. Anisotropic microstructure and superelasticity of additive manufactured NiTi alloy bulk builds using laser directed energy deposition. Mater. Sci. Eng. A 2016, 674, 125–134. [Google Scholar] [CrossRef]
- Bagheri, A.; Mahtabi, M.J.; Shamsaei, N. Fatigue behavior and cyclic deformation of additive manufactured NiTi. J. Mater. Process. Technol. 2017, 252, 440–453. [Google Scholar] [CrossRef]
- Moghaddam, N.S.; Saghaian, S.E.; Amerinatanzi, A.; Ibrahim, H.; Li, P.; Toker, G.P.; Karaca, H.E.; Elahinia, M. Anisotropic tensile and actuation properties of NiTi fabricated with selective laser melting. Mater. Sci. Eng. A 2018, 724, 220–230. [Google Scholar] [CrossRef]
- Shi, G.F.; Li, L.X.; Yu, Z.L.; Liu, R.Y.; Sha, P.W.; Xu, Z.Z.; Guo, Y.T.; Xi, R.; Liu, J.B.; Xin, R.L.; et al. The interaction effect of process parameters on the phase transformation behavior and tensile properties in additive manufacturing of Ni-rich NiTi alloy. J. Manuf. Process. 2022, 77, 539–550. [Google Scholar] [CrossRef]
- Wang, X.B.; Yu, J.Y.; Liu, J.W.; Chen, L.G.; Yang, Q.; Wei, H.L.; Sun, J.; Wang, Z.C.; Zhang, Z.H.; Zhao, G.Q.; et al. Effect of process parameters on the phase transformation behavior and tensile properties of NiTi shape memory alloys fabricated by selective laser melting. Addit. Manuf. 2020, 36, 101545. [Google Scholar] [CrossRef]
- Qiu, P.; Gao, P.P.; Wang, S.Y.; Li, Z.H.; Yang, Y.; Zhang, Q.Q.; Xiong, Z.W.; Hao, S.J. Study on corrosion behavior of the selective laser melted NiTi alloy with superior tensile property and shape memory effect. Corros. Sci. 2020, 175, 108891. [Google Scholar] [CrossRef]
- Dongwoo, K.; Jing, L.; Chang, Y.; Haberstroh, K.M.; Webster, T.J. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium. Biomaterials 2008, 29, 970–983. [Google Scholar]
- Webster, T.J.; Ejiofor, J.U. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef]
- Branemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Q.; Yu, Z.; Ren, L.; Zhao, X.; Wang, J. Study on Osseointegration Capability of β-Type Ti–Nb–Zr–Ta–Si Alloy for Orthopedic Implants. Materials 2024, 17, 472. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.H.; Liu, Q.P.; Ren, L.Q.; Wang, J.C. In vitro evaluation of the biocompatibility and bioactivity of a SLM-fabricated NiTi alloy with superior tensile property. J. Mater. Sci.-Mater. M 2024, 35, 52. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Mikulewicz, M.; Chojnacka, K. Release of metal ions from orthodontic appliances by in vitro studies: A systematic literature review. Biol. Trace Elem. Res. 2011, 139, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Habijan, T.; Haberland, C.; Meier, H.; Frenzel, J.; Wittsiepe, J.; Wuwer, C.; Greulich, C.; Schildhauer, T.A.; Köller, M. The biocompatibility of dense and porous Nickel-Titanium produced by selective laser melting. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, A.; Dobkowska, A.; Kijeńska-Gawrońska, E.; Jakubczak, M.; Krawczyńska, A.; Choińska, E.; Jastrzębska, A.; Dean, D.; Wysocki, B.; Święszkowski, W. Biological and Corrosion Evaluation of In Situ Alloyed NiTi Fabricated through Laser Powder Bed Fusion (LPBF). Int. J. Mol. Sci. 2021, 22, 13209. [Google Scholar] [CrossRef]
- Pelton, A.R.; DiCello, J.; Miyazaki, S. Optimisation of processing and properties of medical grade Nitinol wire. Minim. Invasive Ther. Allied Technol. 2000, 9, 107–118. [Google Scholar] [CrossRef]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef]
- Ren, X.D.; Kiosses, W.B.; Sieg, D.J.; Otey, C.A.; Schwartz, M.A. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 2000, 113 Pt 20, 3673–3678. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Rehman, F.U.; Zhao, C.; Liu, B.; He, N. Recent advances in nano scaffolds for bone repair. Bone Res. 2016, 4, 16050. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Jiang, Y.; Liu, H.; Song, S.; Wang, C.; Li, Z.; Yang, Z.; Liu, H.; Wang, J. Biomimetic Composite Scaffolds to Manipulate Stem Cells for Aiding Rheumatoid Arthritis Management. Adv. Funct. Mater. 2019, 29, 1807860. [Google Scholar] [CrossRef]
- Zou, L.; Zhou, L.; Yang, C.; Qu, S.; Li, Y. Unusual dry sliding tribological behavior of biomedical ultrafine-grained TiNbZrTaFe composites fabricated by powder metallurgy. J. Mater. Res. 2014, 29, 902–909. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Wang, Q.; Han, J.; Wang, W.; Li, S.; Liu, H.; Guo, S.; Zhang, J.; Ge, K.; et al. Graphene Oxide/Chitosan/Hydroxyapatite Composite Membranes Enhance Osteoblast Adhesion and Guided Bone Regeneration. Acs Appl. Bio Mater. 2021, 4, 8049–8059. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Tosa, I.; Yamada, D.; Yasumatsu, M.; Hinoi, E.; Ono, M.; Oohashi, T.; Kuboki, T.; Takarada, T. Postnatal Runx2 deletion leads to low bone mass and adipocyte accumulation in mice bone tissues. Biochem. Biophys. Res. Commun. 2019, 516, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Liu, L.; Mu, H.; Veeraraghavan, V.P. Nobiletin promotes osteogenic differentiation of human osteoblastic cell line (MG-63) through activating the BMP-2/RUNX-2 signaling pathway. Saudi J. Biol. Sci. 2021, 28, 4916–4920. [Google Scholar] [CrossRef]
- Kovacs, B.; Vajda, E.; Nagy, E.E. Regulatory Effects and Interactions of the Wnt and OPG-RANKL-RANK Signaling at the Bone-Cartilage Interface in Osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4653. [Google Scholar] [CrossRef] [PubMed]
- Bagherifard, S.; Ghelichi, R.; Khademhosseini, A.; Guagliano, M. Cell Response to Nanocrystallized Metallic Substrates Obtained through Severe Plastic Deformation. ACS Appl. Mater. Interfaces 2014, 6, 7963–7985. [Google Scholar] [CrossRef]
- Li, H.F.; Nie, F.L.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Valiev, R.Z. Nanocrystalline Ti49.2Ni50.8 shape memory alloy as orthopaedic implant material with better performance. J. Mater. Sci. Technol. 2019, 35, 2156–2162. [Google Scholar] [CrossRef]
| Time | Cell Viability | |
|---|---|---|
| XY-NiTi | XZ-NiTi | |
| Day 2 | 109.24% | 93.42% |
| Day 4 | 105.05% | 95.12% |
| Day 6 | 103.18% | 103.52% |
| Gene Subtype | Oligonucleotide Primers (5′–3′) |
|---|---|
| ALP | F: ATCGGACCCTGCCTTACC R: CTCTTGGGCTTGCTGTCG |
| RUNX2 | F: ACTACCAGCCACCGAGACCA R: ACTGCTTGCAGCCTTAAATGACTCT |
| OPG | F: CAAATGTCCTCCTGGCACCT R: TTCACTGGTGTGCCAAGTGT |
| OCN | F: AGCCACCGAGACACCATGAGA R: AGCCACCGAGACACCATGAGA |
| GAPDH | F: CAATGACCCCTTCATTGACC R: TGGACTCCACGACGTACTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Yu, Z.; Liu, Q.; Ren, L.; Zhao, X.; Wang, J. The Anisotropic Osteoinductive Capacity of a Nickel–Titanium Alloy Fabricated Through Laser Powder Bed Fusion. Int. J. Mol. Sci. 2025, 26, 4640. https://doi.org/10.3390/ijms26104640
Sun Y, Yu Z, Liu Q, Ren L, Zhao X, Wang J. The Anisotropic Osteoinductive Capacity of a Nickel–Titanium Alloy Fabricated Through Laser Powder Bed Fusion. International Journal of Molecular Sciences. 2025; 26(10):4640. https://doi.org/10.3390/ijms26104640
Chicago/Turabian StyleSun, Yu, Zhenglei Yu, Qingping Liu, Luquan Ren, Xin Zhao, and Jincheng Wang. 2025. "The Anisotropic Osteoinductive Capacity of a Nickel–Titanium Alloy Fabricated Through Laser Powder Bed Fusion" International Journal of Molecular Sciences 26, no. 10: 4640. https://doi.org/10.3390/ijms26104640
APA StyleSun, Y., Yu, Z., Liu, Q., Ren, L., Zhao, X., & Wang, J. (2025). The Anisotropic Osteoinductive Capacity of a Nickel–Titanium Alloy Fabricated Through Laser Powder Bed Fusion. International Journal of Molecular Sciences, 26(10), 4640. https://doi.org/10.3390/ijms26104640







