A New Bromelain-Based Polyenzymatic Complex Plus N-Acetylcysteine: A Promising Approach for the Treatment of Urinary Tract Infections
Abstract
1. Introduction
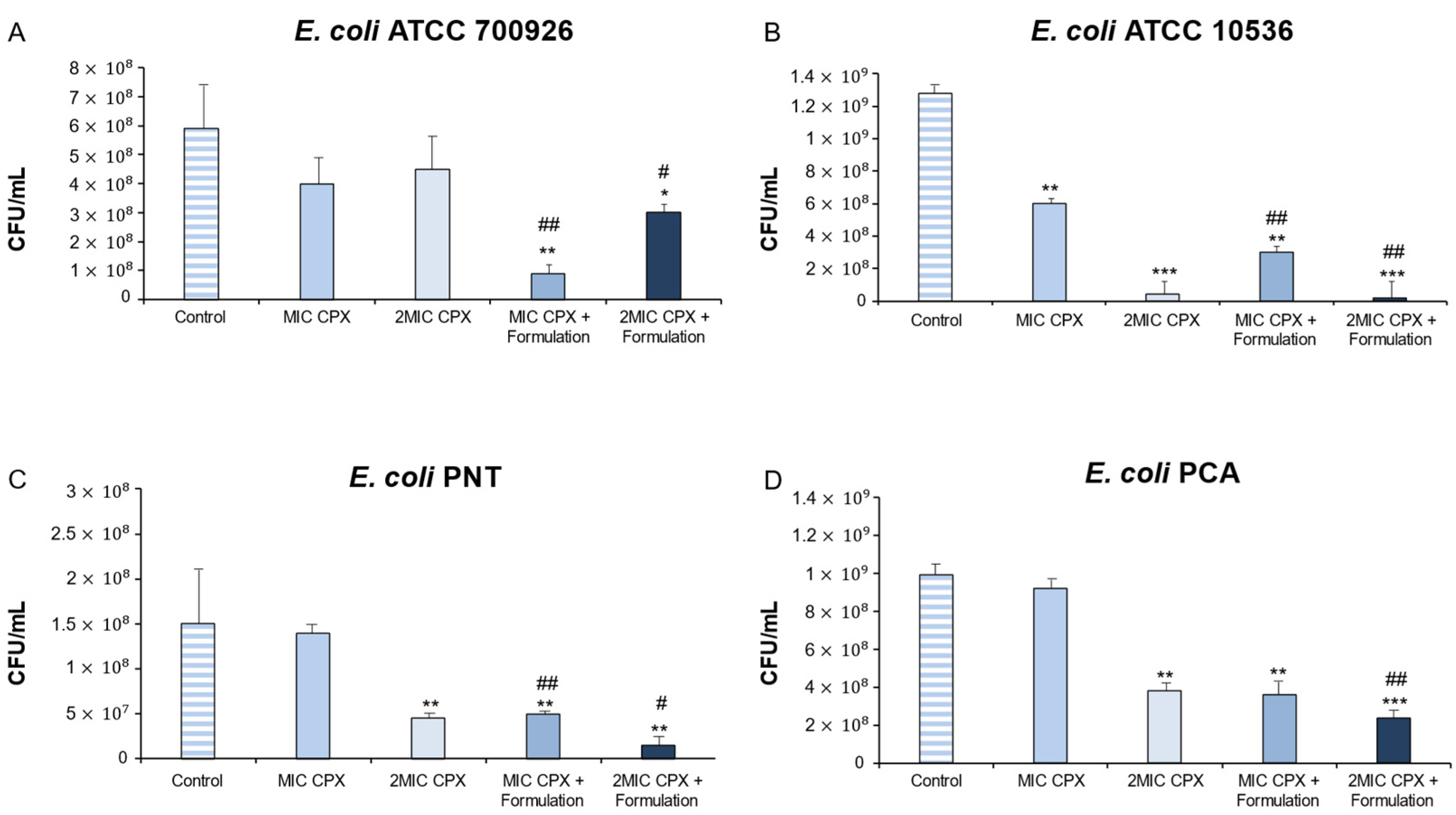
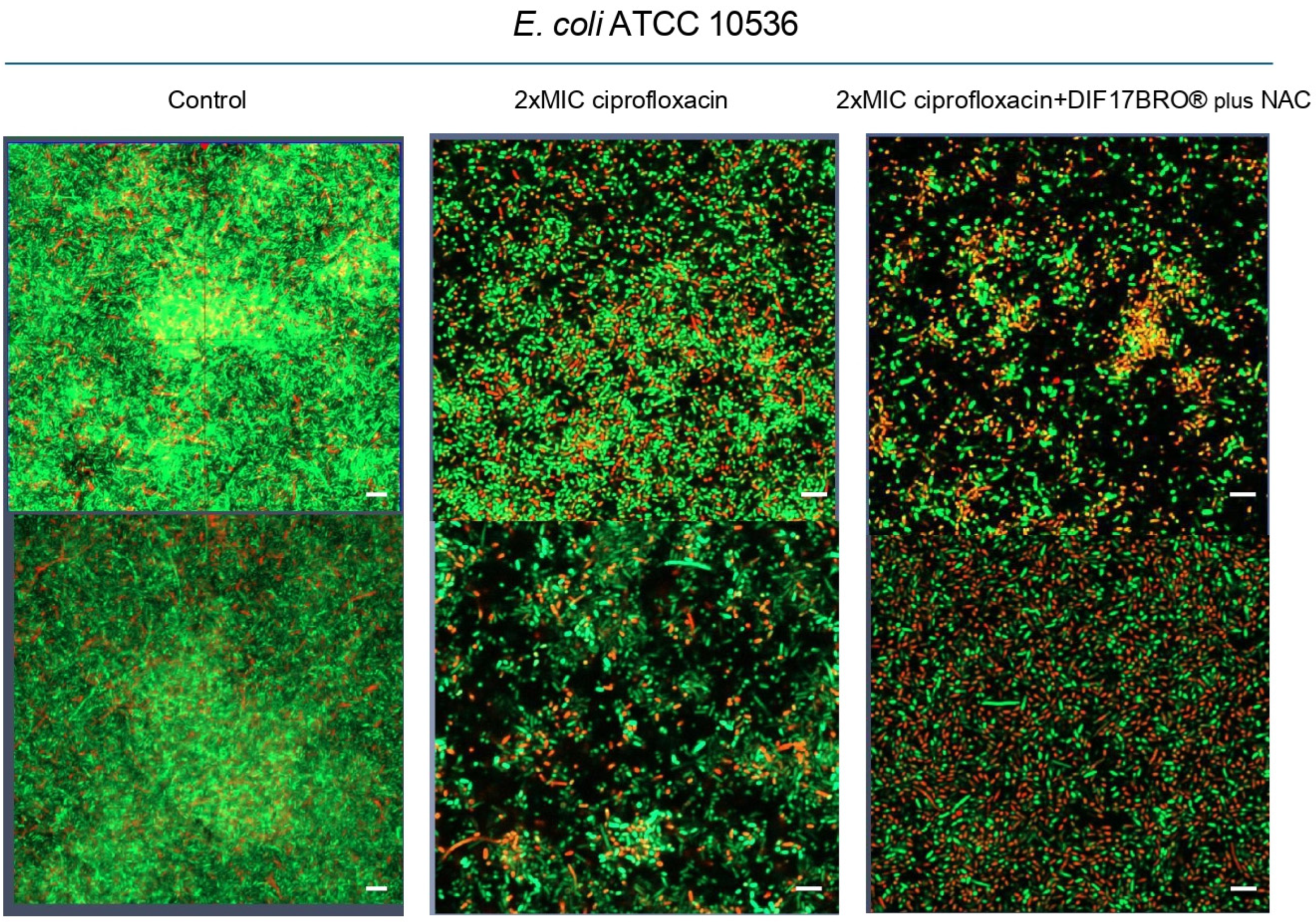
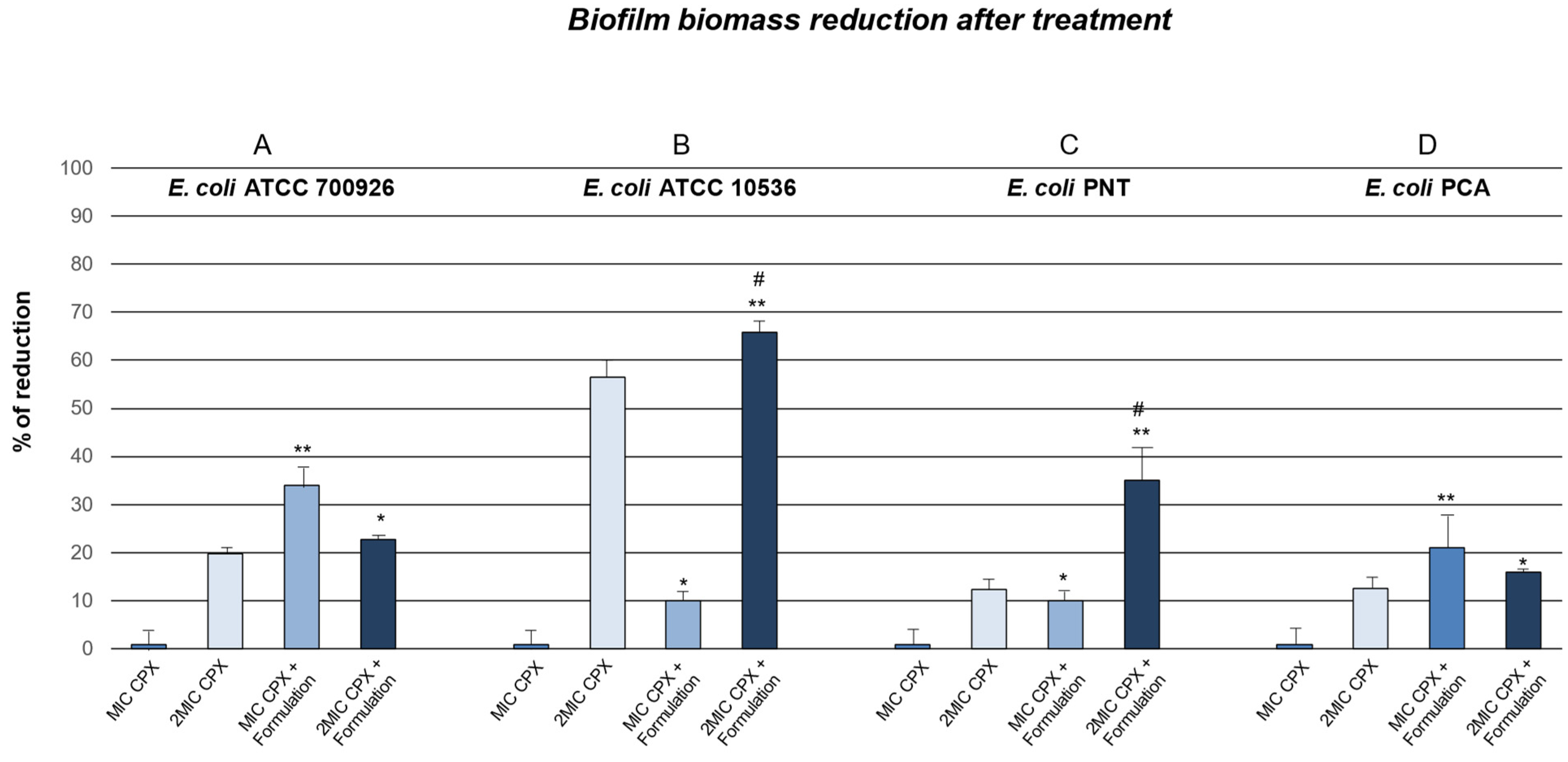
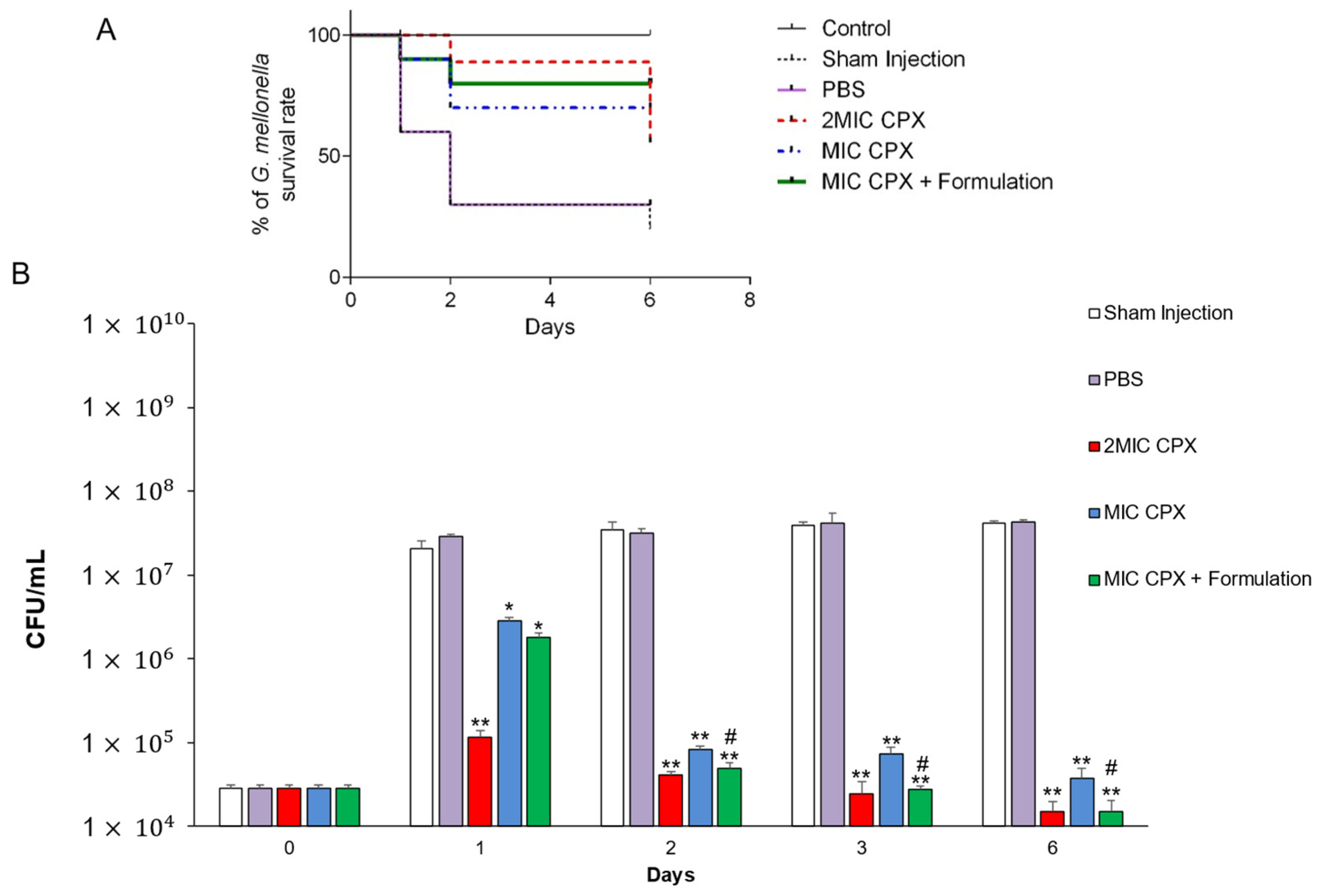
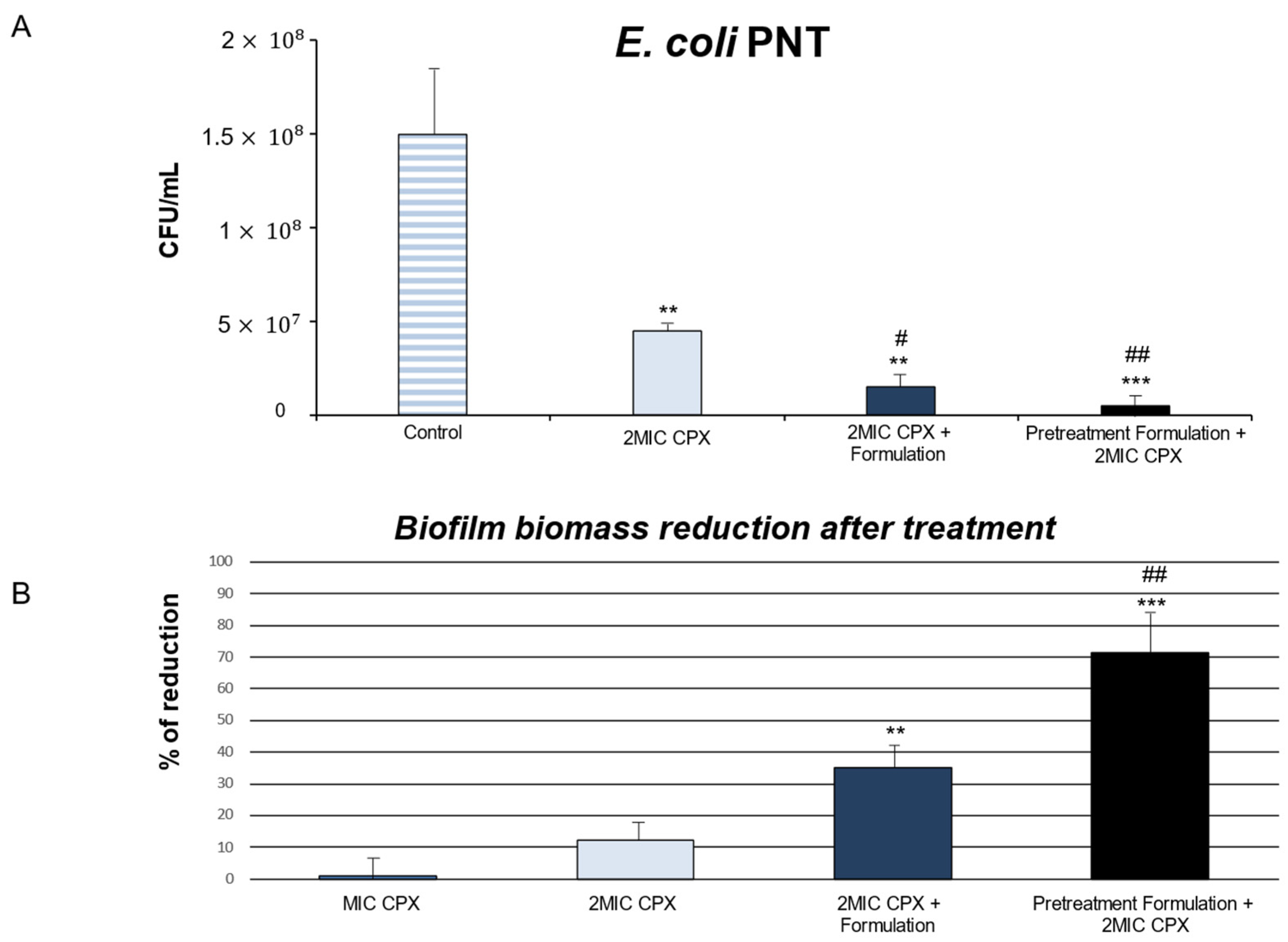
2. Results and Discussion
3. Materials and Methods
3.1. Bacterial Cultures
3.2. Antimicrobial Activity of Ciprofloxacin
3.3. Anti-Biofilm Effect of Ciprofloxacin Alone and in Combination with Formulation (DIF17BRO® Plus NAC)
3.4. In Vivo Galleria Mellonella Infection Assay
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Lila, A.S.A.; Rajab, A.A.H.; Abdallah, M.H.; Rizvi, S.M.D.; Moin, A.; Khafagy, E.-S.; Tabrez, S.; Hegazy, W.A.H. Biofilm Lifestyle in Recurrent Urinary Tract Infections. Life 2023, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From Biofilm Formation to New Antibiofilm Strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ahn, J.; Choi, W.S.; Park, H.K.; Kim, S.; Paick, S.H.; Kim, H.G. What is the Cause of Recurrent Urinary Tract Infection? Contemporary Microscopic Concepts of Pathophysiology. Int. Neurourol. J. 2021, 25, 192–201. [Google Scholar] [CrossRef]
- Sheikhi, R.; Amini, M.E.; Alidoust, L.; Atrkar Roushan, Z.; Nikokar, I. Evaluation of adhesin genes and risk factors associated with urinary tract infections by drug-resistant uropathogenic Escherichia coli in North of Iran. J. Infect. Dev. Ctries. 2024, 18, 761–769. [Google Scholar] [CrossRef]
- Robino, L.; Sauto, R.; Morales, C.; Navarro, N.; González, M.J.; Cruz, E.; Neffa, F.; Zeballos, J.; Scavone, P. Presence of intracellular bacterial communities in uroepithelial cells, a potential reservoir in symptomatic and non-symptomatic people. BMC Infect. Dis. 2024, 24, 590. [Google Scholar] [CrossRef]
- Alshaikh, S.A.; El-Banna, T.; Sonbol, F.; Farghali, M.H. Correlation between antimicrobial resistance, biofilm formation, and virulence determinants in uropathogenic Escherichia coli from Egyptian hospital. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 20. [Google Scholar] [CrossRef]
- Amante, C.; De Soricellis, C.; Luccheo, G.; Luccheo, L.; Russo, P.; Aquino, R.P.; Del Gaudio, P. Flogomicina: A Natural Antioxidant Mixture as an Alternative Strategy to Reduce Biofilm Formation. Life 2023, 13, 1005. [Google Scholar] [CrossRef]
- Mancini, A.; Pucciarelli, S.; Lombardi, F.E.; Barocci, S.; Pauri, P.; Lodolini, S. Differences between Community—And Hospital—Acquired urinary tract infections in a tertiary care hospital. New Microbiol. 2020, 43, 17–21. [Google Scholar]
- Jancel, T.; Dudas, V. Management of Uncomplicated Urinary Tract Infections. West. J. Med. 2002, 176, 51–55. [Google Scholar] [CrossRef]
- Olin, S.J.; Bartges, J.W. Urinary tract infections: Treatment/comparative therapeutics. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 721–746. [Google Scholar] [CrossRef]
- Shaheen, G.; Akram, M.; Jabeen, F.; Ali Shah, S.M.; Munir, N.; Daniyal, M.; Riaz, M.; Tahir, I.M.; Ghauri, A.O.; Sultana, S.; et al. Therapeutic Potential of Medicinal Plants for the Management of Urinary Tract Infection: A Systematic Review. Clin. Exp. Pharmacol. Physiol. 2019, 46, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Das, S. Natural Therapeutics for Urinary Tract Infections-a Review. Future J. Pharm. Sci. 2020, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options. Int. J. Mol. Sci. 2023, 24, 10537. [Google Scholar] [CrossRef]
- Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain a Potential Bioactive Compound: A Comprehensive Overview from a Pharmacological Perspective. Life 2021, 11, 317, Erratum in Life 2024, 14, 483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Recinella, L.; Pinti, M.; Libero, M.L.; Di Lodovico, S.; Veschi, S.; Piro, A.; Generali, D.; Acquaviva, A.; Nilofar, N.; Orlando, G.; et al. Beneficial Effects Induced by a Proprietary Blend of a New Bromelain-Based Polyenzymatic Complex Plus N-Acetylcysteine in Urinary Tract Infections: Results from In Vitro and Ex Vivo Studies. Antibiotics 2024, 13, 985. [Google Scholar] [CrossRef]
- Pecoraro, C.; Carbone, D.; Parrino, B.; Cascioferro, S.; Diana, P. Recent Developments in the Inhibition of Bacterial Adhesion as Promising Anti-Virulence Strategy. Int. J. Mol. Sci. 2023, 24, 4872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flemming, H.-C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Zhang, T.; Ray, S.; Melican, K.; Richter-Dahlfors, A. The maturation of native uropathogenic Escherichia coli biofilms seen through a non-interventional lens. Biofilm 2024, 8, 100212. [Google Scholar] [CrossRef]
- Römling, U. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell. Mol. Life Sci. 2005, 62, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.O.; Richter, A.M.; Hengge, R. Cellulose as an architectural element in spatially structured Escherichia coli biofilms. J. Bacteriol. 2013, 195, 5540–5554. [Google Scholar] [CrossRef]
- Wang, S.L.; Lin, H.T.; Liang, T.W.; Chen, Y.J.; Yen, Y.H.; Guo, S.P. Reclamation of chitinous materials by bromelain for the preparation of antitumor and antifungal materials. Bioresour. Technol. 2008, 99, 4386–4393. [Google Scholar] [CrossRef] [PubMed]
- Jančič, U.; Gorgieva, S. Bromelain and Nisin: The Natural Antimicrobials with High Potential in Biomedicine. Pharmaceutics 2021, 14, 76. [Google Scholar] [CrossRef]
- Carter, C.J.; Pillai, K.; Badar, S.; Mekkawy, A.H.; Akhter, J.; Jefferies, T.; Valle, S.J.; Morris, D.L. Dissolution of Biofilm Secreted by Three Different Strains of Pseudomonas aeruginosa with Bromelain, N-Acetylcysteine, and Their Combinations. Appl. Sci. 2021, 11, 11388. [Google Scholar] [CrossRef]
- El-Feky, M.A.; El-Rehewy, M.S.; Hassan, M.A.; Abolella, H.A.; Abd El-Baky, R.M.; Gad, G.F. Effect of ciprofloxacin and N-acetylcysteine on bacterial adherence and biofilm formation on ureteral stent surfaces. Pol. J. Microbiol. 2009, 58, 261–267. [Google Scholar] [PubMed]
- Tsai, C.J.-Y.; Loh, J.M.S.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef]
- Sugeçti, S. Biochemical and immune responses of model organism Galleria mellonella after infection with Escherichia coli. Entomol. Exp. Appl. 2021, 169, 911–917. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute [CLSI]. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Di Fermo, P.; Ciociola, T.; Di Lodovico, S.; D’ercole, S.; Petrini, M.; Giovati, L.; Conti, S.; Di Giulio, M.; Cellini, L. Antimicrobial Peptide L18R Displays a Modulating Action against Inter-Kingdom Biofilms in the Lubbock Chronic Wound Biofilm Model. Microorganisms 2021, 9, 1779. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Napoli, E.; Di Campli, E.; Di Fermo, P.; Gentile, D.; Ruberto, G.; Nostro, A.; Marini, E.; Cellini, L.; Di Giulio, M. Pistacia vera L. oleoresin and levofloxacin is a synergistic combination against resistant Helicobacter pylori strains. Sci. Rep. 2019, 9, 4646. [Google Scholar] [CrossRef]
- Romano, L.; Napolitano, L.; Crocetto, F.; Sciorio, C.; Priadko, K.; Fonticelli, M.; Federico, A.; Romano, M.; Gravina, A.G. The potential therapeutic role of Hericium erinaceus extract in pathologic conditions involving the urogenital-gut axis: Insights into the involved mechanisms and mediators. J. Physiol. Pharmacol. 2024, 75, 3–9. [Google Scholar] [CrossRef]
- Sha, S.P.; Modak, D.; Sarkar, S.; Roy, S.K.; Sah, S.P.; Ghatani, K.; Bhattacharjee, S. Fruit waste: A current perspective for the sustainable production of pharmacological, nutraceutical, and bioactive resources. Front. Microbiol. 2023, 14, 1260071. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recinella, L.; Pinti, M.; Di Lodovico, S.; Brenciani, A.; Giovanetti, E.; Diban, F.; Di Giulio, M.; Brunetti, L.; Leone, S. A New Bromelain-Based Polyenzymatic Complex Plus N-Acetylcysteine: A Promising Approach for the Treatment of Urinary Tract Infections. Int. J. Mol. Sci. 2025, 26, 4639. https://doi.org/10.3390/ijms26104639
Recinella L, Pinti M, Di Lodovico S, Brenciani A, Giovanetti E, Diban F, Di Giulio M, Brunetti L, Leone S. A New Bromelain-Based Polyenzymatic Complex Plus N-Acetylcysteine: A Promising Approach for the Treatment of Urinary Tract Infections. International Journal of Molecular Sciences. 2025; 26(10):4639. https://doi.org/10.3390/ijms26104639
Chicago/Turabian StyleRecinella, Lucia, Morena Pinti, Silvia Di Lodovico, Andrea Brenciani, Eleonora Giovanetti, Firas Diban, Mara Di Giulio, Luigi Brunetti, and Sheila Leone. 2025. "A New Bromelain-Based Polyenzymatic Complex Plus N-Acetylcysteine: A Promising Approach for the Treatment of Urinary Tract Infections" International Journal of Molecular Sciences 26, no. 10: 4639. https://doi.org/10.3390/ijms26104639
APA StyleRecinella, L., Pinti, M., Di Lodovico, S., Brenciani, A., Giovanetti, E., Diban, F., Di Giulio, M., Brunetti, L., & Leone, S. (2025). A New Bromelain-Based Polyenzymatic Complex Plus N-Acetylcysteine: A Promising Approach for the Treatment of Urinary Tract Infections. International Journal of Molecular Sciences, 26(10), 4639. https://doi.org/10.3390/ijms26104639










