The Plant Alkaloid Harmaline Blocks the Voltage-Gated Sodium Channel Nav1.7: A Study Using an Automated Patch-Clamp
Abstract
1. Introduction
2. Results
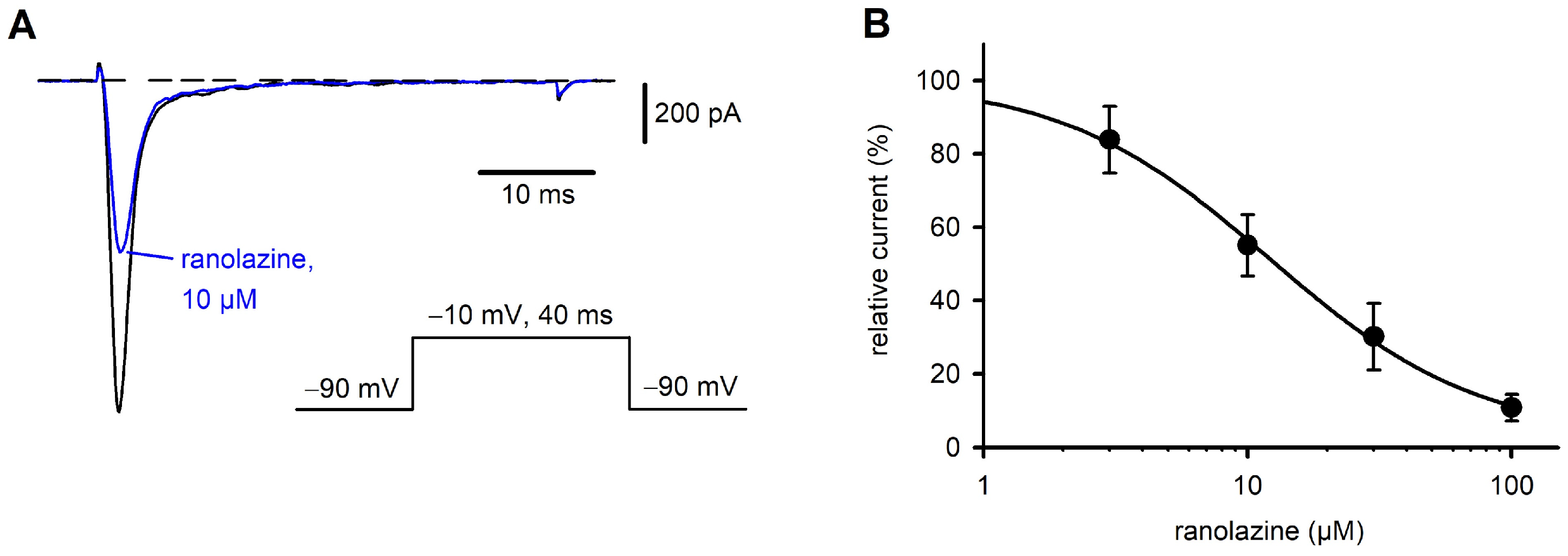
2.1. Characterization of Nav1.7 Currents and Inhibition by Ranolazine
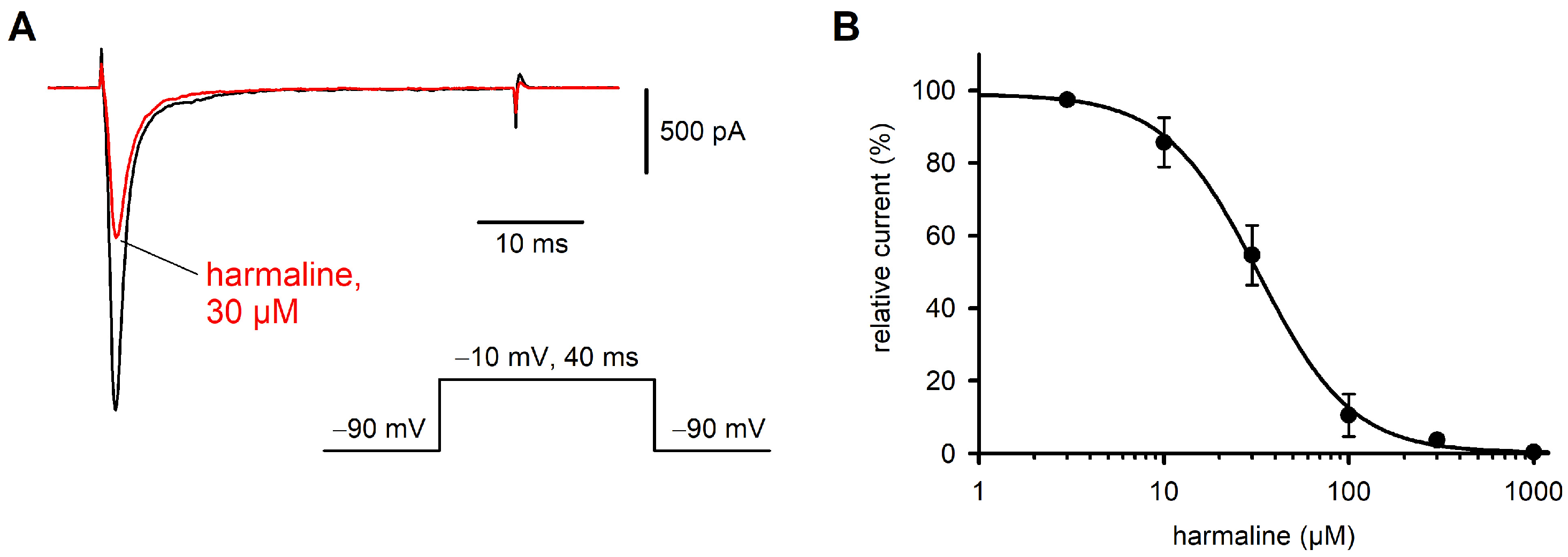
2.2. Effect of Harmaline on Nav1.7 Currents
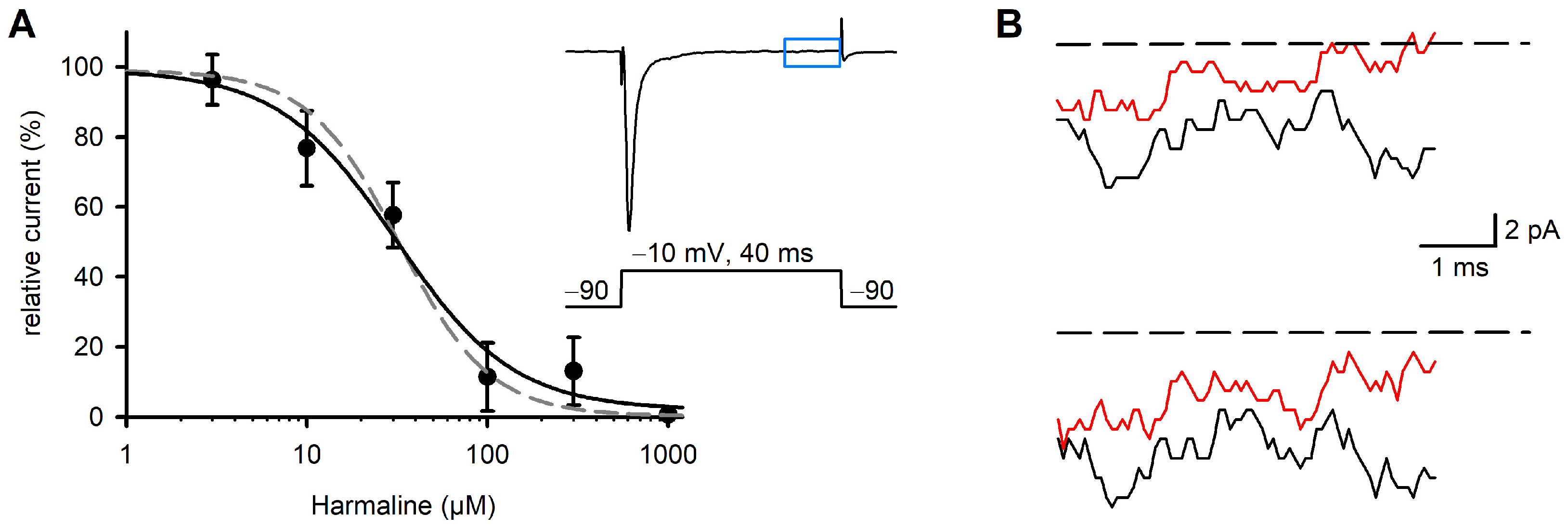
2.3. Effect of Harmaline on Late Na+ Currents
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Solutions and Drugs
4.3. Electrophysiological Recordings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHO | Chinese hamster ovary cells |
| CNS | Central nervous system |
| DRG | Dorsal root ganglion |
| IO | Inferior olive |
| MAOs | Monoamine oxidases |
| Nav1.7 | Voltage-gated sodium channel, α-subunit 9 |
| NO | Nitric oxide |
| SCN9A | Gene, encoding the α-subunit of the voltage-gated sodium channel Nav1.7 |
| TTX | Tetrodotoxin |
References
- Rharrabe, K.; Bakrim, A.; Ghailani, N.; Sayah, F. Bioinsecticidal effect of harmaline on Plodia interpunctella development (Lepidoptera: Pyralidae). Pest. Biochem. Physiol. 2007, 89, 137–145. [Google Scholar] [CrossRef]
- Arshad, N.; Zitterl-Eglseer, K.; Hasnain, S.; Hess, M. Effect of Peganum harmala or its beta-carboline alkaloids on certain antibiotic resistant strains of bacteria and protozoa from poultry. Phytother. Res. 2008, 22, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Farouk, L.; Laroubi, A.; Aboufatima, R.; Benharref, A.; Chait, A. Evaluation of the analgesic effect of alkaloid extract of Peganum harmala L.: Possible mechanisms involved. J. Ethnopharmacol. 2008, 115, 449–454. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Iqbal, Z.; Malik, I. Recent pharmacological developments in beta-carboline alkaloid “harmaline”. Eur. J. Pharmacol. 2013, 721, 391–394. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Wang, Y.; He, X. Cytotoxic indole alkaloids against human leukemia cell lines from the toxic plant Peganum harmala. Toxins 2015, 7, 4507–4518. [Google Scholar] [CrossRef]
- Mina, C.N.; Farzaei, M.H.; Gholamreza, A. Medicinal properties of Peganum harmala L. in traditional Iranian medicine and modern phytotherapy: A review. J. Tradit. Chin. Med. 2015, 35, 104–109. [Google Scholar]
- Berrougui, H.; Martin-Cordero, C.; Khalil, A.; Hmamouchi, M.; Ettaib, A.; Marhuenda, E.; Herrera, M.D. Vasorelaxant effects of harmine and harmaline extracted from Peganum harmala L. seeds in isolated rat aorta. Pharmacol. Res. 2006, 54, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.C.; Liao, J.F.; Chen, C.F. Spasmolytic effects of three harmala alkaloids on guinea-pig isolated trachea. Pharmacol. Toxicol. 2001, 89, 259–264. [Google Scholar] [CrossRef]
- Handforth, A. Harmaline tremor: Underlying mechanisms in a potential animal model of essential tremor. Tremor Other Hyperkinet. Mov. 2012, 2, 02-92-769-1. [Google Scholar] [CrossRef]
- Lee, J.; Kim, I.; Lee, J.; Knight, E.; Cheng, L.; Kang, S.I.; Jang, D.P.; Chang, S.Y. Development of harmaline-induced tremor in a swine model. Tremor Other Hyperkinet. Mov. 2018, 8, 532. [Google Scholar] [CrossRef]
- Zhan, X.; Graf, W.M. Harmaline attenuates voltage—Sensitive Ca2+ currents in neurons of the inferior olive. J. Pharm. Pharm. Sci. 2012, 15, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Do, L.V.; Zou, L.; Zhan, R.S.; Jones, M.; Nawaz, S.; Manaye, K. Harmaline toxicity on dorsal striatal neurons and its role in tremor. Neurotoxicology 2023, 99, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.J.; Houghton, P.J.; Rose, S.; Jenner, P.; Lees, A.D. Activities of extract and constituents of Banisteriopsis caapi relevant to parkinsonism. Pharmacol. Biochem. Behav. 2003, 75, 627–633. [Google Scholar] [CrossRef]
- Monsef, H.R.; Ghobadi, A.; Iranshahi, M.; Abdollahi, M. Antinociceptive effects of Peganum harmala L. alkaloid extract on mouse formalin test. J. Pharm. Pharm. Sci. 2004, 7, 65–69. [Google Scholar]
- Shoaib, M.; Shah, S.W.; Ali, N.; Shah, I.; Ullah, S.; Ghias, M.; Tahir, M.N.; Gul, F.; Akhtar, S.; Ullah, A.; et al. Scientific investigation of crude alkaloids from medicinal plants for the management of pain. BMC Complement. Altern. Med. 2016, 16, 178. [Google Scholar] [CrossRef]
- Abolhassanzadeh, Z.; Aflaki, E.; Yousefi, G.; Mohagheghzadeh, A. Randomized clinical trial of peganum oil for knee osteoarthritis. J. Evid. Based Complement. Altern. Med. 2015, 20, 126–131. [Google Scholar] [CrossRef]
- Bennett, D.L.; Woods, C.G. Painful and painless channelopathies. Lancet Neurol. 2014, 13, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Estacion, M.; Waxman, S.G.; Dib-Hajj, S.D. Effects of ranolazine on wild-type and mutant hNav1.7 channels and on DRG neuron excitability. Mol. Pain. 2010, 6, 35. [Google Scholar] [CrossRef]
- Chevalier, M.; Amuzescu, B.; Gawali, V.; Todt, H.; Knott, T.; Scheel, O.; Abriel, H. Late cardiac sodium current can be assessed using automated patch-clamp. F1000Res 2014, 3, 245. [Google Scholar] [CrossRef][Green Version]
- Eisfeld, J.; Schumacher, M.; Krautwald, M.; Wierschke, S.; Fechtali, T.; Brinkmeier, H. The plant alkaloid harmaline blocks the human voltage-gated sodium channel Nav1.7 expressed in CHO cells: A study using automated patch clamp. Acta Physiol. 2017, 219 (Suppl. S711), 59. [Google Scholar]
- Rajamani, S.; Shryock, J.C.; Belardinelli, L. Block of tetrodotoxin-sensitive, Nav1.7 and tetrodotoxin-resistant, Nav1.8, Na+ channels by ranolazine. Channels 2008, 2, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.K.; Calderon, J.; Wang, S.Y. State- and use-dependent block of muscle Nav1.4 and neuronal Nav1.7 voltage-gated Na+ channel isoforms by ranolazine. Mol. Pharmacol. 2008, 73, 940–948. [Google Scholar] [CrossRef]
- Zhang, Y.; Otto, P.; Qin, L.; Eiber, N.; Hashemolhosseini, S.; Kröger, S.; Brinkmeier, H. Methocarbamol blocks muscular Nav1.4 channels and decreases isometric force of mouse muscles. Muscle Nerve 2021, 63, 141–150. [Google Scholar] [CrossRef]
- Splettstoesser, F.; Bonnet, U.; Wiemann, M.; Bingmann, D.; Busselberg, D. Modulation of voltage-gated channel currents by harmaline and harmane. Br. J. Pharmacol. 2005, 144, 52–58. [Google Scholar] [CrossRef]
- Park, Y.G.; Choi, J.H.; Lee, C.; Kim, S.; Kim, Y.; Chang, K.Y.; Paek, S.H.; Kim, D. Heterogeneity of tremor mechanisms assessed by tremor-related cortical potential in mice. Mol. Brain 2010, 8, 3. [Google Scholar] [CrossRef]
- Karaki, H.; Kishimoto, T.; Ozaki, H.; Sakata, K.; Umeno, H.; Urakawa, N. Inhibition of calcium channels by harmaline and other harmala alkaloids in vascular and intestinal smooth muscles. Br. J. Pharmacol. 1986, 89, 367–375. [Google Scholar] [CrossRef] [PubMed]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-gated sodium channels: Structure, function, pharmacology, and clinical indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef] [PubMed]
- Elajnaf, T.; Baptista-Hon, D.T.; Hales, T.G. Potent inactivation-dependent inhibition of adult and neonatal Nav1.5 channels by lidocaine and levobupivacaine. Anesth. Analg. 2018, 127, 650–660. [Google Scholar] [CrossRef]
- Denomme, N.; Lukowski, A.L.; Hull, J.M.; Jameson, M.B.; Bouza, A.A.; Narayan, A.R.H.; Isom, L.L. The voltage-gated sodium channel inhibitor, 4,9-anhydrotetrodotoxin, blocks human Nav1.1 in addition to Nav1.6. Neurosci. Lett. 2020, 724, 134853. [Google Scholar] [CrossRef]
- Jiang, B.; Meng, L.; Zou, N.; Wang, H.; Li, S.; Huang, L.; Cheng, X.; Wang, Z.; Chen, W.; Wang, C. Mechanism-based pharmacokinetics-pharmacodynamics studies of harmine and harmaline on neurotransmitters regulatory effects in healthy rats: Challenge on monoamine oxidase and acetylcholinesterase inhibition. Phytomedicine 2019, 62, 152967. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisfeld, J.; Schumacher, M.; Krautwald, M.; Wierschke, S.; Qin, L.; Fechtali, T.; Brinkmeier, H. The Plant Alkaloid Harmaline Blocks the Voltage-Gated Sodium Channel Nav1.7: A Study Using an Automated Patch-Clamp. Int. J. Mol. Sci. 2025, 26, 4636. https://doi.org/10.3390/ijms26104636
Eisfeld J, Schumacher M, Krautwald M, Wierschke S, Qin L, Fechtali T, Brinkmeier H. The Plant Alkaloid Harmaline Blocks the Voltage-Gated Sodium Channel Nav1.7: A Study Using an Automated Patch-Clamp. International Journal of Molecular Sciences. 2025; 26(10):4636. https://doi.org/10.3390/ijms26104636
Chicago/Turabian StyleEisfeld, Jörg, Marina Schumacher, Mirjam Krautwald, Stephan Wierschke, Lu Qin, Taoufiq Fechtali, and Heinrich Brinkmeier. 2025. "The Plant Alkaloid Harmaline Blocks the Voltage-Gated Sodium Channel Nav1.7: A Study Using an Automated Patch-Clamp" International Journal of Molecular Sciences 26, no. 10: 4636. https://doi.org/10.3390/ijms26104636
APA StyleEisfeld, J., Schumacher, M., Krautwald, M., Wierschke, S., Qin, L., Fechtali, T., & Brinkmeier, H. (2025). The Plant Alkaloid Harmaline Blocks the Voltage-Gated Sodium Channel Nav1.7: A Study Using an Automated Patch-Clamp. International Journal of Molecular Sciences, 26(10), 4636. https://doi.org/10.3390/ijms26104636




