Apoptosis and G2/M Phase Cell Cycle Arrest Induced by Alkaloid Erythraline Isolated from Erythrina velutina in SiHa Cervical Cancer Cell
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization of the Alkaloid-Enriched Fraction from Erythrina velutina Leaves
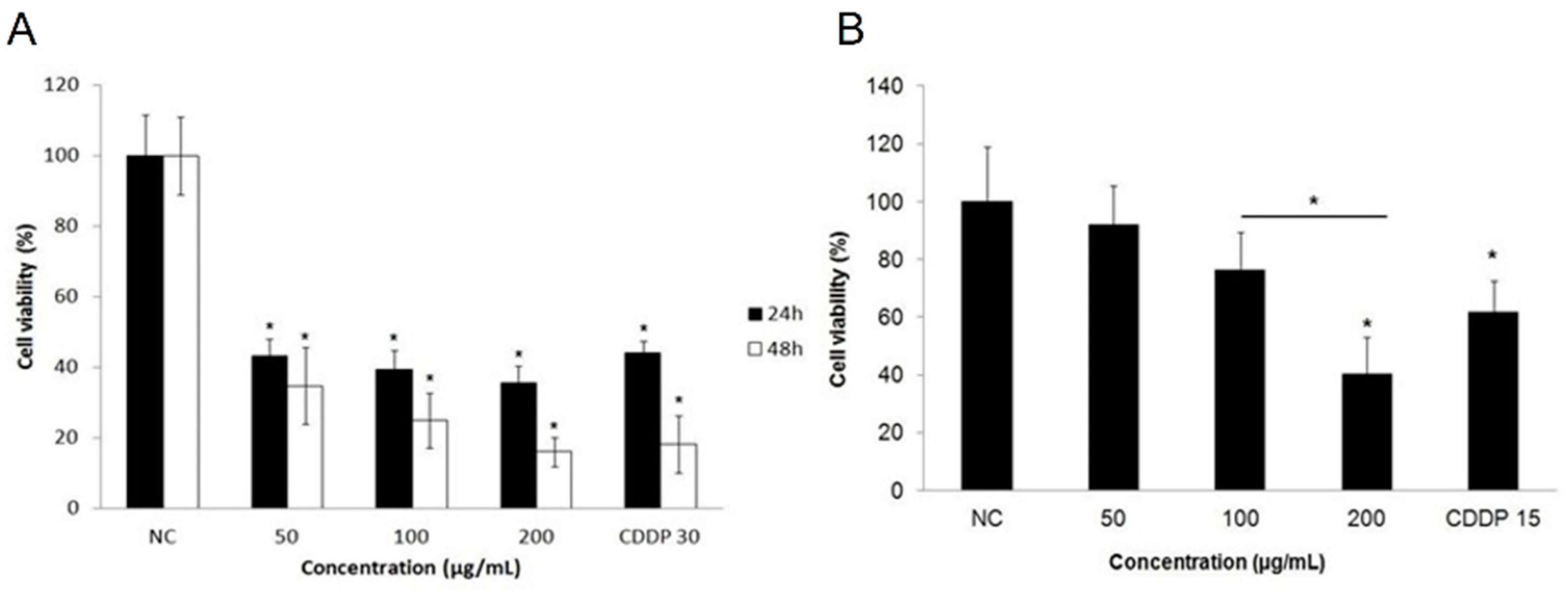
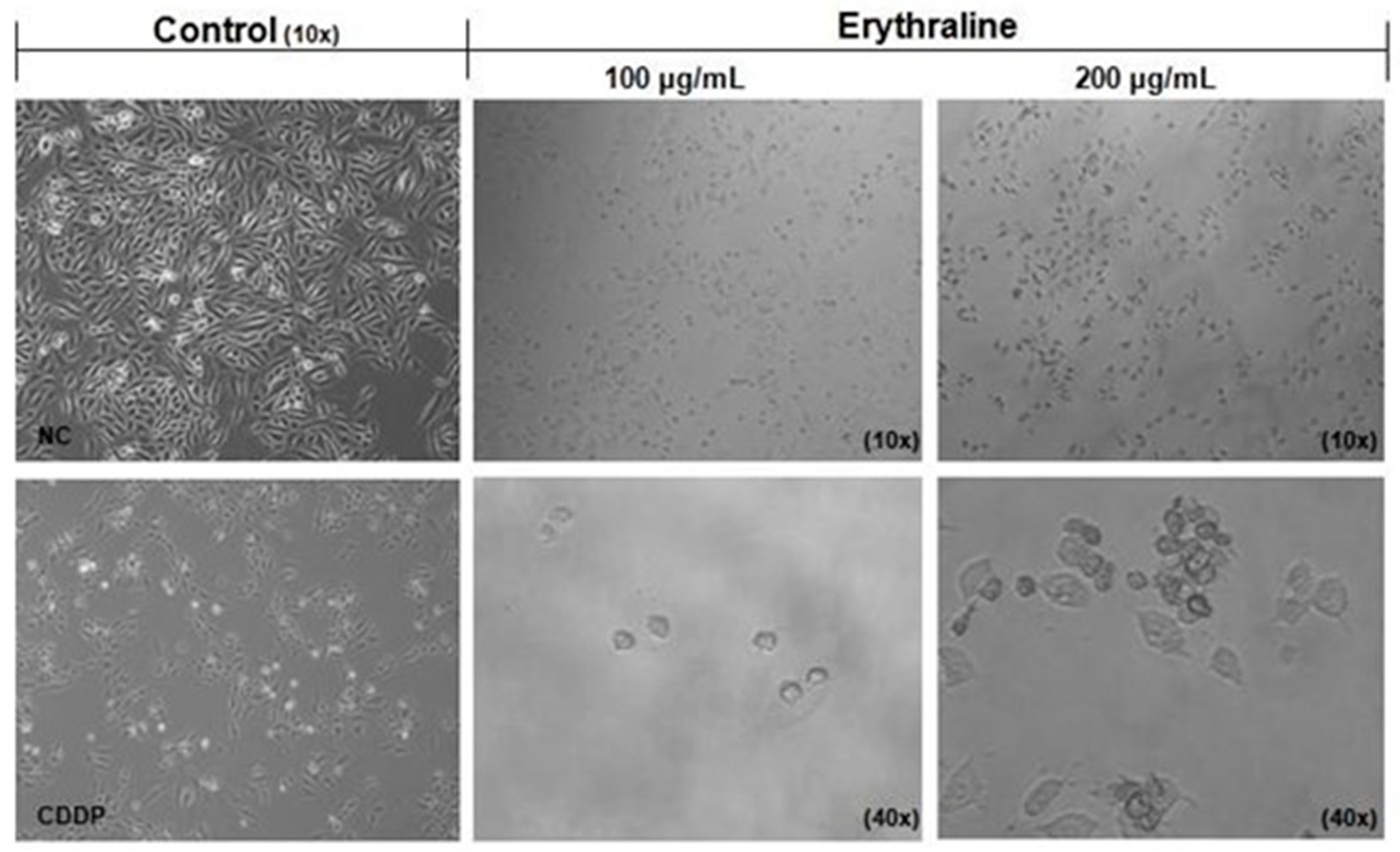
2.2. Cytotoxicity
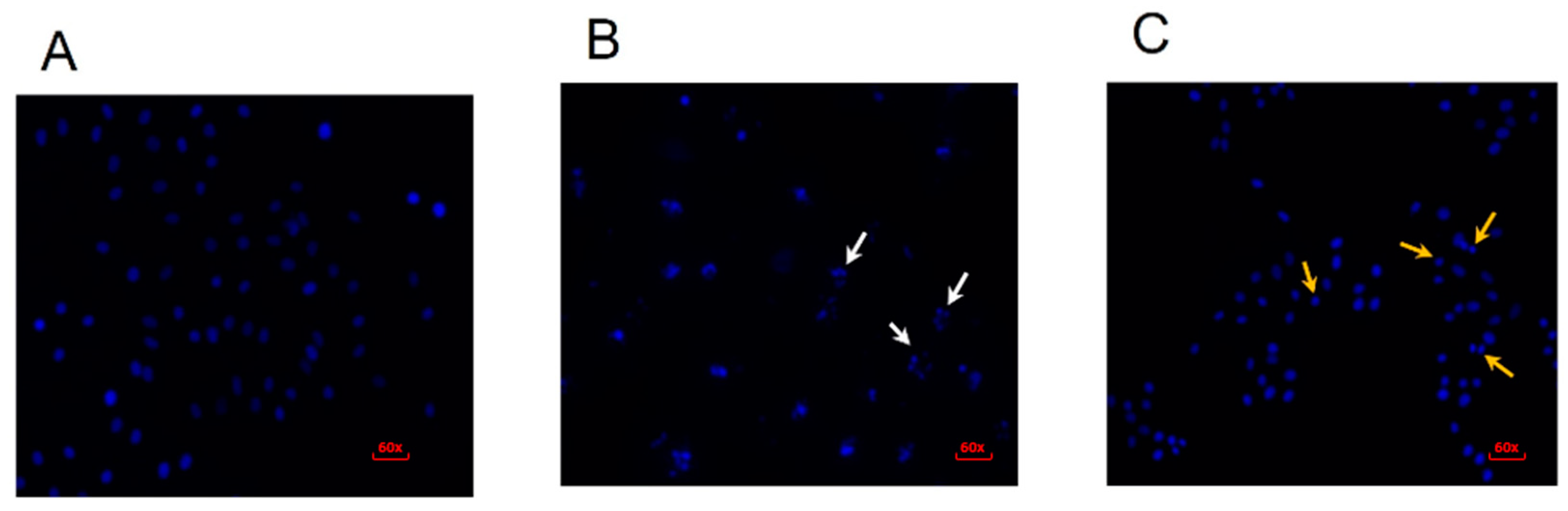
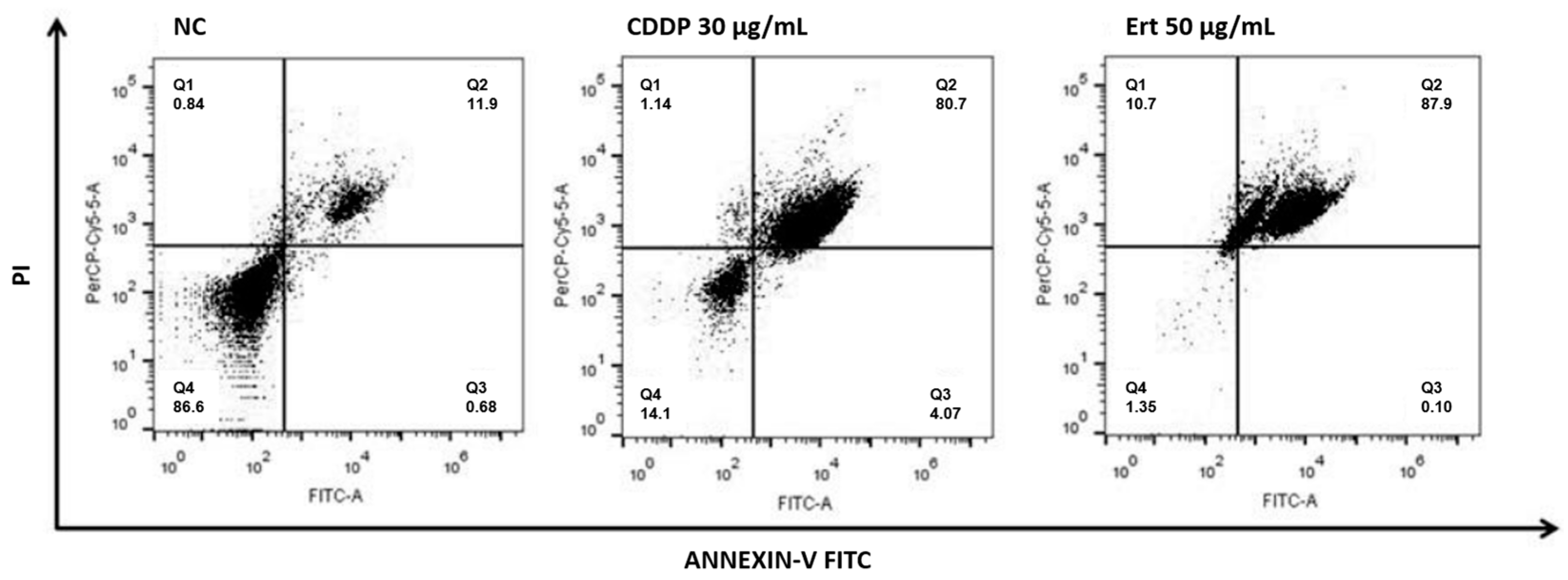
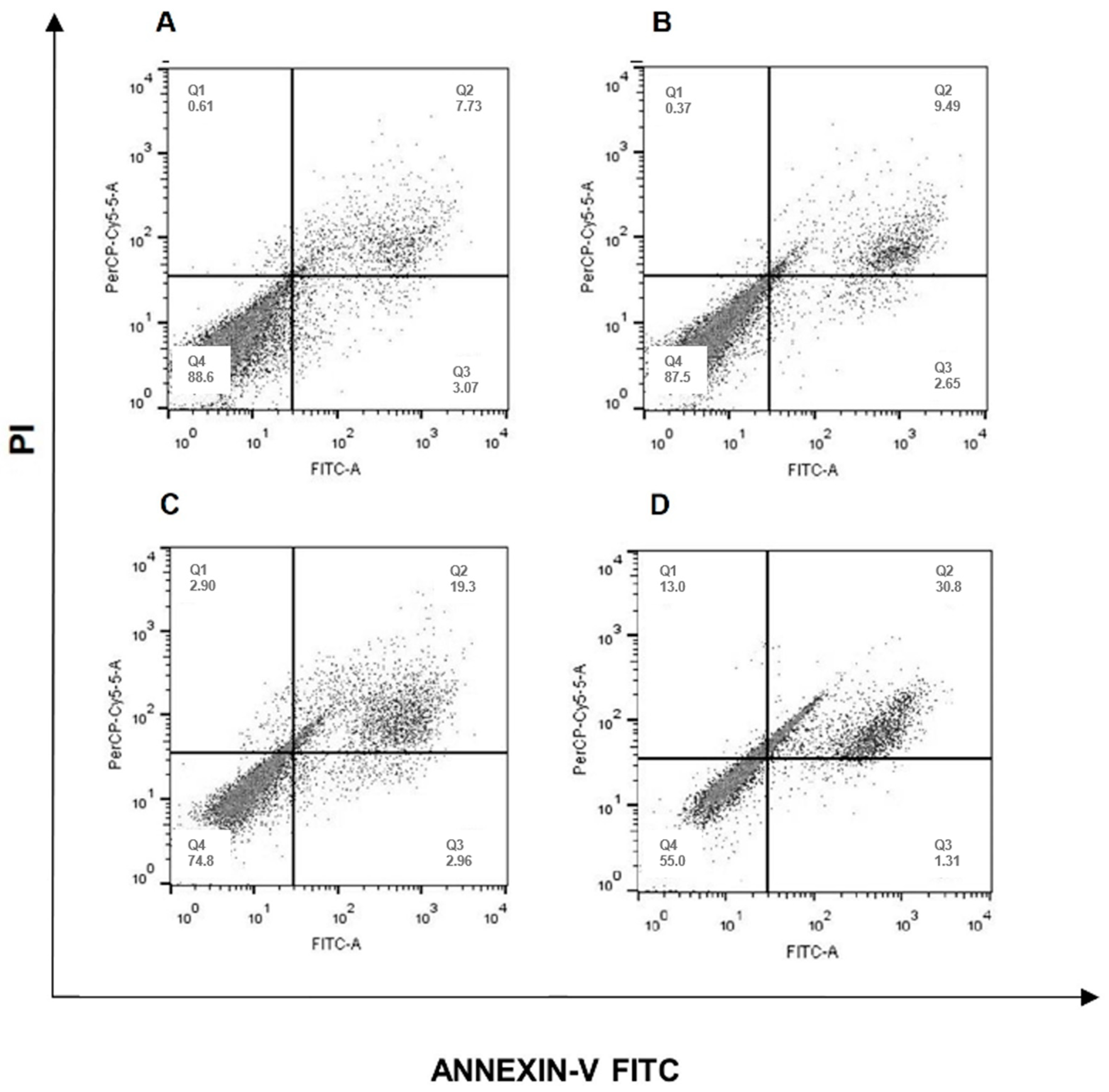
2.3. Erythraline Induces Apoptosis in SiHa Cells
2.4. Caspase Activity Investigation
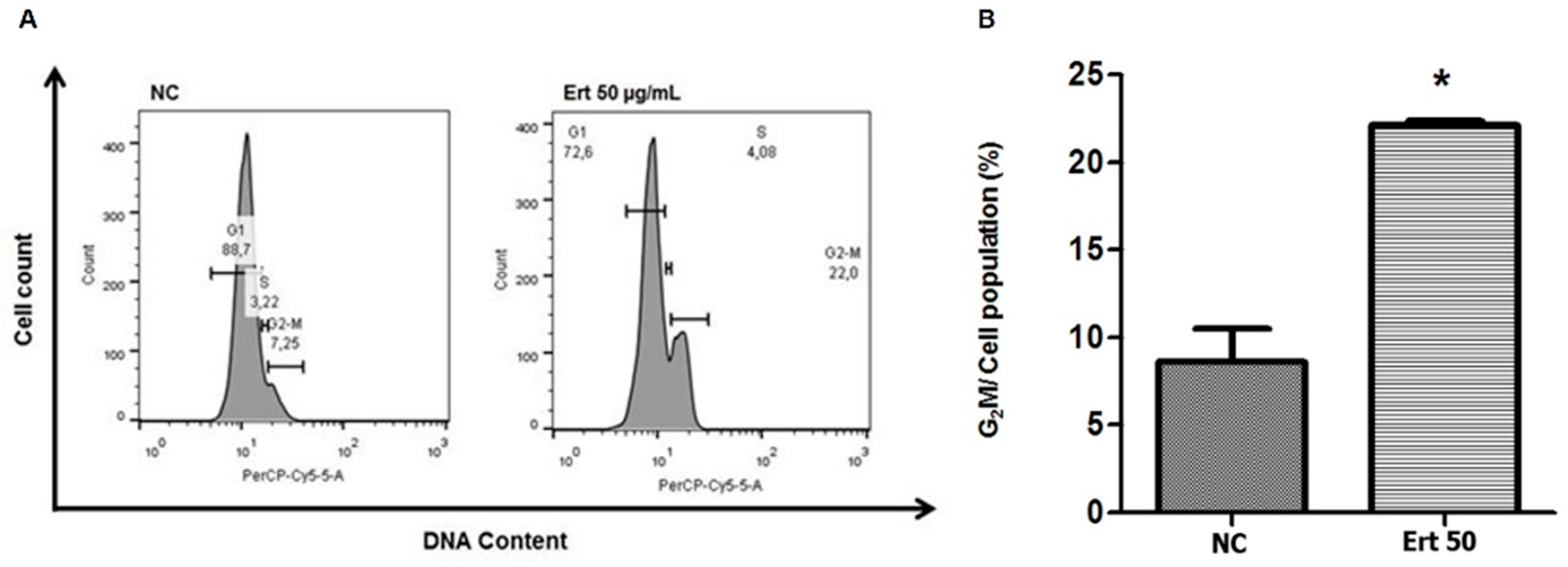
2.5. Erythraline Increases G2/M Arrest of Cell Cycle in SiHa Cells
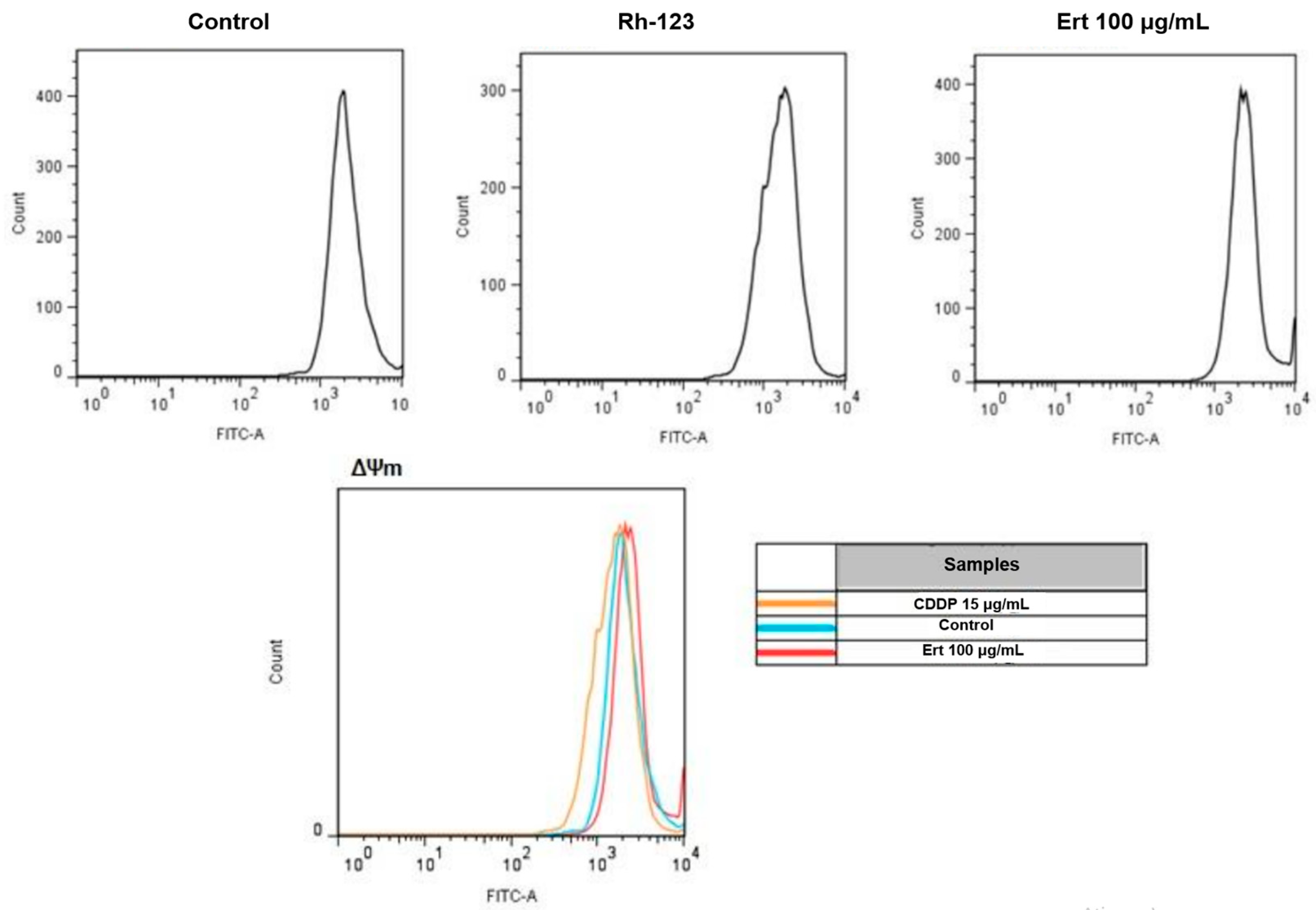
2.6. Analysis of Mitochondrial Membrane Potential with Rhodamine 123
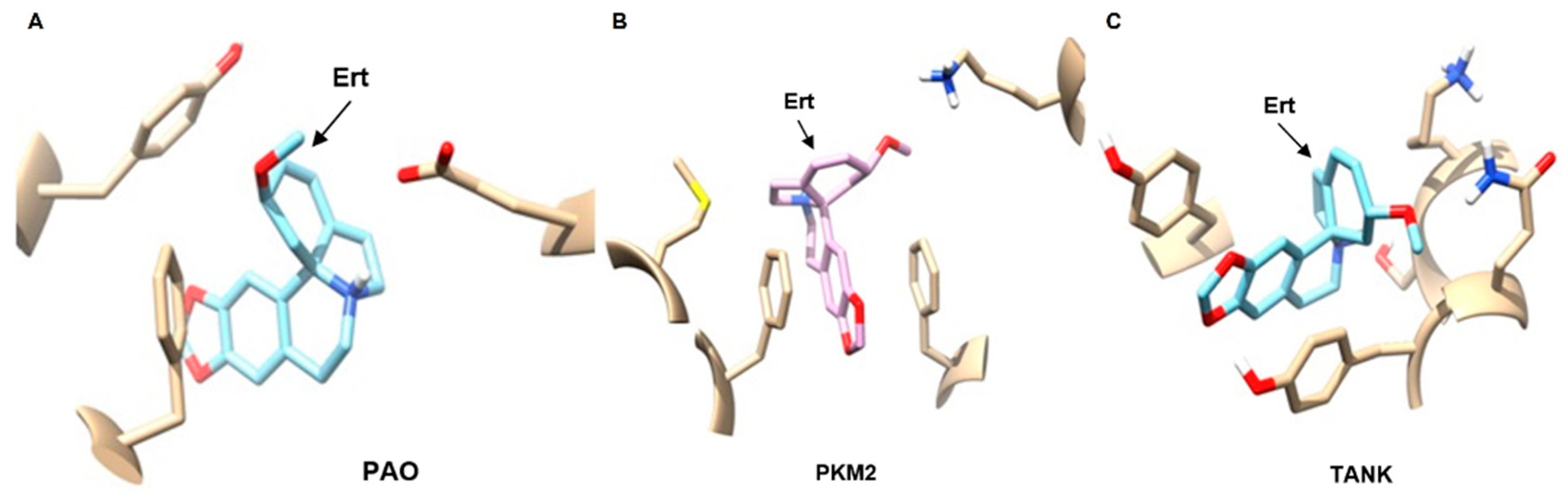
2.7. In Silico Virtual Screening of Erythraline and Antitumor Targets
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. Reagents and Antibodies
4.4. Cell Lines and Culture
4.5. Cell Viability Assay
4.6. Fluorescence Microscopy
4.7. Apoptosis Analysis
4.8. Cell Cycle Analysis
4.9. Rhodamine-123 Retention Assay
4.10. Molecular Protein–Protein Docking
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPV | Human Papillomavirus |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| PBMC | Peripheral blood mononuclear cells |
| CDDP | Cisplatin |
| NC | Control |
| DAPI | 4,6-Diamidino-2-phenylindole |
| Ert | Erythraline |
| PAO | Polyamine oxidase |
| PKM2 | Pyruvate kinase M2 |
| TANK | Tankyrase |
| PARP | Poly (ADP-ribose) polymerase |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide |
| DMSO | Dimethyl sulfoxide |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal bovine serum |
| FITC | Fluorescein isothio-cyanate |
| PI | Propidium iodide |
| PBS | Phosphate-buffered saline |
| Rh-123 | Rhodamine 123 |
| ΔΨm | Mitochondrial membrane potential |
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; Sanjose, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervi-cal cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Mu-ñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Lowy, D.R.; Schiller, J.T. Prophylactic human papillomavirus vaccines. J. Clin. Investig. 2006, 116, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.E.; Simas, N.K.; Kustter, R.M. Phytochemical profiling, antioxidant, and phytotoxic potentials of Erythrina speciosa Andrews leaves. Ciência Nat. 2024, 46, e86537. [Google Scholar] [CrossRef]
- Amaral, P.A.; Antunes, A.R.; Barlow, J.W. Isolation of erythrinan alkaloids from the leaves and flowers of Erythrina speciosa. Rev. Bras. Farm. 2019, 29, 488–490. [Google Scholar] [CrossRef]
- Jiménez-Cabrera, T.; Bautista, M.; Velázquez-González, C.; Jaramillo-Morales, O.A.; Guerrero-Solano, J.A.; Urrutia-Hernández, T.A.; De la O-Arciniega, M. Promising Antioxidant Activity of Erythrina Genus: An Alternative Treatment for Inflammatory Pain? Int. J. Mol. Sci. 2020, 22, 248. [Google Scholar] [CrossRef]
- Patil, V.S.; Meena, H.; Harish, D.R. Erythrina variegata L. bark: An untapped bioactive source harbouring therapeutic proper-ties for the treatment of Alzheimer’s disease. Silico Pharmacol. 2021, 9, 51. [Google Scholar] [CrossRef]
- Lucena, S.V.; Rufino, F.P.; de Dantas Moura, G.E.D.; Rabêlo, L.M.A.; Monteiro, N.K.V.; Ferreira, A.T.; Perales, J.E.A.; Uchôa, A.F.; Justo, G.Z.; de Oliveira, C.F.R.; et al. The Kunitz chymotrypsin inhibitor from Erythrina velutina seeds displays activity against HeLa cells through arrest in cell cycle. 3 Biotech 2022, 12, 19. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Olofinsan, K.; Abrahamse, H.; George, B.P. Therapeutic Role of Alkaloids and Alkaloid Derivatives in Cancer Management. Molecules 2023, 28, 5578. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz-Hajduk, J.; Sparzak-Stefanowska, B.; Krauze-Baranowska, M.; Ochocka, J.R. Securinine from Phyllanthus glaucus induces cell cycle arrest and apoptosis in human cervical cancer HeLa cells. PLoS ONE 2016, 28, e0165372. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, L.G.P.; Guaratini, T.; Lopes, J.L.C.; Lopes, N.P. Application of electron ionization mass spectrometry for Mulungu alkaloid analysis. Quim. Nova 2012, 35, 2177–2180. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Gunatilaka, A.A.L.; Letcher, R.M.; Lobo, A.M.F.T.; Widdowson, D.A. Phenol oxidation and biosynthesis. Part XXII. The alkaloids of Erythrina lysistemon, E. abyssinica, E. poeppigiana, E. fusca, and E. Lithosperma; the structure of erythratidine. J. Chem. Soc. Perkin Trans. 1973, 1, 874–880. [Google Scholar] [CrossRef]
- Amer, M.E.; Shamma, M.; Freyer, A.J. The tetracyclic Erythrina alkaloids. J. Nat. Prod. 1991, 54, 329–363. [Google Scholar] [CrossRef]
- Ito, K. Studies on the alkaloids of Erythrina plants. Yakugaku Zasshi 1999, 119, 340–356. [Google Scholar] [CrossRef]
- Mohammed, M.M.; Ibrahim, N.A.; Awad, N.E.; Matloub, A.A.; Mohamed-Ali, A.G.; Barakat, E.E.; Mohamed, A.E.; Colla, P.L. Anti-HIV-1 and cytotoxicity of the alkaloids of Erythrina abyssinica Lam. growing in Sudan. Nat. Prod. Res. 2012, 26, 25–35. [Google Scholar] [CrossRef]
- Armwoodm, S.; Juma, B.F.; Ombito, J.O.; Majinda, R.R.T.; Gwebu, E.T. Effect of erythrinaline alkaloids from Erythrina lysistemon on human recombinant caspase-3. Afr. J. Pharm. Pharmacol. 2017, 12, 183–187. [Google Scholar]
- Zhao, C.; Lu, E.; Hu, X.; Cheng, H.; Zhang, J.A.; Zhu, X. S100A9 regulates cisplatin chemosensitivity of squamous cervical cancer cells and related mechanism. Cancer Manag. Res. 2018, 10, 3753–3764. [Google Scholar] [CrossRef]
- Gottron, F.J.; Ying, H.S.; Choi, D.W. Caspase inhibition selectively reduces the apoptotic component of oxygen-glucose deprivation-induced cortical neuronal cell death. Mol. Cell Neurosci. 1997, 9, 159–169. [Google Scholar] [CrossRef]
- Santos, L.S.; Silva, V.R.; Menezes, L.R.A.; Soares, M.B.P.; Costa, E.V.; Bezerra, D.P. Xylopine Induces Oxidative Stress and Causes G2/M Phase Arrest, Triggering Caspase-Mediated Apoptosis by p53-Independent Pathway in HCT116 Cells. Oxid. Med. Cell. Longev. 2017, 2017, 7126872. [Google Scholar] [CrossRef]
- Cummings, B.S.; Kinsey, G.R.; Bolchoz, L.J.; Schnellmann, R.G. Identification of caspase-independent apoptosis in epithelial and cancer cells. J. Pharmacol. Exp. Ther. 2004, 310, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, S.; Mancinelli, R.; Miglietta, S.; Cona, A.; Angelini, R.; Canettieri, G.; Spandidos, D.A.; Gaudio, E.; Agostinelli, E. Maize polyamine oxidase in the presence of spermine/spermidine induces the apoptosis of LoVo human colon adenocarci-noma cells. Int. J. Oncol. 2019, 54, 2080–2094. [Google Scholar]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Liang, L.J.; Yang, F.Y.; Wang, D.; Zhang, Y.F.; Yu, H.; Wang, Z.; Sun, B.B.; Liu, Y.T.; Wang, G.Z.; Zhou, G.B. CIP2A induces PKM2 tetramer formation and oxidative phosphorylation in non-small cell lung cancer. Cell Discov. 2024, 10, 13. [Google Scholar] [CrossRef]
- Wu, B.; Liang, Z.; Lan, H.; Teng, X.; Wang, C. The role of PKM2 in cancer progression and its structural and biological basis. J. Physiol. Biochem. 2024, 80, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.; Wang, Y.F.; Liu, B.; Zhang, W.F.; Zhao, Y.F.; Kulkarni, A.B.; Sun, Z.J. Dual induction of apoptotic and autophagic cell death by targeting survivin in head neck squamous cell carcinoma. Cell Death Dis. 2015, 6, e1771. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.J. Tankyrase 2 promotes lung cancer cell malignancy. World J. Clin. Oncol. 2024, 15, 755–764. [Google Scholar] [CrossRef]

| Peak | Tr (min) | Alkaloid | m/z (IE-MS) | m/z (IE-MS) Literature |
|---|---|---|---|---|
| 1 | 30 | Erythrinin | 295 (38), 280 (49), 264 (100), 262 (24), 224 (23), 211 (21) | 295 (32), 280 (37), 265 (26), 264 (100), 262 (19), 224 (12), 211 (28) [13] |
| 2 | 30.8 | Erythraline | 297 (29), 282 (14), 267 (17), 266 (100), 226 (20), 213 (21) | 297 (35), 282 (31), 267 (26), 266 (100), 264 (21), 239 (11), 226 (13), 213 (22) [13] |
| 3 | 34.9 | Erythratidine | 331 (3), 300 (20), 273 (83), 272 (37), 258 (32), 257 (100), 256 (34), 244 (84) | 331, 300, 273, 257 (100), 244b, 331 (5), 300 (13), 273 (50), 272 (15), 258 (27), 257 (100), 256 (54), 244 (60), 242 (15) [14] |
| 4 | 36.4 | Erythroculin | 343 (80), 328 (21), 286 (66), 285 (100), 271 (24), 256 (37), 255 (71) | 343 (71), 328 (0.5), 312 (13), 285 (100) [15] |
| 5 | 36.6 | Crystamidine | 309 (30), 294 (21), 278 (39), 277 (40), 276 (100), 266 (26), 250 (57) | 309, 294, 278, 276 (100) [16] |
| 6 | 39.7 | Oxo-erythraline | 311 (74), 296 (40), 280 (45), 279 (59), 278 (100), 268 (13), 251 (26), 250 (54) | 311 (81), 296 (42), 280 (57), 279 (51), 278 (100), 268 (20), 266 (17), 250 (48) [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, C.A.N.; Souza, A.T.B.d.; Soares, A.K.M.d.C.; Bernardes-Oliveira, E.; Rocha, H.A.O.; Barbosa, E.G.; Guaratini, T.; Lucena-Silva, N.; Cobucci, R.N.; Giordani, R.B.; et al. Apoptosis and G2/M Phase Cell Cycle Arrest Induced by Alkaloid Erythraline Isolated from Erythrina velutina in SiHa Cervical Cancer Cell. Int. J. Mol. Sci. 2025, 26, 4627. https://doi.org/10.3390/ijms26104627
Miranda CAN, Souza ATBd, Soares AKMdC, Bernardes-Oliveira E, Rocha HAO, Barbosa EG, Guaratini T, Lucena-Silva N, Cobucci RN, Giordani RB, et al. Apoptosis and G2/M Phase Cell Cycle Arrest Induced by Alkaloid Erythraline Isolated from Erythrina velutina in SiHa Cervical Cancer Cell. International Journal of Molecular Sciences. 2025; 26(10):4627. https://doi.org/10.3390/ijms26104627
Chicago/Turabian StyleMiranda, Cleine Aglacy Nunes, Amaxsell Thiago Barros de Souza, Ana Katarina Menezes da Cruz Soares, Emanuelly Bernardes-Oliveira, Hugo Alexandre Oliveira Rocha, Euzébio Guimarães Barbosa, Thais Guaratini, Norma Lucena-Silva, Ricardo Ney Cobucci, Raquel Brandt Giordani, and et al. 2025. "Apoptosis and G2/M Phase Cell Cycle Arrest Induced by Alkaloid Erythraline Isolated from Erythrina velutina in SiHa Cervical Cancer Cell" International Journal of Molecular Sciences 26, no. 10: 4627. https://doi.org/10.3390/ijms26104627
APA StyleMiranda, C. A. N., Souza, A. T. B. d., Soares, A. K. M. d. C., Bernardes-Oliveira, E., Rocha, H. A. O., Barbosa, E. G., Guaratini, T., Lucena-Silva, N., Cobucci, R. N., Giordani, R. B., & Crispim, J. C. d. O. (2025). Apoptosis and G2/M Phase Cell Cycle Arrest Induced by Alkaloid Erythraline Isolated from Erythrina velutina in SiHa Cervical Cancer Cell. International Journal of Molecular Sciences, 26(10), 4627. https://doi.org/10.3390/ijms26104627








