Circulating Cytokines Mediate the Protective Effect of Physical Activity on Cardiovascular Diseases: A Mendelian Randomization Mediation Analysis
Abstract
1. Introduction
2. Results
2.1. Selection of IVs
2.2. Causal Association Between Physical Activity and Cardiovascular Diseases
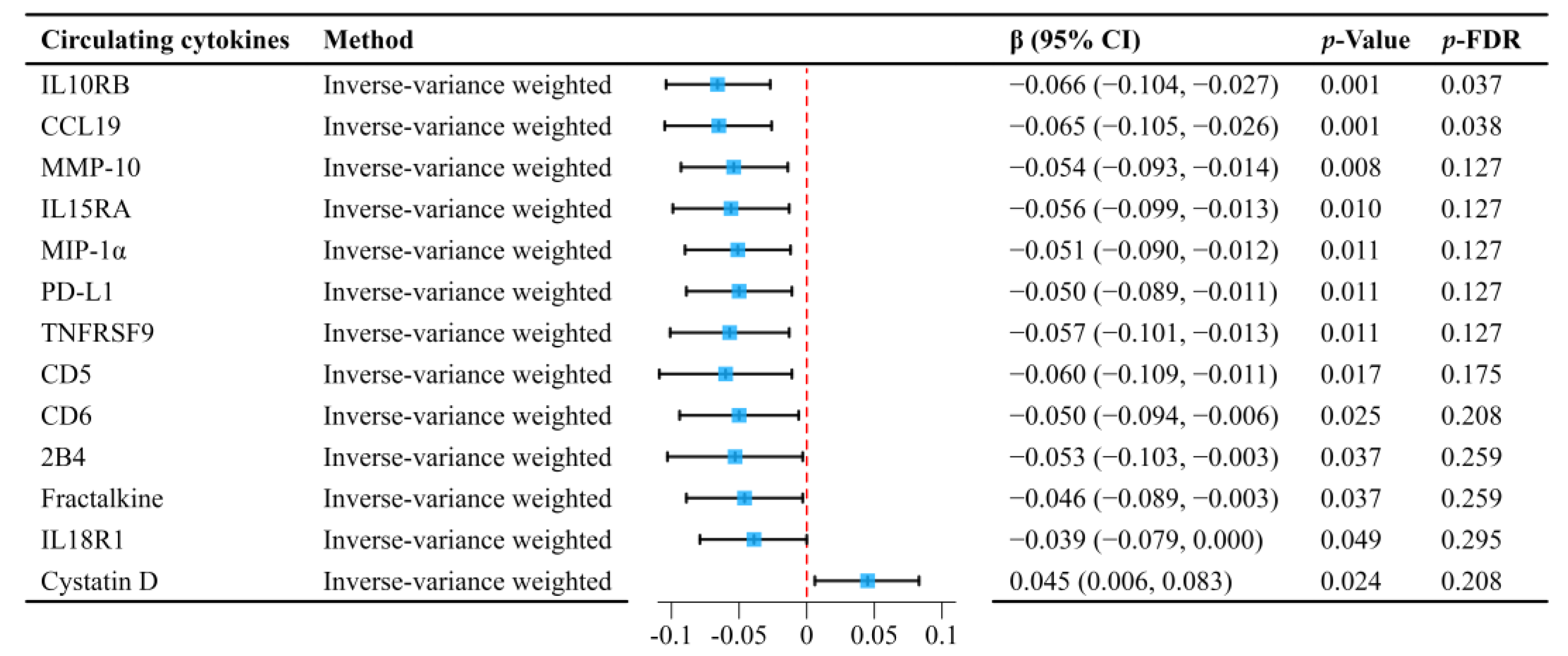
2.3. Mediation MR Analyses of Circulating Cytokines
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Data Sources
4.3. Selection of Genetic Instruments
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCL19 | C-C motif chemokine 19 |
| CVDs | Cardiovascular diseases |
| CI | Confidence interval |
| CAD | Coronary artery disease |
| FDR | False discovery rate |
| GWAS | Genome-wide association study |
| HF | Heart failure |
| IL-10 | Interleukin-10 |
| IL10RB | Interleukin-10 receptor subunit beta |
| IL15RA | Interleukin-15 receptor subunit alpha |
| IVs | Instrumental variables |
| IVW | Inverse-variance weighted |
| LD | Linkage disequilibrium |
| MMP-10 | Matrix metalloproteinase-10 |
| MR | Mendelian randomization |
| pQTL | Protein quantitative trait locus |
| SNPs | Single nucleotide polymorphisms |
| IHD | Ischemic heart disease |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Zhang, K.; Kan, C.; Chen, J.; Shi, J.; Ma, Y.; Wang, X.; Li, X.; Cai, W.; Pan, R.; Zhang, J.; et al. Epidemiology of 369 diseases and injuries attributable to 84 risk factors: 1990–2019 with 2040 projection. iScience 2024, 27, 109508. [Google Scholar] [CrossRef] [PubMed]
- Winzer, E.B.; Woitek, F.; Linke, A. Physical Activity in the Prevention and Treatment of Coronary Artery Disease. J. Am. Heart Assoc. 2018, 7, e007725. [Google Scholar] [CrossRef] [PubMed]
- Kushima, T.; Yamagishi, K.; Kihara, T.; Tamakoshi, A.; Iso, H. Physical Activity and Risk of Mortality from Heart Failure among Japanese Population. J. Atheroscler. Thromb. 2022, 29, 1076–1084. [Google Scholar] [CrossRef]
- Cha, J.; Kim, J.; Hong, K. Association Between Types of Physical Activity and Risk of Ischemic Heart Disease Based on US Guidelines: A Systematic Review and Meta-Analysis. SAGE Open 2024, 14, 21582440241297817. [Google Scholar] [CrossRef]
- Sattar, N.; Preiss, D. Reverse Causality in Cardiovascular Epidemiological Research. Circulation 2017, 135, 2369–2372. [Google Scholar] [CrossRef]
- Ertek, S.; Cicero, A. Impact of physical activity on inflammation: Effects on cardiovascular disease risk and other inflammatory conditions. Arch. Med. Sci. 2012, 8, 794–804. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.-X.; Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: How to proceed? Nat. Rev. Cardiol. 2022, 19, 522–542. [Google Scholar] [CrossRef]
- Docherty, S.; Harley, R.; McAuley, J.J.; Crowe, L.A.N.; Pedret, C.; Kirwan, P.D.; Siebert, S.; Millar, N.L. The effect of exercise on cytokines: Implications for musculoskeletal health: A narrative review. BMC Sports Sci. Med. Rehabil. 2022, 14, 5. [Google Scholar] [CrossRef]
- Cho, J.M.; Koh, J.H.; Kim, S.G.; Lee, S.; Kim, Y.; Cho, S.; Kim, K.; Kim, Y.C.; Han, S.S.; Lee, H.; et al. Mendelian randomization uncovers a protective effect of interleukin-1 receptor antagonist on kidney function. Commun. Biol. 2023, 6, 722. [Google Scholar] [CrossRef] [PubMed]
- Rontogianni, M.O.; Gill, D.; Bouras, E.; Asimakopoulos, A.G.; Tzoulaki, I.; Karhunen, V.; Lehtimäki, T.; Raitakari, O.; Wielscher, M.; Salomaa, V.; et al. Association of inflammatory cytokines with lung function, chronic lung diseases, and COVID-19. iScience 2024, 27, 110704. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Correction: Why Most Published Research Findings Are False. PLoS Med. 2022, 19, e1004085. [Google Scholar] [CrossRef] [PubMed]
- Richmond, R.C.; Davey Smith, G. Mendelian Randomization: Concepts and Scope. Cold Spring Harb. Perspect. Med. 2022, 12, a040501. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Burgess, S.; Daniel, R.M.; Butterworth, A.S.; Thompson, S.G.; EPIC-InterAct Consortium. Network Mendelian randomization: Using genetic variants as instrumental variables to investigate mediation in causal pathways. Int. J. Epidemiol. 2014, 44, 484–495. [Google Scholar] [CrossRef]
- Mora, S.; Cook, N.; Buring, J.E.; Ridker, P.M.; Lee, I.M. Physical activity and reduced risk of cardiovascular events: Potential mediating mechanisms. Circulation 2007, 116, 2110–2118. [Google Scholar] [CrossRef]
- Kazemi, A.; Soltani, S.; Aune, D.; Hosseini, E.; Mokhtari, Z.; Hassanzadeh, Z.; Jayedi, A.; Pitanga, F.; Akhlaghi, M. Leisure-time and occupational physical activity and risk of cardiovascular disease incidence: A systematic-review and dose-response meta-analysis of prospective cohort studies. Int. J. Behav. Nutr. Phys. Act. 2024, 21, 45. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; apos; Garra, A. Interleukin-10 and the Interleukin-10 Receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in Atherosclerosis: From Pathophysiology to Practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Bruunsgaard, H. Physical activity and modulation of systemic low-level inflammation. J. Leukoc. Biol. 2005, 78, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.J.; Fegers-Wustrow, I.; Halle, M.; Haykowsky, M.J.; Chung, E.H.; Kovacic, J.C. Exercise for Primary and Secondary Prevention of Cardiovascular Disease. JACC 2022, 80, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Groux, H.; Cottrez, F. The complex role of interleukin-10 in autoimmunity. J. Autoimmun. 2003, 20, 281–285. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; Liu, J.; Ye, J.; Xu, Y.; Wang, Z.; Yu, J.; Ye, D.; Zhao, M.; Feng, Y.; et al. The role of interleukin-10 family members in cardiovascular diseases. Int. Immunopharmacol. 2021, 94, 107475. [Google Scholar] [CrossRef]
- Boef, A.G.; Dekkers, O.M.; le Cessie, S. Mendelian randomization studies: A review of the approaches used and the quality of reporting. Int. J. Epidemiol. 2015, 44, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Palmer, T.M.; Lawlor, D.A.; Harbord, R.M.; Sheehan, N.A.; Tobias, J.H.; Timpson, N.J.; Smith, G.D.; Sterne, J.A. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat. Methods Med. Res. 2012, 21, 223–242. [Google Scholar] [CrossRef]
- Au Yeung, S.L.; Gill, D. Standardizing the reporting of Mendelian randomization studies. BMC Med. 2023, 21, 187. [Google Scholar] [CrossRef]
- Klimentidis, Y.C.; Raichlen, D.A.; Bea, J.; Garcia, D.O.; Wineinger, N.E.; Mandarino, L.J.; Alexander, G.E.; Chen, Z.; Going, S.B. Genome-wide association study of habitual physical activity in over 377,000 UK Biobank participants identifies multiple variants including CADM2 and APOE. Int. J. Obes. 2018, 42, 1161–1176. [Google Scholar] [CrossRef]
- van der Harst, P.; Verweij, N. Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ. Res. 2018, 122, 433–443. [Google Scholar] [CrossRef]
- Shah, S.; Henry, A.; Roselli, C.; Lin, H.; Sveinbjörnsson, G.; Fatemifar, G.; Hedman, Å.K.; Wilk, J.B.; Morley, M.P.; Chaffin, M.D.; et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat. Commun. 2020, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Stacey, D.; Eriksson, N.; Macdonald-Dunlop, E.; Hedman, Å.K.; Kalnapenkis, A.; Enroth, S.; Cozzetto, D.; Digby-Bell, J.; Marten, J.; et al. Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat. Immunol. 2023, 24, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019, 35, 4851–4853. [Google Scholar] [CrossRef]
- Pierce, B.L.; Ahsan, H.; Vanderweele, T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011, 40, 740–752. [Google Scholar] [CrossRef]
- Burgess, S.; Foley, C.N.; Allara, E.; Staley, J.R.; Howson, J.M.M. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat. Commun. 2020, 11, 376. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hong, X.; Gao, W.; Luo, S.; Cai, J.; Liu, G.; Huang, Y. Causal relationship between physical activity, leisure sedentary behaviors and COVID-19 risk: A Mendelian randomization study. J. Transl. Med. 2022, 20, 216. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Zhao, Q.; Lawlor, D.A.; Sheehan, N.A.; Thompson, J.; Davey Smith, G. Improving the accuracy of two-sample summary-data Mendelian randomization: Moving beyond the NOME assumption. Int. J. Epidemiol. 2019, 48, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Rothmann, M.D.; Tsou, H.-H. On Non-inferiority Analysis Based On Delta-method Confidence Intervals. J. Biopharm. Stat. 2003, 13, 565–583. [Google Scholar] [CrossRef]
- Sobel, M.E. Asymptotic Confidence Intervals for Indirect Effects in Structural Equation Models. Sociol. Methodol. 1982, 13, 290–312. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 2018, 57, 289–300. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Liu, Y. Circulating Cytokines Mediate the Protective Effect of Physical Activity on Cardiovascular Diseases: A Mendelian Randomization Mediation Analysis. Int. J. Mol. Sci. 2025, 26, 4615. https://doi.org/10.3390/ijms26104615
Sun Y, Liu Y. Circulating Cytokines Mediate the Protective Effect of Physical Activity on Cardiovascular Diseases: A Mendelian Randomization Mediation Analysis. International Journal of Molecular Sciences. 2025; 26(10):4615. https://doi.org/10.3390/ijms26104615
Chicago/Turabian StyleSun, Yulin, and Yining Liu. 2025. "Circulating Cytokines Mediate the Protective Effect of Physical Activity on Cardiovascular Diseases: A Mendelian Randomization Mediation Analysis" International Journal of Molecular Sciences 26, no. 10: 4615. https://doi.org/10.3390/ijms26104615
APA StyleSun, Y., & Liu, Y. (2025). Circulating Cytokines Mediate the Protective Effect of Physical Activity on Cardiovascular Diseases: A Mendelian Randomization Mediation Analysis. International Journal of Molecular Sciences, 26(10), 4615. https://doi.org/10.3390/ijms26104615


_Kim.png)


