Phase Separation: Orchestrating Biological Adaptations to Environmental Fluctuations
Abstract
1. Introduction
2. The Basic Principle of Phase Separation
2.1. The Concept and Properties of Phase Separation
2.2. Multivalent Interactions in Phase Separation
2.3. The Role of IDRs in Phase Separation
2.4. The Environment and the Occurrence of Phase Separation
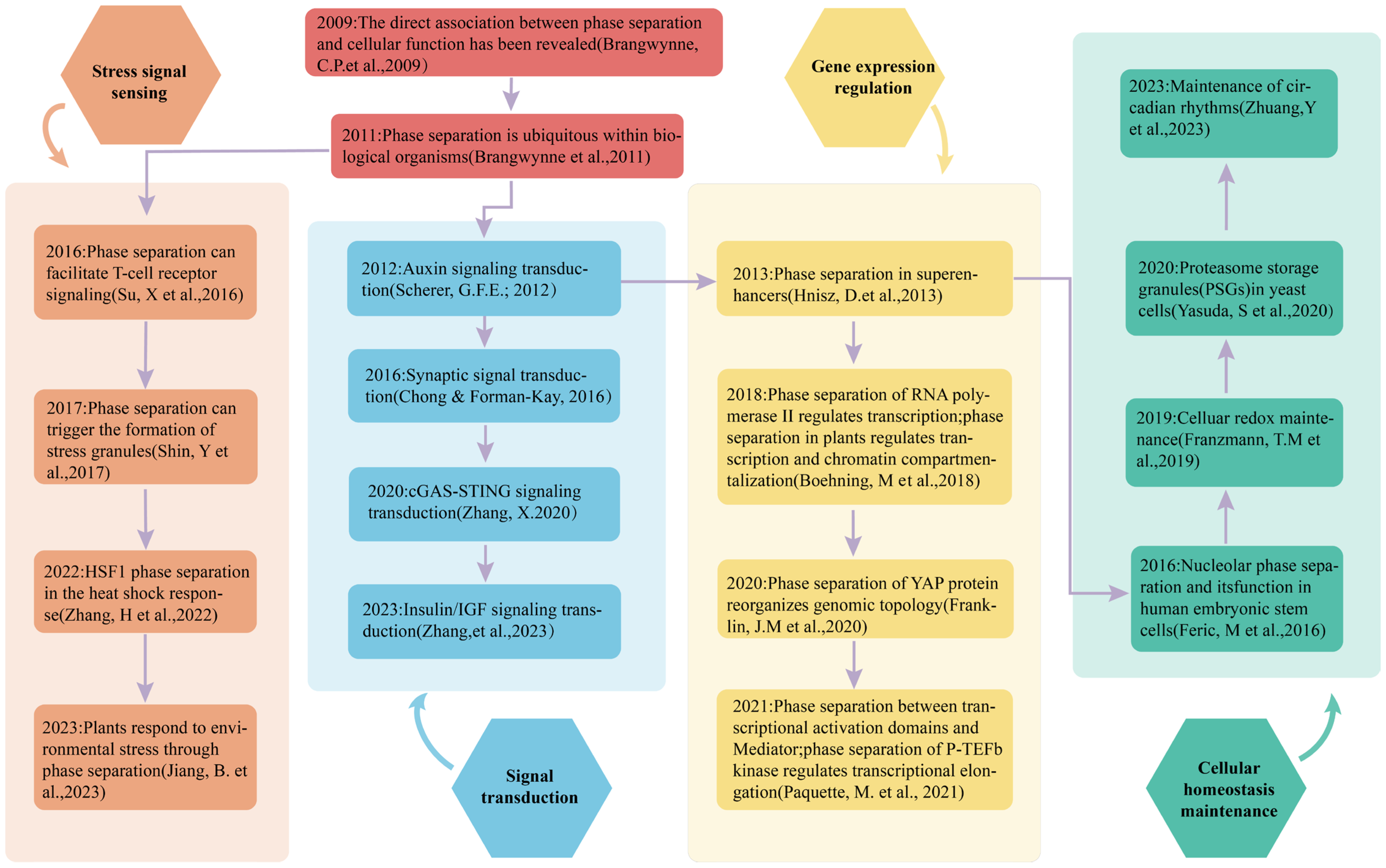
3. The Role of Phase Separation in Detecting and Transducing Environmental Signals
3.1. Phase Separation for Phenotypic Plasticity in Response to Environmental Changes
3.1.1. Phase Separation in Plants: Regulating Phenotypic Plasticity in Response to Environmental Variations
3.1.2. Phase Separation in Animals: Regulating Phenotypic Plasticity to Adapt to Environmental Changes
3.1.3. Phase Separation in Microorganisms: Regulating Phenotypic Plasticity to Adapt to Environmental Fluctuations
3.2. The Regulation of Stress Responses by Phase Separation
3.2.1. Phase Separation in Stress Signal Perception
3.2.2. Phase Separation in Signal Transduction
3.2.3. Phase Separation in Stress Response Gene Expression
3.3. Phase Separation in Cellular Homeostasis During Environmental Variation
3.3.1. Phase Separation in Constructing Intracellular Functional Zones for Cellular Homeostasis
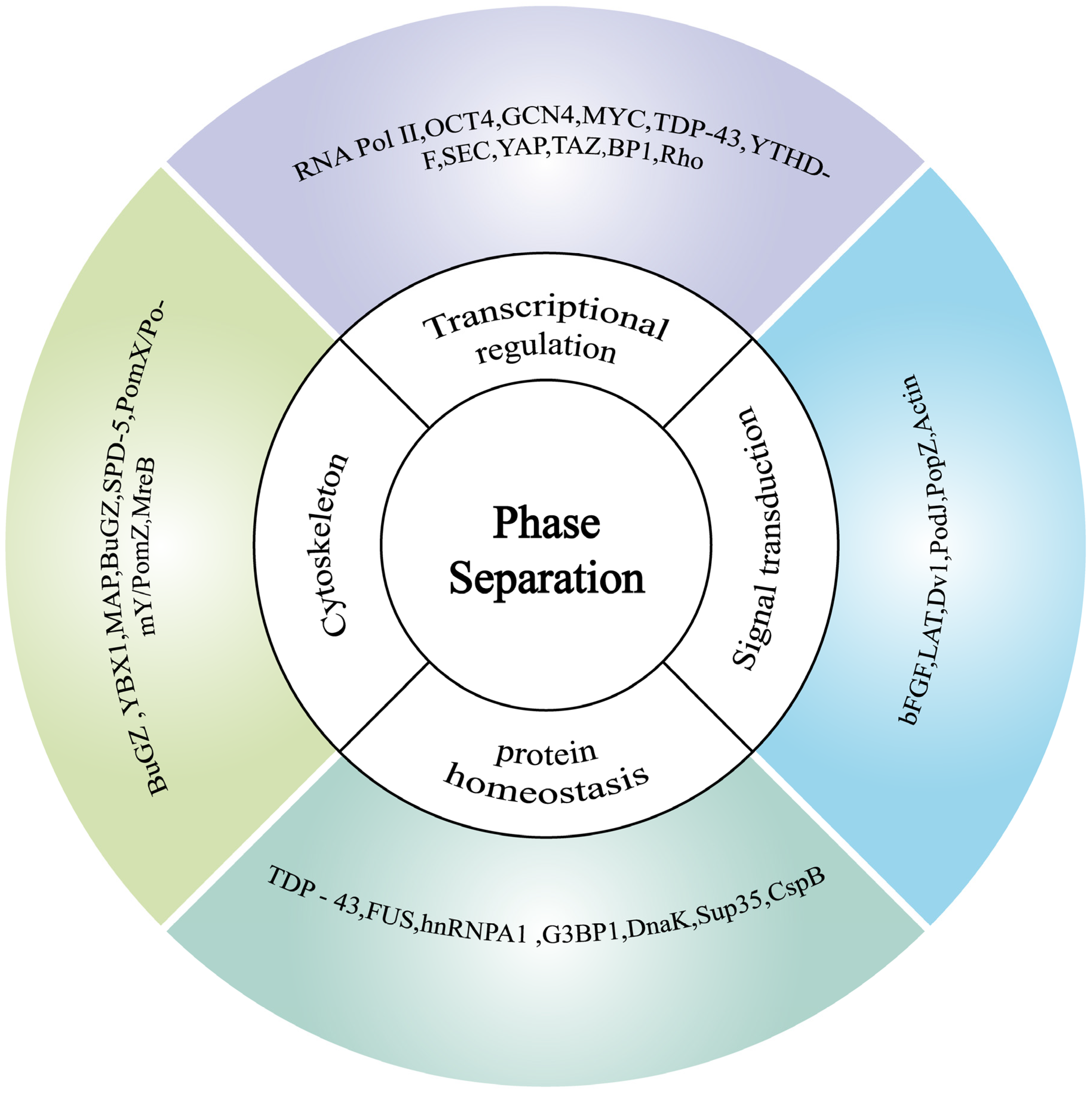
3.3.2. The Role of Phase Separation in Protein Homeostasis
3.3.3. Phase Separation in Gene Transcription for Cellular Homeostasis
4. Phase Separation in Biological Adaptation and Evolution
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Langemeyer, L.; Frohlich, F.; Ungermann, C. Rab GTPase Function in Endosome and Lysosome Biogenesis. Trends Cell Biol. 2018, 28, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Repnik, U.; Cesen, M.H.; Turk, B. The endolysosomal system in cell death and survival. Cold Spring Harbor Perspect. Biol. 2013, 5, a8755. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef]
- Snead, W.T.; Gladfelter, A.S. The Control Centers of Biomolecular Phase Separation: How Membrane Surfaces, PTMs, and Active Processes Regulate Condensation. Mol. Cell 2019, 76, 295–305. [Google Scholar] [CrossRef]
- Dorone, Y.; Boeynaems, S.; Flores, E.; Jin, B.; Hateley, S.; Bossi, F.; Lazarus, E.; Pennington, J.G.; Michiels, E.; De Decker, M.; et al. A prion-like protein regulator of seed germination undergoes hydration-dependent phase separation. Cell 2021, 184, 4284–4298. [Google Scholar] [CrossRef]
- Gonzalez, A.; Mannen, T.; Çağatay, T.; Fujiwara, A.; Matsumura, H.; Niesman, A.B.; Brautigam, C.A.; Chook, Y.M.; Yoshizawa, T. Mechanism of karyopherin-β2 binding and nuclear import of ALS variants FUS(P525L) and FUS(R495X). Sci. Rep. 2021, 11, 3754. [Google Scholar] [CrossRef]
- Ishiguro, A.; Lu, J.; Ozawa, D.; Nagai, Y.; Ishihama, A. ALS-linked FUS mutations dysregulate G-quadruplex-dependent liquid-liquid phase separation and liquid-to-solid transition. J. Biol. Chem. 2021, 297, 101284. [Google Scholar] [CrossRef]
- Girdhar, A.; Bharathi, V.; Tiwari, V.R.; Abhishek, S.; Deeksha, W.; Mahawar, U.S.; Raju, G.; Singh, S.K.; Prabusankar, G.; Rajakumara, E.; et al. Computational insights into mechanism of AIM4-mediated inhibition of aggregation of TDP-43 protein implicated in ALS and evidence for in vitro inhibition of liquid-liquid phase separation (LLPS) of TDP-43(2C)-A315T by AIM4. Int. J. Biol. Macromol. 2020, 147, 117–130. [Google Scholar] [CrossRef]
- Watanabe, S.; Inami, H.; Oiwa, K.; Murata, Y.; Sakai, S.; Komine, O.; Sobue, A.; Iguchi, Y.; Katsuno, M.; Yamanaka, K. Aggresome formation and liquid-liquid phase separation independently induce cytoplasmic aggregation of TAR DNA-binding protein 43. Cell Death Dis. 2020, 11, 909. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, L.; Shen, J.; Zhai, Y.; Jiang, Q.; Yi, M.; Deng, X.; Ruan, Z.; Fang, R.; Chen, Z.; et al. The STING phase-separator suppresses innate immune signalling. Nat. Cell Biol. 2021, 23, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Franzmann, T.M.; Jahnel, M.; Pozniakovsky, A.; Mahamid, J.; Holehouse, A.S.; Nuske, E.; Richter, D.; Baumeister, W.; Grill, S.W.; Pappu, R.V.; et al. Phase separation of a yeast prion protein promotes cellular fitness. Science 2018, 359, eaao5654. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, I.; Nüske, E.; Munder, M.C.; Kulasegaran, G.; Malinovska, L.; Kroschwald, S.; Richter, D.; Fahmy, K.; Gibson, K.; Verbavatz, J.; et al. Filament formation by metabolic enzymes is a specific adaptation to an advanced state of cellular starvation. eLife 2014, 3, e02409. [Google Scholar] [CrossRef]
- Parry, B.R.; Surovtsev, I.V.; Cabeen, M.T.; O’Hern, C.S.; Dufresne, E.R.; Jacobs-Wagner, C. The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell 2014, 156, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Julicher, F.; Hyman, A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef]
- Brangwynne, C.P.; Mitchison, T.J.; Hyman, A.A. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 4334–4339. [Google Scholar] [CrossRef]
- Soding, J.; Zwicker, D.; Sohrabi-Jahromi, S.; Boehning, M.; Kirschbaum, J. Mechanisms for Active Regulation of Biomolecular Condensates. Trends Cell Biol. 2020, 30, 4–14. [Google Scholar] [CrossRef]
- Boeynaems, S.; Alberti, S.; Fawzi, N.L.; Mittag, T.; Polymenidou, M.; Rousseau, F.; Schymkowitz, J.; Shorter, J.; Wolozin, B.; Van Den Bosch, L.; et al. Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol. 2018, 28, 420–435. [Google Scholar] [CrossRef]
- Wang, J.; Choi, J.M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maharana, S.; Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 2018, 174, 688–699. [Google Scholar] [CrossRef]
- Yan, K.; Ji, Q.; Zhao, D.; Li, M.; Sun, X.; Wang, Z.; Liu, X.; Liu, Z.; Li, H.; Ding, Y.; et al. SGF29 nuclear condensates reinforce cellular aging. Cell Discov. 2023, 9, 110. [Google Scholar] [CrossRef]
- Aguzzi, A.; Altmeyer, M. Phase Separation: Linking Cellular Compartmentalization to Disease. Trends Cell Biol. 2016, 26, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Langdon, E.M.; Gladfelter, A.S. A New Lens for RNA Localization: Liquid-Liquid Phase Separation. Annu. Rev. Microbiol. 2018, 72, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Hyman, A.A.; Weber, C.A.; Julicher, F. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Cai, S.; Shi, R.; Li, X.; Deng, B.; Li, R.; Yang, S.; Huang, J.; Liang, Y.; Lu, P.; et al. HERD-1 mediates multiphase condensate immiscibility to regulate small RNA-driven transgenerational epigenetic inheritance. Nat. Cell Biol. 2024, 26, 1958–1970. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Li, P.; Lin, Y. A brief guideline for studies of phase-separated biomolecular condensates. Nat. Chem. Biol. 2022, 18, 1307–1318. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Huai, J.; Peng, F.; Wu, J.; Lin, R.; Fang, X. Condensation of SEUSS promotes hyperosmotic stress tolerance in Arabidopsis. Nat. Chem. Biol. 2022, 18, 1361–1369. [Google Scholar] [CrossRef]
- Tong, J.; Ren, Z.; Sun, L.; Zhou, S.; Yuan, W.; Hui, Y.; Ci, D.; Wang, W.; Fan, L.M.; Wu, Z.; et al. ALBA proteins confer thermotolerance through stabilizing HSF messenger RNAs in cytoplasmic granules. Nat. Plants 2022, 8, 778–791. [Google Scholar] [CrossRef]
- Chong, P.A.; Forman-Kay, J.D. Liquid-liquid phase separation in cellular signaling systems. Curr. Opin. Struct. Biol. 2016, 41, 180–186. [Google Scholar] [CrossRef]
- Frey, S.; Richter, R.P.; Görlich, D. FG-Rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 2006, 314, 815–817. [Google Scholar] [CrossRef]
- Kato, M.; McKnight, S.L. A solid-state conceptualization of information transfer from gene to message to protein. Annu. Rev. Biochem. 2018, 87, 351–390. [Google Scholar] [CrossRef]
- Martin, E.W.; Holehouse, A.S.; Peran, I.; Farag, M.; Incicco, J.J.; Bremer, A.; Grace, C.R.; Soranno, A.; Pappu, R.V.; Mittag, T. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 2020, 367, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Bremer, A.; Farag, M.; Borcherds, W.M.; Peran, I.; Martin, E.W.; Pappu, R.V.; Mittag, T. Deciphering how naturally occurring sequence features impact the phase behaviours of disordered prion-like domains. Nat. Chem. 2022, 14, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Vernon, R.M.; Chong, P.A.; Tsang, B.; Kim, T.H.; Bah, A.; Farber, P.; Lin, H.; Forman-Kay, J.D. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. eLife 2018, 7, e31486. [Google Scholar] [CrossRef] [PubMed]
- Fare, C.M.; Villani, A.; Drake, L.E.; Shorter, J. Higher-order organization of biomolecular condensates. Open Biol. 2021, 11, 210137. [Google Scholar] [CrossRef]
- Jakobi, A.J.; Huber, S.T.; Mortensen, S.A.; Schultz, S.W.; Palara, A.; Kuhm, T.; Shrestha, B.K.; Lamark, T.; Hagen, W.; Wilmanns, M.; et al. Structural basis of p62/SQSTM1 helical filaments and their role in cellular cargo uptake. Nat. Commun. 2020, 11, 440. [Google Scholar] [CrossRef]
- Lu, N.; Duvall, S.W.; Zhao, G.; Kowallis, K.A.; Zhang, C.; Tan, W.; Sun, J.; Petitjean, H.N.; Tomares, D.T.; Zhao, G.P.; et al. Scaffold-scaffold interaction facilitates cell polarity development in Caulobacter crescentus. mBio 2023, 14, e321822. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Cheng, S.; Li, Y.; Li, X.Y.; Lu, N.; Sun, J.; Tang, G.; Yang, Y.; Cai, K.; Li, X.; et al. Phase separation modulates the assembly and dynamics of a polarity-related scaffold-signaling hub. Nat. Commun. 2022, 13, 7181. [Google Scholar] [CrossRef]
- Brodsky, S.; Jana, T.; Barkai, N. Order through disorder: The role of intrinsically disordered regions in transcription factor binding specificity. Curr. Opin. Struct. Biol. 2021, 71, 110–115. [Google Scholar] [CrossRef]
- Fuxreiter, M.; Tompa, P.; Simon, I.; Uversky, V.N.; Hansen, J.C.; Asturias, F.J. Malleable machines take shape in eukaryotic transcriptional regulation. Nat. Chem. Biol. 2008, 4, 728–737. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, X.C.; Chen, Z.J. Structures and mechanisms in the cGAS-STING innate immunity pathway. Immunity 2020, 53, 43–53. [Google Scholar] [CrossRef]
- Cuylen, S.; Blaukopf, C.; Politi, A.Z.; Muller-Reichert, T.; Neumann, B.; Poser, I.; Ellenberg, J.; Hyman, A.A.; Gerlich, D.W. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature 2016, 535, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Pelham, J.F.; Dunlap, J.C.; Hurley, J.M. Intrinsic disorder is an essential characteristic of components in the conserved circadian circuit. Cell Commun. Signal. 2020, 18, 181. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P.; Csermely, P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J. 2004, 18, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Altmeyer, M.; Neelsen, K.J.; Teloni, F.; Pozdnyakova, I.; Pellegrino, S.; Grofte, M.; Rask, M.D.; Streicher, W.; Jungmichel, S.; Nielsen, M.L.; et al. Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). Nat. Commun. 2015, 6, 8088. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef]
- Payliss, B.J.; Tse, Y.W.E.; Reichheld, S.E.; Lemak, A.; Yun, H.Y.; Houliston, S.; Patel, A.; Arrowsmith, C.H.; Sharpe, S.; Wyatt, H.D.M. Phosphorylation of the DNA repair scaffold SLX4 drives folding of the SAP domain and activation of the MUS81-EME1 endonuclease. Cell Rep. 2022, 41, 111537. [Google Scholar] [CrossRef]
- Witus, S.R.; Tuttle, L.M.; Li, W.; Zelter, A.; Wang, M.; Kermoade, K.E.; Wilburn, D.B.; Davis, T.N.; Brzovic, P.S.; Zhao, W.; et al. BRCA1/BARD1 intrinsically disordered regions facilitate chromatin recruitment and ubiquitylation. EMBO J. 2023, 42, e113565. [Google Scholar] [CrossRef]
- Chattaraj, A.; Shakhnovich, E.I. Separation of sticker-spacer energetics governs the coalescence of metastable condensates. Biophys. J. 2025, 124, 428–439. [Google Scholar] [CrossRef]
- Alshareedah, I.; Moosa, M.M.; Pham, M.; Potoyan, D.A.; Banerjee, P.R. Programmable viscoelasticity in protein-RNA condensates with disordered sticker-spacer polypeptides. Nat. Commun. 2021, 12, 6620. [Google Scholar] [CrossRef]
- Zhuang, Y.; Li, Z.; Xiong, S.; Sun, C.; Li, B.; Wu, S.A.; Lyu, J.; Shi, X.; Yang, L.; Chen, Y.; et al. Circadian clocks are modulated by compartmentalized oscillating translation. Cell 2023, 186, 3245–3260. [Google Scholar] [CrossRef]
- Tao, H.; Rigoni, C.; Li, H.; Koistinen, A.; Timonen, J.; Zhou, J.; Kontturi, E.; Rojas, O.J.; Chu, G. Thermodynamically controlled multiphase separation of heterogeneous liquid crystal colloids. Nat. Commun. 2023, 14, 5277. [Google Scholar] [CrossRef]
- Fritsch, A.W.; Diaz-Delgadillo, A.F.; Adame-Arana, O.; Hoege, C.; Mittasch, M.; Kreysing, M.; Leaver, M.; Hyman, A.A.; Julicher, F.; Weber, C.A. Local thermodynamics govern formation and dissolution of Caenorhabditis elegans P granule condensates. Proc. Natl. Acad. Sci. USA 2021, 118, e2102772118. [Google Scholar] [CrossRef]
- Paquette, M.; El-Houjeiri, L.; Zirden, L.C.; Puustinen, P.; Blanchette, P.; Jeong, H.; Dejgaard, K.; Siegel, P.M.; Pause, A. AMPK-dependent phosphorylation is required for transcriptional activation of TFEB and TFE3. Autophagy 2021, 17, 3957–3975. [Google Scholar] [CrossRef]
- Eck, R.J.; Kraemer, B.C.; Liachko, N.F. Regulation of TDP-43 phosphorylation in aging and disease. GeroScience 2021, 43, 1605–1614. [Google Scholar] [CrossRef]
- Kedersha, N.; Ivanov, P.; Anderson, P. Stress granules and cell signaling: More than just a passing phase? Trends Biochem. Sci. 2013, 38, 494–506. [Google Scholar] [CrossRef]
- Kilic, S.; Lezaja, A.; Gatti, M.; Bianco, E.; Michelena, J.; Imhof, R.; Altmeyer, M. Phase separation of 53BP1 determines liquid-like behavior of DNA repair compartments. EMBO J. 2019, 38, e101379. [Google Scholar] [CrossRef]
- Yang, Y.; Gomez, N.; Infarinato, N.; Adam, R.C.; Sribour, M.; Baek, I.; Laurin, M.; Fuchs, E. The pioneer factor SOX9 competes for epigenetic factors to switch stem cell fates. Nat. Cell Biol. 2023, 25, 1185–1195. [Google Scholar] [CrossRef]
- Parra, A.S.; Johnston, C.A. Phase separation as a driver of stem cell organization and function during development. J. Dev. Biol. 2023, 11, 45. [Google Scholar] [CrossRef]
- Su, X.; Ditlev, J.A.; Hui, E.; Xing, W.; Banjade, S.; Okrut, J.; King, D.S.; Taunton, J.; Rosen, M.K.; Vale, R.D. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 2016, 352, 595–599. [Google Scholar] [CrossRef]
- Botterbusch, S.; Baumgart, T. Interactions between Phase-Separated Liquids and Membrane Surfaces. Appl. Sci. 2021, 11, 1288. [Google Scholar] [CrossRef]
- Jung, J.H.; Barbosa, A.D.; Hutin, S.; Kumita, J.R.; Gao, M.; Derwort, D.; Silva, C.S.; Lai, X.; Pierre, E.; Geng, F.; et al. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 2020, 585, 256–260. [Google Scholar] [CrossRef]
- Han, X.; Kui, M.; Xu, T.; Ye, J.; Du, J.; Yang, M.; Jiang, Y.; Hu, Y. CO interacts with JAZ repressors and bHLH subgroup IIId factors to negatively regulate jasmonate signaling in Arabidopsis seedlings. Plant Cell 2023, 35, 852–873. [Google Scholar] [CrossRef]
- Huang, X.; Ma, Z.; He, D.; Han, X.; Liu, X.; Dong, Q.; Tan, C.; Yu, B.; Sun, T.; Nordenskiold, L.; et al. Molecular condensation of the CO/NF-YB/NF-YC/FT complex gates floral transition in Arabidopsis. EMBO J. 2025, 44, 225–250. [Google Scholar] [CrossRef]
- Fang, X.; Wang, L.; Ishikawa, R.; Li, Y.; Fiedler, M.; Liu, F.; Calder, G.; Rowan, B.; Weigel, D.; Li, P.; et al. Arabidopsis FLL2 promotes liquid-liquid phase separation of polyadenylation complexes. Nature 2019, 569, 265–269. [Google Scholar] [CrossRef]
- Cui, S.; Song, P.; Wang, C.; Chen, S.; Hao, B.; Xu, Z.; Cai, L.; Chen, X.; Zhu, S.; Gan, X.; et al. The RNA binding protein EHD6 recruits the m(6)A reader YTH07 and sequesters OsCOL4 mRNA into phase-separated ribonucleoprotein condensates to promote rice flowering. Mol. Plant 2024, 17, 935–954. [Google Scholar] [CrossRef]
- Ouyang, M.; Li, X.; Zhang, J.; Feng, P.; Pu, H.; Kong, L.; Bai, Z.; Rong, L.; Xu, X.; Chi, W.; et al. Liquid-Liquid phase transition drives intra-chloroplast cargo sorting. Cell 2020, 180, 1144–1159. [Google Scholar] [CrossRef]
- Jiang, B. Light-induced cryptochrome 2 liquid-liquid phase separation and mRNA methylation. New Phytol. 2024, 244, 2163–2169. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, H.; Pan, Y.; Lyu, M.; Yang, Z.; Kou, X.; Deng, X.W.; Zhong, S. Sensory circuitry controls cytosolic calcium-mediated phytochrome B phototransduction. Cell 2023, 186, 1230–1243. [Google Scholar] [CrossRef]
- Wang, H.; Yan, X.; Aigner, H.; Bracher, A.; Nguyen, N.D.; Hee, W.Y.; Long, B.M.; Price, G.D.; Hartl, F.U.; Hayer-Hartl, M. Rubisco condensate formation by CcmM in beta-carboxysome biogenesis. Nature 2019, 566, 131–135. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Li, Y.; Zhen, W.; Wang, C.; Wang, X.; Xie, Z.; Xu, X.; Guo, S.; Botella, J.R.; et al. Sequestration of DBR1 to stress granules promotes lariat intronic RNAs accumulation for heat-stress tolerance. Nat. Commun. 2024, 15, 7696. [Google Scholar] [CrossRef]
- Shen, L. Epitranscriptomic regulation through phase separation in plants. Trends Plant Sci. 2024, 19, 1360–1385. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, D.; He, Y. FRIGIDA establishes a local chromosomal environment for FLOWERING LOCUS C mRNA production. Nat. Plants 2018, 4, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Du, Q.; Guo, Y.; Wang, Y.; Jiao, Y. Condensation of STM is critical for shoot meristem maintenance and salt tolerance in Arabidopsis. Mol. Plant 2023, 16, 1445–1459. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, H.W.; Xu, H.; Singh, R.M.; Yao, T.; Jang, I.C.; Chua, N.H. Nutrient status regulates MED19a phase separation for ORESARA1-dependent senescence. New Phytol. 2022, 236, 1779–1795. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Q.; Zhang, D.; Lu, Y.; Wang, Y.; Pan, Y.; Qiu, Y.; Men, Y.; Yan, W.; Xiao, Z.; et al. A cytoplasmic osmosensing mechanism mediated by molecular crowding-sensitive DCP5. Science 2024, 386, k9067. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.; Dutta, D. Membrane proteins in plant salinity stress perception, sensing, and response. J. Membr. Biol. 2023, 256, 109–124. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef]
- Cao-Pham, A.H.; Urano, D.; Ross-Elliott, T.J.; Jones, A.M. Nudge-nudge, WNK-WNK (kinases), say no more? New Phytol. 2018, 220, 35–48. [Google Scholar] [CrossRef]
- Protter, D.; Parker, R. Principles and properties of stress granules. Trends Cell Biol. 2016, 26, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Franzmann, T.M.; Alberti, S. Protein phase separation as a stress survival strategy. Cold Spring Harbor Perspect. Biol. 2019, 11, a034058. [Google Scholar] [CrossRef]
- Peng, L.; Li, E.M.; Xu, L.Y. From start to end: Phase separation and transcriptional regulation. Biochim. Biophys. Acta-Gene Regul. Mech. 2020, 1863, 194641. [Google Scholar] [CrossRef]
- Boija, A.; Klein, I.A.; Sabari, B.R.; Dall’Agnese, A.; Coffey, E.L.; Zamudio, A.V.; Li, C.H.; Shrinivas, K.; Manteiga, J.C.; Hannett, N.M.; et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 2018, 175, 1842–1855. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chung, C.I.; Koach, J.; Liu, H.; Navalkar, A.; He, H.; Ma, Z.; Zhao, Q.; Yang, X.; He, L.; et al. MYC phase separation selectively modulates the transcriptome. Nat. Struct. Mol. Biol. 2024, 31, 1567–1579, Author Correction in: Nat. Struct. Mol. Biol. 2024, 31, 1808. [Google Scholar] [CrossRef]
- Conicella, A.E.; Dignon, G.L.; Zerze, G.H.; Schmidt, H.B.; D’Ordine, A.M.; Kim, Y.C.; Rohatgi, R.; Ayala, Y.M.; Mittal, J.; Fawzi, N.L. TDP-43 alpha-helical structure tunes liquid-liquid phase separation and function. Proc. Natl. Acad. Sci. USA 2020, 117, 5883–5894. [Google Scholar] [CrossRef]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 608–624. [Google Scholar] [CrossRef]
- Guo, C.; Che, Z.; Yue, J.; Xie, P.; Hao, S.; Xie, W.; Luo, Z.; Lin, C. ENL initiates multivalent phase separation of the super elongation complex (SEC) in controlling rapid transcriptional activation. Sci. Adv. 2020, 6, y4858. [Google Scholar] [CrossRef]
- Franklin, J.M.; Guan, K.L. YAP/TAZ phase separation for transcription. Nat. Cell Biol. 2020, 22, 357–358. [Google Scholar] [CrossRef]
- Tang, G.; Xia, H.; Huang, Y.; Guo, Y.; Chen, Y.; Ma, Z.; Liu, W. Liquid-liquid phase separation of H3K27me3 reader BP1 regulates transcriptional repression. Genome Biol. 2024, 25, 67. [Google Scholar] [CrossRef]
- Bement, W.M.; Goryachev, A.B.; Miller, A.L.; von Dassow, G. Patterning of the cell cortex by Rho GTPases. Nat. Rev. Mol. Cell Biol. 2024, 25, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Deng, K.; Liu, M.; Yang, S.; Xu, W.; Feng, T.; Jie, M.; Liu, Z.; Sheng, X.; Chen, H.; et al. Phase separation of BuGZ regulates gut regeneration and aging through interaction with m6A regulators. Nat. Commun. 2023, 14, 6700. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shen, H.; He, J.; Jin, B.; Tian, Y.; Li, W.; Hou, L.; Zhao, W.; Nan, J.; Zhao, J.; et al. Cytoskeleton remodeling mediated by circRNA-YBX1 phase separation suppresses the metastasis of liver cancer. Proc. Natl. Acad. Sci. USA 2023, 120, e2074671176. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Wegmann, S. Biomolecular condensation involving the cytoskeleton. Brain Res. Bull. 2023, 194, 105–117. [Google Scholar] [CrossRef]
- Woodruff, J.B.; Ferreira, G.B.; Widlund, P.O.; Mahamid, J.; Honigmann, A.; Hyman, A.A. The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. Cell 2017, 169, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Ramm, B.; Schumacher, D.; Harms, A.; Heermann, T.; Klos, P.; Muller, F.; Schwille, P.; Sogaard-Andersen, L. Biomolecular condensate drives polymerization and bundling of the bacterial tubulin FtsZ to regulate cell division. Nat. Commun. 2023, 14, 3825. [Google Scholar] [CrossRef]
- Bratton, B.P.; Shaevitz, J.W.; Gitai, Z.; Morgenstein, R.M. MreB polymers and curvature localization are enhanced by RodZ and predict E. coli’s cylindrical uniformity. Nat. Commun. 2018, 9, 2797. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, F.; Zhao, T.; Zhao, H.; Wang, X.; Chen, L.; Li, J.P.; Luo, S.Z. Phase separation on cell surface facilitates bFGF signal transduction with heparan sulphate. Nat. Commun. 2022, 13, 1112. [Google Scholar] [CrossRef] [PubMed]
- Povarova, O.I.; Antifeeva, I.A.; Fonin, A.V.; Turoverov, K.K.; Kuznetsova, I.M. The role of liquid-liquid phase separation in actin polymerization. Int. J. Mol. Sci. 2023, 24, 3281. [Google Scholar] [CrossRef]
- Patel, A.; Lee, H.O.; Jawerth, L.; Maharana, S.; Jahnel, M.; Hein, M.Y.; Stoynov, S.; Mahamid, J.; Saha, S.; Franzmann, T.M.; et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 2015, 162, 1066–1077. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Y.; Li, F.; Yang, Y.; Zhang, H.; Liu, X.; Xie, X.; Chen, X.; Shi, Y.; Zhang, L. Phase separation of hnRNPA1 and TERRA regulates telomeric stability. J. Mol. Cell Biol. 2024, 16, mjae037. [Google Scholar] [CrossRef]
- Guillen-Boixet, J.; Kopach, A.; Holehouse, A.S.; Wittmann, S.; Jahnel, M.; Schlussler, R.; Kim, K.; Trussina, I.; Wang, J.; Mateju, D.; et al. RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell 2020, 181, 346–361. [Google Scholar] [CrossRef]
- Calloni, G.; Chen, T.; Schermann, S.M.; Chang, H.; Genevaux, P.; Agostini, F.; Tartaglia, G.G.; Hayer-Hartl, M.; Hartl, F.U. DnaK functions as a central hub in the e. coli chaperone network. Cell Rep. 2012, 1, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Willimsky, G.; Bang, H.; Fischer, G.; Marahiel, M.A. Characterization of cspB, a Bacillus subtilis inducible cold shock gene affecting cell viability at low temperatures. J. Bacteriol. 1992, 174, 6326–6335. [Google Scholar] [CrossRef] [PubMed]
- Scherer, G.F.E.; Labusch, C.; Effendi, Y. Phospholipases and the network of auxin signal transduction with ABP1 and TIR1 as two receptors: A comprehensive and provocative model. Front. Plant Sci. 2012, 3, 56. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, X.; Zeng, M.; Zhang, M. Phase separation as a mechanism for assembling dynamic postsynaptic density signalling complexes. Curr. Opin. Neurobiol. 2019, 57, 1–8. [Google Scholar] [CrossRef]
- Zeng, M.; Chen, X.; Guan, D.; Xu, J.; Wu, H.; Tong, P.; Zhang, M. Reconstituted postsynaptic density as a molecular platform for understanding synapse formation and plasticity. Cell 2018, 174, 1172–1187. [Google Scholar] [CrossRef]
- Wu, X.; Cai, Q.; Shen, Z.; Chen, X.; Zeng, M.; Du, S.; Zhang, M. RIM and RIM-BP form presynaptic active-zone-like condensates via phase separation. Mol. Cell 2019, 73, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Zeng, M.; Zhang, M.; Huganir, R.L. Rapid dispersion of SynGAP from synaptic spines triggers AMPA receptor insertion and spine enlargement during LTP. Neuron 2015, 85, 173–189. [Google Scholar] [CrossRef]
- Vogel, J.; Luisi, B.F. Hfq and its constellation of RNA. Nat. Rev. Microbiol. 2011, 9, 578–589. [Google Scholar] [CrossRef]
- Riback, J.A.; Katanski, C.D.; Kear-Scott, J.L.; Pilipenko, E.V.; Rojek, A.E.; Sosnick, T.R.; Drummond, D.A. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell 2017, 168, 1028–1040. [Google Scholar] [CrossRef]
- Chakrabortee, S.; Kayatekin, C.; Newby, G.A.; Mendillo, M.L.; Lancaster, A.; Lindquist, S. Luminidependens (LD) is an Arabidopsis protein with prion behavior. Proc. Natl. Acad. Sci. USA 2016, 113, 6065–6070. [Google Scholar] [CrossRef]
- Jiang, B.; Zhong, Z.; Gu, L.; Zhang, X.; Wei, J.; Ye, C.; Lin, G.; Qu, G.; Xiang, X.; Wen, C.; et al. Light-induced LLPS of the CRY2/SPA1/FIO1 complex regulating mRNA methylation and chlorophyll homeostasis in Arabidopsis. Nat. Plants 2023, 9, 2042–2058. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, S.; Zeng, Y.; Wang, X.; Qin, Y.; Ren, Q.; Xiang, S.; Wang, Y.; Xiao, J.; Sun, Y. Reversible phase separation of HSF1 is required for an acute transcriptional response during heat shock. Nat. Cell Biol. 2022, 24, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Tsuchiya, H.; Kaiho, A.; Guo, Q.; Ikeuchi, K.; Endo, A.; Arai, N.; Ohtake, F.; Murata, S.; Inada, T.; et al. Stress- and ubiquitylation-dependent phase separation of the proteasome. Nature 2020, 578, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Hnisz, D.; Abraham, B.J.; Lee, T.I.; Lau, A.; Saint-Andre, V.; Sigova, A.A.; Hoke, H.A.; Young, R.A. Super-enhancers in the control of cell identity and disease. Cell 2013, 155, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Boehning, M.; Dugast-Darzacq, C.; Rankovic, M.; Hansen, A.S.; Yu, T.; Marie-Nelly, H.; McSwiggen, D.T.; Kokic, G.; Dailey, G.M.; Cramer, P.; et al. RNA polymerase II clustering through carboxy-terminal domain phase separation. Nat. Struct. Mol. Biol. 2018, 25, 833–840. [Google Scholar] [CrossRef]
- Feric, M.; Vaidya, N.; Harmon, T.S.; Mitrea, D.M.; Zhu, L.; Richardson, T.M.; Kriwacki, R.W.; Pappu, R.V.; Brangwynne, C.P. Coexisting liquid phases underlie nucleolar subcompartments. Cell 2016, 165, 1686–1697. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, B.; Gu, L.; Chen, Y.; Mora, M.; Zhu, M.; Noory, E.; Wang, Q.; Lin, C. A photoregulatory mechanism of the circadian clock in Arabidopsis. Nat. Plants 2021, 7, 1397–1408. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, M.; Liu, Y.; Li, C.; Zhang, S.; Duan, J.; Chen, J.; Qi, L.; Liu, Y.; Li, H.; et al. Liquid-liquid phase separation of TZP promotes PPK-mediated phosphorylation of the phytochrome A photoreceptor. Nat. Plants 2024, 10, 798–814. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, S.; Tu, Y.; Liu, E.; Li, Y.; Wang, X.; Chen, C.; Zhai, S.; Qi, J.; Wu, C.; et al. Proteasome resides in and dismantles plant heat stress granules constitutively. Mol. Cell 2024, 84, 3320–3335. [Google Scholar] [CrossRef]
- Shi, C.; Yang, X.; Hou, Y.; Jin, X.; Guo, L.; Zhou, Y.; Zhang, C.; Yin, H. USP15 promotes cGAS activation through deubiquitylation and liquid condensation. Nucleic Acids Res. 2022, 50, 11093–11108. [Google Scholar] [CrossRef]
- Vamadevan, V.; Chaudhary, N.; Maddika, S. Ubiquitin-assisted phase separation of dishevelled-2 promotes Wnt signalling. J. Cell Sci. 2022, 135, jcs260284. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lin, L.; Wang, C.; Zhu, J. Promotion of row 1-specific tip complex condensates by Gpsm2-Galphai provides insights into row identity of the tallest stereocilia. Sci. Adv. 2022, 8, n4556. [Google Scholar] [CrossRef]
- Boyd-Shiwarski, C.R.; Shiwarski, D.J.; Griffiths, S.E.; Beacham, R.T.; Norrell, L.; Morrison, D.E.; Wang, J.; Mann, J.; Tennant, W.; Anderson, E.N.; et al. WNK kinases sense molecular crowding and rescue cell volume via phase separation. Cell 2022, 185, 4488–4506. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, D.; Xu, Q.; Cheng, J.; Yu, Z.; Li, G.; Qiao, S.; Pan, J.; Wang, H.; Shi, J.; et al. G-quadruplexes promote the motility in MAZ phase-separated condensates to activate CCND1 expression and contribute to hepatocarcinogenesis. Nat. Commun. 2024, 15, 1045. [Google Scholar] [CrossRef]
- Wibisana, J.N.; Inaba, T.; Shinohara, H.; Yumoto, N.; Hayashi, T.; Umeda, M.; Ebisawa, M.; Nikaido, I.; Sako, Y.; Okada, M. Enhanced transcriptional heterogeneity mediated by NF-kappaB super-enhancers. PLoS Genet. 2022, 18, e1010235. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, L.; Cai, S.; Zhuang, Z.; Zhao, Z.; Jin, S.; Xie, W.; Zhou, L.; Zhang, L.; Zhao, J.; et al. RNA-induced liquid phase separation of SARS-CoV-2 nucleocapsid protein facilitates NF-kappaB hyper-activation and inflammation. Signal Transduct. Target. Ther. 2021, 6, 167. [Google Scholar] [CrossRef]
- Gaglia, G.; Rashid, R.; Yapp, C.; Joshi, G.N.; Li, C.G.; Lindquist, S.L.; Sarosiek, K.A.; Whitesell, L.; Sorger, P.K.; Santagata, S. HSF1 phase transition mediates stress adaptation and cell fate decisions. Nat. Cell Biol. 2020, 22, 151–158. [Google Scholar] [CrossRef]
- Yang, P.; Mathieu, C.; Kolaitis, R.M.; Zhang, P.; Messing, J.; Yurtsever, U.; Yang, Z.; Wu, J.; Li, Y.; Pan, Q.; et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 2020, 181, 325–345. [Google Scholar] [CrossRef]
- Youn, J.Y.; Dunham, W.H.; Hong, S.J.; Knight, J.; Bashkurov, M.; Chen, G.I.; Bagci, H.; Rathod, B.; MacLeod, G.; Eng, S.; et al. High-density proximity mapping reveals the subcellular organization of mRNA-associated granules and bodies. Mol. Cell 2018, 69, 517–532. [Google Scholar] [CrossRef]
- Cui, Q.; Bi, H.; Lv, Z.; Wu, Q.; Hua, J.; Gu, B.; Huo, C.; Tang, M.; Chen, Y.; Chen, C.; et al. Diverse CMT2 neuropathies are linked to aberrant G3BP interactions in stress granules. Cell 2023, 186, 803–820. [Google Scholar] [CrossRef]
- Wang, X.; Fan, X.; Zhang, J.; Wang, F.; Chen, J.; Wen, Y.; Wang, L.; Li, T.; Li, H.; Gu, H.; et al. hnRNPA2B1 represses the disassembly of arsenite-induced stress granules and is essential for male fertility. Cell Rep. 2024, 43, 113769. [Google Scholar] [CrossRef]
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 2016, 164, 487–498. [Google Scholar] [CrossRef]
- Wheeler, J.R.; Matheny, T.; Jain, S.; Abrisch, R.; Parker, R. Distinct stages in stress granule assembly and disassembly. eLife 2016, 5, e18413. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yang, F.; Yang, T.; Wang, Y.; Ding, M.; Li, L.; Xu, P.; Liu, S.; Dai, M.; Chi, C.; et al. Phase separation of EB1 guides microtubule plus-end dynamics. Nat. Cell Biol. 2023, 25, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Li, X.; Li, C.; Bai, H.; Chen, J.; Gan, L.; Zhu, J.; Oh, T.; Yan, X.; Zhu, J.; et al. SERRATE drives phase separation behaviours to regulate m6A modification and miRNA biogenesis. Nat. Cell Biol. 2024, 26, 2129–2143. [Google Scholar] [CrossRef]
- Bajczyk, M.; Lange, H.; Bielewicz, D.; Szewc, L.; Bhat, S.S.; Dolata, J.; Kuhn, L.; Szweykowska-Kulinska, Z.; Gagliardi, D.; Jarmolowski, A. SERRATE interacts with the nuclear exosome targeting (NEXT) complex to degrade primary miRNA precursors in Arabidopsis. Nucleic Acids Res. 2020, 48, 6839–6854. [Google Scholar] [CrossRef] [PubMed]
- See, Y.X.; Chen, K.; Fullwood, M.J. MYC overexpression leads to increased chromatin interactions at super-enhancers and MYC binding sites. Genome Res. 2022, 32, 629–642. [Google Scholar] [CrossRef]
- Qian, H.; Zhu, M.; Tan, X.; Zhang, Y.; Liu, X.; Yang, L. Super-enhancers and the super-enhancer reader BRD4: Tumorigenic factors and therapeutic targets. Cell Death Discov. 2023, 9, 470. [Google Scholar] [CrossRef]
- Zheng, Z.Z.; Xia, L.; Hu, G.S.; Liu, J.Y.; Hu, Y.H.; Chen, Y.J.; Peng, J.Y.; Zhang, W.J.; Liu, W. Super-enhancer-controlled positive feedback loop BRD4/ERalpha-RET-ERalpha promotes ERalpha-positive breast cancer. Nucleic Acids Res. 2022, 50, 10230–10248. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, M.; Guo, F.; Liu, X.; Zhang, Q.; Yang, S.; Yeung, Y.T.; Yang, R.; Wang, K.; Wu, Q.; et al. A novel lncRNA MTAR1 promotes cancer development through IGF2BPs mediated post-transcriptional regulation of c-MYC. Oncogene 2022, 41, 4736–4753. [Google Scholar] [CrossRef]
- Rao, S.; Huang, S.C.; Glenn, S.H.B.; Engreitz, J.M.; Perez, E.M.; Kieffer-Kwon, K.R.; Sanborn, A.L.; Johnstone, S.E.; Bascom, G.D.; Bochkov, I.D.; et al. Cohesin loss eliminates all loop domains. Cell 2017, 171, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Zhang, Y.; Wei, Y.; Zhang, C.; Lin, H.; He, J.; Wang, J.; Guo, W.; Li, H.; Chen, Q.; et al. METTL3/METTL14 maintain human nucleoli integrity by mediating SUV39H1/H2 degradation. Nat. Commun. 2024, 15, 7186. [Google Scholar] [CrossRef]
- Li, P.; Chen, P.; Qi, F.; Shi, J.; Zhu, W.; Li, J.; Zhang, P.; Xie, H.; Li, L.; Lei, M.; et al. High-throughput and proteome-wide discovery of endogenous biomolecular condensates. Nat. Chem. 2024, 16, 1101–1112. [Google Scholar] [CrossRef]
- Chen, D.; Lyu, M.; Kou, X.; Li, J.; Yang, Z.; Gao, L.; Li, Y.; Fan, L.M.; Shi, H.; Zhong, S. Integration of light and temperature sensing by liquid-liquid phase separation of phytochrome B. Mol. Cell 2022, 82, 3015–3029. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Gu, J.; Yao, J.; Li, Y.; Zhang, Z.; Xia, W.; Wang, Z.; Gui, X.; Li, L.; Li, D.; et al. Liquid-liquid phase separation of RBGD2/4 is required for heat stress resistance in Arabidopsis. Dev. Cell 2022, 57, 583–597. [Google Scholar] [CrossRef]
- Zavaliev, R.; Mohan, R.; Chen, T.; Dong, X. Formation of NPR1 condensates promotes cell survival during the plant immune response. Cell 2020, 182, 1093–1108. [Google Scholar] [CrossRef]
- Zhu, P.; Lister, C.; Dean, C. Cold-induced Arabidopsis FRIGIDA nuclear condensates for FLC repression. Nature 2021, 599, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, D.; Wang, Z.; Xu, S.; Batistic, O.; Steinhorst, L.; Li, H.; Weng, Y.; Ren, D.; Kudla, J.; et al. Cold-induced calreticulin OsCRT3 conformational changes promote OsCIPK7 binding and temperature sensing in rice. EMBO J. 2023, 42, e110518. [Google Scholar] [CrossRef]
- Liu, M.J.; Yeh, F.J.; Yvon, R.; Simpson, K.; Jordan, S.; Chambers, J.; Wu, H.M.; Cheung, A.Y. Extracellular pectin-RALF phase separation mediates FERONIA global signaling function. Cell 2024, 187, 312–330. [Google Scholar] [CrossRef]
- Piccinno, R.; Minneker, V.; Roukos, V. 53BP1-DNA repair enters a new liquid phase. EMBO J. 2019, 38, e102871. [Google Scholar] [CrossRef]
- Iserman, C.; Desroches, A.C.; Jegers, C.; Friedrich, U.; Zarin, T.; Fritsch, A.W.; Mittasch, M.; Domingues, A.; Hersemann, L.; Jahnel, M.; et al. Condensation of Ded1p promotes a translational switch from housekeeping to stress protein production. Cell 2020, 181, 818–831. [Google Scholar] [CrossRef]
- Reimann, T.M.; Mudsam, C.; Schachtler, C.; Ince, S.; Sticht, H.; Herrmann, C.; Sturzl, M.; Kost, B. The large GTPase AtGBPL3 links nuclear envelope formation and morphogenesis to transcriptional repression. Nat. Plants 2023, 9, 766–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pei, G.; Wang, Y.; Wu, D.; Liu, X.; Li, G.; He, J.; Zhang, X.; Shan, X.; Li, P.; et al. Phase separation of the nuclear pore complex facilitates selective nuclear transport to regulate plant defense against pathogen and pest invasion. Mol. Plant 2023, 16, 1016–1030. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, C.; Lu, D.; Song, C.P.; Zhang, L. Phase separation in plants: New insights into cellular compartmentalization. J. Integr. Plant Biol. 2021, 63, 1835–1855. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.T.; Spradling, A.C. A Balbiani body and the fusome mediate mitochondrial inheritance during Drosophila oogenesis. Development 2003, 130, 1579–1590. [Google Scholar] [CrossRef]
- Levone, B.R.; Lenzken, S.C.; Antonaci, M.; Maiser, A.; Rapp, A.; Conte, F.; Reber, S.; Mechtersheimer, J.; Ronchi, A.E.; Muhlemann, O.; et al. FUS-dependent liquid-liquid phase separation is important for DNA repair initiation. J. Cell Biol. 2021, 220, e202008030. [Google Scholar] [CrossRef] [PubMed]
- Staples, M.I.; Frazer, C.; Fawzi, N.L.; Bennett, R.J. Phase separation in fungi. Nat. Microbiol. 2023, 8, 375–386. [Google Scholar] [CrossRef]
- Nandana, V.; Schrader, J.M. Roles of liquid-liquid phase separation in bacterial RNA metabolism. Curr. Opin. Microbiol. 2021, 61, 91–98. [Google Scholar] [CrossRef]
- van der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef]
- Holehouse, A.S.; Kragelund, B.B. The molecular basis for cellular function of intrinsically disordered protein regions. Nat. Rev. Mol. Cell Biol. 2024, 25, 187–211. [Google Scholar] [CrossRef]
- Wegmann, S.; Eftekharzadeh, B.; Tepper, K.; Zoltowska, K.M.; Bennett, R.E.; Dujardin, S.; Laskowski, P.R.; MacKenzie, D.; Kamath, T.; Commins, C.; et al. Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J. 2018, 37, e98049. [Google Scholar] [CrossRef] [PubMed]
- Mateju, D.; Franzmann, T.M.; Patel, A.; Kopach, A.; Boczek, E.E.; Maharana, S.; Lee, H.O.; Carra, S.; Hyman, A.A.; Alberti, S. An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J. 2017, 36, 1669–1687. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Weihs, T.; Wurm, C.A.; Jansen, I.; Rehman, J.; Sahl, S.J.; Hell, S.W. MINFLUX nanometer-scale 3D imaging and microsecond-range tracking on a common fluorescence microscope. Nat. Commun. 2021, 12, 1478. [Google Scholar] [CrossRef]
- Riback, J.A.; Zhu, L.; Ferrolino, M.C.; Tolbert, M.; Mitrea, D.M.; Sanders, D.W.; Wei, M.T.; Kriwacki, R.W.; Brangwynne, C.P. Composition-dependent thermodynamics of intracellular phase separation. Nature 2020, 581, 209–214. [Google Scholar] [CrossRef]
- Strauss, S.; Nickels, P.C.; Strauss, M.T.; Jimenez, S.V.; Ellenberg, J.; Carter, J.D.; Gupta, S.; Janjic, N.; Jungmann, R. Modified aptamers enable quantitative sub-10-nm cellular DNA-PAINT imaging. Nat. Methods 2018, 15, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Shahzadi, K.; Jin, Y.; Li, R.; Momanyi, B.M.; Zulfiqar, H.; Ning, L.; Lin, H. Identification of RNA-dependent liquid-liquid phase separation proteins using an artificial intelligence strategy. Proteomics 2024, 24, 2400044. [Google Scholar] [CrossRef]
- Jia, L.; Gao, S.; Qiao, Y. Optical control over liquid–liquid phase separation. Small Methods 2024, 8, 2301724. [Google Scholar] [CrossRef]
- Shuyu, S.; Wen, S.; Xiaoyi, O.; Ping, W. Phase separation in synthetic biology. Quant. Biol. 2021, 9, 378–399. [Google Scholar] [CrossRef]



| Type | Protein Name | Features Associated with Phase-Separation Capacity | Nature of Stress | References |
|---|---|---|---|---|
| Plants | OsCRT3, OsCIPK7 | Cold stress triggers secondary structural changes in OsCRT3 and enhances its binding affinity with OsCIPK7, which finally boosts its kinase activity. | Cold | [148] |
| Plants | TANDEM ZINC-FINGER/PLUS3 (TZP) | TZP’s phase separation promotes PPKs and phyA colocalization and interaction, enhancing PPK-mediated phyA phosphorylation in FR light, crucial for plant photomorphogenic development in FR-rich conditions like canopy shade. | Light | [118] |
| Plants | CRY2/SPA1/FIO1 | The blue light receptor cryptochrome 2 (CRY2) and the METTL16-type m6A writer FIONA1 (FIO1) regulate chlorophyll homeostasis in response to blue light. | Light | [111] |
| Plants | ELF3 | The ability of temperature to rapidly shift ELF3 between active and inactive states via phase transition represents a previously unknown thermosensory mechanism. | Heat | [61] |
| Plants | RALF | RALF-pectin phase separation mediates an exoskeletal mechanism to broadly activate FER-LLG1-dependent cell surface responses to mediate the global role of FER in plant growth and survival. | Salt, heat | [149] |
| Plants | PhyB | The N-terminal extension’s disorder and C-terminal structure’s oligomerization drive phase separation, with the NTE sensing temperature signals. | Temperature light | [144] |
| Plants | SEU | The condensation of SEU is essential for plant tolerance to osmotic stress. | Osmotic pressure | [2] |
| Plants | RBGD2/4 | Tyrosine enrichment in the low-complexity domain (LCD) drives phase separation, recruiting heat-tolerant proteins and transcripts to stress granules (SGs). | Heat | [3] |
| Plants | NPR1 | Conserved cysteine clusters in IDRs mediate phase separation to form SINCs, enriching proteins that regulate cell death and stress responses, thus promoting cell survival under stress. | Salt | [4] |
| Plants | ALBA4/5/6 | Heat stress induces ALBA’s phase separation, aiding HSF mRNA recruitment to SGs and P-bodies, inhibiting HSF mRNA degradation. | Heat | [5] |
| Plants | FRI | Low-temperature signals induce FRI nuclear condensates, dissociating FRI from the FLC gene. COOLAIR RNA promotes FRI condensate accumulation, stabilizing FRI proteins for rapid temperature response. | Temperature | [6] |
| Plants | STM | Under salt stress, it enhances transcriptional regulation, promoting meristematic activity and salt tolerance. | Salt | [7] |
| Plants | FLOE1 | FLOE1 mediates the response to water stress and regulates seed germination. | Water | [8] |
| Animal/microorganisms | RNA polymerase (Pol) II | Pol II forms clusters or hubs at active genes through interactions between CTDs and activators, and CTD phosphorylation liberates Pol II enzymes from hubs for promoter escape and transcription elongation. | Heat | [115] |
| Animals | TIFA | As a sensor of upstream signals, the phase separation process of TIFA leads to the formation of membrane-less condensates within the ALPK1-TIFA-TRAF6 pathway, providing a potential application direction for the development of therapeutic biotechnology. | Disease | [10] |
| Animals | WNK kinases | WNK kinases are physiological crowding sensors that phase separate to coordinate a cell volume rescue response. | Hyperosmotic stress-induced | [11] |
| Animals | FUS | FUS is enriched in the nucleus and involved in transcription, DNA repair, and RNA biogenesis | Disease | [98] |
| Animals | Glycyl-tRNA synthetase (GlyRS) | GlyRS is translocated from the cytoplasm into SGs upon stress, where the mutant GlyRS perturbs the G3BP-centric SG network by aberrantly binding to G3BP. This disrupts SG-mediated stress responses, leading to increased stress vulnerability in motoneurons. Disrupting this aberrant interaction rescues SG abnormalities and alleviates motor deficits in CMT2D mice. | Peripheral neuropathy | [13] |
| Animals | MeCP2 | MeCP2 enhances the separation of heterochromatin and euchromatin through its condensate partitioning properties, and the disruption of condensates may be a common consequence of mutations in MeCP2 that cause Rett syndrome. | Disease | [14] |
| Microorganisms | 53BP1 | This liquid droplet-like behavior of 53BP1 compartments might help to coordinate local lesion recognition with global gene activation in response to DNA damage. | Ionizing radiation | [150] |
| Microorganisms | Ded1p | Ded1p condensation inactivates Ded1p, represses housekeeping mRNA translation, and promotes stress mRNA translation. It is adaptive and fine-tuned to the organism’s maximum growth temperature, and part of an extended heat shock response that selectively inhibits housekeeping mRNA translation to enhance survival in severe heat stress. | Heat | [17,151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Han, F.; Qi, Z.; Yan, C.; Su, B.; Wang, J. Phase Separation: Orchestrating Biological Adaptations to Environmental Fluctuations. Int. J. Mol. Sci. 2025, 26, 4614. https://doi.org/10.3390/ijms26104614
Wang W, Han F, Qi Z, Yan C, Su B, Wang J. Phase Separation: Orchestrating Biological Adaptations to Environmental Fluctuations. International Journal of Molecular Sciences. 2025; 26(10):4614. https://doi.org/10.3390/ijms26104614
Chicago/Turabian StyleWang, Wenxiu, Fangbing Han, Zhi Qi, Chunxia Yan, Bodan Su, and Jin Wang. 2025. "Phase Separation: Orchestrating Biological Adaptations to Environmental Fluctuations" International Journal of Molecular Sciences 26, no. 10: 4614. https://doi.org/10.3390/ijms26104614
APA StyleWang, W., Han, F., Qi, Z., Yan, C., Su, B., & Wang, J. (2025). Phase Separation: Orchestrating Biological Adaptations to Environmental Fluctuations. International Journal of Molecular Sciences, 26(10), 4614. https://doi.org/10.3390/ijms26104614






