Ovarian Cancer—Epidemiology, Classification, Pathogenesis, Treatment, and Estrogen Receptors’ Molecular Backgrounds
Abstract
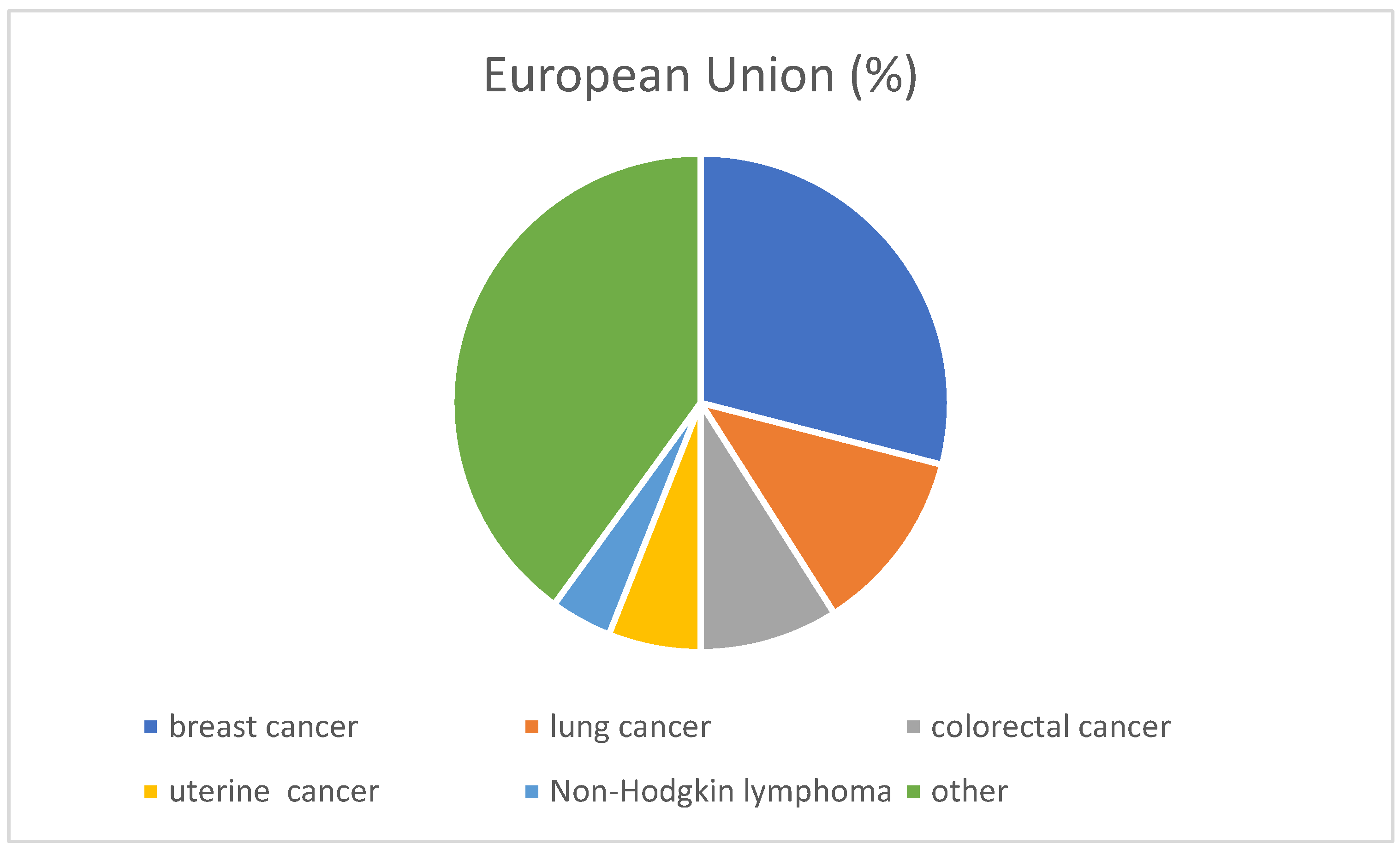
1. Epidemiology of Ovarian Cancer
2. Risk Factors for Ovarian Cancer
3. Ovarian Cancer Prevention
4. Symptoms of Ovarian Cancer
5. Ovarian Cancer Diagnosis
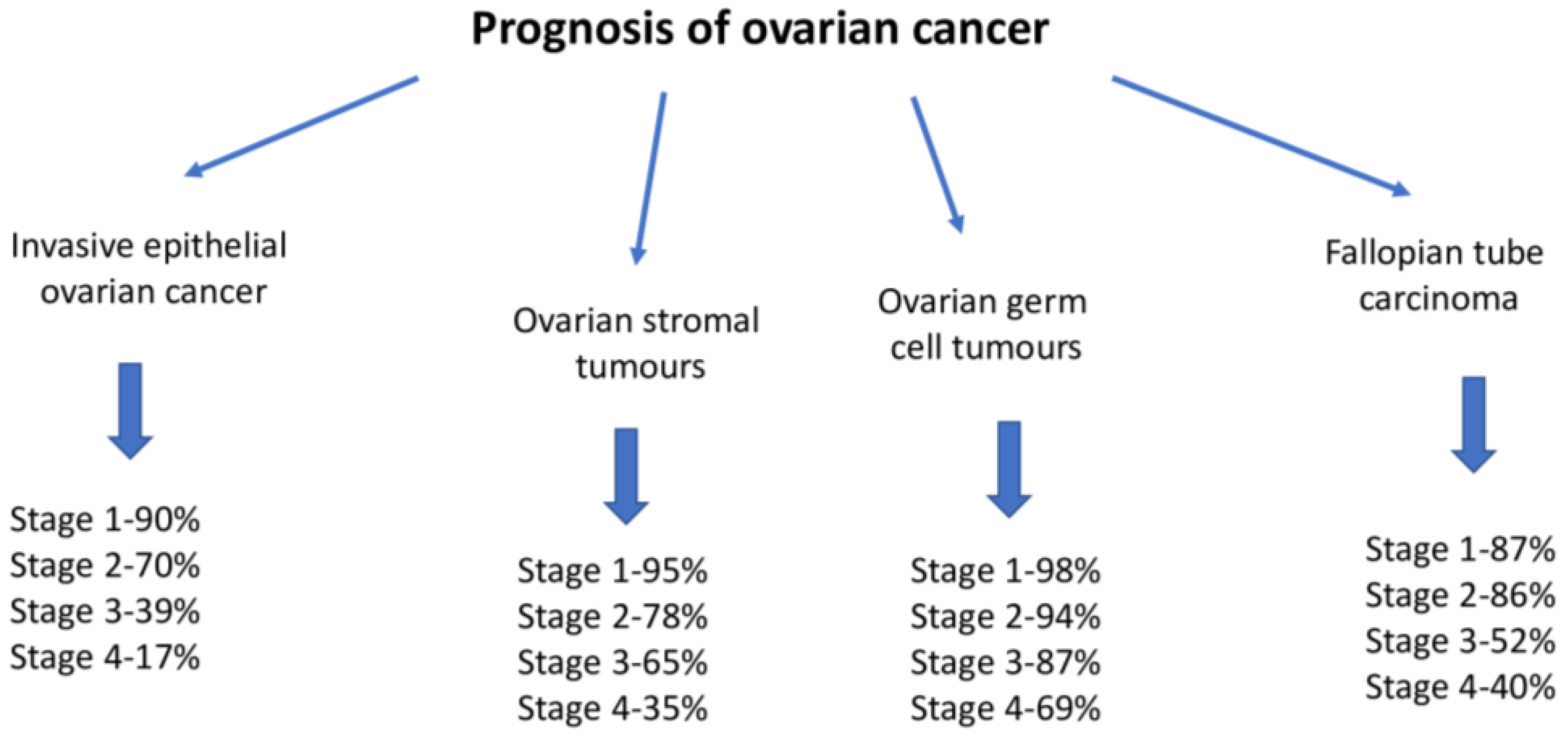
6. Morphological Types of Ovarian Cancer
- -
- Gonadal dysgenesis, with the 46 XY karyotype and sex-determining region Y (SRY) gene encoding a protein that determines testicular development.
- -
- Androgen insensitivity syndrome.
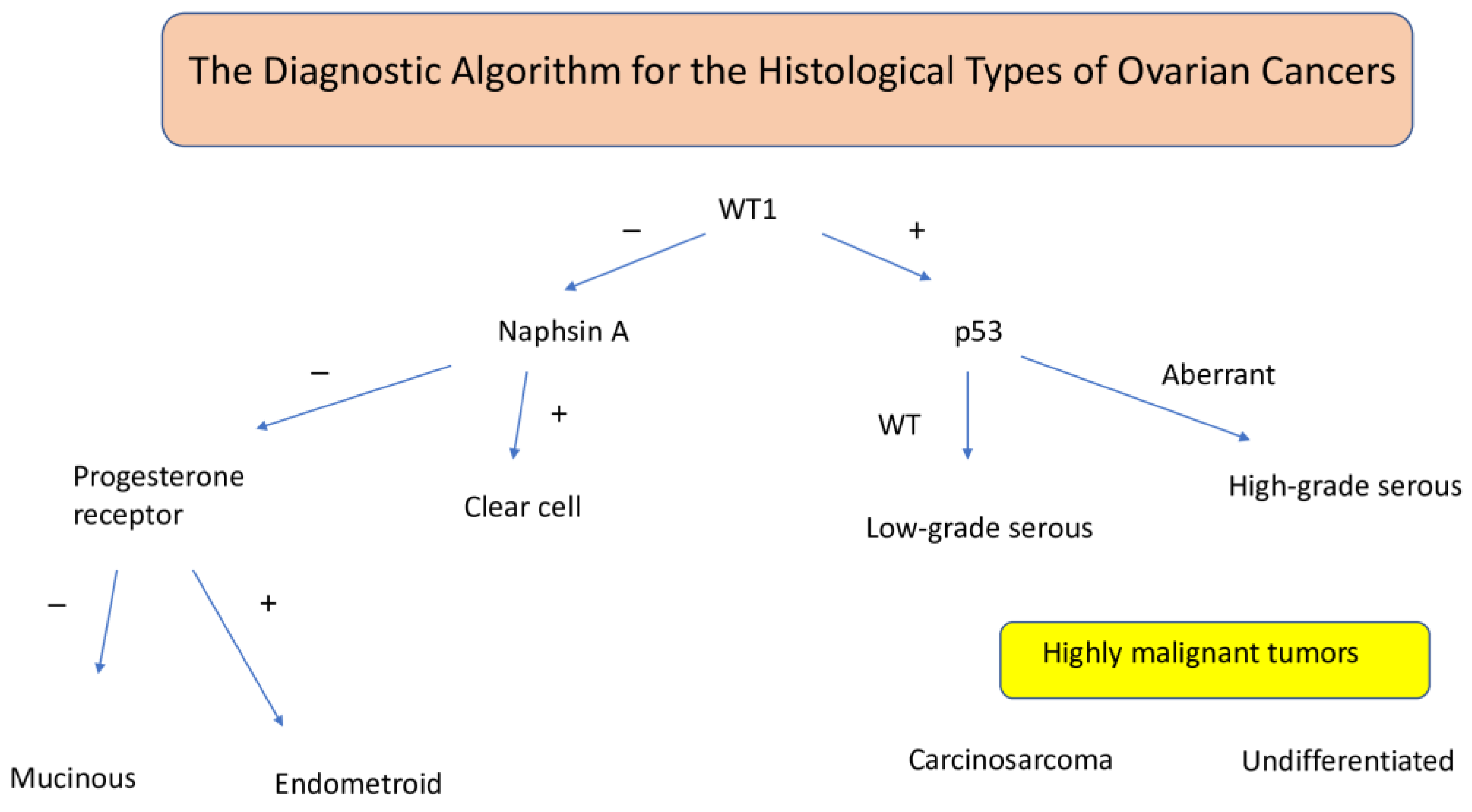
7. Histopathological Classification of Ovarian Cancer
- (1)
- High-grade serous carcinomas;
- (2)
- Endometrioid carcinomas;
- (3)
- Clear cell carcinomas, which often arise in the ovary and are often associated with endometriosis;
- (4)
- Mucinous carcinomas;
- (5)
- Low-grade serous carcinomas [35].
- G1—well-differentiated cancer (undifferentiated cells < 5%);
- G2—moderately differentiated cancer (50% of undifferentiated cells);
- G3—undifferentiated cancer (undifferentiated cells > 50%).
8. Ovarian Cancer Treatment
- -
- The clinical stage (Figure 4).
- -
- Whether a complete cytoreduction procedure is possible.
- -
- Sensitivity to platinum derivatives in systemic therapy.
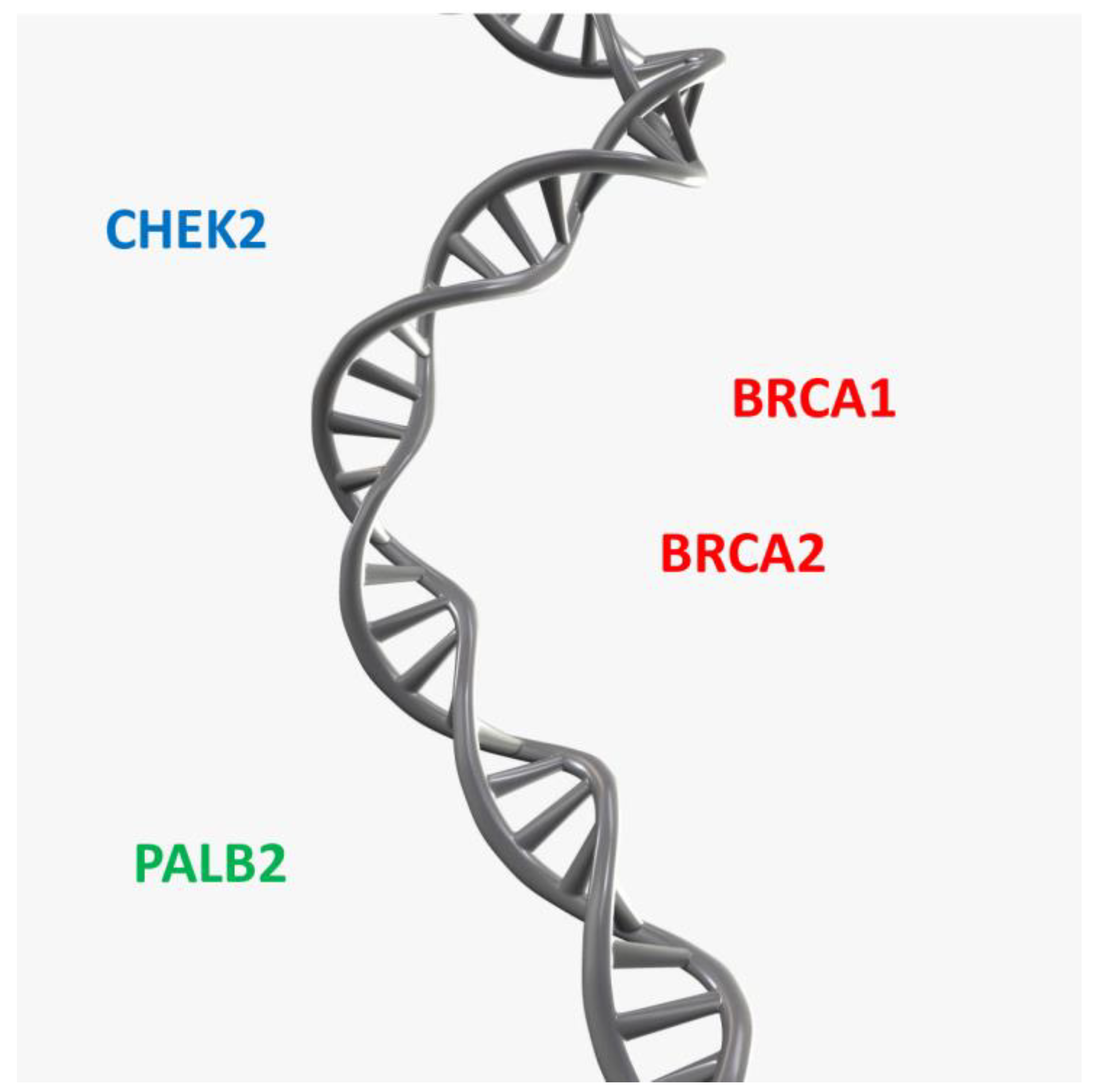
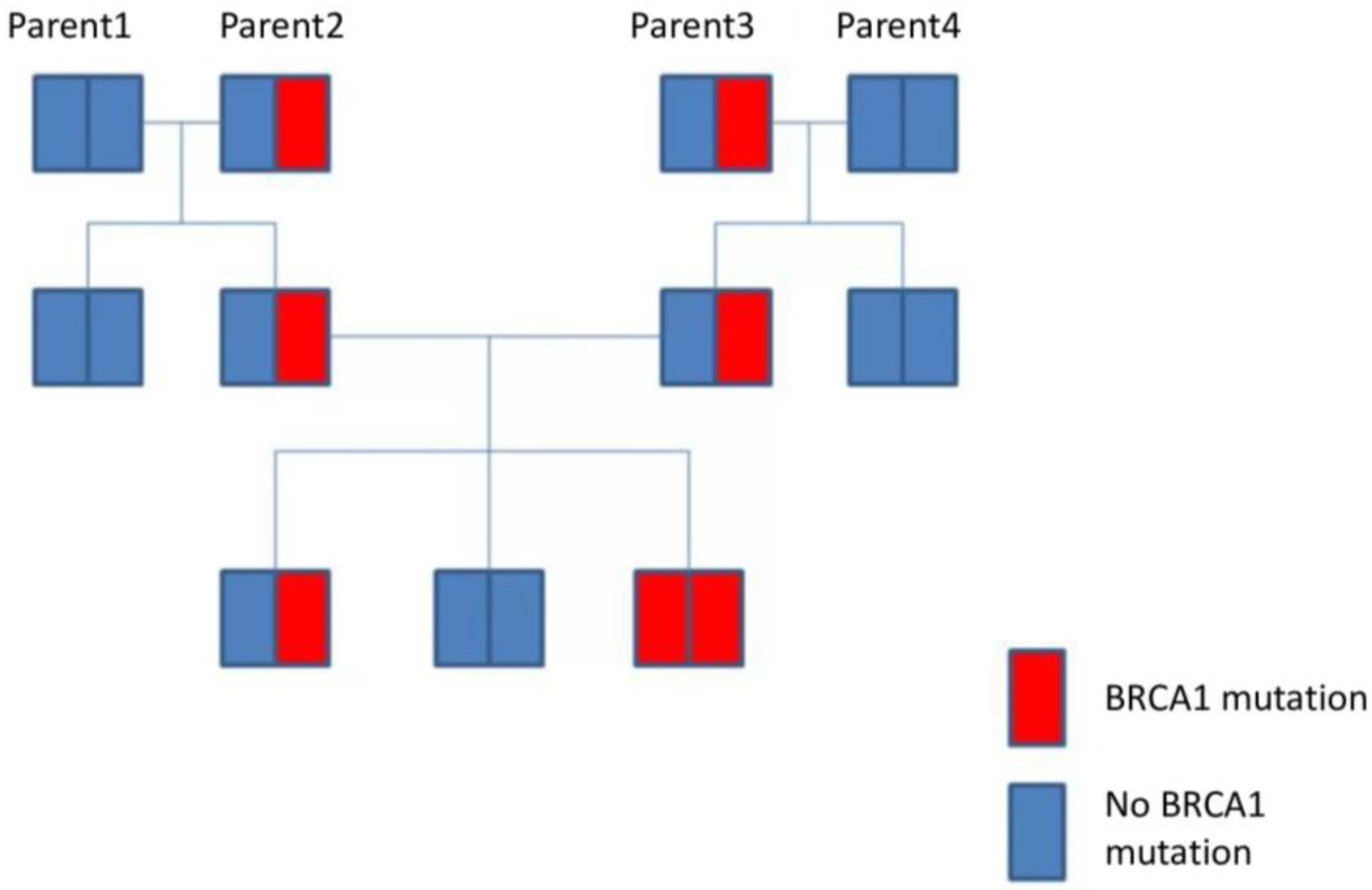
9. Genetic Factors
10. The Role of Estrogen Receptors in Ovarian Cancer Oncogenesis
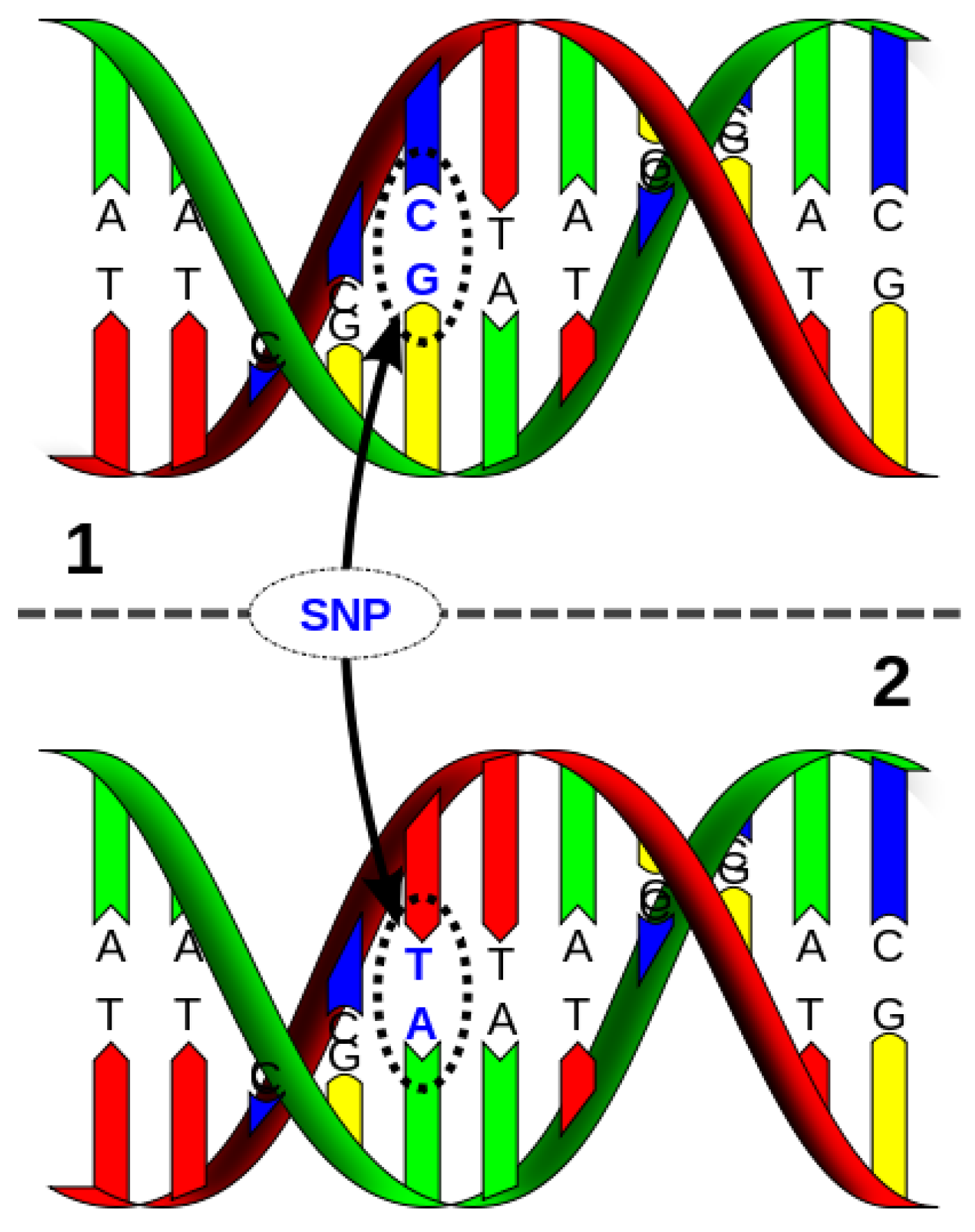
11. Single Nucleotide Polymorphisms
12. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Didkowska, J.; Wojciechowska, U.; Michalek, I.M.; Caetano Dos Santos, F.L. Cancer incidence and mortality in Poland in 2019. Sci. Rep. 2022, 12, 10875. [Google Scholar] [CrossRef]
- Wojciechowska, U.; Barańska, K.; Michałek, I.; Olasek, P.; Miklewska, M.; Didkowska, J.A. Nowotwory Złośliwe w Polsce w 2020 Roku; Krajowy Rejestr Nowotworów: Warszawa, Poland, 2022. [Google Scholar]
- The Organization for Economic Co-Operation and Development. Krajowe profile dotyczące nowotworów: Polska 2023. In EU Country Cancer Profiles; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- OECD; Europejskie Obserwatorium Polityki i Systemów Opieki Zdrowotnej. Polska: Profil Systemu Ochrony Zdrowia; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Trama, A.; Bielska-Lasota, M. Rzadkie nowotwory złośliwe—Narastający problem w Europie i w Polsce. Med. Prakt. Onkol. 2013, 1, 53–58. [Google Scholar]
- Ali, A.T.; Al-Ani, O.; Al-Ani, F. Epidemiology and risk factors for ovarian cancer. Prz. Menopauzalny 2023, 22, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S. FIGO staging in ovarian carcinoma and histological subtypes. J. Gynecol. Oncol. 2020, 31, e70. [Google Scholar] [CrossRef]
- Barili, V.; Ambrosini, E.; Bortesi, B.; Minari, R.; De Sensi, E.; Cannizzaro, I.R.; Taiani, A.; Michiara, M.; Sikokis, A.; Boggiani, D.; et al. Genetic Basis of Breast and Ovarian Cancer: Approaches and Lessons Learned from Three Decades of Inherited Predisposition Testing. Genes 2024, 15, 219. [Google Scholar] [CrossRef]
- Gambini, D.; Ferrero, S.; Kuhn, E. Lynch Syndrome: From Carcinogenesis to Prevention Interventions. Cancers 2022, 14, 4102. [Google Scholar] [CrossRef]
- Daly, M.B.; Dresher, C.W.; Yates, M.S.; Jeter, J.M.; Karlan, B.Y.; Alberts, D.S.; Lu, K.H. Salpingectomy as a means to reduce ovarian cancer risk. Cancer Prev. Res. 2015, 8, 342–348. [Google Scholar] [CrossRef]
- Dixon-Suen, S.C.; Webb, P.M.; Wilson, L.F.; Tuesley, K.; Stewart, L.M.; Jordan, S.J. The Association Between Hysterectomy and Ovarian Cancer Risk: A Population-Based Record-Linkage Study. J. Natl. Cancer Inst. 2019, 111, 1097–1103. [Google Scholar] [CrossRef]
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: An individual participant meta-analysis of 27 276 women with endometrial cancer from 36 epidemiological studies. Lancet. Oncol. 2015, 16, 1061–1070. [Google Scholar] [CrossRef]
- Norheim, O.F.; Jha, P.; Admasu, K.; Godal, T.; Hum, R.J.; Kruk, M.E.; Gómez-Dantés, O.; Mathers, C.D.; Pan, H.; Sepúlveda, J.; et al. Avoiding 40% of the premature deaths in each country, 2010–2030: Review of national mortality trends to help quantify the UN sustainable development goal for health. Lancet 2015, 385, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Stover, J.; Winfrey, W. The effects of family planning and other factors on fertility, abortion, miscarriage, and stillbirths in the Spectrum model. BMC Public. Health 2017, 17, 775. [Google Scholar] [CrossRef]
- Wojciechowska, P.; Milska Musa, K.A. Quality of life and mental state of women trying to conceive using the in vitro method. Eur. Transl. Clin. Med. 2024, 7, 106–112. [Google Scholar] [CrossRef]
- Aune, D. Plant Foods, Antioxidant Biomarkers, and the Risk of Cardiovascular Disease, Cancer, and Mortality: A Review of the Evidence. Adv. Nutr. 2019, 10, S404–S421. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, M.S. Nutrition and cancer: A review of the evidence for an anti-cancer diet. Nutr. J. 2004, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, G. Endometriosis-associated Ovarian Clear Cell Carcinoma: A Special Entity? J. Cancer 2021, 12, 6773–6786. [Google Scholar] [CrossRef]
- Hablase, R.; Kyrou, I.; Randeva, H.; Karteris, E.; Chatterjee, J. The “Road” to Malignant Transformation from Endometriosis to Endometriosis-Associated Ovarian Cancers (EAOCs): An mTOR-Centred Review. Cancers 2024, 16, 2160. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Hediger, M.L.; Peterson, C.M.; Croughan, M.; Sundaram, R.; Stanford, J.; Chen, Z.; Fujimoto, V.Y.; Varner, M.W.; Trumble, A.; et al. ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: The ENDO study. Fertil. Steril. 2011, 96, 360–365. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Mahamat-Saleh, Y.; Farland, L.V.; Shigesi, N.; Terry, K.L.; Harris, H.R.; Roman, H.; Becker, C.M.; As-Sanie, S.; Zondervan, K.T.; et al. Endometriosis and cancer: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 393–420. [Google Scholar] [CrossRef]
- Neff, R.T.; Senter, L.; Salani, R. BRCA mutation in ovarian cancer: Testing, implications and treatment considerations. Ther. Adv. Med. Oncol. 2017, 9, 519–531. [Google Scholar] [CrossRef]
- AlHilli, M.M.; Al-Hilli, Z. Perioperative Management of Women Undergoing Risk-reducing Surgery for Hereditary Breast and Ovarian Cancer. J. Minim. Invasive Gynecol. 2019, 26, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.P.; Lubinski, J.; Møller, P.; Singer, C.F.; Karlan, B.; Senter, L.; Rosen, B.; Maehle, L.; Ghadirian, P.; Cybulski, C.; et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J. Clin. Oncol. 2014, 32, 1547–1553. [Google Scholar] [CrossRef]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Evans, D.G.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Association of risk-reducing surgery in BRCA1 26or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010, 304, 967–975. [Google Scholar] [CrossRef]
- Liberto, J.M.; Chen, S.Y.; Shih, I.M.; Wang, T.H.; Wang, T.L.; Pisanic, T.R., 2nd. Current and Emerging Methods for Ovarian Cancer Screening and Diagnostics: A Comprehensive Review. Cancers 2022, 14, 2885. [Google Scholar] [CrossRef]
- Bankhead, C.R.; Collins, C.; Stokes-Lampard, H.; Rose, P.; Wilson, S.; Clements, A.; Mant, D.; Kehoe, S.T.; Austoker, J. Identifying symptoms of ovarian cancer: A qualitative and quantitative study. BJOG 2008, 115, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.U.; Li, H.; Zhang, B. The diagnostic value of serum HE4 and CA-125 and ROMA index in ovarian cancer. Biomed. Rep. 2016, 5, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Doubeni, C.A.; Doubeni, A.R.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician 2016, 93, 937–944. [Google Scholar]
- Levanon, K.; Crum, C.; Drapkin, R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J. Clin. Oncol. 2008, 26, 5284–5293. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef]
- Kim, J.; Park, E.Y.; Kim, O.; Schilder, J.M.; Coffey, D.M.; Cho, C.H.; Bast, R.C., Jr. Cell Origins of High-Grade Serous Ovarian Cancer. Cancers 2018, 10, 433. [Google Scholar] [CrossRef]
- Wu, R.; Baker, S.J.; Hu, T.C.; Norman, K.M.; Fearon, E.R.; Cho, K.R. Type I to type II ovarian carcinoma progression: Mutant Trp53 or Pik3ca confers a more aggressive tumor phenotype in a mouse model of ovarian cancer. Am. J. Pathol. 2013, 182, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Kordowitzki, P.; Lange, B.; Elias, K.M.; Haigis, M.C.; Mechsner, S.; Braicu, I.E.; Sehouli, J. Transforming treatment paradigms: Focus on personalized medicine for high-grade serous ovarian cancer. CA Cancer J. Clin. 2025. [Google Scholar] [CrossRef] [PubMed]
- Prat, J.; FIGO Committee on Gynecologic Oncology. FIGO’s staging classification for cancer of the ovary fallopian tube and peritoneum: Abridged republication. J. Gynecol. Oncol. 2015, 26, 87–89. [Google Scholar] [CrossRef]
- Zalewski, K.; Misiek, M.; Góźdź, S.; Bidziński, M. Nowy system klasyfikacji zaawansowania nowotworów jajnika, jajowodu i otrzewnej—Stan na 2014 rok. Onkol. W Prakt. Klin. 2015, 11, 129–134. [Google Scholar]
- Marth, C.; Reimer, D.; Zeimet, A.G. Front-line therapy of advanced epithelial ovarian cancer: Standard treatment. Ann. Oncol. 2017, 28, viii36–viii39. [Google Scholar] [CrossRef]
- Macchia, G.; Titone, F.; Restaino, S.; Arcieri, M.; Pellecchia, G.; Andreetta, C.; Driul, L.; Vizzielli, G.; Pezzulla, D. Is It Time to Reassess the Role of Radiotherapy Treatment in Ovarian Cancer? Healthcare 2023, 11, 2413. [Google Scholar] [CrossRef]
- Durno, K.; Powell, M.E. The role of radiotherapy in ovarian cancer. Int. J. Gynecol. Cancer 2022, 32, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Hatae, M.; Watanabe, Y.; Yaegashi, N.; Ishiko, O.; Kodama, S.; Yamaguchi, S.; Ochiai, K.; Takano, M.; Yokota, H.; et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: A proposal for patient selection. J. Clin. Oncol. 2010, 28, 1727–1732. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Wang, Y.; Zhao, X.; Qi, X. Hormone therapy for ovarian cancer: Emphasis on mechanisms and applications (Review). Oncol. Rep. 2021, 46, 223. [Google Scholar] [CrossRef]
- Hirschl, N.; Leveque, W.; Granitto, J.; Sammarco, V.; Fontillas, M.; Penson, R.T. PAR Inhibitors: Strategic Use and Optimal Management in Ovarian Cancer. Cancers 2024, 16, 932. [Google Scholar] [CrossRef]
- Basta, P.; Bidziński, M.; Kluz, T.; Nowak-Markwitz, E.; Olejek, A.; Sawicki, W. Zasady postępowania z chorymi z podejrzeniem i rozpoznaniem raka jajnika—Rekomendacje Polskiego Towarzystwa Ginekologicznego. Ginekol. I Perinatol. Prakt. 2016, 1, 127–129. [Google Scholar]
- Zamwar, U.M.; Anjankar, A.P. Aetiology, Epidemiology, Histopathology, Classification, Detailed Evaluation, and Treatment of Ovarian Cancer. Cureus 2022, 14, e30561. [Google Scholar] [CrossRef]
- La Vecchia, C. Ovarian cancer: Epidemiology and risk factors. Eur J Cancer Prev. 2017, 26, 55–62. [Google Scholar] [CrossRef]
- Paluch-Shimon, S.; Cardoso, F.; Sessa, C.; Balmana, J.; Cardoso, M.J.; Gilbert, F.; Senkus, E. ESMO Guidelines Committee. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 2016, 27, v103–v110. [Google Scholar] [CrossRef]
- Walsh, T.; Casadei, S.; Lee, M.K.; Pennil, C.C.; Nord, A.S.; Thornton, A.M.; Roeb, W.; Agnew, K.J.; Stray, S.M.; Wickramanayake, A.; et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc. Natl. Acad. Sci. USA 2011, 108, 18032–18037. [Google Scholar] [CrossRef]
- Chudecka-Głaz, A. Bezpieczeństwo leczenia niraparybem pacjentek z rakiem jajnika. Onkol. W Prakt. Klin. 2023, 9, 368–378. [Google Scholar]
- Doraczyńska-Kowalik, A.; Janus-Szymańska, G.; Matkowski, R.; Gabalewicz, K.; Michałowska, D.; Sąsiadek, M.M. Genetyka i onkologia (część 2.)Podstawy medycyny personalizowanej w leczeniu raka piersi i raka jajnika. Biul. Pol. Tow. Onkol. Nowotw. 2020, 5, 255–272. [Google Scholar] [CrossRef]
- Boon, W.C.; Chow, J.D.Y.; Simpson, E.R. The multiple roles of estrogens and the enzyme aromatase. Prog. Brain Res. 2010, 181, 209–232. [Google Scholar] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein. Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar]
- Prossnitz, E.R.; Barton, M. Estrogen biology: New insights into GPER function and clinical opportunities. Mol. Cell Endocrinol. 2014, 389, 71–83. [Google Scholar] [CrossRef]
- Fujiwara, S.; Terai, Y.; Kawaguchi, H.; Takai, M.; Yoo, S.; Tanaka, Y.; Tanaka, T.; Tsunetoh, S.; Sasaki, H.; Kanemura, M.; et al. GPR30 regulates the EGFR-Akt cascade and predicts lower survival in patients with ovarian cancer. J. Ovarian Res. 2012, 5, 35. [Google Scholar] [CrossRef]
- Smith, H.O.; Arias-Pulido, H.; Kuo, D.Y.; Howard, T.; Qualls, C.R.; Lee, S.J.; Verschraegen, C.F.; Hathaway, H.J.; Joste, N.E.; Prossnitz, E.R. GPR30 predicts poor survival for ovarian cancer. Gynecol. Oncol. 2009, 114, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, M.J.; Piastowska-Ciesielska, A.W. Estrogens, Estrogen Receptors and Tumor Microenvironment in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 14673. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Green, S.; Stack, G.; Berry, M.; Jin, J.R.; Chambon, P. Functional domains of the human estrogen receptor. Cell 1987, 51, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Mosselman, S.; Polman, J.; Dijkema, R. ER beta: Identification and characterization of a novel human estrogen receeptor. FEBS Lett. 1996, 392, 49–53. [Google Scholar] [CrossRef]
- Zivadinovic, D.; Watson, C.S. Membrane estrogen receptor-alpha levels predict estrogen-induced ERK1/2 activation in MCF-7 cells. Breast Cancer Res. 2005, 7, 130–144. [Google Scholar] [CrossRef]
- Boyapati, S.M.; Shu, X.O.; Ruan, Z.X.; Cai, Q.; Smith, J.R.; Wen, W.; Gao, Y.T.; Zheng, W. Polymorphism in ER gene interactwith estrogen receptor status in breast cancer survival. Clin. Res. 2005, 11, 1093–1098. [Google Scholar]
- Levin, E.R. Integration of the extranuclear and nuclear actions of estrogen. Mol. Endocrinol. 2005, 19, 1951–1959. [Google Scholar] [CrossRef]
- Leung, P.C.; Choi, J.H. Endocrine signaling in ovarian surface epithelium and cancer. Hum. Reprod. Update 2007, 2, 143–162. [Google Scholar] [CrossRef]
- Gallo, D.; Ferlini, C.; Scambia, G. The epithelial-mesenchymal transition and the estrogen-signaling in ovarian cancer. Curr. Drug Targets 2010, 11, 474–481. [Google Scholar] [CrossRef]
- Johansson, Å.; Schmitz, D.; Höglund, J.; Hadizadeh, F.; Karlsson, T.; Ek, W.E. Investigating the Effect of Estradiol Levels on the Risk of Breast, Endometrial, and Ovarian Cancer. J. Endocr. Soc. 2022, 6, bvac100. [Google Scholar] [CrossRef]
- Skrzypczak, M.; Wolinska, E.; Adaszek, Ł.; Ortmann, O.; Treeck, O. Epigenetic Modulation of Estrogen Receptor Signaling in Ovarian Cancer. Int. J. Mol. Sci. 2024, 26, 166. [Google Scholar] [CrossRef] [PubMed]
- Kocanova, S.; Mazaheri, M.; Caze-Subra, S.; Bystricky, K. Ligands specify estrogen receptor alpha nuclear localization and degradation. BMC Cell Biol. 2010, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Hermenegildo, C. Modulation of the oestrogenreceptor: A process with distinct sussceptible steps. Hum. Reprod. 2000, 6, 207–211. [Google Scholar]
- Paech, K.; Webb, P.; Kuiper, G.G.; Nilsson, S.; Gustafsson, J.; Kushner, P.J.; Scanlan, T.S. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science 1997, 277, 1508–1510. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Hidaka, T.; Kataaoka, K.; Ymakawa, Y.; Akada, S.; Terenishi, A.; Saito, S. Absence of estrrogen receptor-alpha expression in human ovarian clear cell adenocarcinoma compared with ovarian serous, endometrioid, and mucinous adenocarcinoma. Am. J. Surg. Pathol. 2001, 25, 667–672. [Google Scholar] [CrossRef]
- Rutherford, T.; Brown, W.D.; Sapi, E.; Aschkenazi, S.; Munoz, A.; Mor, G. Absence of estrogen receptor—β expression in metastatic ovarian disease. Obs. Gynecol. 2000, 96, 417–421. [Google Scholar]
- Schüler-Toprak, S.; Weber, F.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Expression of estrogen-related receptors in ovarian cancer and impact on survival. J. Cancer Res. Clin. Oncol. 2021, 147, 2555–2567. [Google Scholar] [CrossRef]
- Borella, F.; Fucina, S.; Mangherini, L.; Cosma, S.; Carosso, A.R.; Cusato, J.; Cassoni, P.; Bertero, L.; Katsaros, D.; Benedetto, C. Hormone Receptors and Epithelial Ovarian Cancer: Recent Advances in Biology and Treatment Options. Biomedicines 2023, 11, 2157. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, H.; Zhang, Y.; Li1, Z.; Meng, W.; Xia, J.; Lei, W.; Meng, K.; Guo, Y. Research Progress of Estrogen Receptor in Ovarian Cancer. Clin. Exp. Obs. Gynecol. 2023, 50, 199. [Google Scholar] [CrossRef]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef] [PubMed]
- Mal, R.; Magner, A.; David, J.; Datta, J.; Vallabhaneni, M.; Kassem, M.; Manouchehri, J.; Willingham, N.; Stover, D.; Vandeusen, J.; et al. Estrogen Receptor Beta (ERbeta): A Ligand Activated Tumor Suppressor. Front. Oncol. 2020, 10, 587386. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. A systematic review and meta-analysis of hormone receptor expression in low-grade serous ovarian carcinoma. Eur. J. Obs. Gynecol. Reprod. Biol. 2021, 256, 172–178. [Google Scholar] [CrossRef]
- Zhou, X.; Gu, Y.; Wang, D.N.; Ni, S.; Yan, J. Eight functional polymorphisms in the estrogen receptor 1 gene and endometrial cancer risk: A meta-analysis. PLoS ONE 2013, 8, e60851. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.H.; Su, J.; Mu, Y.Y.; Zhang, X.Q.; Li, H.Z.; He, X.F.; He, X.F. Association between the ESR1 and ESR2 polymorphisms and osteoporosis risk: An updated meta-analysis. Medicine 2023, 102, e35461. [Google Scholar] [CrossRef]
- Ren, H.; Liu, H.; Huang, L.; Xie, W.; Lin, D.; Luo, D. Association of ESR1 and ESR2 Polymorphisms with Osteoporosis: A Meta-Analysis from 36 Studies. J. Clin. Densitom. 2022, 25, 699–711. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, X.; Zhang, L.; Hu, Q.; Guo, Y.; Jiang, H.; Shi, S.; Zhang, X. Association of estrogen receptor αPvuII and XbaI polymorphisms with prostate cancer susceptibility and risk stratification: A meta-analysis from case-control studies. Oncol. Targets Ther. 2017, 10, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Aboelroos, S.A.; Eltamany, E.H.M.; Mohamed, F.A.M.; Suliman, M.A. Association between estrogen receptor alpha and aryl hydrocarbon receptor gene polymorphisms in the prognosis of breast cancer in Egypt. Egypt. J. Immunol. 2024, 31, 87–92. [Google Scholar]
- Vega-García, S.; Saucedo-García, R.; Basurto-Acevedo, M.L.; Vargas-Gutiérrez, C.; Galván-Duarte, R.E.; Reyes-Maldonado, E.; Aguilar-Gallegos, U.I.; Avelar-Garnica, F.; Galván-Plata, M.E. Alpha estrogen receptor polymorphisms and their association with breast density. Rev. Med. Inst. Mex. Seguro Soc. 2020, 58, S13–S20. [Google Scholar]
- Wang, B.; Yuan, F. Comment on “Estrogen receptor alpha (ERS1) SNPs c454-397T>C (PvuII) and c454-351A>G (XbaI) are risk biomarkers for breast cancer development”. Mol. Biol. Rep. 2019, 46, 5. [Google Scholar] [CrossRef]
- Pemmaraju, S.; Amidyala, L.; Vottery, R.; Nallari, P.; Akka, J.; Ananthapur, V. Association of ER-α gene PvuII polymorphism with ovarian cancer. Cancer Treat Res. Commun. 2018, 14, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Boyle, B.; Dallaire, N.; MacKay, J. Evaluation of the impact of single nucleotide polymorphisms and primer mismatches on quantitative PCR. BMC Biotechnol. 2009, 9, 75. [Google Scholar] [CrossRef]
- Tomita-Mitchell, A.; Muniappan, B.P.; Herrero-Jimenez, P.; Zarbl, H.; Thilly, W.G. Single nucleotide polymorphism spectra in newborns and centenarians: Identification of genes coding for rise of mortal disease. Gene 1998, 223, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, H.C.; Chanock, S.J. SNPs in cancer research and treatment. Br. J. Cancer 2004, 90, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Shastry, B.S. SNPs in disease gene mapping, medicinal drug development and evolution. J. Hum. Genet. 2007, 52, 871–880. [Google Scholar] [CrossRef]
- Shastry, B.S. SNPs: Impact on genefunction and phenotype. Methods Mol. Biol. 2009, 578, 3–22. [Google Scholar]
- Antontseva, E.V.; Degtyareva, A.O.; Korbolina, E.E.; Damarov, I.S.; Merkulova, T.I. Human-genome single nucleotide polymorphisms affecting transcription factor binding and their role in pathogenesis. Vavilovskii Zhurnal Genet. Sel. 2023, 27, 662–675. [Google Scholar] [CrossRef]
- Dakal, T.C.; Dhabhai, B.; Pant, A.; Moar, K.; Chaudhary, K.; Yadav, V.; Ranga, V.; Sharma, N.K.; Kumar, A.; Maurya, P.K.; et al. Oncogenes and tumor suppressor genes: Functions and roles in cancers. Med. Comm. 2020 2024, 5, e582. [Google Scholar] [CrossRef]
- Deng, N.; Zhou, H.; Fan, H.; Yuan, Y. Single nucleotide polymorphisms and cancer susceptibility. Oncotarget 2017, 8, 110635–110649. [Google Scholar] [CrossRef]
- Turczynowicz, A.; Niedźwiecka, K.; Panasiuk, D.; Pużyńska, W.; Luchowski, K.; Kondracka, J.; Jakubów, P. Single nucleotide polymorphisms as predictors of treatment efficacy and adverse effects of morphine in palliative medicine: A literature review. Palliat. Med. Pract. 2023, 17, 29–38. [Google Scholar] [CrossRef]
- Marc, J. The many faces of estrogen signaling. Biochem. Med. (Zagreb) 2014, 24, 329–342. [Google Scholar]
- Yaşar, P.; Ayaz, G.; User, S.D.; Güpür, G.; Muyan, M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod. Med. Biol. 2016, 16, 4–20. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for estrogen receptor expression in human cancer. Exp. Hematol. Oncol. 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Coons, L.A.; Houtman, R.; Carlson, K.E.; Martin, T.A.; Mayne, C.G.; Melchers, D.; Jefferson, T.B.; Ramsey, J.T.; Katzenellenbogen, J.A.; et al. A mutant form of ERα associated with estrogen insensitivity affects the coupling between ligand binding and coactivator recruitment. Sci. Signal 2020, 13, eaaw4653. [Google Scholar] [CrossRef]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef]
- Zuo, J. Estrogen-related receptors: Novel potential regulators of osteoarthritis pathogenesis. Mol. Med. 2021, 27, 5. [Google Scholar]
- Gan, X.; Dai, G.; Li, Y.; Xu, L.; Liu, G. Intricate roles of estrogen and estrogen receptors in digestive system cancers: A systematic review. Cancer Biol. Med. 2024, 21, 898–915. [Google Scholar] [CrossRef]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef]
- Su, X.; Xu, X.; Li, G.; Lin, B.; Cao, J.; Teng, L. ER-α36: A novel biomarker and potential therapeutic target in breast cancer. Oncol. Targets Ther. 2014, 7, 1525–1533. [Google Scholar]
- Sundermann, E.; Maki, P.; Bishop, J. A Review of Estrogen Receptor α Gene (ESR1) Polymorphisms, Mood, and Cognition. Menopause 2010, 17, 874–886. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Yin, L. Estrogen receptor alpha-36 (ER-α36): A new player in human breast cancer. Mol. Cell Endocrinol. 2015, 418 Pt 3, 193–206. [Google Scholar] [CrossRef]
- Ding, S.; Yu, J.; Chen, S.; Hsu, G.C.; Hsu, H.M.; Ho, J.Y.; Lin, Y.H.; Chang, C.C.; Fann, C.S.; Cheng, C.W.; et al. Diverse associations between ESR1 polymorphism and breast cancer development and progression. Clin. Cancer Res. 2010, 16, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Herrington, D.M.; Howard, T.D.; Brosnihan, K.B.; McDonnell, D.P.; Li, X.; Hawkins, G.A.; Reboussin, D.M.; Xu, J.; Zheng, S.L.; Meyers, D.A.; et al. Common estrogen receptor polymorphism augments effects of hormone replacement therapy on E-selectin but not C-reactive protein. Circulation 2002, 105, 1879–1882. [Google Scholar] [CrossRef]
- Swartz, C.D.; Afshari, C.A.; Yu, L.; Hall, K.E.; Dixon, D. Estrogen-induced changes in IGF-I, Myb family and MAP kinase pathway genes in human uterine leiomyoma and normal uterine smooth muscle cell lines. Mol. Hum. Reprod. 2005, 11, 441–450. [Google Scholar] [CrossRef]
- Wang, Y.M.; Liu, Z.W.; Guo, J.B.; Wang, X.F.; Zhao, X.X.; Zheng, X. ESR1 Gene Polymorphisms and Prostate Cancer Risk: A HuGE Review and Meta-Analysis. PLoS ONE 2013, 8, e66999. [Google Scholar] [CrossRef] [PubMed]
- Cahua, J.; Cruz, M.; Méndez, A.; Antúnez-Ortiz, D.L.; Vences-Velázquez, A.; del Carmen Alarcón-Romero, L.; Parra, E.J.; Tello-Flores, V.A.; Leyva-Vázquez, M.A.; Valladares-Salgado, A.; et al. Polymorphisms in the LPL and CETP Genes and Haplotype in the ESR1 Gene Are Associated with Metabolic Syndrome in Women from Southwestern Mexico. Int. J. Mol. Sci. 2015, 16, 21539–21554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Yuan, X.; Zhang, Z.; Zhang, P.; Chao, H.; Jiang, L.; Jiang, J. Association Between ESR1 PvuII, XbaI, and P325P Polymorphisms andBreast Cancer Susceptibility: A Meta-Analysis. Med. Sci Monit. 2015, 21, 2986–2996. [Google Scholar] [CrossRef]
- Li, L.W.; Xu, L. Menopausal status modifies breast cancer risk associated with ESR1 PvuII and XbaI polymorphisms in Asian women: A HuGE review and meta-analysis. Asian Pac. J. Cancer Prev. 2012, 13, 5105–5111. [Google Scholar] [CrossRef]
- Aboelroos, S.A.; Segaey, D.G.E.; Elgawad, A.K.A.; Orabi, M.; Mohamed, M.H.; Hassan, N.R. Association of Estrogen Receptor-α and Aryl Hydrocarbon Receptor Gene Polymorphisms with Ischemic Stroke in an Egyptian Population: A Pilot Study. J. Mol. Neurosci. 2024, 74, 85. [Google Scholar] [CrossRef]
- Madeira, K.P.; Daltoé, R.D.; Sirtoli, G.M.; Carvalho, A.A.; Rangel, L.B.; Silva, I.V. Estrogen receptor alpha (ERS1) SNPs c454-397T>C (PvuII) and c454-351A>G (XbaI) are risk biomarkers for breast cancer development. Mol. Biol. Rep. 2014, 41, 5459–5466. [Google Scholar] [CrossRef]
- Smolarz, B.; Nowak, A.Z.; Bryś, M.; Forma, E.; Łukasiewicz, H.; Samulak, D.; Langner, S.; Romanowicz, H. Analysis of Single Nucleotide Polymorphisms (SNPs) rs2234693 and rs9340799 of the ESR1 Gene and the Risk of Breast Cancer. In Vivo 2024, 38, 2134–2143. [Google Scholar] [CrossRef] [PubMed]
- Seremak-Mrozikiewicz, A.; Drews, K.; Bartkowiak-Wieczorek, J.; Kurzawińska, G.; Pieńkowski, W.; Spaczyński, M.; Mrozikiewicz, P.M. PvuII genetic polymorphism of estrogen receptor alpha in the group of postmenopausal women with osteopenia and osteoporosis. Ginekol. Pol. 2005, 76, 679–686. [Google Scholar]
- de Mattos, C.S.; Trevisan, C.M.; Peluso, C.; Adami, F.; Cordts, E.B.; Christofolini, D.M.; Barbosa, C.P.; Bianco, B. ESR1 and ESR2 gene polymorphisms are associated with human reproduction outcomes in Brazilian women. J. Ovarian Res. 2014, 7, 114. [Google Scholar] [CrossRef]
- Pramesti, R.I.; Sanif, R.; Abadi, A.; Saleh, I. Polymorphism Estrogen Receptor α Gene of Epithelial Ovarian Carcinoma. Indones. J. Obstet. Gynecol. 2011, 35, 218–224. [Google Scholar]
- Liu, X.; Huang, J.; Lin, H.; Xiong, L.; Ma, Y. Lao HESR1PvuII(rs2234693 T>C) polymorphism and cancer susceptibility: Evidence from 80 studies. J. Cancer 2018, 9, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Kutilin, D.S.; Tsandekova, M.R.; Porkhanova, N.V. Features of the Copy Number Variation of Certain Genes in Tumor Cells in Patients with Serous Ovarian Adenocarcinoma. Bull. Exp. Biol. Med. 2021, 170, 332–339. [Google Scholar] [CrossRef]
- Gaillard, S.; Bayable, A.; Deshmukh, S.K.; Nayar, U.; Xiu, J.; Ingram, L.; Hubbard, G.; Sledge, G.W., Jr.; Herzog, T.J.; Thaker, P.H.; et al. Characterization of ESR1 mutations in endometrial and ovarian cancers. J. Clin. Oncol. 2024, 42, 16. [Google Scholar] [CrossRef]
- Iyshwarya, B.K.; Mohammed, V.; Veerabathiran, R. Genetics of endometriosis and its association with ovarian cancer. Gynecol. Obstet. Clin. Med. 2021, 1, 177–185. [Google Scholar] [CrossRef]
- Barnard, M.E.; Farland, L.V.; Yan, B.; Wang, J.; Trabert, B.; Doherty, J.A.; Meeks, H.D.; Madsen, M.; Guinto, E.; Collin, L.J.; et al. Endometriosis Typology Ovarian Cancer Risk. JAMA 2024, 332, 482–489. [Google Scholar] [CrossRef]
- Eldafira, E.; Prasasty, V.D.; Abinawanto, A.; Syahfirdi, L.; Pujianto, D.A. Polymorphisms of Estrogen Receptor-α and Estrogen Receptor-β Genes and its Expression in Endometriosis. Turk. J. Pharm. Sci. 2021, 18, 91–95. [Google Scholar] [CrossRef]
- Wang, J.; Hu, R.; Wang, J.; He, Q. Pvu II and Xba I in Estrogen Receptor 1 (ESR1) Polymorphisms and Susceptibility to Endometriosis Risk. Clin. Lab. 2020, 1, 66. [Google Scholar]



| Value of ROMA Test | Risk of Ovarian Cancer |
|---|---|
| premenopausal women | |
| <11.4% | Low |
| >11.4% | High |
| postmenopausal women | |
| <29.9% | Low |
| >29.9% | High |
| Degree of Advancement | Description |
|---|---|
| I | The cancer is limited to the ovaries or fallopian tubes. |
| IA | A tumor confined to one ovary or fallopian tube (intact continuity of the tumor capsule), no lesions on the surface of the ovary or the fallopian tube, an absence of tumor cells from fluid or peritoneal lavages. |
| IB | Tumors limited to 2 ovaries or fallopian tubes (intact continuity of the tumor capsule), no lesions on the surface of the ovary or the fallopian tube, no tumor cells in the fluid or peritoneal lavages. |
| IC | Tumors confined to 1 or 2 ovaries or fallopian tubes with the following: |
| IC1 | An intraoperative capsular injury; |
| IC2 | Impaired capsule continuity prior to surgery or the presence of a tumor on the surface of the ovary or fallopian tube; |
| IC3 | The presence of cancer cells in the fluid or peritoneal lavage. |
| II | Cancer limited to the ovaries or fallopian tubes with the involvement of the pelvic structure (below the plane of pelvic entry) or primary peritoneal cancer. |
| IIA | Involvement and/or implantation on the surface of the uterus and/or fallopian tube and/or ovaries. |
| IIB | The involvement of other pelvic structures. |
| III | Neoplasm involving 1 or 2 ovaries/fallopian tubes or primary peritoneal cancer with metastases to the peritoneum outside the pelvis and/or metastases to retroperitoneal lymph nodes. |
| IIIA1 | Neoplastic metastases are present only in the retroperitoneal lymph nodes (histopathologically confirmed). |
| IIIA1(i) | Metastases to the retroperitoneal lymph nodes in the largest dimension ≤ 10 mm. |
| IIIA1(ii) | Metastases to the retroperitoneal lymph nodes in the largest dimension > 10 mm. |
| IIIA2 | Microscopic peritoneal metastases outside the lesser pelvis (above the plane of pelvic entry), with or without retroperitoneal lymph node metastases. |
| IIIB | Macroscopic peritoneal metastases outside the lesser pelvis with a diameter of ≤2 cm in the largest dimension, with or without retroperitoneal lymph node metastases. |
| IIIC | Macroscopic peritoneal metastases outside the pelvis with a diameter of >2 cm in the largest dimension, with or without retroperitoneal lymph node metastases (including the involvement of the liver capsule and the spleen by neoplasm, without the infiltration of the parenchyma of the organ). |
| IV | The presence of distant metastases (including peritoneal metastases). |
| IVA | Pleural effusion with cytologically confirmed neoplasm. |
| IVB | Interstitial liver metastases and organ metastases outside the abdomen (including inguinal lymph nodes and lymph nodes outside the abdominal cavity). |
| HTH | Mechanism of Action | Options |
|---|---|---|
| Gonadotropin-releasing hormone receptor analogs | Competitively binds GnRH-R and reduces the secretion of follicle-stimulating hormone and luteinizing hormone | GnRH I agonists Triptorelin Goserelin Histrelin Leuprolide acetate GnRH II antagonists Cetrorelix Degarelix acetate |
| Estrogen | Estrogen receptor blockade | Antiestrogens: Tamoxifen Toremifene |
| Estrogen synthesis suppression | Aromatase inhibitors: Anastrozole Exemestane Letrozole | |
| Estrogen receptor downregulation | ER antagonist: Fulvestrant | |
| Hormonal ablation | Surgery Radiation (infrequently used) | |
| Androgen | Androgen receptor blockade | Anti-androgens: Flutamide Bicalutamide Enzalutamide |
| Progesterone | Progesterone receptor blockade | PR antagonists: Mifepristone Medroxyprogesterone Megestrol acetate |
| Increasing progesterone levels | Oral contraceptive pills Pregnancy Breastfeeding |
| Medicines | Drug Doses | Indicate |
|---|---|---|
| Olaparib | 200–800 mg per day | Indicated for the maintenance therapy of recurrent serous ovarian cancer. The presence of BRCA mutations should be confirmed prior to use. |
| Lynparz | 300 mg twice daily | Indicated as monotherapy for
|
| Tamoxifen (Selective Estrogen Receptor Modulators) | 20–40 mg per day | It is one of the types of hormonal drugs, i.e., the so-called antiestrogens, and is used in the treatment of hormone-dependent cancers. These include breast cancer (breast cancer), ovarian cancer, and prostate cancer. |
| Letrozole (Aromatase Inhibitor) | 2.5 mg daily | Letrozole is a drug from the group of aromatase inhibitors. It works by blocking the aromatase enzyme, which leads to a decrease in the production of estrogen in the body. Estrogen is a hormone that can accelerate the growth of certain cancers. By lowering estrogen levels, letrozole can slow or stop the growth of these cancer cells. |
| Cetrorelix (Antagonist of GnRH) | 0.25 mg per day | Its main function is to inhibit the secretion of luteinizing hormone from the anterior pituitary gland, thereby preventing ovulation and inhibiting the production of sex steroids. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolarz, B.; Biernacka, K.; Łukasiewicz, H.; Samulak, D.; Piekarska, E.; Romanowicz, H.; Makowska, M. Ovarian Cancer—Epidemiology, Classification, Pathogenesis, Treatment, and Estrogen Receptors’ Molecular Backgrounds. Int. J. Mol. Sci. 2025, 26, 4611. https://doi.org/10.3390/ijms26104611
Smolarz B, Biernacka K, Łukasiewicz H, Samulak D, Piekarska E, Romanowicz H, Makowska M. Ovarian Cancer—Epidemiology, Classification, Pathogenesis, Treatment, and Estrogen Receptors’ Molecular Backgrounds. International Journal of Molecular Sciences. 2025; 26(10):4611. https://doi.org/10.3390/ijms26104611
Chicago/Turabian StyleSmolarz, Beata, Karolina Biernacka, Honorata Łukasiewicz, Dariusz Samulak, Ewa Piekarska, Hanna Romanowicz, and Marianna Makowska. 2025. "Ovarian Cancer—Epidemiology, Classification, Pathogenesis, Treatment, and Estrogen Receptors’ Molecular Backgrounds" International Journal of Molecular Sciences 26, no. 10: 4611. https://doi.org/10.3390/ijms26104611
APA StyleSmolarz, B., Biernacka, K., Łukasiewicz, H., Samulak, D., Piekarska, E., Romanowicz, H., & Makowska, M. (2025). Ovarian Cancer—Epidemiology, Classification, Pathogenesis, Treatment, and Estrogen Receptors’ Molecular Backgrounds. International Journal of Molecular Sciences, 26(10), 4611. https://doi.org/10.3390/ijms26104611






