Zebrafish cdh23 Affects Rod Cell Phototransduction Through Regulating Ca2+ Transport and MAPK Signaling Pathway
Abstract
1. Introduction
2. Results
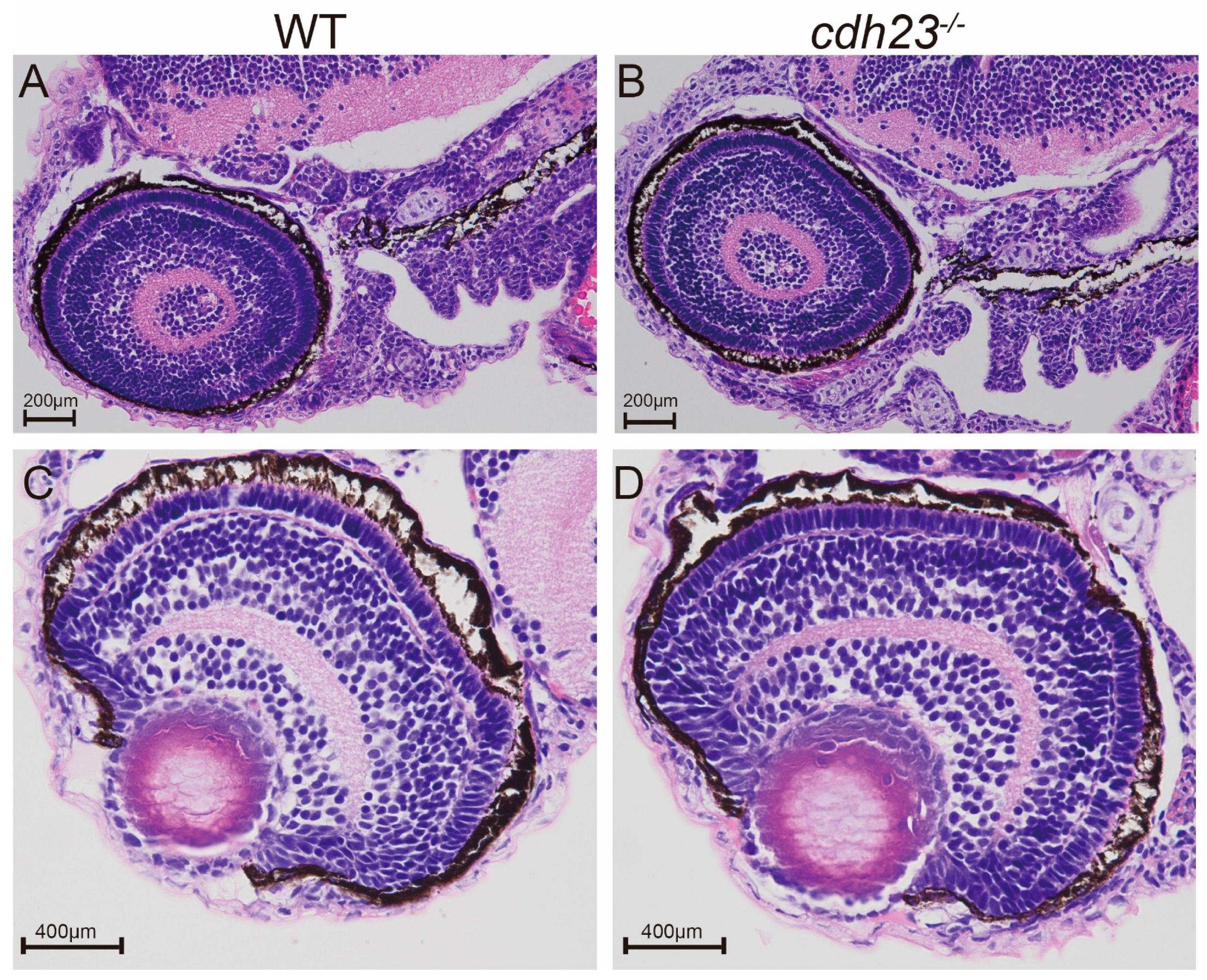
2.1. Histological Analysis of Retina in cdh23−/− Zebrafish Mutants
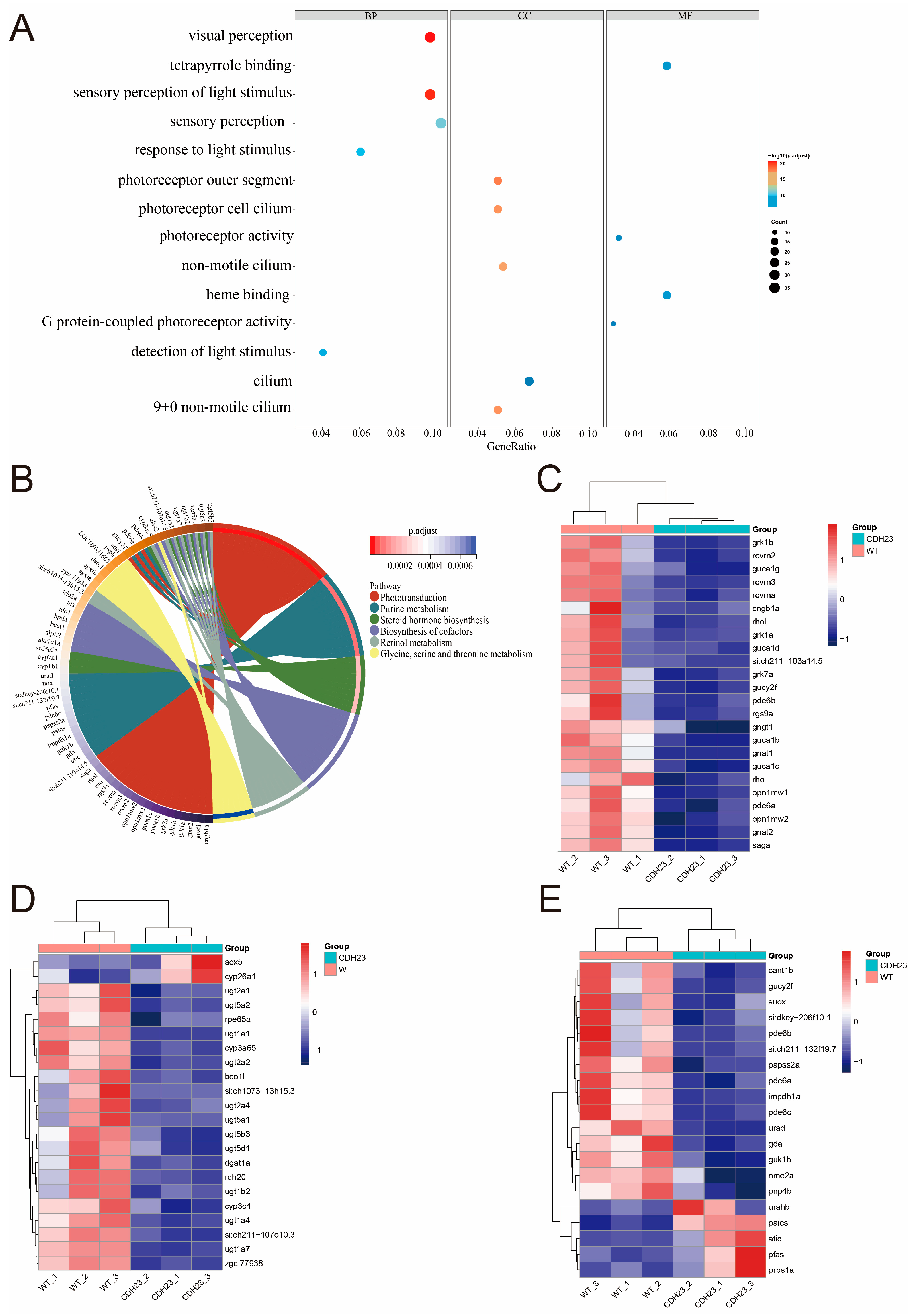
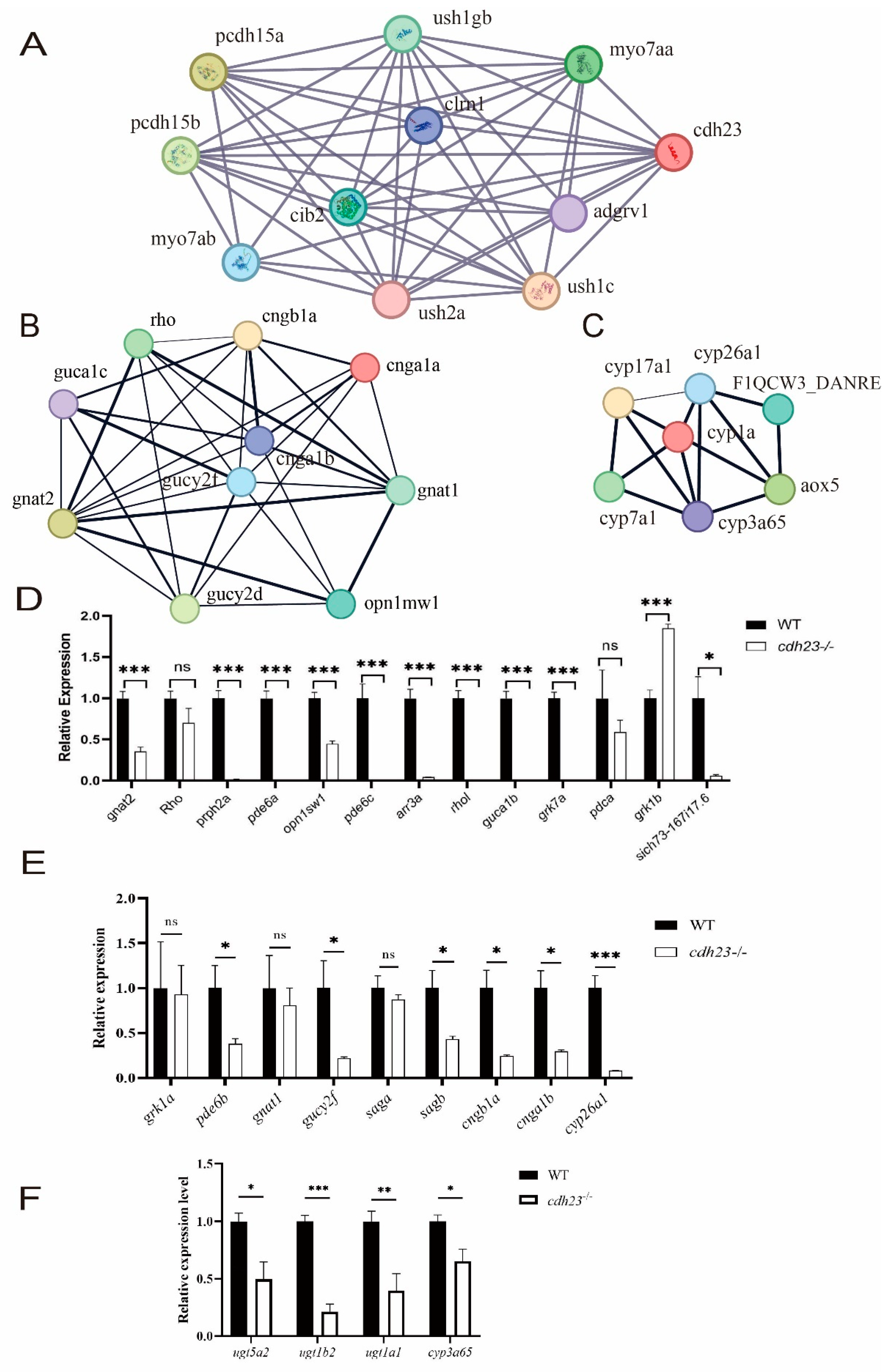
2.2. Analysis of Differential Gene Expression Between Control and cdh23−/−
2.3. Role of Cilia in Photoreceptor Function
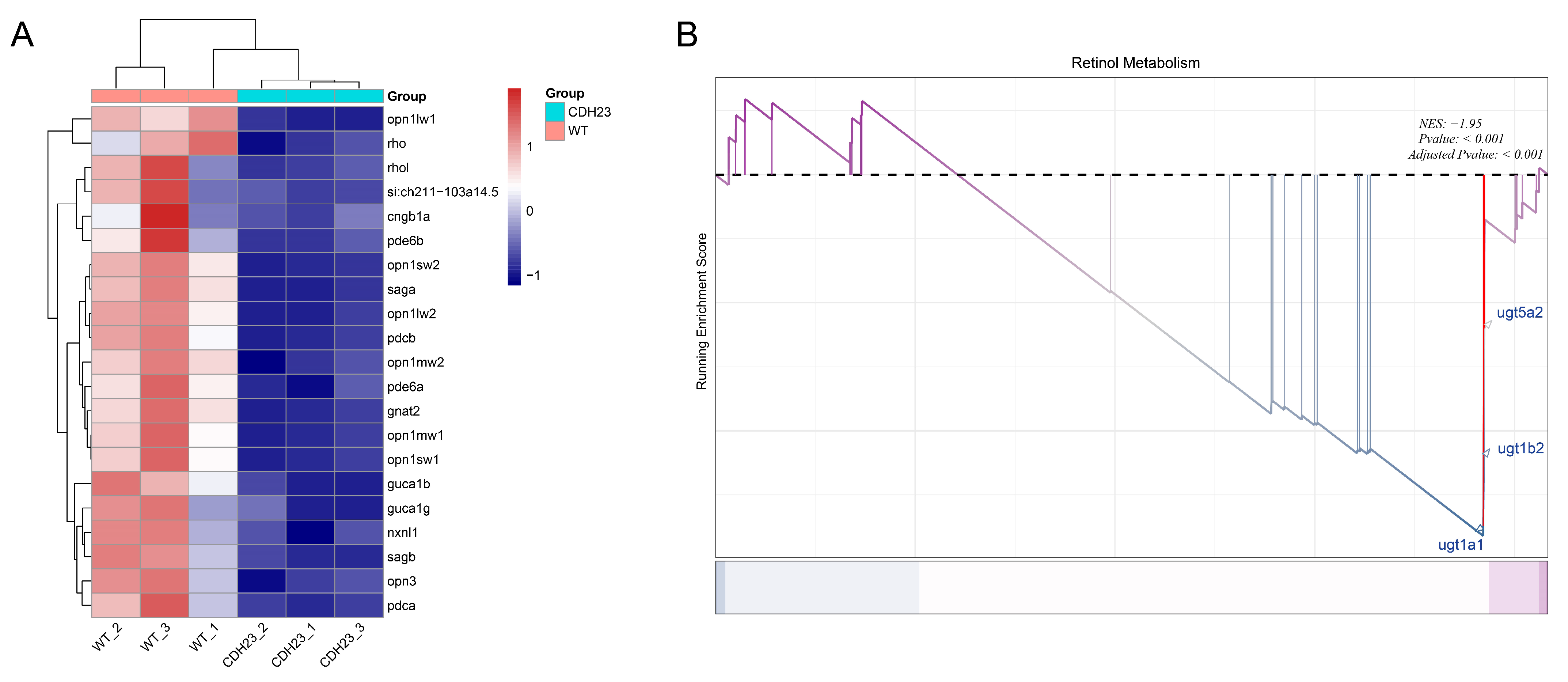
2.4. The Precursor Substance of Rhodopsin, Retinol, Plays an Important Role in Light Conduction
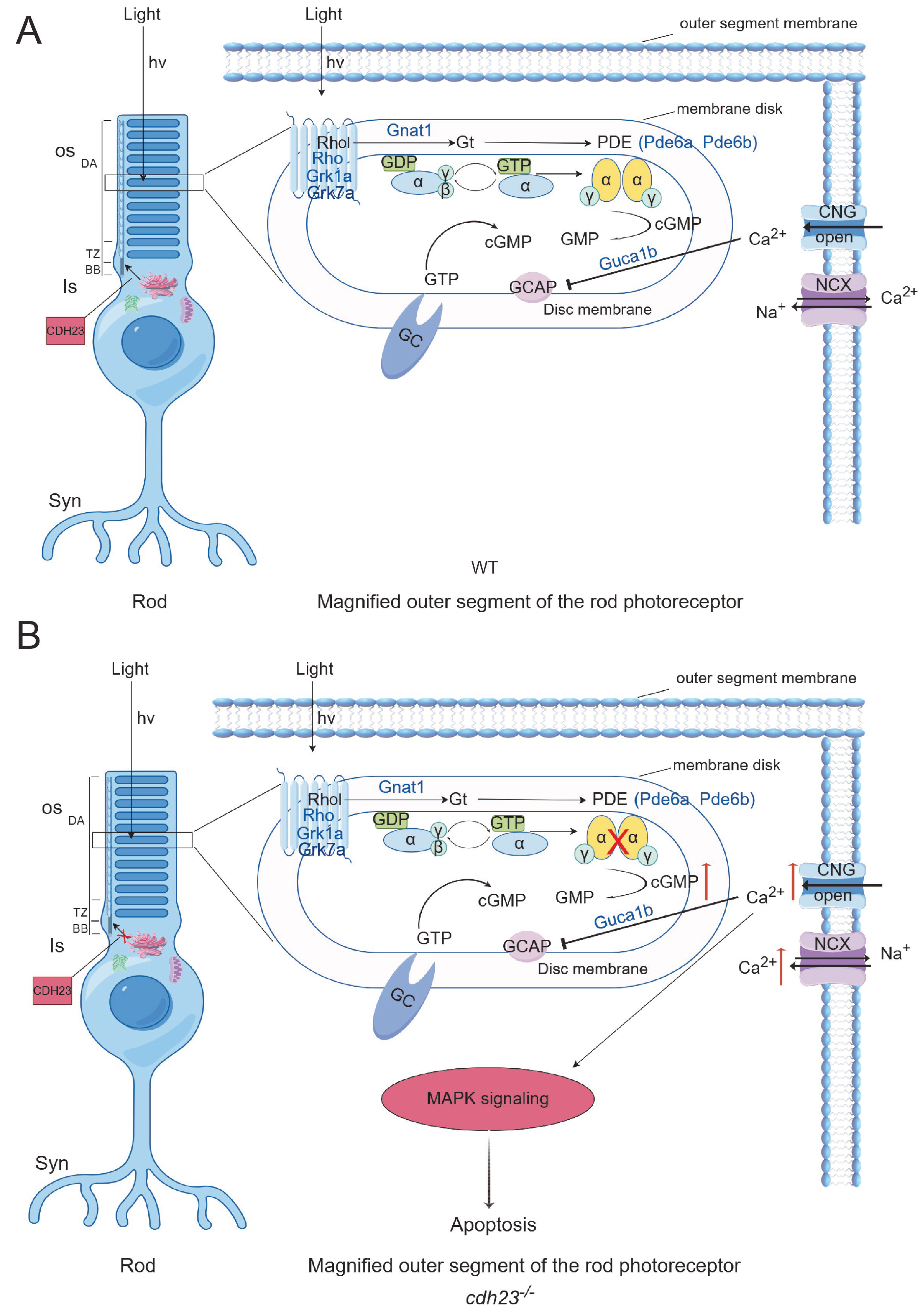
2.5. Abnormal cGMP Metabolism in cdh23−/− Zebrafish Retinas Generates Action Potentials in Ganglion Cells
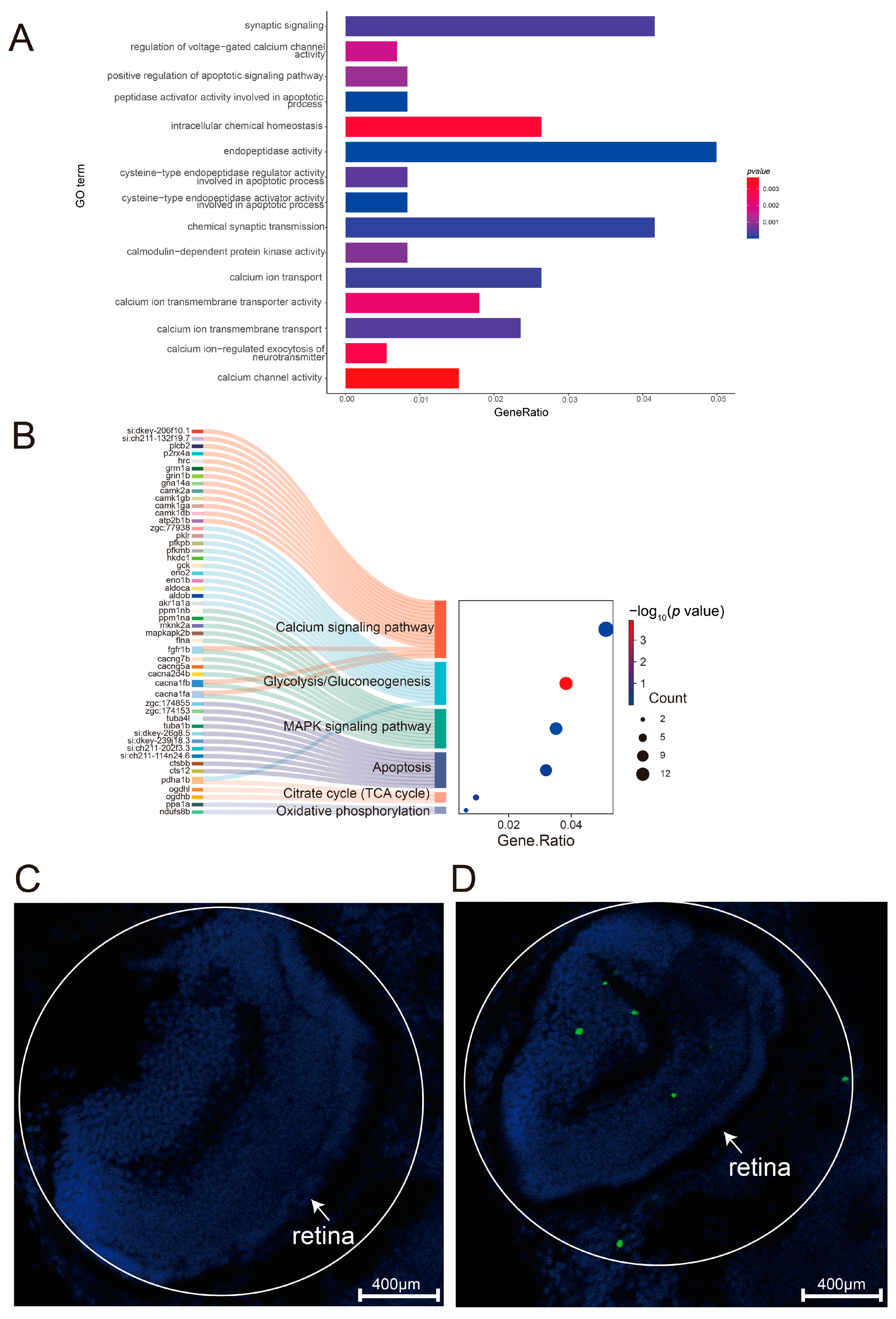
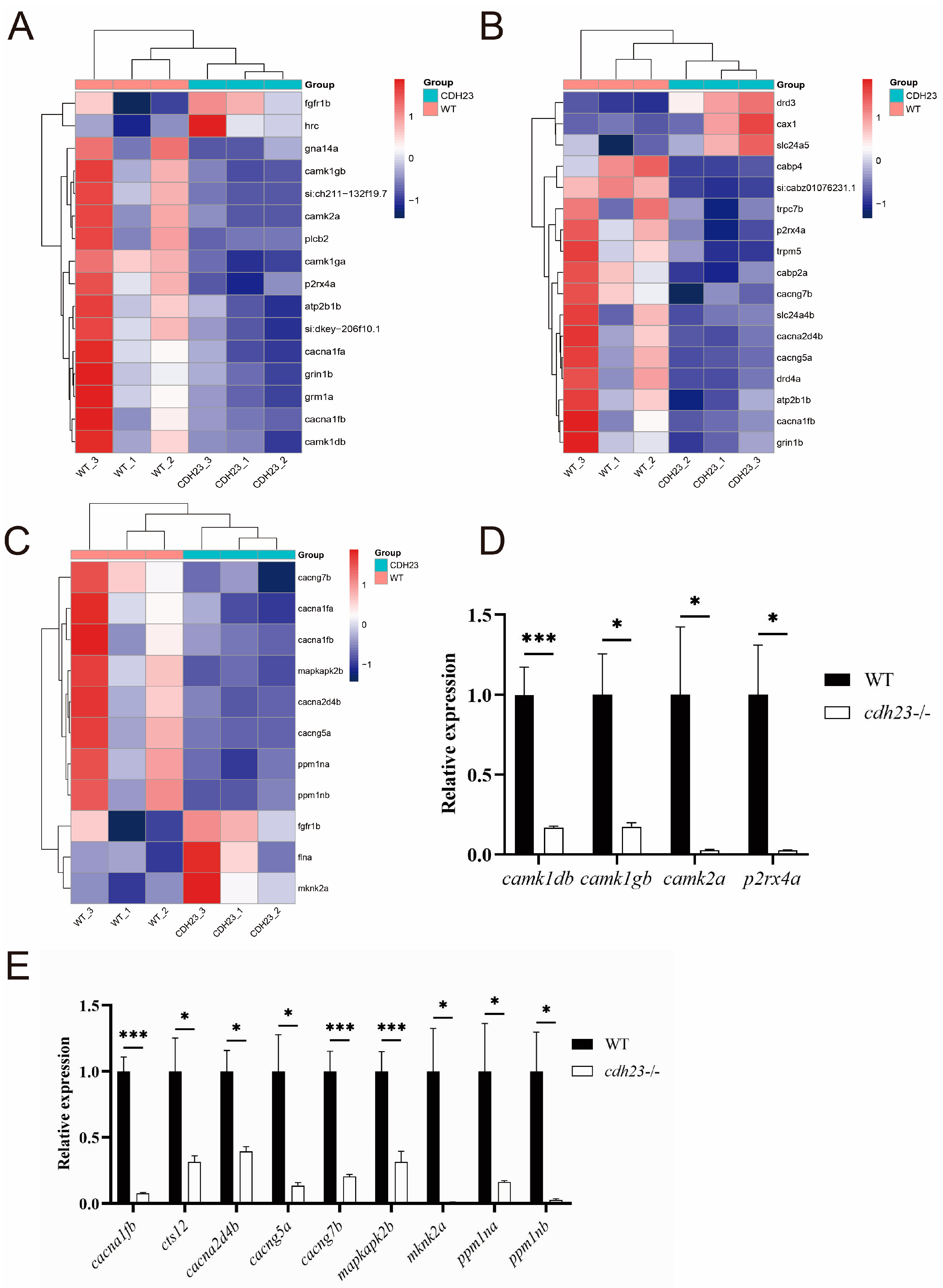
2.6. Expression of Ca2+ Pathway Plays Crucial Role in Photoreceptor Degeneration and Apoptosis Pathway Activation
2.7. Ca2+-Dependent MAPK Pathway Regulates Energy Metabolism and Cell Death
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. RNA-Seq Data Analysis
4.3. Functional Enrichment Analysis
4.4. PPI Network Construction and Analysis
4.5. Cryosection
4.6. Paraffin Section
4.7. Hematoxylin and Eosin Staining
4.8. TUNEL Assay
4.9. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
4.10. Zebrafish Maintenance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Priest, A.V.; Shafraz, O.; Sivasankar, S. Biophysical Basis of Cadherin Mediated Cell-Cell Adhesion. Exp. Cell Res. 2017, 358, 10–13. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Garrett, A.M.; MacLaren, R.E.; Charbel Issa, P. Retinal Cadherins and the Retinal Cadherinopathies: Current Concepts and Future Directions. Prog. Retin. Eye Res. 2022, 90, 101038. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.D.; Holme, R.H.; Bryda, E.C.; Belyantseva, I.A.; Pellegrino, R.; Kachar, B.; Steel, K.P.; Noben-Trauth, K. Mutations in Cdh23, Encoding a New Type of Cadherin, Cause Stereocilia Disorganization in Waltzer, the Mouse Model for Usher Syndrome Type 1D. Nat. Genet. 2001, 27, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Bork, J.M.; Peters, L.M.; Riazuddin, S.; Bernstein, S.L.; Ahmed, Z.M.; Ness, S.L.; Polomeno, R.; Ramesh, A.; Schloss, M.; Srisailpathy, C.R.S.; et al. Usher Syndrome 1D and Nonsyndromic Autosomal Recessive Deafness DFNB12 Are Caused by Allelic Mutations of the Novel Cadherin-Like Gene CDH23. Am. J. Hum. Genet. 2001, 68, 26–37. [Google Scholar] [CrossRef]
- Oshima, A.; Jaijo, T.; Aller, E.; Millan, J.M.; Carney, C.; Usami, S.; Moller, C.; Kimberling, W.J. Mutation Profile of the CDH23 Gene in 56 Probands with Usher Syndrome Type I. Hum. Mutat. 2008, 29, E37–E46. [Google Scholar] [CrossRef]
- Di Palma, F.; Pellegrino, R.; Noben-Trauth, K. Genomic Structure, Alternative Splice Forms and Normal and Mutant Alleles of Cadherin 23 (Cdh23). Gene 2001, 281, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Michel, V.; Goodyear, R.J.; Weil, D.; Marcotti, W.; Perfettini, I.; Wolfrum, U.; Kros, C.J.; Richardson, G.P.; Petit, C. Cadherin 23 Is a Component of the Transient Lateral Links in the Developing Hair Bundles of Cochlear Sensory Cells. Dev. Biol. 2005, 280, 281–294. [Google Scholar] [CrossRef]
- Siemens, J.; Kazmierczak, P.; Reynolds, A.; Sticker, M.; Littlewood-Evans, A.; Müller, U. The Usher Syndrome Proteins Cadherin 23 and Harmonin Form a Complex by Means of PDZ-Domain Interactions. Proc. Natl. Acad. Sci. USA 2002, 99, 14946–14951. [Google Scholar] [CrossRef]
- Fuster-García, C.; García-Bohórquez, B.; Rodríguez-Muñoz, A.; Aller, E.; Jaijo, T.; Millán, J.M.; García-García, G. Usher Syndrome: Genetics of a Human Ciliopathy. Int. J. Mol. Sci. 2021, 22, 6723. Available online: https://www.mdpi.com/1422-0067/22/13/6723 (accessed on 7 April 2025). [CrossRef]
- Jaiganesh, A.; Narui, Y.; Araya-Secchi, R.; Sotomayor, M. Beyond Cell–Cell Adhesion: Sensational Cadherins for Hearing and Balance. Cold Spring Harb. Perspect. Biol. 2018, 10, a029280. [Google Scholar] [CrossRef]
- Sahly, I.; Dufour, E.; Schietroma, C.; Michel, V.; Bahloul, A.; Perfettini, I.; Pepermans, E.; Estivalet, A.; Carette, D.; Aghaie, A.; et al. Localization of Usher 1 Proteins to the Photoreceptor Calyceal Processes, Which Are Absent from Mice. J. Cell Biol. 2012, 199, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Schietroma, C.; Parain, K.; Estivalet, A.; Aghaie, A.; Boutet de Monvel, J.; Picaud, S.; Sahel, J.A.; Perron, M.; El-Amraoui, A.; Petit, C. Usher Syndrome Type 1–Associated Cadherins Shape the Photoreceptor Outer Segment. J. Cell Biol. 2017, 216, 1849–1864. Available online: https://rupress.org/jcb/article/216/6/1849/38992/Usher-syndrome-type-1-associated-cadherins-shape (accessed on 7 April 2025). [CrossRef] [PubMed]
- Lagziel, A.; Ahmed, Z.M.; Schultz, J.M.; Morell, R.J.; Belyantseva, I.A.; Friedman, T.B. Spatiotemporal Pattern and Isoforms of Cadherin 23 in Wild Type and Waltzer Mice during Inner Ear Hair Cell Development. Dev. Biol. 2005, 280, 295–306. [Google Scholar] [CrossRef]
- Söllner, C.; Rauch, G.J.; Siemens, J.; Geisler, R.; Schuster, S.C. Mutations in Cadherin 23 Affect Tip Links in Zebrafish Sensory Hair Cells. Nature 2004, 428, 955–959. Available online: https://www.nature.com/articles/nature02484 (accessed on 8 January 2025). [CrossRef]
- Boëda, B.; El-Amraoui, A.; Bahloul, A.; Goodyear, R.; Daviet, L.; Blanchard, S.; Perfettini, I.; Fath, K.R.; Shorte, S.; Reiners, J.; et al. Myosin VIIa, Harmonin and Cadherin 23, Three Usher I Gene Products That Cooperate to Shape the Sensory Hair Cell Bundle. EMBO J. 2002, 21, 6689–6699. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Regulation of Cadherin-Mediated Adhesion in Morphogenesis. Nat. Rev. Mol. Cell biology 2005, 6, 622–634. Available online: https://www.nature.com/articles/nrm1699 (accessed on 8 January 2025). [CrossRef]
- Halbleib, J.M.; Nelson, W.J. Cadherins in Development: Cell Adhesion, Sorting, and Tissue Morphogenesis. Genes. Dev. 2006, 20, 3199–3214. [Google Scholar] [CrossRef] [PubMed]
- Brusés, J.L. Cadherin-Mediated Adhesion at the Interneuronal Synapse. Curr. Opin. Cell Biol. 2000, 12, 593–597. [Google Scholar] [CrossRef]
- Ayton, L.N.; Galvin, K.L.; Johansen, L.; O’Hare, F.; Shepard, E.R. Awareness of Usher Syndrome and the Need for Multidisciplinary Care: A Cross-Occupational Survey of Allied Health Clinicians. J. Multidiscip. Heal. 2023, 16, 1927–1936. [Google Scholar] [CrossRef]
- Toms, M.; Pagarkar, W.; Moosajee, M. Usher Syndrome: Clinical Features, Molecular Genetics and Advancing Therapeutics. Ther. Adv. Ophthalmol. 2020, 12, 2515841420952194. [Google Scholar] [CrossRef]
- Cross, N.; van Steen, C.; Zegaoui, Y.; Satherley, A.; Angelillo, L. Current and Future Treatment of Retinitis Pigmentosa. Clin. Ophthalmol. 2022, 16, 2909–2921. [Google Scholar] [CrossRef] [PubMed]
- Delmaghani, S.; El-Amraoui, A. The Genetic and Phenotypic Landscapes of Usher Syndrome: From Disease Mechanisms to a New Classification. Hum. Genet. 2022, 141, 709–735. [Google Scholar] [CrossRef] [PubMed]
- Okano, S.; Makita, Y.; Katada, A.; Harabuchi, Y.; Kohmoto, T.; Naruto, T.; Masuda, K.; Imoto, I. Novel Compound Heterozygous CDH23 Variants in a Patient with Usher Syndrome Type I. Hum. Genome Var. 2019, 6, 8. Available online: https://www.nature.com/articles/s41439-019-0037-y (accessed on 31 March 2025). [CrossRef]
- Bonnet, C.; Riahi, Z.; Chantot-Bastaraud, S.; Smagghe, L.; Letexier, M.; Marcaillou, C.; Lefèvre, G.M.; Hardelin, J.P.; El-Amraoui, A.; Singh-Estivalet, A.; et al. An Innovative Strategy for the Molecular Diagnosis of Usher Syndrome Identifies Causal Biallelic Mutations in 93% of European Patients. Eur. J. Hum. Genet. 2016, 24, 1730–1738. Available online: https://www.nature.com/articles/ejhg201699 (accessed on 20 April 2025). [CrossRef] [PubMed]
- Jouret, G.; Poirsier, C.; Spodenkiewicz, M.; Jaquin, C.; Gouy, E.; Arndt, C.; Labrousse, M.; Gaillard, D.; Doco-Fenzy, M.; Lebre, A.-S. Genetics of Usher Syndrome: New Insights From a Meta-Analysis. Otol. Neurotol. 2019, 40, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.P.; de Monvel, J.B.; Petit, C. How the Genetics of Deafness Illuminates Auditory Physiology. Annu. Rev. 2011, 73, 311–334. Available online: https://www.annualreviews.org/content/journals/10.1146/annurev-physiol-012110-142228 (accessed on 7 April 2025). [CrossRef]
- Bolz, H.; von Brederlow, B.; Ramírez, A.; Bryda, E.C.; Kutsche, K.; Nothwang, H.G.; Seeliger, M.; Cabrera, M.D.C.; Vila, M.C.; Molina, O.P.; et al. Mutation of CDH23, Encoding a New Member of the Cadherin Gene Family, Causes Usher Syndrome Type 1D. Nat. Genet. 2001, 27, 108–112. [Google Scholar] [CrossRef]
- Williams, D.S. Usher Syndrome: Animal Models, Retinal Function of Usher Proteins, and Prospects for Gene Therapy. Vis. Res. 2008, 48, 433–441. [Google Scholar] [CrossRef]
- Millán, J.M.; Aller, E.; Jaijo, T.; Blanco-Kelly, F.; Gimenez-Pardo, A.; Ayuso, C. An Update on the Genetics of Usher Syndrome. J. Ophthalmol. 2011, 2011, 417217. [Google Scholar] [CrossRef]
- Flores-Guevara, R.; Renault, F.; Loundon, N.; Marlin, S.; Pelosse, B.; Momtchilova, M.; Auzoux-Chevé, M.; Vermersch, A.I.; Richard, P. Usher Syndrome Type 1: Early Detection of Electroretinographic Changes. Eur. J. Paediatr. Neurol. 2009, 13, 505–507. [Google Scholar] [CrossRef]
- Malm, E.; Ponjavic, V.; Möller, C.; Kimberling, W.J.; Andréasson, S. Phenotypes in defined genotypes including siblings with Usher syndrome. Ophthalmic Genet. 2011, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Reiners, J.; Nagel-Wolfrum, K.; Jürgens, K.; Märker, T.; Wolfrum, U. Molecular Basis of Human Usher Syndrome: Deciphering the Meshes of the Usher Protein Network Provides Insights into the Pathomechanisms of the Usher Disease. Exp. Eye Res. 2006, 83, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, S.; Westerveld, A.; De Jong, P.T.V.M.; Bleeker-Wagemakers, E.M.; Bergen, A.A.B. Retinitis Pigmentosa: Defined From a Molecular Point of View. Surv. Ophthalmol. 1999, 43, 321–334. [Google Scholar] [CrossRef]
- Liu, X.; Udovichenko, I.P.; Brown, S.D.; Steel, K.P.; Williams, D.S. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J. Neurosci. 1999, 19, 6267–6274. [Google Scholar] [CrossRef]
- Wasfy, M.M.; Matsui, J.I.; Miller, J.; Dowling, J.E.; Perkins, B.D. Myosin 7aa−/− Mutant Zebrafish Show Mild Photoreceptor Degeneration and Reduced Electroretinographic Responses. Exp. Eye Res. 2014, 122, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xie, B.-L.; Dong, X.-P.; Wang, L.-X.; Zhu, G.-H.; Wang, T.; Wu, W.-J.; Lai, R.-S.; Tao, R.; Guan, M.-X.; et al. Cdh23 Affects Congenital Hearing Loss through Regulating Purine Metabolism. Front. Mol. Neurosci. 2023, 16, 1079529. [Google Scholar] [CrossRef]
- Besharse, J.C.; Horst, C.J. The Photoreceptor Connecting Cilium A Model for the Transition Zone. In Ciliary and Flagellar Membranes; Bloodgood, R.A., Ed.; Springer US: Boston, MA, USA, 1990; pp. 389–417. ISBN 978-1-4612-7845-0. [Google Scholar]
- Crane, R.; Conley, S.M.; Al-Ubaidi, M.R.; Naash, M.I. Gene Therapy to the Retina and the Cochlea. Front. Neurosci. 2021, 15, 652215. [Google Scholar] [CrossRef]
- Botto, C.; Dalkara, D.; El-Amraoui, A. Progress in Gene Editing Tools and Their Potential for Correcting Mutations Underlying Hearing and Vision Loss. Front. Genome Ed. 2021, 3, 737632. [Google Scholar] [CrossRef]
- Holmgren, M.; Sheets, L. Using the Zebrafish Lateral Line to Understand the Roles of Mitochondria in Sensorineural Hearing Loss. Front. Cell Dev. Biol. 2020, 8, 628712. [Google Scholar] [CrossRef]
- Young, R.W. The renewal of photoreceptor cell outer segments. J. Cell Biol. 1967, 33, 61–72. [Google Scholar] [CrossRef]
- Besharse, J.C.; Wetzel, M.G. Immunocytochemical Localization of Opsin in Rod Photoreceptors during Periods of Rapid Disc Assembly. J. Neurocytol. 1995, 24, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Dooley, K.; Zon, L.I. Zebrafish: A Model System for the Study of Human Disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The Zebrafish Eye—A Paradigm for Investigating Human Ocular Genetics. Eye 2017, 31, 68–86. [Google Scholar] [CrossRef]
- Kundu, T. Zebrafish: A Metamorphosis in Ophthalmological Research—A Literature Review; Springer: Singapore, 2022; Available online: https://link.springer.com/chapter/10.1007/978-981-16-5217-2_13 (accessed on 8 January 2025).
- Cogné, B.; Latypova, X.; Senaratne, L.D.S.; Martin, L.; Koboldt, D.C.; Kellaris, G.; Fievet, L.; Le Meur, G.; Caldari, D.; Debray, D.; et al. Mutations in the Kinesin-2 Motor KIF3B Cause an Autosomal-Dominant Ciliopathy. Am. J. Hum. Genet. 2020, 106, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.G.S.; Lopes-Ferreira, M.; Lima, C. An Overview towards Zebrafish Larvae as a Model for Ocular Diseases. Int. J. Mol. Sci. 2023, 24, 5387. Available online: https://www.mdpi.com/1422-0067/24/6/5387 (accessed on 8 January 2025). [CrossRef]
- Manjang, K.; Tripathi, S.; Yli-Harja, O.; Dehmer, M.; Emmert-Streib, F. Graph-Based Exploitation of Gene Ontology Using GOxploreR for Scrutinizing Biological Significance. Sci. Rep. 2020, 10, 16672. Available online: https://www.nature.com/articles/s41598-020-73326-3 (accessed on 8 January 2025). [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Bujakowska, K.M.; Liu, Q.; Pierce, E.A. Photoreceptor Cilia and Retinal Ciliopathies. Cold Spring Harb. Perspect. Biol. 2017, 9, a028274. [Google Scholar] [CrossRef]
- Molday, R.S.; Moritz, O.L. Photoreceptors at a Glance. J. Cell Sci. 2015, 128, 4039–4045. [Google Scholar] [CrossRef]
- Pearring, J.N.; Salinas, R.Y.; Baker, S.A.; Arshavsky, V.Y. Protein Sorting, Targeting and Trafficking in Photoreceptor Cells. Prog. Retin. Eye Res. 2013, 36, 24–51. [Google Scholar] [CrossRef]
- Sjostrand, F.S. The Ultrastructure of the Outer Segments of Rods and Cones of the Eye as Revealed by the Electron Microscope. J. Cell Comp. Physiol. 1953, 42, 15–44. [Google Scholar] [CrossRef]
- Nickell, S.; Park, P.S.-H.; Baumeister, W.; Palczewski, K. Three-Dimensional Architecture of Murine Rod Outer Segments Determined by Cryoelectron Tomography. J. Cell Biol. 2007, 177, 917–925. [Google Scholar] [CrossRef]
- Kono, M.; Goletz, P.W.; Crouch, R.K. 11-Cis- and All-Trans-Retinols Can Activate Rod Opsin: Rational Design of the Visual Cycle. Biochemistry 2008, 47, 7567–7571. Available online: https://pubmed.ncbi.nlm.nih.gov/18563917/ (accessed on 21 April 2025). [CrossRef] [PubMed]
- Dowling, J.E.; Wald, G. Vitamin a deficiency and night blindness. Proc. Natl. Acad. Sci. USA 1958, 44, 648–661. [Google Scholar] [CrossRef]
- Dowling, J.E.; Wald, G. The biological function of vitamin a acid. Proc. Natl. Acad. Sci. USA 1960, 46, 587–608. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Steinman, J.; Li, D.; Lor, A.; Gruesen, A.; Sadah, A.; van Kuijk, F.J.; Montezuma, S.R.; Kondkar, A.A.; Radhakrishnan, R.; et al. The Logistical Backbone of Photoreceptor Cell Function: Complementary Mechanisms of Dietary Vitamin A Receptors and Rhodopsin Transporters. Int. J. Mol. Sci. 2024, 25, 4278. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, D.; Aguila, M.; Bellingham, J.; Li, W.; McCulley, C.; Reeves, P.J.; Cheetham, M.E. The Molecular and Cellular Basis of Rhodopsin Retinitis Pigmentosa Reveals Potential Strategies for Therapy. Prog. Retin. Eye Res. 2018, 62, 1–23. [Google Scholar] [CrossRef]
- Zhen, F.; Zou, T.; Wang, T.; Zhou, Y.; Dong, S.; Zhang, H. Rhodopsin-Associated Retinal Dystrophy: Disease Mechanisms and Therapeutic Strategies. Front. Neurosci. 2023, 17, 1132179. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis Pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Zhang, Q. Retinitis Pigmentosa: Progress and Perspective. Asia Pac J Ophthalmol. 2016, 5, 265–271. [Google Scholar] [CrossRef]
- Perea-Romero, I.; Gordo, G.; Iancu, I.F.; Del Pozo-Valero, M.; Almoguera, B.; Blanco-Kelly, F.; Carreño, E.; Jimenez-Rolando, B.; Lopez-Rodriguez, R.; Lorda-Sanchez, I.; et al. Genetic Landscape of 6089 Inherited Retinal Dystrophies Affected Cases in Spain and Their Therapeutic and Extended Epidemiological Implications. Sci. Rep. 2021, 11, 1526. [Google Scholar] [CrossRef]
- Carrigan, M.; Duignan, E.; Humphries, P.; Palfi, A.; Kenna, P.F.; Farrar, G.J. A Novel Homozygous Truncating GNAT1 Mutation Implicated in Retinal Degeneration. Br. J. Ophthalmol. 2016, 100, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, U.B.; Seifert, R. Cyclic Nucleotide-Gated Ion Channels. Physiol. Rev. 2002, 82, 769–824. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gao, X.; Goswami, D.; Hou, L.; Pal, K.; Yin, Y.; Zhao, G.; Ernst, O.P.; Griffin, P.; Melcher, K.; et al. Molecular Assembly of Rhodopsin with G Protein-Coupled Receptor Kinases. Cell Res. 2017, 27, 728–747. Available online: https://www.nature.com/articles/cr201772 (accessed on 8 January 2025). [CrossRef]
- Peshenko, I.V.; Dizhoor, A.M. Guanylyl Cyclase-Activating Proteins (GCAPs) Are Ca2+/Mg2+ Sensors: Implications for Photoreceptor Guanylyl Cyclase (RetGC) Regulation in Mammalian Photoreceptors. J. Biol. Chem. 2004, 279, 16903–16906. [Google Scholar] [CrossRef]
- McLaughlin, M.E.; Sandberg, M.A.; Berson, E.L.; Dryja, T.P. Recessive Mutations in the Gene Encoding the β–Subunit of Rod Phosphodiesterase in Patients with Retinitis Pigmentosa. Nat. Genet. 1993, 4, 130–134. Available online: https://www.nature.com/articles/ng0693-130 (accessed on 8 January 2025). [CrossRef]
- Huang, S.H.; Pittler, S.J.; Huang, X.; Oliveira, L.; Berson, E.L.; Dryja, T.P. Autosomal Recessive Retinitis Pigmentosa Caused by Mutations in the Alpha Subunit of Rod cGMP Phosphodiesterase. Nat. Genet. 1995, 11, 468–471. [Google Scholar] [CrossRef]
- Cote, R.H. Characteristics of Photoreceptor PDE (PDE6): Similarities and Differences to PDE5. Int. J. Impot. Res. 2004, 16, S28–S33. [Google Scholar] [CrossRef]
- Arango-Gonzalez, B.; Trifunović, D.; Sahaboglu, A.; Kranz, K.; Michalakis, S.; Farinelli, P.; Koch, S.; Koch, F.; Cottet, S.; Janssen-Bienhold, U.; et al. Identification of a Common Non-Apoptotic Cell Death Mechanism in Hereditary Retinal Degeneration. PLoS ONE 2014, 9, e112142. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Wong-Riley, M.T.T. Energy Metabolism of the Visual System. Eye Brain 2010, 2, 99–116. [Google Scholar] [CrossRef]
- Scimeca, J.C.; Servant, M.J.; Dyer, J.O.; Meloche, S. Essential Role of Calcium in the Regulation of MAP Kinase Phosphatase-1 Expression. Oncogene 1997, 15, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.J.; Murphy, G.G. The Role of L-Type Calcium Channels in Neuronal Excitability and Aging. Neurobiol. Learn. Mem. 2020, 173, 107230. [Google Scholar] [CrossRef]
- Racioppi, L.; Means, A.R. Calcium/Calmodulin-Dependent Protein Kinase Kinase 2: Roles in Signaling and Pathophysiology. J. Biol. Chem. 2012, 287, 31658–31665. [Google Scholar] [CrossRef] [PubMed]
- Deretic, D.; Williams, A.H.; Ransom, N.; Morel, V.; Hargrave, P.A.; Arendt, A. Rhodopsin C Terminus, the Site of Mutations Causing Retinal Disease, Regulates Trafficking by Binding to ADP-Ribosylation Factor 4 (ARF4). Proc. Natl. Acad. Sci. USA 2005, 102, 3301–3306. Available online: https://www.pnas.org/doi/10.1073/pnas.0500095102 (accessed on 8 January 2025). [CrossRef]
- Wang, J.; Deretic, D. Molecular Complexes That Direct Rhodopsin Transport to Primary Cilia. Prog. Retin. Eye Res. 2014, 38, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.; Shlaer, S.; Pirenne, M.H. Energy, quanta, and vision. J. Gen. Physiol. 1942, 25, 819–840. [Google Scholar] [CrossRef]
- Khateb, S.; Nassisi, M.; Bujakowska, K.M.; Méjécase, C.; Condroyer, C.; Antonio, A.; Foussard, M.; Démontant, V.; Mohand-Saïd, S.; Sahel, J.-A.; et al. Longitudinal Clinical Follow-up and Genetic Spectrum of Patients With Rod-Cone Dystrophy Associated With Mutations in PDE6A and PDE6B. JAMA Ophthalmol. 2019, 137, 669–679. [Google Scholar] [CrossRef]
- Tykocki, N.R.; Jackson, W.F.; Watts, S.W. Reverse-Mode Na+/Ca2+ Exchange Is an Important Mediator of Venous Contraction. Pharmacol. Res. 2012, 66, 544–554. [Google Scholar] [CrossRef]
- Bagur, R.; Hajnóczky, G. Intracellular Ca2+ Sensing: Role in Calcium Homeostasis and Signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef]
- Frasson, M.; Sahel, J.A.; Fabre, M.; Simonutti, M.; Dreyfus, H.; Picaud, S. Retinitis Pigmentosa: Rod Photoreceptor Rescue by a Calcium-Channel Blocker in the Rd Mouse. Nat. Med. 1999, 5, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Günter, A.; Das, S.; Mühlfriedel, R.; Michalakis, S.; Jiao, K.; Seeliger, M.W.; Paquet-Durand, F. Inherited Retinal Degeneration: PARP-Dependent Activation of Calpain Requires CNG Channel Activity. Biomolecules 2022, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Schnetkamp, P.P.M. The SLC24 Gene Family of Na+/Ca2+–K+ Exchangers: From Sight and Smell to Memory Consolidation and Skin Pigmentation. Mol. Asp. Med. 2013, 34, 455–464. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Xie, B.; Chen, D.; Jiang, J.; Zeng, T.; Xiong, L.; Shi, Q.; Xie, H.; Cai, Y.; Liang, J.; et al. Zebrafish cdh23 Affects Rod Cell Phototransduction Through Regulating Ca2+ Transport and MAPK Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 4604. https://doi.org/10.3390/ijms26104604
Zheng X, Xie B, Chen D, Jiang J, Zeng T, Xiong L, Shi Q, Xie H, Cai Y, Liang J, et al. Zebrafish cdh23 Affects Rod Cell Phototransduction Through Regulating Ca2+ Transport and MAPK Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(10):4604. https://doi.org/10.3390/ijms26104604
Chicago/Turabian StyleZheng, Xiaoying, Binling Xie, Dingrui Chen, Jifan Jiang, Ting Zeng, Lei Xiong, Qingying Shi, Hao Xie, Yisheng Cai, Jiaxin Liang, and et al. 2025. "Zebrafish cdh23 Affects Rod Cell Phototransduction Through Regulating Ca2+ Transport and MAPK Signaling Pathway" International Journal of Molecular Sciences 26, no. 10: 4604. https://doi.org/10.3390/ijms26104604
APA StyleZheng, X., Xie, B., Chen, D., Jiang, J., Zeng, T., Xiong, L., Shi, Q., Xie, H., Cai, Y., Liang, J., Chen, S., Qu, X., & Xie, H. (2025). Zebrafish cdh23 Affects Rod Cell Phototransduction Through Regulating Ca2+ Transport and MAPK Signaling Pathway. International Journal of Molecular Sciences, 26(10), 4604. https://doi.org/10.3390/ijms26104604




