Targeting Mitochondrial Dysfunction to Prevent Endothelial Dysfunction and Atherosclerosis in Diabetes: Focus on the Novel Uncoupler BAM15
Abstract
1. Introduction
2. Endothelial Dysfunction and Atherosclerosis in Diabetes: The Role of Nitric Oxide, Insulin Signaling, and Metabolic Dysregulation
2.1. Impaired Nitric Oxide Signaling in Endothelial Cells: A Key Contributor to Vascular Dysfunction
2.2. Disrupted Insulin Signaling in Endothelial Cells and Its Impact on Vascular Homeostasis
2.3. Mitochondrial Dysfunction in Endothelial Cells: A Nexus Between Oxidative Stress and Atherosclerosis
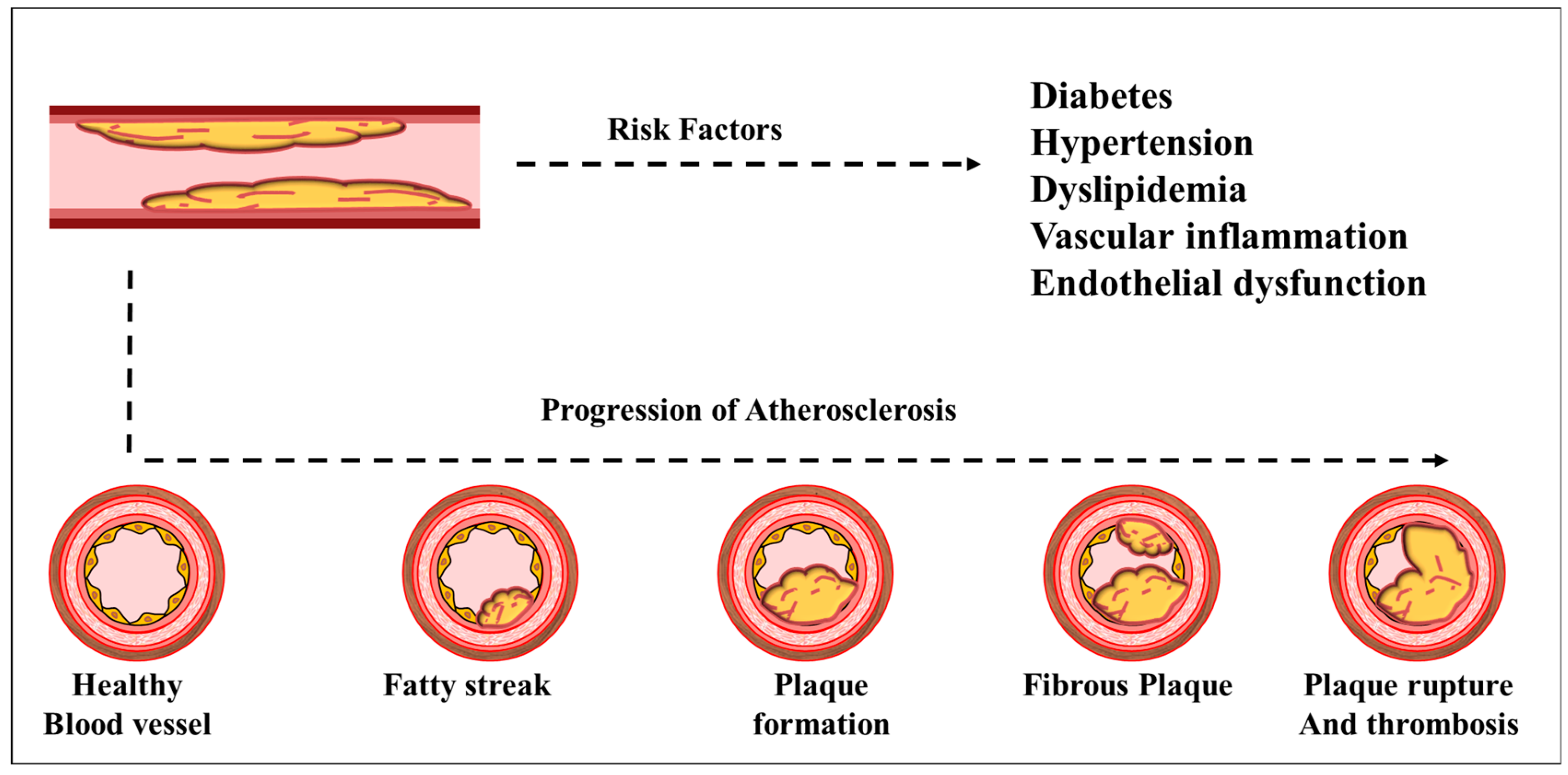
2.4. Diabetes-Induced Atherosclerosis: Mechanistic Insights into Lipid Accumulation and Plaque Formation
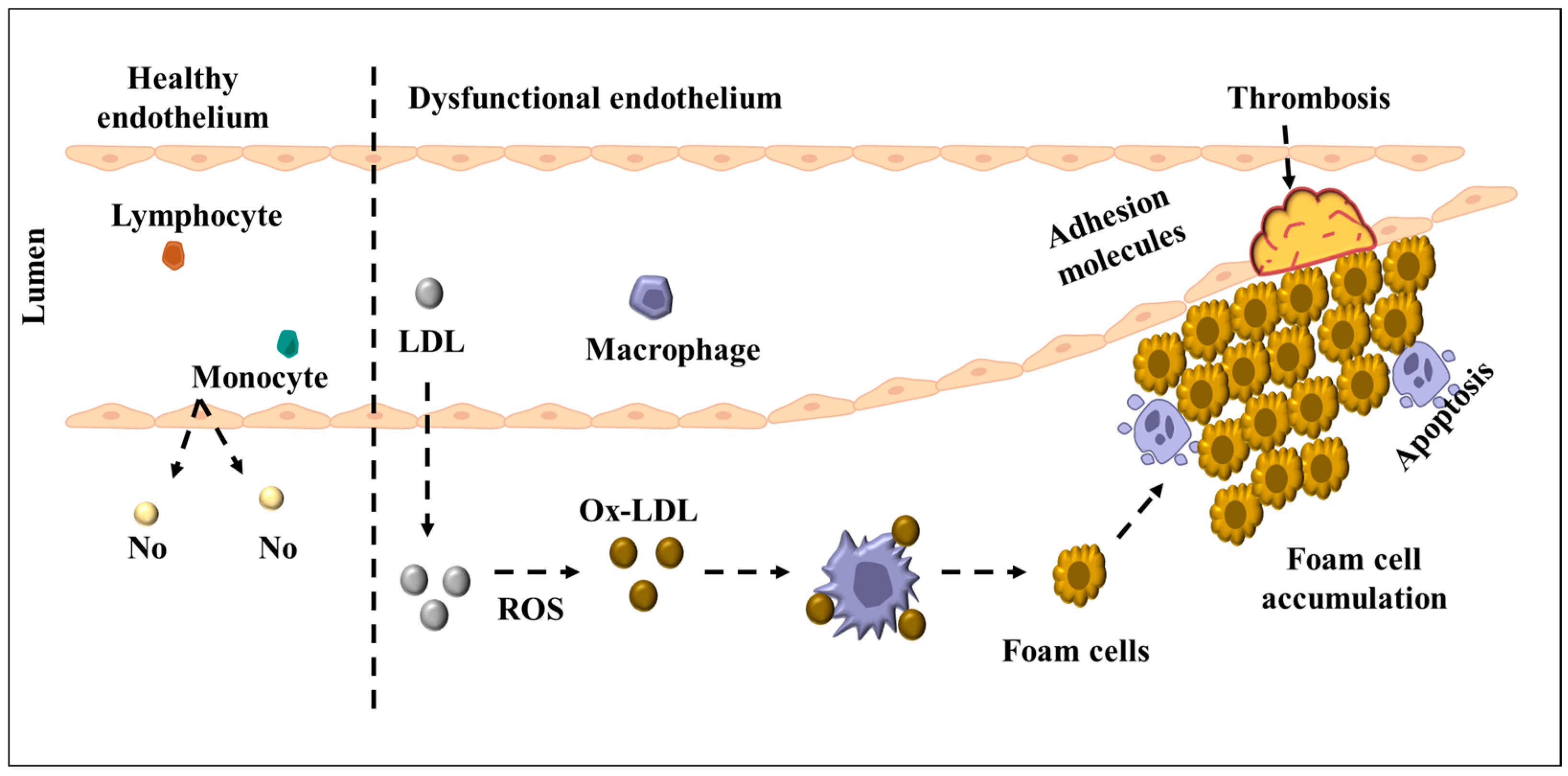
2.5. Endothelial Dysfunction as a Precursor to Atherosclerosis: The Role of Chronic Inflammation and Oxidative Stress
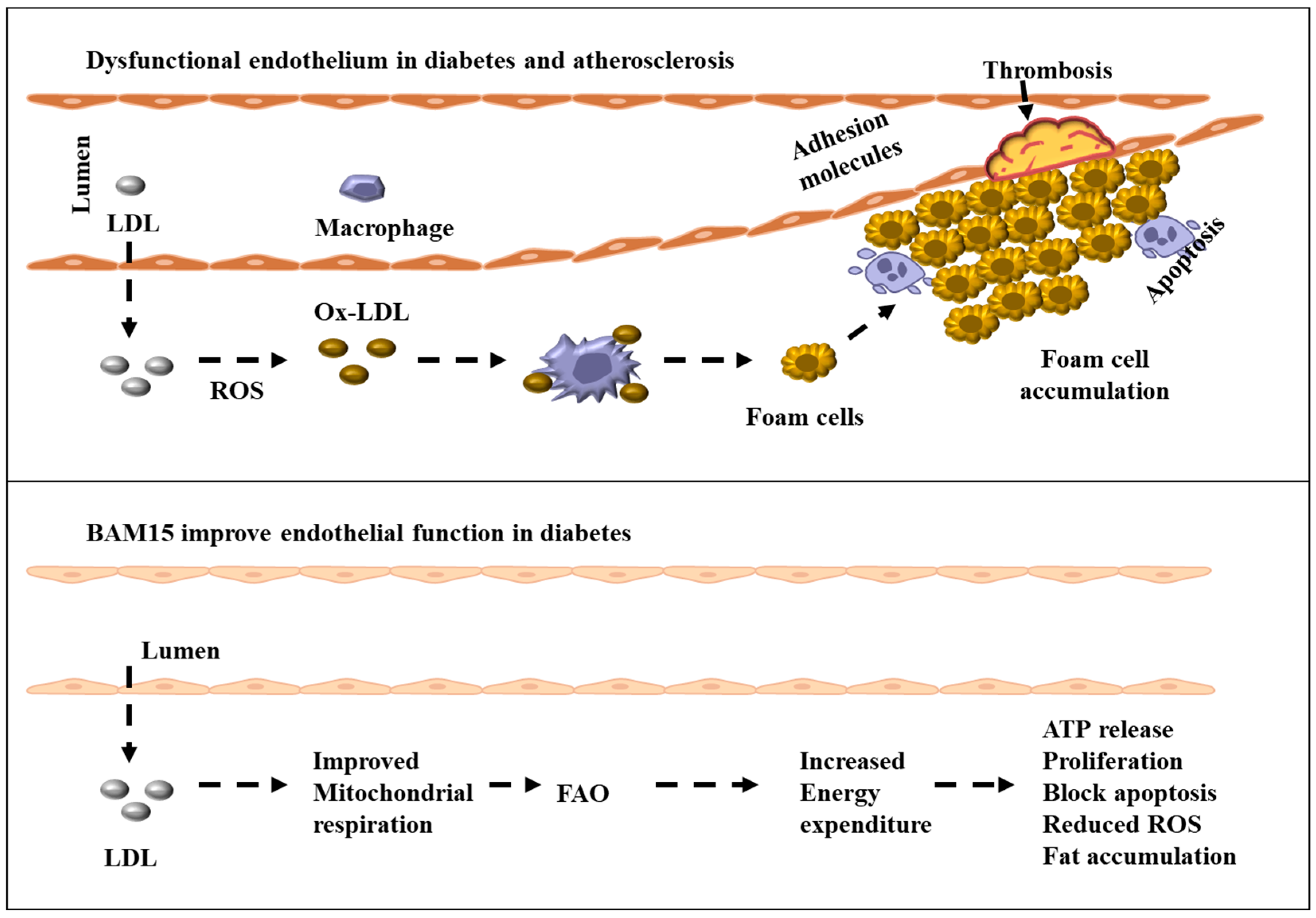
3. Targeting Mitochondrial Dysfunction in Endothelial Cells: Therapeutic Potential of BAM15 and Mitochondrial Uncoupling in Diabetes-Induced Atherosclerosis
3.1. Metabolic Reprogramming in Endothelial Cells: Implications for Vascular Health
3.2. Dysregulated Fatty Acid Metabolism in Endothelial Dysfunction and Atherosclerosis
3.3. Angiogenic Metabolism in Endothelial Cells: Balancing Energy Demand and Vascular Growth
3.4. Lipid-Lowering Therapeutics: Current Strategies and Their Limitations in Diabetes-Associated Atherosclerosis
3.5. Mitochondrial Uncoupling as a Novel Therapeutic Approach: Potential of BAM15 in Restoring Endothelial Function
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. S1), S62–S67. [CrossRef] [PubMed]
- Taylor, R. Type 2 diabetes: Etiology and reversibility. Diabetes Care 2013, 36, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2014, 38, S8–S16. [Google Scholar] [CrossRef]
- Khawandanah, J. Double or hybrid diabetes: A systematic review on disease prevalence, characteristics and risk factors. Nutr. Diabetes 2019, 9, 33. [Google Scholar] [CrossRef]
- Herder, C.; Roden, M. A novel diabetes typology: Towards precision diabetology from pathogenesis to treatment. Diabetologia 2022, 65, 1770–1781. [Google Scholar] [CrossRef]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2021, 17, 150–161. [Google Scholar] [CrossRef]
- Houeiss, P.; Luce, S.; Boitard, C. Environmental Triggering of Type 1 Diabetes Autoimmunity. Front. Endocrinol. 2022, 13, 933965. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, V.L.; MacDonald, P.E.; Klip, A. The cell biology of systemic insulin function. J. Cell Biol. 2018, 217, 2273–2289. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Vithian, K.; Hurel, S. Microvascular complications: Pathophysiology and management. Clin. Med. 2010, 10, 505–509. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef]
- Cannarile, F.; Valentini, V.; Mirabelli, G.; Alunno, A.; Terenzi, R.; Luccioli, F.; Gerli, R.; Bartoloni, E. Cardiovascular disease in systemic sclerosis. Ann. Transl. Med. 2015, 3, 8. [Google Scholar]
- Carris, N.W.; Magness, R.R.; Labovitz, A.J. Prevention of Diabetes Mellitus in Patients With Prediabetes. Am. J. Cardiol. 2019, 123, 507–512. [Google Scholar] [CrossRef]
- Hadi, H.A.; Suwaidi, J.A. Endothelial dysfunction in diabetes mellitus. Vasc. Health Risk Manag. 2007, 3, 853–876. [Google Scholar]
- Kolluru, G.K.; Bir, S.C.; Kevil, C.G. Endothelial dysfunction and diabetes: Effects on angiogenesis, vascular remodeling, and wound healing. Int. J. Vasc. Med. 2012, 2012, 918267. [Google Scholar] [CrossRef]
- Trimm, E.; Red-Horse, K. Vascular endothelial cell development and diversity. Nat. Rev. Cardiol. 2023, 20, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Malik, M.A. Vascular endothelial function. In PanVascular Medicine; Lanzer, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–37. [Google Scholar]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.C.; Loscalzo, J. Vascular Nitric Oxide: Formation and Function. J. Blood Med. 2010, 2010, 147–162. [Google Scholar] [CrossRef]
- Su, Y. Regulation of endothelial nitric oxide synthase activity by protein-protein interaction. Curr. Pharm. Des. 2014, 20, 3514–3520. [Google Scholar] [CrossRef]
- Li, C.; Huang, W.; Harris, M.B.; Goolsby, J.M.; Venema, R.C. Interaction of the endothelial nitric oxide synthase with the CAT-1 arginine transporter enhances NO release by a mechanism not involving arginine transport. Biochem. J. 2005, 386, 567–574. [Google Scholar] [CrossRef]
- Sun, J.; Liao, J.K. Functional interaction of endothelial nitric oxide synthase with a voltage-dependent anion channel. Proc. Natl. Acad. Sci. USA 2002, 99, 13108–13113. [Google Scholar] [CrossRef]
- Morel, C.; Lemerle, E.; Tsai, F.C.; Obadia, T.; Srivastava, N.; Marechal, M.; Salles, A.; Albert, M.; Stefani, C.; Benito, Y.; et al. Caveolin-1 protects endothelial cells from extensive expansion of transcellular tunnel by stiffening the plasma membrane. eLife 2024, 12, RP92078. [Google Scholar] [CrossRef]
- Frank, P.G. Endothelial caveolae and caveolin-1 as key regulators of atherosclerosis. Am. J. Pathol. 2010, 177, 544–546. [Google Scholar] [CrossRef]
- Jin, Z.G. Where is endothelial nitric oxide synthase more critical: Plasma membrane or Golgi? Arterioscler. Thromb. Vasc. Biol. 2006, 26, 959–961. [Google Scholar] [CrossRef]
- Peterson, T.E.; Poppa, V.; Ueba, H.; Wu, A.; Yan, C.; Berk, B.C. Opposing effects of reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. Circ. Res. 1999, 85, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. (Landmark Ed.) 2022, 27, 105. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Vijayakumar, S.; Kalogeroupoulos, A.; Butler, J. Multiple Avenues of Modulating the Nitric Oxide Pathway in Heart Failure Clinical Trials. Curr. Heart Fail. Rep. 2018, 15, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.R.; Wedgwood, S.; Czech, L.; Kim, G.A.; Lakshminrusimha, S.; Schumacker, P.T.; Steinhorn, R.H.; Farrow, K.N. Cyclic stretch induces inducible nitric oxide synthase and soluble guanylate cyclase in pulmonary artery smooth muscle cells. Int. J. Mol. Sci. 2013, 14, 4334–4348. [Google Scholar] [CrossRef]
- Hussain, M.B.; Hobbs, A.J.; MacAllister, R.J. Autoregulation of nitric oxide-soluble guanylate cyclase-cyclic GMP signalling in mouse thoracic aorta. Br. J. Pharmacol. 1999, 128, 1082–1088. [Google Scholar] [CrossRef]
- Feron, O.; Balligand, J.-L. Caveolins and the regulation of endothelial nitric oxide synthase in the heart. Cardiovasc. Res. 2006, 69, 788–797. [Google Scholar] [CrossRef]
- Ray, A.; Maharana, K.C.; Meenakshi, S.; Singh, S. Endothelial dysfunction and its relation in different disorders: Recent update. Health Sci. Rev. 2023, 7, 100084. [Google Scholar] [CrossRef]
- Denicola, A.; Batthyány, C.; Lissi, E.; Freeman, B.A.; Rubbo, H.; Radi, R. Diffusion of Nitric Oxide into Low Density Lipoprotein. J. Biol. Chem. 2002, 277, 932–936. [Google Scholar] [CrossRef]
- Kawashima, S.; Yokoyama, M. Dysfunction of Endothelial Nitric Oxide Synthase and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 998–1005. [Google Scholar] [CrossRef]
- Chen, J.Y.; Ye, Z.X.; Wang, X.F.; Chang, J.; Yang, M.W.; Zhong, H.H.; Hong, F.F.; Yang, S.L. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 2018, 97, 423–428. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2020, 11, 613780. [Google Scholar] [CrossRef] [PubMed]
- Dhananjayan, R.; Koundinya, K.S.; Malati, T.; Kutala, V.K. Endothelial Dysfunction in Type 2 Diabetes Mellitus. Indian J. Clin. Biochem. 2016, 31, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Gratton, J.-P.; Bernatchez, P.; Sessa, W.C. Caveolae and Caveolins in the Cardiovascular System. Circ. Res. 2004, 94, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Popov, D. Endothelial cell dysfunction in hyperglycemia: Phenotypic change, intracellular signaling modification, ultrastructural alteration, and potential clinical outcomes. Int. J. Diabetes Mellit. 2010, 2, 189–195. [Google Scholar] [CrossRef]
- Wu, W.-Z.; Bai, Y.-P. Endothelial GLUTs and vascular biology. Biomed. Pharmacother. 2023, 158, 114151. [Google Scholar] [CrossRef]
- Veys, K.; Fan, Z.; Ghobrial, M.; Bouché, A.; García-Caballero, M.; Vriens, K.; Conchinha, N.V.; Seuwen, A.; Schlegel, F.; Gorski, T.; et al. Role of the GLUT1 Glucose Transporter in Postnatal CNS Angiogenesis and Blood-Brain Barrier Integrity. Circ. Res. 2020, 127, 466–482. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Li, S.; Lv, J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 851941. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, M.; Singh, J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: Molecular insights and therapeutic strategies. Cardiovasc. Diabetol. 2018, 17, 121. [Google Scholar] [CrossRef]
- Kim, J.-a.; Wei, Y.; Sowers, J.R. Role of Mitochondrial Dysfunction in Insulin Resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Dludla, P.V.; Nyambuya, T.M.; Yakobi, S.H.; Mxinwa, V.; Nkambule, B.B. Monocyte-mediated inflammation and cardiovascular risk factors in type 2 diabetes mellitus: A systematic review and meta-analysis of pre-clinical and clinical studies. JRSM Cardiovasc. Dis. 2020, 9, 2048004019900748. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Asmis, R. Reactive oxygen species and thiol redox signaling in the macrophage biology of atherosclerosis. Antioxid. Redox Signal. 2012, 17, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Sterling, K.; Wang, Z.; Zhang, Y.; Song, W. The role of inflammasomes in human diseases and their potential as therapeutic targets. Signal Transduct. Target. Ther 2024, 9, 10. [Google Scholar] [CrossRef]
- Medrano-Bosch, M.; Simon-Codina, B.; Jimenez, W.; Edelman, E.R.; Melgar-Lesmes, P. Monocyte-endothelial cell interactions in vascular and tissue remodeling. Front. Immunol. 2023, 14, 1196033. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Hill, R.B. Mitochondrial regulation of diabetic vascular disease: An emerging opportunity. Transl. Res. 2018, 202, 83–98. [Google Scholar] [CrossRef]
- San-Millán, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants 2023, 12, 782. [Google Scholar] [CrossRef]
- Diano, S.; Horvath, T.L. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol. Med. 2012, 18, 52–58. [Google Scholar] [CrossRef]
- Nishikawa, T.; Araki, E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2007, 9, 343–353. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Roy Chowdhury, S.K.; Sangle, G.V.; Xie, X.; Stelmack, G.L.; Halayko, A.J.; Shen, G.X. Effects of extensively oxidized low-density lipoprotein on mitochondrial function and reactive oxygen species in porcine aortic endothelial cells. Am. J. Physiol. Endocrinol. Metab 2010, 298, E89–E98. [Google Scholar] [CrossRef] [PubMed]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodríguez Guzmán, R.; Centofanti, F.; Doldo, E.; Céspedes Miranda, E.M.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Lu, Y.; Cui, X.; Zhang, L.; Wang, X.; Xu, Y.; Qin, Z.; Liu, G.; Wang, Q.; Tian, K.; Lim, K.S.; et al. The Functional Role of Lipoproteins in Atherosclerosis: Novel Directions for Diagnosis and Targeting Therapy. Aging Dis. 2022, 13, 491–520. [Google Scholar] [CrossRef]
- Gaggini, M.; Gorini, F.; Vassalle, C. Lipids in Atherosclerosis: Pathophysiology and the Role of Calculated Lipid Indices in Assessing Cardiovascular Risk in Patients with Hyperlipidemia. Int. J. Mol. Sci. 2023, 24, 75. [Google Scholar] [CrossRef]
- Heinecke, J.W. Lipoprotein oxidation in cardiovascular disease: Chief culprit or innocent bystander? J. Exp. Med. 2006, 203, 813–816. [Google Scholar] [CrossRef]
- Sunil, B.; Ashraf, A.P. Dyslipidemia in Pediatric Type 2 Diabetes Mellitus. Curr. Diab. Rep. 2020, 20, 53. [Google Scholar] [CrossRef]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Sukhorukov, V.N.; Surkova, R.; Orekhov, N.A.; Orekhov, A.N. Glycation of LDL: AGEs, impact on lipoprotein function, and involvement in atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1094188. [Google Scholar] [CrossRef] [PubMed]
- Alique, M.; Luna, C.; Carracedo, J.; Ramírez, R. LDL biochemical modifications: A link between atherosclerosis and aging. Food Nutr. Res. 2015, 59, 29240. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Yang, X.; Zhou, F.; Wu, P.H.; Xing, S.; Xu, G.; Li, W.; Chi, J.; Ouyang, C.; Zhang, Y.; et al. C-reactive protein promotes atherosclerosis by increasing LDL transcytosis across endothelial cells. Br. J. Pharmacol. 2014, 171, 2671–2684. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Ballester, M.; Herrero-Cervera, A.; Vinué, Á.; Martínez-Hervás, S.; González-Navarro, H. Impact of Cholesterol Metabolism in Immune Cell Function and Atherosclerosis. Nutrients 2020, 12, 2021. [Google Scholar] [CrossRef]
- Xiao, Q.; Hou, R.; Xie, L.; Niu, M.; Pan, X.; Zhu, X. Macrophage metabolic reprogramming and atherosclerotic plaque microenvironment: Fostering each other? Clin. Transl. Med. 2023, 13, e1257. [Google Scholar] [CrossRef]
- Ye, J.; Li, L.; Wang, M.; Ma, Q.; Tian, Y.; Zhang, Q.; Liu, J.; Li, B.; Zhang, B.; Liu, H.; et al. Diabetes Mellitus Promotes the Development of Atherosclerosis: The Role of NLRP3. Front. Immunol. 2022, 13, 900254. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Mallick, R.; Duttaroy, A.K. Modulation of endothelium function by fatty acids. Mol. Cell. Biochem. 2022, 477, 15–38. [Google Scholar] [CrossRef]
- Zechner, R.; Zimmermann, R.; Eichmann, T.O.; Kohlwein, S.D.; Haemmerle, G.; Lass, A.; Madeo, F. FAT SIGNALS—Lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012, 15, 279–291. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Tarshoby, M.; Monestel, R.; Hook, G.; Cronin, J.; Johnson, A.; Bayazeed, B.; Baron, A.D. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J. Clin. Investig. 1997, 100, 1230–1239. [Google Scholar] [CrossRef]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Ley, K. Adhesion molecules and atherogenesis. Acta Physiol. Scand. 2001, 173, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Bhatt, N.; Cortassa, S.C. Mitochondrial and cellular mechanisms for managing lipid excess. Front. Physiol. 2014, 5, 282. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef]
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef]
- Eelen, G.; de Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef]
- Du, W.; Ren, L.; Hamblin, M.H.; Fan, Y. Endothelial Cell Glucose Metabolism and Angiogenesis. Biomedicines 2021, 9, 147. [Google Scholar] [CrossRef]
- Potente, M.; Carmeliet, P. The Link Between Angiogenesis and Endothelial Metabolism. Annu. Rev. Physiol. 2017, 79, 43–66. [Google Scholar] [CrossRef]
- Liu, B.; Dai, Z. Fatty Acid Metabolism in Endothelial Cell. Genes 2022, 13, 2301. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.; Ye, L. The Role of Amino Acids in Endothelial Biology and Function. Cells 2022, 11, 1372. [Google Scholar] [CrossRef] [PubMed]
- Mallick, R.; Basak, S.; Duttaroy, A.K. Fatty acids and evolving roles of their proteins in neurological, cardiovascular disorders and cancers. Prog. Lipid Res. 2021, 83, 101116. [Google Scholar] [CrossRef] [PubMed]
- Abumrad, N.A.; Cabodevilla, A.G.; Samovski, D.; Pietka, T.; Basu, D.; Goldberg, I.J. Endothelial Cell Receptors in Tissue Lipid Uptake and Metabolism. Circ. Res. 2021, 128, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Gugliucci, A. Triglyceride-Rich Lipoprotein Metabolism: Key Regulators of Their Flux. J. Clin. Med. 2023, 12, 4399. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, J.; Li, H.; Chen, C.; Wang, Y. CD36 Signaling in Diabetic Cardiomyopathy. Aging Dis. 2021, 12, 826–840. [Google Scholar] [CrossRef]
- Rekhi, U.R.; Omar, M.; Alexiou, M.; Delyea, C.; Immaraj, L.; Elahi, S.; Febbraio, M. Endothelial Cell CD36 Reduces Atherosclerosis and Controls Systemic Metabolism. Front. Cardiovasc. Med. 2021, 8, 768481. [Google Scholar] [CrossRef]
- Son, N.H.; Basu, D.; Samovski, D.; Pietka, T.A.; Peche, V.S.; Willecke, F.; Fang, X.; Yu, S.Q.; Scerbo, D.; Chang, H.R.; et al. Endothelial cell CD36 optimizes tissue fatty acid uptake. J. Clin. Investig. 2018, 128, 4329–4342. [Google Scholar] [CrossRef]
- Biswas, S.; Gao, D.; Altemus, J.B.; Rekhi, U.R.; Chang, E.; Febbraio, M.; Byzova, T.V.; Podrez, E.A. Circulating CD36 is increased in hyperlipidemic mice: Cellular sources and triggers of release. Free Radic. Biol. Med. 2021, 168, 180–188. [Google Scholar] [CrossRef]
- Black, P.N.; Sandoval, A.; Arias-Barrau, E.; DiRusso, C.C. Targeting the fatty acid transport proteins (FATP) to understand the mechanisms linking fatty acid transport to metabolism. Immunol. Endocr. Metab. Agents Med. Chem. 2009, 9, 11–17. [Google Scholar] [CrossRef]
- Inouye, K.E.; Prentice, K.J.; Lee, A.; Wang, Z.B.; Dominguez-Gonzalez, C.; Chen, M.X.; Riveros, J.K.; Burak, M.F.; Lee, G.Y.; Hotamışlıgil, G.S. Endothelial-derived FABP4 constitutes the majority of basal circulating hormone and regulates lipolysis-driven insulin secretion. JCI Insight 2023, 8, e164642. [Google Scholar] [CrossRef] [PubMed]
- Elmasri, H.; Karaaslan, C.; Teper, Y.; Ghelfi, E.; Weng, M.; Ince, T.A.; Kozakewich, H.; Bischoff, J.; Cataltepe, S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009, 23, 3865–3873. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Liang, X.; Lipsky, S.; Karaaslan, C.; Kozakewich, H.; Hotamisligil, G.S.; Bischoff, J.; Cataltepe, S. Dual role of fatty acid-binding protein 5 on endothelial cell fate: A potential link between lipid metabolism and angiogenic responses. Angiogenesis 2016, 19, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Iso, T.; Maeda, K.; Hanaoka, H.; Suga, T.; Goto, K.; Syamsunarno, M.R.; Hishiki, T.; Nagahata, Y.; Matsui, H.; Arai, M.; et al. Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2549–2557. [Google Scholar] [CrossRef]
- Yang, A.; Mottillo, E.P. Adipocyte lipolysis: From molecular mechanisms of regulation to disease and therapeutics. Biochem. J. 2020, 477, 985–1008. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Hirano, T. Pathophysiology of Diabetic Dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef]
- Ebbert, J.O.; Jensen, M.D. Fat Depots, Free Fatty Acids, and Dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar] [CrossRef]
- Xiong, J. Fatty Acid Oxidation in Cell Fate Determination. Trends Biochem. Sci. 2018, 43, 854–857. [Google Scholar] [CrossRef]
- Schoors, S.; Bruning, U.; Missiaen, R.; Queiroz, K.C.; Borgers, G.; Elia, I.; Zecchin, A.; Cantelmo, A.R.; Christen, S.; Goveia, J.; et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 2015, 520, 192–197. [Google Scholar] [CrossRef]
- Sebastián, D.; Guitart, M.; García-Martínez, C.; Mauvezin, C.; Orellana-Gavaldà, J.M.; Serra, D.; Gómez-Foix, A.M.; Hegardt, F.G.; Asins, G. Novel role of FATP1 in mitochondrial fatty acid oxidation in skeletal muscle cells. J. Lipid Res. 2009, 50, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Liu, J.; Yu, Z.X.; Yamazaki, T.; Yan, Y.; Kawagishi, H.; Rovira, I.I.; Liu, C.; Wolfgang, M.J.; Mukouyama, Y.S.; et al. Macrophage fatty acid oxidation inhibits atherosclerosis progression. J. Mol. Cell. Cardiol. 2019, 127, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Bruning, U.; Morales-Rodriguez, F.; Kalucka, J.; Goveia, J.; Taverna, F.; Queiroz, K.C.S.; Dubois, C.; Cantelmo, A.R.; Chen, R.; Loroch, S.; et al. Impairment of Angiogenesis by Fatty Acid Synthase Inhibition Involves mTOR Malonylation. Cell Metab. 2018, 28, 866–880.e815. [Google Scholar] [CrossRef] [PubMed]
- Kalucka, J.; Bierhansl, L.; Conchinha, N.V.; Missiaen, R.; Elia, I.; Brüning, U.; Scheinok, S.; Treps, L.; Cantelmo, A.R.; Dubois, C.; et al. Quiescent Endothelial Cells Upregulate Fatty Acid β-Oxidation for Vasculoprotection via Redox Homeostasis. Cell Metab. 2018, 28, 881–894.e13. [Google Scholar] [CrossRef]
- Patella, F.; Schug, Z.T.; Persi, E.; Neilson, L.J.; Erami, Z.; Avanzato, D.; Maione, F.; Hernandez-Fernaud, J.R.; Mackay, G.; Zheng, L.; et al. Proteomics-based metabolic modeling reveals that fatty acid oxidation (FAO) controls endothelial cell (EC) permeability. Mol. Cell. Proteom. 2015, 14, 621–634. [Google Scholar] [CrossRef]
- Yao, H.; Gong, J.; Peterson, A.L.; Lu, X.; Zhang, P.; Dennery, P.A. Fatty Acid Oxidation Protects against Hyperoxia-induced Endothelial Cell Apoptosis and Lung Injury in Neonatal Mice. Am. J. Respir. Cell Mol. Biol. 2019, 60, 667–677. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Dludla, P.V.; Mkandla, Z.; Mutize, T.; Nyambuya, T.M.; Mxinwa, V.; Nkambule, B.B. Differential expression of glycoprotein IV on monocyte subsets following high-fat diet feeding and the impact of short-term low-dose aspirin treatment. Metabol. Open 2020, 7, 100047. [Google Scholar] [CrossRef]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef]
- Wong, B.W.; Marsch, E.; Treps, L.; Baes, M.; Carmeliet, P. Endothelial cell metabolism in health and disease: Impact of hypoxia. EMBO J. 2017, 36, 2187–2203. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Sewduth, R.; Santoro, M.M. “Decoding” Angiogenesis: New Facets Controlling Endothelial Cell Behavior. Front. Physiol. 2016, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Lamalice, L.; Boeuf, F.L.; Huot, J. Endothelial Cell Migration During Angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Chápuli, R.; Quesada, A.R.; Angel Medina, M. Angiogenesis and signal transduction in endothelial cells. Cell. Mol. Life Sci. 2004, 61, 2224–2243. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; de Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef]
- Leung, S.W.S.; Shi, Y. The glycolytic process in endothelial cells and its implications. Acta Pharmacol. Sin. 2022, 43, 251–259. [Google Scholar] [CrossRef]
- Xu, Y.; An, X.; Guo, X.; Habtetsion, T.G.; Wang, Y.; Xu, X.; Kandala, S.; Li, Q.; Li, H.; Zhang, C.; et al. Endothelial PFKFB3 Plays a Critical Role in Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1231–1239. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Draoui, N.; de Zeeuw, P.; Carmeliet, P. Angiogenesis revisited from a metabolic perspective: Role and therapeutic implications of endothelial cell metabolism. Open Biol 2017, 7, 170219. [Google Scholar] [CrossRef]
- Kim, B.; Li, J.; Jang, C.; Arany, Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 2017, 36, 2321–2333. [Google Scholar] [CrossRef]
- Teuwen, L.-A.; Geldhof, V.; Carmeliet, P. How glucose, glutamine and fatty acid metabolism shape blood and lymph vessel development. Dev. Biol. 2019, 447, 90–102. [Google Scholar] [CrossRef]
- Rosenson, R.S. Statins in atherosclerosis: Lipid-lowering agents with antioxidant capabilities. Atherosclerosis 2004, 173, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.K.; Ali, S.; Sanghera, R.S. Pharmacological Options in Atherosclerosis: A Review of the Existing Evidence. Cardiol. Ther. 2019, 8, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Kreisberg, R.A.; Oberman, A. Lipids and Atherosclerosis: Lessons Learned from Randomized Controlled Trials of Lipid Lowering and Other Relevant Studies. J. Clin. Endocrinol. Metab. 2002, 87, 423–437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Q.; Liao, J.K. Statins and cardiovascular diseases: From cholesterol lowering to pleiotropy. Curr. Pharm. Des. 2009, 15, 467–478. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Merz, C.N.B.; Brewer, H.B.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith, S.C.; Stone, N.J. Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e149–e161. [Google Scholar] [CrossRef]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.-C. Mechanism of Action of Fibrates on Lipid and Lipoprotein Metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef]
- Zhang, B.; Kuipers, F.; de Boer, J.F.; Kuivenhoven, J.A. Modulation of Bile Acid Metabolism to Improve Plasma Lipid and Lipoprotein Profiles. J. Clin. Med. 2021, 11, 4. [Google Scholar] [CrossRef]
- Kamanna, V.S.; Kashyap, M.L. Mechanism of action of niacin. Am. J. Cardiol. 2008, 101, 20b–26b. [Google Scholar] [CrossRef]
- McKenney, J. New Perspectives on the Use of Niacin in the Treatment of Lipid Disorders. Arch. Intern. Med. 2004, 164, 697–705. [Google Scholar] [CrossRef]
- Schachter, M. Strategies for Modifying High-Density Lipoprotein Cholesterol: A Role for Nicotinic Acid. Cardiovasc. Drugs Ther. 2005, 19, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, S.J.; Chen, S.Y.; Brandon, A.E.; Salamoun, J.M.; Byrne, F.L.; Garcia, C.J.; Beretta, M.; Olzomer, E.M.; Shah, D.P.; Philp, A.M.; et al. Mitochondrial uncoupler BAM15 reverses diet-induced obesity and insulin resistance in mice. Nat. Commun. 2020, 11, 2397. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Johnson, E.; Byrne, F.L. Exploring the therapeutic potential of mitochondrial uncouplers in cancer. Mol. Metab. 2021, 51, 101222. [Google Scholar] [CrossRef] [PubMed]
- Goedeke, L.; Shulman, G.I. Therapeutic potential of mitochondrial uncouplers for the treatment of metabolic associated fatty liver disease and NASH. Mol. Metab. 2021, 46, 101178. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Divakaruni, A.S.; Jastroch, M.; Brand, M.D. Mitochondrial uncoupling and lifespan. Mech. Ageing Dev. 2010, 131, 463–472. [Google Scholar] [CrossRef]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef]
- Axelrod, C.L.; King, W.T.; Davuluri, G.; Noland, R.C.; Hall, J.; Hull, M.; Dantas, W.S.; Zunica, E.R.; Alexopoulos, S.J.; Hoehn, K.L.; et al. BAM15-mediated mitochondrial uncoupling protects against obesity and improves glycemic control. EMBO Mol. Med. 2020, 12, e12088. [Google Scholar] [CrossRef]
- Dang, C.P.; Issara-Amphorn, J.; Charoensappakit, A.; Udompornpitak, K.; Bhunyakarnjanarat, T.; Saisorn, W.; Sae-Khow, K.; Leelahavanichkul, A. BAM15, a Mitochondrial Uncoupling Agent, Attenuates Inflammation in the LPS Injection Mouse Model: An Adjunctive Anti-Inflammation on Macrophages and Hepatocytes. J. Innate Immun. 2021, 13, 359–375. [Google Scholar] [CrossRef]
- Dantas, W.S.; Zunica, E.R.M.; Heintz, E.C.; Vandanmagsar, B.; Floyd, Z.E.; Yu, Y.; Fujioka, H.; Hoppel, C.L.; Belmont, K.P.; Axelrod, C.L.; et al. Mitochondrial uncoupling attenuates sarcopenic obesity by enhancing skeletal muscle mitophagy and quality control. J. Cachexia Sarcopenia Muscle 2022, 13, 1821–1836. [Google Scholar] [CrossRef]
- Cho, I.; Song, H.O.; Ji, H.E.; Yang, S.; Cho, J.H. BAM15 Relieves Neurodegeneration in Aged Caenorhabditis elegans and Extends Lifespan. Metabolites 2022, 12, 1129. [Google Scholar] [CrossRef]
- Hiengrach, P.; Visitchanakun, P.; Tongchairawewat, P.; Tangsirisatian, P.; Jungteerapanich, T.; Ritprajak, P.; Wannigama, D.L.; Tangtanatakul, P.; Leelahavanichkul, A. Sepsis Encephalopathy Is Partly Mediated by miR370-3p-Induced Mitochondrial Injury but Attenuated by BAM15 in Cecal Ligation and Puncture Sepsis Male Mice. Int. J. Mol. Sci. 2022, 23, 5445. [Google Scholar] [CrossRef] [PubMed]
- Udompornpitak, K.; Bhunyakarnjanarat, T.; Saisorn, W.; Manipuntee, C.; Plengplang, K.; Sittichaitaweekul, S.; Jenphatanapong, P.; Udomkarnjananun, S.; Kaewduangduen, W.; Ariya-Anandech, K.; et al. Polymeric Particle BAM15 Targeting Macrophages Attenuates the Severity of LPS-Induced Sepsis: A Proof of Concept for Specific Immune Cell-Targeted Therapy. Pharmaceutics 2023, 15, 2695. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Tsuji, T.; Yamashita, T.; Hayase, N.; Hu, X.; Yuen, P.S.; Star, R.A. BAM15 treats mouse sepsis and kidney injury, linking mortality, mitochondrial DNA, tubule damage, and neutrophils. J. Clin. Investig. 2023, 133, e152401. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Telfser, A.J.; Olzomer, E.M.; Vancuylenberg, C.S.; Zhou, M.; Beretta, M.; Li, C.; Alexopoulos, S.J.; Turner, N.; Byrne, F.L.; et al. Beneficial effects of simultaneously targeting calorie intake and calorie efficiency in diet-induced obese mice. Clin. Sci. 2024, 138, 173–187. [Google Scholar] [CrossRef]
- Taylor, A.L.; Dubuisson, O.; Pandey, P.; Zunica, E.R.M.; Vandanmagsar, B.; Dantas, W.S.; Johnson, A.; Axelrod, C.L.; Kirwan, J.P. Restricting bioenergetic efficiency enhances longevity and mitochondrial redox capacity in Drosophila melanogaster. Aging Cell 2024, 23, e14107. [Google Scholar] [CrossRef]
- Ma, M.; Zhong, J.; Tai, Y.; Xu, S.; Pei, Z.; Wang, X. Combining RNA-seq, molecular docking and experimental verification to explore the mechanism of BAM15 as a potential drug for atherosclerosis. Sci. Rep. 2025, 15, 13347. [Google Scholar] [CrossRef]
- Bugger, H.; Abel, E.D. Mitochondria in the diabetic heart. Cardiovasc. Res. 2010, 88, 229–240. [Google Scholar] [CrossRef]
- Duncan, J.G. Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2011, 1813, 1351–1359. [Google Scholar] [CrossRef]
| Author (Year) | Model | BAM15 Dose and Duration | Key Findings | Functional Outcomes | Ref. |
|---|---|---|---|---|---|
| Stephanie J Alexopoulos et al. (2020) | Diet-induced obesity in male C57BL/6J mice | 0.05–0.15% w/w in diet for 8 days (prevention) | BAM15 is orally bioavailable; reduces body fat, hepatic fat, and inflammatory lipids; improves insulin sensitivity without affecting food intake or lean mass; increases nutrient oxidation | BAM15 is a unique mitochondrial uncoupler that effectively prevents and reverses diet-induced obesity without reducing food intake or compromising lean body mass | [143] |
| Christopher L Axelrod et al. (2020) | 4-week-old male C57BL/6J mice; high-fat diet (HFD) | 0.1% w/w in diet (chronic exposure) | BAM15 increased energy expenditure, improved glucose and lipid metabolism, enhanced AMPK activation, and improved insulin sensitivity | Protection against diet-induced obesity and improved glycemic control independent of weight loss | [148] |
| Cong Phi Dang et al. (2021) | LPS-induced systemic inflammation in mice | 1 mg/kg i.p., 3 h before LPS (4 mg/kg) | Reduced serum and tissue pro-inflammatory cytokines; enhanced hepatic AMPK activation; reduced inflammatory monocyte infiltration in liver | Attenuated organ injury (liver enzymes, creatinine); improved inflammatory and metabolic profile in sepsis | [149] |
| Wagner S Dantas et al. (2022) | Sarcopenic obesity in aged male C57BL/6J mice (80 weeks) | 0.1% w/w in high-fat diet for 10 weeks | BAM15 reduced body weight and fat mass, enhanced energy expenditure, increased skeletal muscle mass and strength, improved mitochondrial quality control, and reduced ER stress and apoptosis | Attenuated sarcopenic obesity; improved muscle function and metabolic health in aged mice | [150] |
| Injeong Cho et al. (2022) | Caenorhabditis elegans (wild-type and ucp-4 mutants) | 50 µM (treatment during aging) | BAM15 treatment reduced mechanosensory neuronal defects and preserved touch responses and short-term memory in aging nematodes; it also extended the lifespan of both wild-type and ucp-4 mutants | Reduced neurodegeneration and extended lifespan in C. elegans | [151] |
| Pratsanee Hiengrach et al. (2022) | Male C57BL/6J mice, cecal ligation and puncture (CLP) sepsis model | 5 mg/kg (administered before and 6 h post-CLP surgery) | BAM15 attenuated sepsis by reducing organ damage, systemic inflammation, mitochondrial injury, and neuronal miR370-3p upregulation; it also reduced blood–brain barrier damage and apoptosis in spleen and brain | Reduced mitochondrial injury, decreased systemic inflammation, reduced neuronal miR370-3p, improved blood–brain barrier integrity, and alleviated brain injury and encephalopathy | [152] |
| Kanyarat Udompornpitak et al. (2023) | C57BL/6 mice with LPS-induced sepsis | BAM15: 2 mg/kg i.p.; BAM15 particles: 2 mg/kg i.p. (before LPS) | BAM15 particles specifically targeted macrophages, reduced inflammation, and improved mitochondrial activity; BAM15 and BAM15 particles reduced LPS-induced liver injury and sepsis severity | Reduced inflammation and liver injury and improved mitochondrial activity in macrophages | [153] |
| Naoko Tsuji et al. (2023) | Male 6-week-old mice, CLP-induced sepsis | 1 mg/kg i.p. at 0, 6, or 12 h post-CLP + antibiotics/fluids | Reduced mortality, kidney damage, and splenic apoptosis; decreased plasma/urine mtDNA and mtROS in tubule cells | BAM15 prevented neutrophil apoptosis and mtDNA release; mtDNA injection reversed effects, linking mtROS and mtDNA with sepsis pathology | [154] |
| Sing-Young Chen et al. (2024) | Male C57BL/6J mice fed a high-fat Western diet (diet-induced obesity) | 0.05% (w/w) in food; 4 weeks of treatment | Combining BAM15 with semaglutide (low or high dose) improved body fat reduction and glucose control while preventing lean mass loss and liver TG accumulation | Improved weight loss and glucose homeostasis and reduced body fat without lean mass loss | [155] |
| Analisa L Taylor et al. (2024) | Drosophila melanogaster on normal diet (ND) or HFD | 0.036% (w/w) supplemented with diet (ND or HFD) for the duration of lifespan study | BAM15 extended lifespan by 9% on ND and 25% on HFD, improved locomotor activity, enhanced oxidative phosphorylation, and upregulated mitochondrial function and antioxidant defense | Extended lifespan, enhanced locomotor function, and improved mitochondrial redox capacity and fitness | [156] |
| Minghui Ma et al. (2025) | ApoE (−/−) mice fed a Western diet to establish atherosclerosis model | 85 mg/kg/day (oral, 6 times a week) | BAM15 inhibited atherosclerosis in WD-fed ApoE (−/−) mice; improved hyperlipidemia; reduced ALT, AST, liver TC, and TG levels; inhibited macrophage invasion and lipid accumulation in vitro | Reduced atherosclerosis progression; improved lipid profile and macrophage function | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, W.B.; Rethineswaran, V.K.; Kwon, S.-M. Targeting Mitochondrial Dysfunction to Prevent Endothelial Dysfunction and Atherosclerosis in Diabetes: Focus on the Novel Uncoupler BAM15. Int. J. Mol. Sci. 2025, 26, 4603. https://doi.org/10.3390/ijms26104603
Jang WB, Rethineswaran VK, Kwon S-M. Targeting Mitochondrial Dysfunction to Prevent Endothelial Dysfunction and Atherosclerosis in Diabetes: Focus on the Novel Uncoupler BAM15. International Journal of Molecular Sciences. 2025; 26(10):4603. https://doi.org/10.3390/ijms26104603
Chicago/Turabian StyleJang, Woong Bi, Vinoth Kumar Rethineswaran, and Sang-Mo Kwon. 2025. "Targeting Mitochondrial Dysfunction to Prevent Endothelial Dysfunction and Atherosclerosis in Diabetes: Focus on the Novel Uncoupler BAM15" International Journal of Molecular Sciences 26, no. 10: 4603. https://doi.org/10.3390/ijms26104603
APA StyleJang, W. B., Rethineswaran, V. K., & Kwon, S.-M. (2025). Targeting Mitochondrial Dysfunction to Prevent Endothelial Dysfunction and Atherosclerosis in Diabetes: Focus on the Novel Uncoupler BAM15. International Journal of Molecular Sciences, 26(10), 4603. https://doi.org/10.3390/ijms26104603





