Phosphoproteomics Reveals L1CAM-Associated Signaling Networks in High-Grade Serous Ovarian Carcinoma: Implications for Radioresistance and Tumorigenesis
Abstract
1. Introduction
2. Results
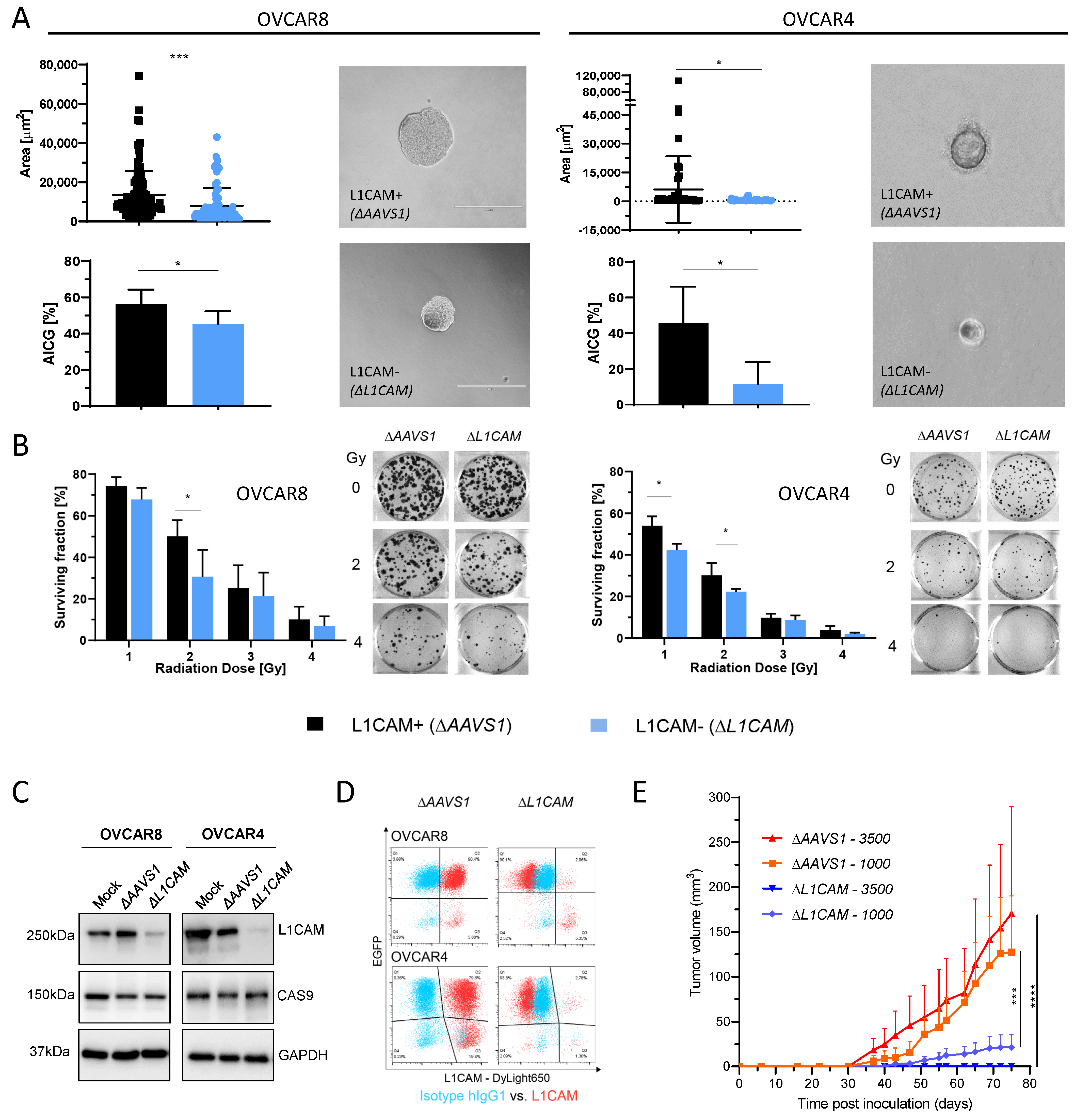
2.1. L1CAM Regulates Anchorage-Independent Cell Growth (AICG) and Radioresistance
2.2. L1CAM-Dependent Tumorigenesis in HGSOC Animal Model
2.3. Phosphoproteomics Analysis Uncovers L1CAM-Regulated Phosphorylation
2.4. Identification of L1CAM-Associated Signaling Networks in HGSOC
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Generation of ΔL1CAM Knockout Cells
4.2. Generation of ΔL1CAM Knockout Cells
4.3. Anchorage-Independent Cell Growth (AICG) and Radiation Response Assay
4.4. Western Blot (WB) Analysis
4.5. Flow Cytometry
4.6. Limiting Dilution Analysis (LDA)
4.7. Preparation of Tryptic Peptides and Phosphopeptide Enrichment
4.8. Tandem Mass Tag (TMT) Labeling and Peptide Fractionation
4.9. Proteomics and Phosphoproteomics Data Analysis and Statistics
4.10. Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AICG | Anchorage-independent cell growth |
| CSCs | Cancer stem cells |
| DDR | DNA damage response |
| DNAPKC | DNA-dependent protein kinase catalytic subunit |
| HGSOC | High-grade serous ovarian carcinoma |
| L1CAM | L1 cell adhesion molecule |
| LDA | Limiting dilution analysis |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| NHEJ | Non-homologous end-joining |
| OC | Ovarian cancer |
| PRKDC | Protein kinase DNA-activated catalytic subunit |
| TMT | Tandem mass tag |
References
- Giordano, M.; Cavallaro, U. Different Shades of L1CAM in the Pathophysiology of Cancer Stem Cells. J. Clin. Med. 2020, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Decio, A.; Battistini, C.; Baronio, M.; Bianchi, F.; Villa, A.; Bertalot, G.; Freddi, S.; Lupia, M.; Jodice, M.G.; et al. L1CAM promotes ovarian cancer stemness and tumor initiation via FGFR1/SRC/STAT3 signaling. J. Exp. Clin. Cancer Res. 2021, 40, 319. [Google Scholar] [CrossRef] [PubMed]
- Terraneo, N.; Jacob, F.; Peitzsch, C.; Dubrovska, A.; Krudewig, C.; Huang, Y.L.; Heinzelmann-Schwarz, V.; Schibli, R.; Béhé, M.; Grünberg, J. L1 Cell Adhesion Molecule Confers Radioresistance to Ovarian Cancer and Defines a New Cancer Stem Cell Population. Cancers 2020, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Basnet, H.; Kaygusuz, Y.; Laughney, A.M.; He, L.; Sharma, R.; O’Rourke, K.P.; Reuter, V.P.; Huang, Y.H.; Turkekul, M.; et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat. Cancer 2020, 1, 28–45. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; Li, Z.; Sathornsumetee, S.; Wang, H.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008, 68, 6043–6048. [Google Scholar] [CrossRef]
- Held-Feindt, J.; Schmelz, S.; Hattermann, K.; Mentlein, R.; Mehdorn, H.M.; Sebens, S. The neural adhesion molecule L1CAM confers chemoresistance in human glioblastomas. Neurochem. Int. 2012, 61, 1183–1191. [Google Scholar] [CrossRef]
- Chen, J.; Gao, F.; Liu, N. L1CAM promotes epithelial to mesenchymal transition and formation of cancer initiating cells in human endometrial cancer. Exp. Ther. Med. 2018, 15, 2792–2797. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, T.; Zhang, Z.; Payne, S.H.; Zhang, B.; McDermott, J.E.; Zhou, J.Y.; Petyuk, V.A.; Chen, L.; Ray, D.; et al. Integrated Proteogenomic Characterization of Human High-Grade Serous Ovarian Cancer. Cell 2016, 166, 755–765. [Google Scholar] [CrossRef]
- Francavilla, C.; Lupia, M.; Tsafou, K.; Villa, A.; Kowalczyk, K.; Rakownikow Jersie-Christensen, R.; Bertalot, G.; Confalonieri, S.; Brunak, S.; Jensen, L.J.; et al. Phosphoproteomics of Primary Cells Reveals Druggable Kinase Signatures in Ovarian Cancer. Cell Rep. 2017, 18, 3242–3256. [Google Scholar] [CrossRef]
- Coelho, R.; Tozzi, A.; Disler, M.; Lombardo, F.; Fedier, A.; López, M.N.; Freuler, F.; Jacob, F.; Heinzelmann-Schwarz, V. Overlapping gene dependencies for PARP inhibitors and carboplatin response identified by functional CRISPR-Cas9 screening in ovarian cancer. Cell Death Dis. 2022, 13, 909. [Google Scholar] [CrossRef]
- Mitra, A.K.; Davis, D.A.; Tomar, S.; Roy, L.; Gurler, H.; Xie, J.; Lantvit, D.D.; Cardenas, H.; Fang, F.; Liu, Y.; et al. In vivo tumor growth of high-grade serous ovarian cancer cell lines. Gynecol. Oncol. 2015, 138, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Abdel Azim, S.; Duggan-Peer, M.; Sprung, S.; Reimer, D.; Fiegl, H.; Soleiman, A.; Marth, C.; Zeimet, A.G. Clinical impact of L1CAM expression measured on the transcriptome level in ovarian cancer. Oncotarget 2016, 7, 37205–37214. [Google Scholar] [CrossRef] [PubMed]
- Rached, J.; Nasr, Z.; Abdallah, J.; Abou-Antoun, T. L1-CAM knock-down radiosensitizes neuroblastoma IMR-32 cells by simultaneously decreasing MycN, but increasing PTEN protein expression. Int. J. Oncol. 2016, 49, 1722–1730. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, Q.; Huang, Z.; Guryanova, O.A.; Huang, Q.; Shou, W.; Rich, J.N.; Bao, S. L1CAM regulates DNA damage checkpoint response of glioblastoma stem cells through NBS1. EMBO J. 2011, 30, 800–813. [Google Scholar] [CrossRef]
- Rinne, N.; Christie, E.L.; Ardasheva, A.; Kwok, C.H.; Demchenko, N.; Low, C.; Tralau-Stewart, C.; Fotopoulou, C.; Cunnea, P. Targeting the PI3K/AKT/mTOR pathway in epithelial ovarian cancer, therapeutic treatment options for platinum-resistant ovarian cancer. Cancer Drug Resist. 2021, 4, 573–595. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Sun, D.; Zhou, H.; Zhu, J.; Zhang, X.; Hou, H. Next-generation sequencing identifies potential novel therapeutic targets in Chinese HGSOC patients. Pathol. Res. Pract. 2022, 238, 154074. [Google Scholar] [CrossRef]
- Yue, X.; Bai, C.; Xie, D.; Ma, T.; Zhou, P.K. DNA-PKcs: A Multi-Faceted Player in DNA Damage Response. Front. Genet. 2020, 11, 607428. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Huang, T.T.; Brill, E.; Nair, J.R.; Zhang, X.; Wilson, K.M.; Chen, L.; Thomas, C.J.; Lee, J.M. Targeting the PI3K/mTOR Pathway Augments CHK1 Inhibitor-Induced Replication Stress and Antitumor Activity in High-Grade Serous Ovarian Cancer. Cancer Res. 2020, 80, 5380–5392. [Google Scholar] [CrossRef]
- David-West, G.; Ernlund, A.; Gadi, A.; Schneider, R.J. mTORC1/2 inhibition re-sensitizes platinum-resistant ovarian cancer by disrupting selective translation of DNA damage and survival mRNAs. Oncotarget 2018, 9, 33064–33076. [Google Scholar] [CrossRef]
- Liang, W.; Lin, Z.; Du, C.; Qiu, D.; Zhang, Q. mRNA modification orchestrates cancer stem cell fate decisions. Mol. Cancer 2020, 19, 38. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, M.; Zheng, Y. Role of Rho GTPases in stem cell regulation. Biochem. Soc. Trans. 2021, 49, 2941–2955. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Hu, Y.; Smyth, G.K. ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 2009, 347, 70–78. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Turker, C.; Akal, F.; Joho, D.; Panse, C.; Barkow-Oesterreicher, S.; Rehrauer, H.; Schlapbach, R. B-fabric: The Swiss army knife for life sciences. In Proceedings of the 13th International Conference on Extending Database Technology, Lausanne, Switzerland, 22–26 March 2010; Volume 13, pp. 717–720. [Google Scholar] [CrossRef]
- Wolski, W.E.; Nanni, P.; Grossmann, J.; d’Errico, M.; Schlapbach, R.; Panse, C. prolfqua: A Comprehensive R-Package for Proteomics Differential Expression Analysis. J. Proteome Res. 2023, 22, 1092–1104. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Kohler, D.; Tsai, T.H.; Verschueren, E.; Huang, T.; Hinkle, T.; Phu, L.; Choi, M.; Vitek, O. MSstatsPTM: Statistical Relative Quantification of Posttranslational Modifications in Bottom-Up Mass Spectrometry-Based Proteomics. Mol. Cell. Proteom. 2023, 22, 100477. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]

| Terms and Identified Hypo-Phosphorylated Proteins | Protein No. | Fold. Enr. | p | FDR |
|---|---|---|---|---|
| DNA repair | ||||
| 9 | 3.5 | 4.1 × 10−3 | 6.2 × 10−2 |
| mTORC1-mediated signaling | ||||
| 4 | 23.4 | 6.1 × 10−4 | 3.3 × 10−2 |
| Metabolism of RNA | ||||
| 21 | 4.1 | 8.6 × 10−8 | 3.2 × 10−5 |
| mRNA processing | ||||
| 19 | 6.6 | 2.8 × 10−10 | 6.3 × 10−9 |
| Processing of capped intron-containing pre-mRNA | ||||
| 12 | 5.9 | 4.5 × 10−6 | 4.2 × 10−4 |
| mRNA Splicing | ||||
| 18 | 8 | 4.6 × 10−11 | 2.0 × 10−9 |
| Activation of the mRNA upon binding of the cap-binding complex and eIFs, and subsequent binding to 43S | ||||
| 5 | 11.7 | 8.2 × 10−4 | 3.8 × 10−2 |
| Signaling by Rho GTPases | ||||
| 16 | 3.2 | 1.0 × 10−4 | 7.5 × 10−3 |
| RHO GTPase cycle | ||||
| 11 | 3.4 | 1.1 × 10−3 | 4.8 × 10−2 |
| Cell division | ||||
| 9 | 3.0 | 9.3 × 10−3 | 1.0 × 10−1 |
| Terms and Identified Hyper-Phosphorylated Proteins | Protein No. | Fold. Enr. | p | FDR |
| Transcription regulation | ||||
| 11 | 2.3 | 9.0 × 10−3 | 6.3 × 10−2 |
| mRNA processing | ||||
| 6 | 7.4 | 8.5 × 10−4 | 9.7 × 10−3 |
| mRNA splicing | ||||
| 6 | 9.5 | 2.7 × 10−4 | 6.1 × 10−3 |
| RHO GTPase cycle | ||||
| 7 | 7.9 | 1.4 × 10−4 | 9.4 × 10−3 |
| Signaling by Rho GTPases | ||||
| 7 | 5 | 1.5 × 10−3 | 7.1 × 10−2 |
| RHOBTB GTPase cycle | ||||
| 4 | 57.6 | 3.7 × 10−5 | 3.8 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorov, T.Z.; Coelho, R.; Jacob, F.; Heinzelmann-Schwarz, V.; Schibli, R.; Béhé, M.; Grünberg, J.; Grzmil, M. Phosphoproteomics Reveals L1CAM-Associated Signaling Networks in High-Grade Serous Ovarian Carcinoma: Implications for Radioresistance and Tumorigenesis. Int. J. Mol. Sci. 2025, 26, 4585. https://doi.org/10.3390/ijms26104585
Todorov TZ, Coelho R, Jacob F, Heinzelmann-Schwarz V, Schibli R, Béhé M, Grünberg J, Grzmil M. Phosphoproteomics Reveals L1CAM-Associated Signaling Networks in High-Grade Serous Ovarian Carcinoma: Implications for Radioresistance and Tumorigenesis. International Journal of Molecular Sciences. 2025; 26(10):4585. https://doi.org/10.3390/ijms26104585
Chicago/Turabian StyleTodorov, Tihomir Zh, Ricardo Coelho, Francis Jacob, Viola Heinzelmann-Schwarz, Roger Schibli, Martin Béhé, Jürgen Grünberg, and Michal Grzmil. 2025. "Phosphoproteomics Reveals L1CAM-Associated Signaling Networks in High-Grade Serous Ovarian Carcinoma: Implications for Radioresistance and Tumorigenesis" International Journal of Molecular Sciences 26, no. 10: 4585. https://doi.org/10.3390/ijms26104585
APA StyleTodorov, T. Z., Coelho, R., Jacob, F., Heinzelmann-Schwarz, V., Schibli, R., Béhé, M., Grünberg, J., & Grzmil, M. (2025). Phosphoproteomics Reveals L1CAM-Associated Signaling Networks in High-Grade Serous Ovarian Carcinoma: Implications for Radioresistance and Tumorigenesis. International Journal of Molecular Sciences, 26(10), 4585. https://doi.org/10.3390/ijms26104585







