Feline Panleukopenia Virus ZZ202303 Strain: Molecular Characterization and Structural Implications of the VP2 Gene Phylogenetic Divergence
Abstract
1. Introduction
2. Results
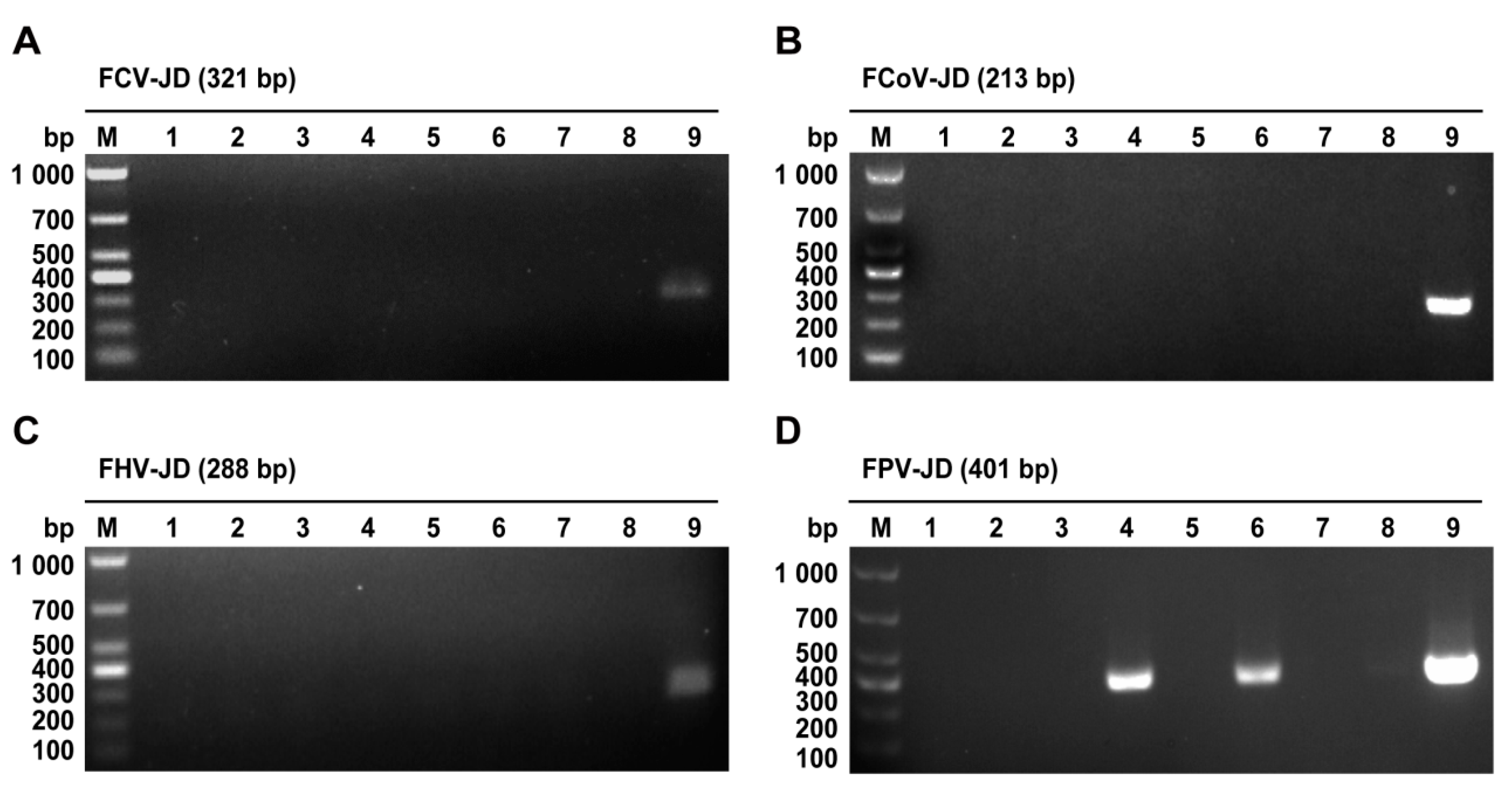
2.1. PCR-Based Identification of FPV
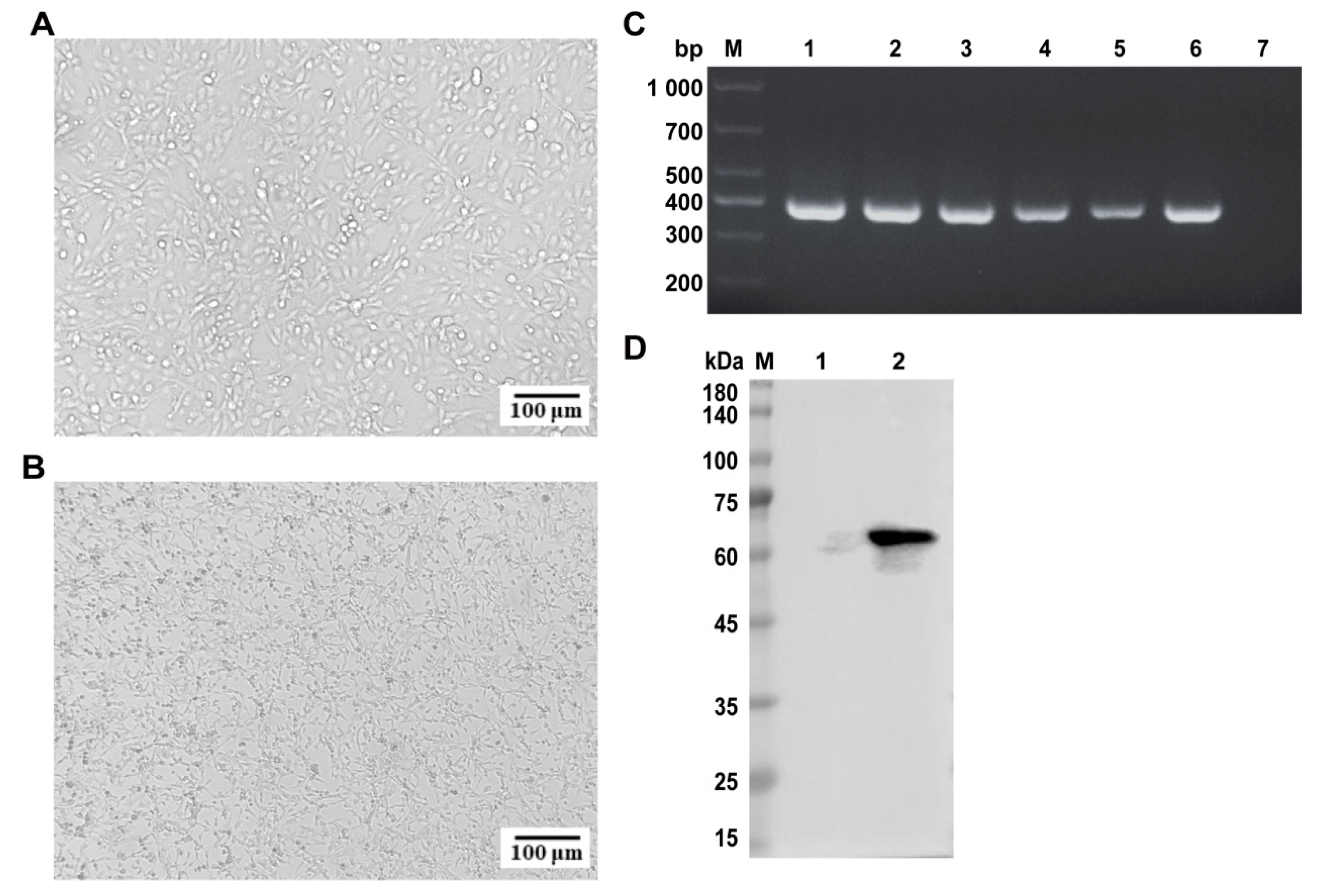
2.2. Isolation and Identification of FPV
2.3. Indirect Immunofluorescence Assay (IFA) of FPV ZZ202303
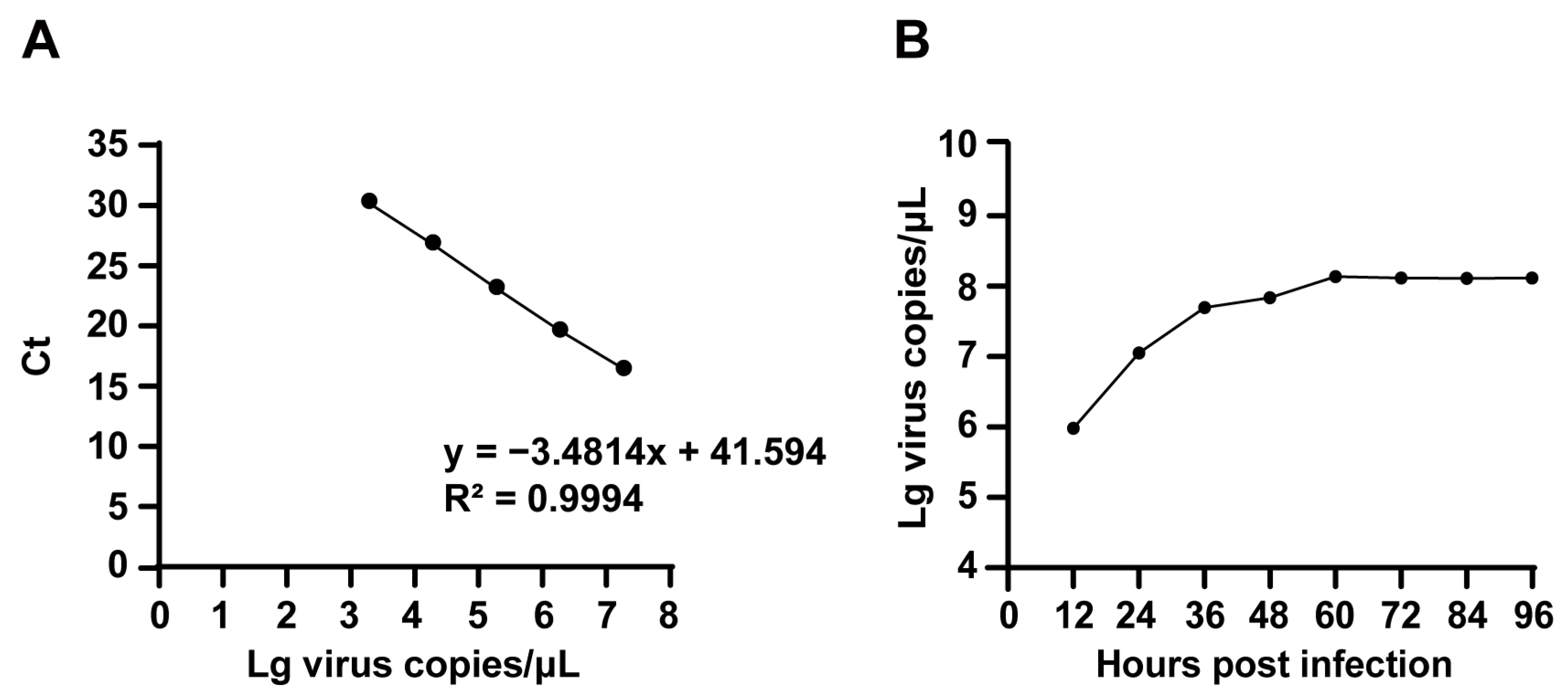
2.4. TCID50 Determination of FPV ZZ202303
2.5. Proliferation Kinetics of FPV ZZ202303
2.6. Nucleotide Sequence Similarity Analysis of the FPV ZZ202303-VP2 Gene
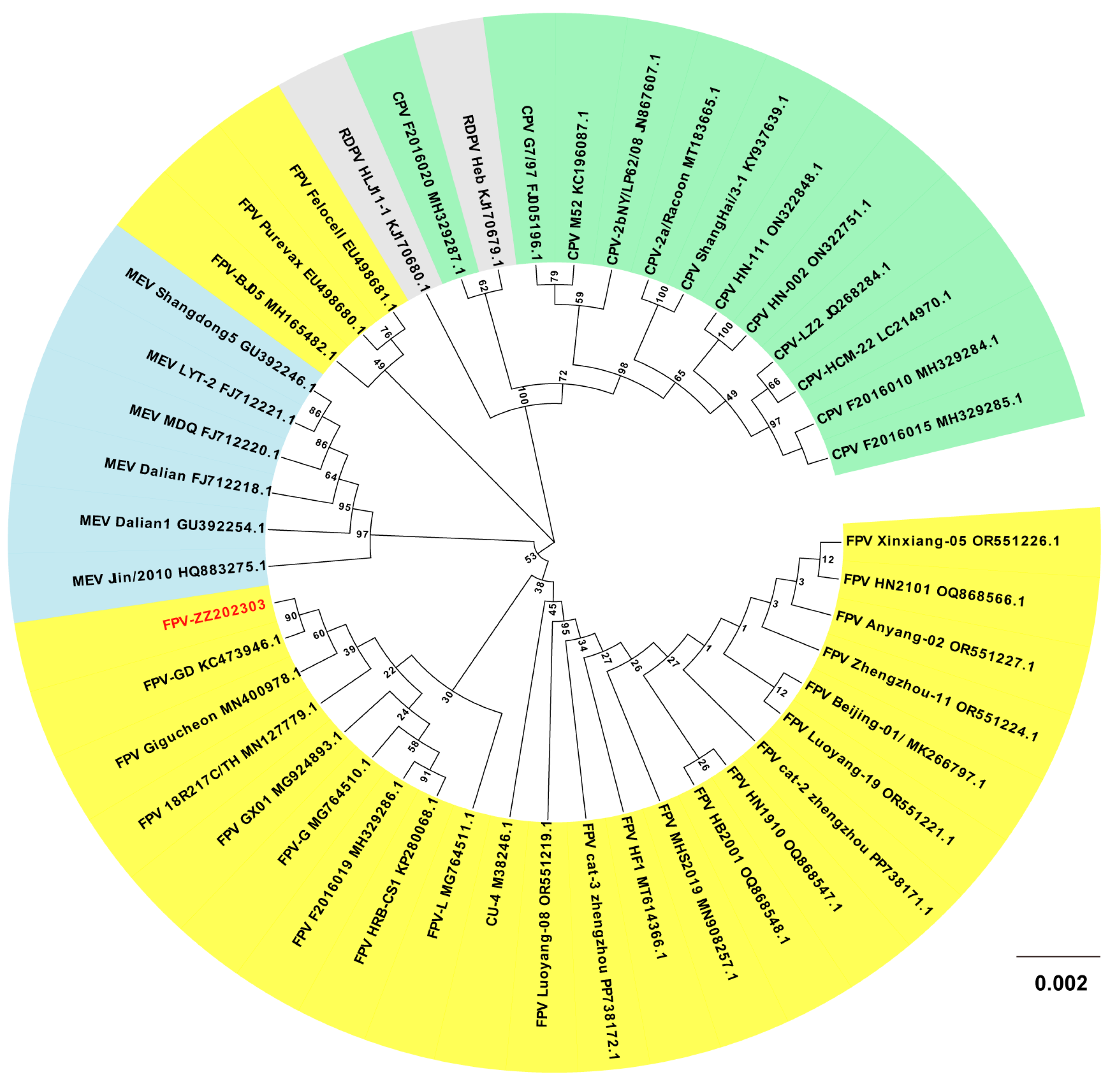
2.7. Phylogenetic Analysis of the FPV ZZ202303-VP2 Gene
2.8. Analysis of Amino Acid Mutation Sites of the FPV ZZ202303-VP2 Protein
3. Discussion
4. Materials and Methods
4.1. Sample Source
4.2. Primer Synthesis
4.3. Primary Reagents
4.4. Sample Treatment
4.5. PCR Identification of FPV
4.6. Isolation and Cultivation of FPV
4.7. Immunoblotting Analysis of FPV
4.8. IFA Identification of FPV
4.9. TCID50 Determination of FPV
4.10. Proliferation Kinetics Analysis of FPV
4.10.1. Establishing a Standard Curve
4.10.2. Collecting Virus Liquid Samples at Different Time Points
4.10.3. Phylogenetic Divergence of FPV
4.11. Statistical Analysis
4.12. Statement of Informed Consent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikeda, Y.; Shinozuka, J.; Miyazawa, T.; Kurosawa, K.; Izumiya, Y.; Nishimura, Y.; Nakamura, K.; Cai, J.; Fujita, K.; Doi, K.; et al. Apoptosis in feline panleukopenia virus-infected lymphocytes. J. Virol. 1998, 72, 6932–6936. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Habib, T.; Chouhan, C.S.; Hassan, J.; Rahman, A.K.M.A.; Nazir, K.H.M.N.H. Epidemiology and molecular characterization of Feline panleukopenia virus from suspected domestic cats in selected Bangladesh regions. PLoS ONE 2023, 18, e0282559. [Google Scholar] [CrossRef] [PubMed]
- Reif, J.S. Seasonality, natality and herd immunity in feline panleukopenia. Am. J. Epidemiol. 1976, 103, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Barrs, V.R. Feline Panleukopenia: A Re-emergent Disease. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 651–670. [Google Scholar] [CrossRef]
- Stuetzer, B.; Hartmann, K. Feline parvovirus infection and associated diseases. Veter- J. 2014, 201, 150–155. [Google Scholar] [CrossRef]
- Zhao, S.; Hu, H.; Lan, J.; Yang, Z.; Peng, Q.; Yan, L.; Luo, L.; Wu, L.; Lang, Y.; Yan, Q. Characterization of a fatal feline panleukopenia virus derived from giant panda with broad cell tropism and zoonotic potential. Front. Immunol. 2023, 14, 1237630. [Google Scholar] [CrossRef]
- Christensen, J.; Tattersall, P. Parvovirus initiator protein NS1 and RPA coordinate replication fork progression in a reconstituted DNA replication system. J. Virol. 2002, 76, 6518–6531. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.-M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef]
- Poncelet, L.; Garigliany, M.; Ando, K.; Franssen, M.; Desmecht, D.; Brion, J.-P. Cell cycle S phase markers are expressed in cerebral neuron nuclei of cats infected by the Feline Panleukopenia Virus. Cell Cycle 2016, 15, 3482–3489. [Google Scholar] [CrossRef]
- Raab, U.; Beckenlehner, K.; Lowin, T.; Niller, H.H.; Doyle, S.; Modrow, S. NS1 protein of parvovirus B19 interacts directly with DNA sequences of the p6 promoter and with the cellular transcription factors Sp1/Sp3. Virology 2002, 293, 86–93. [Google Scholar] [CrossRef]
- DiGangi, B.A.; Levy, J.K.; Griffin, B.; McGorray, S.P.; Dubovi, E.J.; Dingman, P.A.; Tucker, S.J. Prevalence of serum antibody titers against feline panleukopenia virus, feline herpesvirus 1, and feline calicivirus in cats entering a Florida animal shelter. J. Am. Veter- Med. Assoc. 2012, 241, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Parrish, C.R. Canine parvovirus host range is determined by the specific conformation of an additional region of the capsid. J. Virol. 1997, 71, 9214–9222. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.A.; Chandrasekar, V.; Hébert, B.; Sullivan, G.M.; Rossmann, M.G.; Parrish, C.R. Host range and variability of calcium binding by surface loops in the capsids of canine and feline parvoviruses. J. Mol. Biol. 2000, 300, 597–610. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Pan, Y.; Li, H.; He, T.; Dong, Q.; Song, W.; Zhang, W.; Zhang, L.; Kareem, K.; et al. Evolutionary Dynamics and Pathogenicity Analysis of Feline Panleukopenia Virus in Xinjiang, China. Microorganisms 2024, 12, 2205. [Google Scholar] [CrossRef]
- Arora, R.; Malla, W.A.; Tyagi, A.; Mahajan, S.; Sajjanar, B.; Tiwari, A.K. Canine Parvovirus and Its Non-Structural Gene 1 as Oncolytic Agents: Mechanism of Action and Induction of Anti-Tumor Immune Response. Front. Oncol. 2021, 11, 648873. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.R. Structures and functions of parvovirus capsids and the process of cell infection. Curr. Top Microbiol. Immunol. 2010, 343, 149–176. [Google Scholar]
- Chung, H.-C.; Kim, S.-J.; Nguyen, V.G.; Shin, S.; Kim, J.Y.; Lim, S.-K.; Park, Y.H.; Park, B. New genotype classification and molecular characterization of canine and feline parvoviruses. J. Veter-Sci. 2020, 21, e43. [Google Scholar] [CrossRef]
- Hoelzer, K.; Parrish, C.R. The emergence of parvoviruses of carnivores. Veter- Res. 2010, 41, 39. [Google Scholar] [CrossRef]
- Ikeda, Y.; Nakamura, K.; Miyazawa, T.; Tohya, Y.; Takahashi, E.; Mochizuki, M. Feline host range of Canine parvovirus: Recent emergence of new antigenic types in cats. Emerg. Infect. Dis. 2002, 8, 341–346. [Google Scholar] [CrossRef]
- Xue, H.; Hu, C.; Ma, H.; Song, Y.; Zhu, K.; Fu, J.; Mu, B.; Gao, X. Isolation of feline panleukopenia virus from Yanji of China and molecular epidemiology from 2021 to 2022. J. Veter-Sci. 2023, 24, e29. [Google Scholar] [CrossRef]
- Hueffer, K.; Parrish, C.R. Parvovirus host range, cell tropism and evolution. Curr. Opin. Microbiol. 2003, 6, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Callaway, H.M.; Welsch, K.; Weichert, W.; Allison, A.B.; Hafenstein, S.L.; Huang, K.; Iketani, S.; Parrish, C.R. Complex and Dynamic Interactions between Parvovirus Capsids, Transferrin Receptors, and Antibodies Control Cell Infection and Host Range. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Turiso, J.A.L.d.; Cortés, E.; Martínez, C.; de Ybáñez, R.R.; Simarro, I.; Vela, C.; Casal, I. Recombinant vaccine for canine parvovirus in dogs. J. Virol. 1992, 66, 2748–2753. [Google Scholar] [CrossRef]
- Wei, J.; Shi, Y.; Wang, X.; He, S.; Qi, X.; Lu, R.; Gao, Y.; Liu, Z.; Wang, Y.; Wu, Y.; et al. The first outbreak of feline panleukopenia virus infection in captive Pallas’s cats in Xining Wildlife Park. Front. Veter-Sci. 2024, 11, 1418553. [Google Scholar] [CrossRef]
- Huang, S.; Li, X.; Xie, W.; Guo, L.; You, D.; Xu, H.; Liu, D.; Wang, Y.; Hou, Z.; Zeng, X.; et al. Molecular Detection of Parvovirus in Captive Siberian Tigers and Lions in Northeastern China From 2019 to 2021. Front. Microbiol. 2022, 13, 898184. [Google Scholar] [CrossRef]
- Aeffner, F.; Ulrich, R.; Schulze-Rückamp, L.; Beineke, A. Cerebellar hypoplasia in three sibling cats after intrauterine or early postnatal parvovirus infection. Dtsch. Tierarztl. Wochenschr. 2006, 113, 403–406. [Google Scholar] [PubMed]
- Wen, Y.; Tang, Z.; Wang, K.; Geng, Z.; Yang, S.; Guo, J.; Chen, Y.; Wang, J.; Fan, Z.; Chen, P.; et al. Epidemiological and Molecular Investigation of Feline Panleukopenia Virus Infection in China. Viruses 2024, 16, 1967. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Dewangan, H.K. Nanoparticles as Adjuvants in Vaccine Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2020, 37, 183–204. [Google Scholar] [CrossRef]
- Lee, H.; Callaway, H.M.; Cifuente, J.O.; Bator, C.M.; Parrish, C.R.; Hafenstein, S.L. Transferrin receptor binds virus capsid with dynamic motion. Proc. Natl. Acad. Sci. USA 2019, 116, 20462–20471. [Google Scholar] [CrossRef]
- Organtini, L.J.; Lee, H.; Iketani, S.; Huang, K.; Ashley, R.E.; Makhov, A.M.; Conway, J.F.; Parrish, C.R.; Hafenstein, S. Near-Atomic Resolution Structure of a Highly Neutralizing Fab Bound to Canine Parvovirus. J. Virol. 2016, 90, 9733–9742. [Google Scholar] [CrossRef]
- Hoang, M.; Wu, C.N.; Lin, C.F.; Nguyen, H.T.T.; Le, V.P.; Chiou, M.-T.; Lin, C.N. Genetic characterization of feline panleukopenia virus from dogs in Vietnam reveals a unique Thr101 mutation in VP2. PeerJ 2020, 8, e9752. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Liu, S.; Meng, X.; Huang, P.; Cao, Z.; Jin, H.; Wang, J.; Hu, G.; Lan, J.; Zhang, D.; et al. Feline Panleukopenia Virus With G299E Substitution in the VP2 Protein First Identified From a Captive Giant Panda in China. Front. Cell. Infect. Microbiol. 2021, 11, 820144. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, J.; Zeng, Y.; Wang, Y.; Li, L.; Cao, Y.; Cao, L.; Chen, Q.; Ye, Z.; Zhou, D.; et al. Isolation of a feline-derived feline panleukopenia virus with an A300P substitution in the VP2 protein and confirmation of its pathogenicity in dogs. Anim. Dis. 2024, 4, 5. [Google Scholar] [CrossRef]
- DiGangi, B.A.; Levy, J.K.; Griffin, B.; Reese, M.J.; Dingman, P.A.; Tucker, S.J.; Dubovi, E.J. Effects of maternally-derived antibodies on serologic responses to vaccination in kittens. J. Feline Med. Surg. 2012, 14, 118–123. [Google Scholar] [CrossRef]
- Yang, M.; Jiao, Y.; Li, L.; Yan, Y.; Fu, Z.; Liu, Z.; Hu, X.; Li, M.; Shi, Y.; He, J.; et al. A potential dual protection vaccine: Recombinant feline herpesvirus-1 expressing feline parvovirus VP2 antigen. Veter-Microbiol. 2024, 290, 109978. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Zhang, H.; Liu, W.; Jin, H.; Liu, D.; Zhao, J.; Feng, N.; Zhang, C.; Shi, J. Construction and Immunogenicity of Virus-Like Particles of Feline Parvovirus from the Tiger. Viruses 2020, 12, 315. [Google Scholar] [CrossRef]
- Chang, D.; Liu, Y.; Chen, Y.; Hu, X.; Burov, A.; Puzyr, A.; Bondar, V.; Yao, L. Study of the immunogenicity of the VP2 protein of canine parvovirus produced using an improved Baculovirus expression system. BMC Veter-Res. 2020, 16, 202. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C.; Barrs, V.R. Canine parvovirus vaccination and immunisation failures: Are we far from disease eradication? Vet. Microbiol. 2020, 247, 108760. [Google Scholar] [CrossRef]



| Number | Strain |
GenBank
A ccession No. |
Isolation
Location | Isolation Time |
|---|---|---|---|---|
| 1 | F2016010 | MH329284.1 | Luoyang, China | 2016 |
| 2 | F2016015 | MH329285.1 | Luoyang, China | 2016 |
| 3 | F2016020 | MH329287.1 | Luoyang, China | 2016 |
| 4 | G7/97 | FJ005196.1 | Bari, Italy | 1997 |
| 5 | HN-002 | ON322751.1 | Zhengzhou, China | 2020 |
| 6 | HN-111 | ON322848.1 | Zhengzhou, China | 2020 |
| 7 | M52 | KC196087.1 | Montevideo, Uruguay | 2006 |
| 8 | ShangHai/3-1/2016 | KY937639.1 | ShangHai, China | 2016 |
| 9 | CPV/dog/HCM/22/2013 | LC214970.1 | Tokyo, Japan | 2013 |
| 10 | CPV-LZ2 | JQ268284.1 | Lanzhou, China | 2011 |
| 11 | CPV-2a/Racoon_dog/QHD/2/19 | MT183665.1 | Jilin, China | 2019 |
| 12 | CPV-2b/Dog/NY/LP62/08 | JN867607.1 | Ithaca, USA | 2008 |
| 13 | Shangdong5 | GU392246.1 | Shangdong, China | 2007 |
| 14 | MDQ | FJ712220.1 | Jilin, China | 2008 |
| 15 | LYT-2 | FJ712221.1 | Jilin, China | 2008 |
| 16 | Jlin/2010 | HQ883275.1 | Jilin, China | 2010 |
| 17 | Dalian1 | GU392254.1 | Dalian, China | 2009 |
| 18 | Dalian | FJ712218.1 | Dalian, China | 2008 |
| 19 | RDPV HLJ11-1 | KJ170680.1 | Jilin, China | 2011 |
| 20 | RDPV Heb10-2 | KJ170679.1 | Baerbin, China | 2010 |
| 21 | 18R217C/TH/2018 | MN127779.1 | Thailand | 2018 |
| 22 | Anyang-02 | OR551227.1 | Anyang, China | 2021 |
| 23 | Beijing-01/2018 | MK266797.1 | Beijing, China | 2017 |
| 24 | cat-2 | PP738171.1 | Zhengzhou, China | 2021 |
| 25 | cat-3 | PP738172.1 | Zhengzhou, China | 2021 |
| 26 | F2016019 | MH329286.1 | Zhengzhou, China | 2019 |
| 27 | Gigucheon | MN400978.1 | Seoul, Korea | 2017 |
| 28 | GX01 | MG924893.1 | Guilin, China | 2016 |
| 29 | HB2001 | OQ868548.1 | Baoding, China | 2020 |
| 30 | HF1 | MT614366.1 | Hefei, China | 2019 |
| 31 | HN1910 | OQ868547.1 | Zhengzhou, China | 2019 |
| 32 | HN2101 | OQ868566.1 | Zhengzhou, China | 2021 |
| 33 | HRB-CS1 | KP280068.1 | Harbin, China | 2014 |
| 34 | Luoyang-08 | OR551219.1 | Luoyan, China | 2020 |
| 35 | Luoyang-19 | OR551221.1 | Luoyan, China | 2020 |
| 36 | MHS2019 | MN908257.1 | Longyan, China | 2019 |
| 37 | Xinxiang-05 | OR551226.1 | Xinxiang, China | 2022 |
| 38 | Zhengzhou-11 | OR551224.1 | Zhengzhou, China | 2021 |
| 39 | FPV-BJ05 | MH165482.1 | Beijing, China | 2014 |
| 40 | FPV-GD(12/09/YGP) | KC473946.1 | Guangdong, China | 2012 |
| 41 | FPV-G | MG764510.1 | Chengdu, China | 1999 |
| 42 | FPV-L | MG764511.1 | Chengdu, China | 2015 |
| 43 | Felocell | EU498681.1 | Bari, Italy | 2008 |
| 44 | Purevax | EU498680.1 | Bari, Italy | 2008 |
| 45 | CU-4 | M38246.1 | Ithaca, USA | 1996 |
| Strain | Amino Acid Mutation Site | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 16 | 19 | 80 | 93 | 101 | 103 | 305 | 323 | 350 | 562 | 564 | 568 | |
| FPV ZZ202303 | D | R | R | K | K | F | V | D | D | Q | V | N | A |
| CU-4 (M38246.1) | D | R | R | K | K | I | V | D | D | Q | V | N | A |
| Felocell (EU498681.1) | D | R | R | K | K | F | V | D | D | Q | L | N | A |
| Purevax (EU498680.1) | D | R | R | K | K | I | V | D | D | Q | L | N | A |
| Gigucheon (MN400978.1) | N | K | K | K | K | F | V | D | D | Q | V | N | A |
| FPV-GD (KC473946.1) | D | R | R | K | K | F | V | D | D | R | V | N | A |
| G7/97 (FJ005196.1) | D | R | R | R | N | F | A | Y | N | Q | V | S | G |
| Case Number | Gender | Months of Age | Breed | Vaccination Status | Immunity Status |
|---|---|---|---|---|---|
| 1 | Female | 2 | British Shorthair | No | Unimmunized |
| 2 | Female | 7 | Ragdoll | Yes | Complete immunity |
| 3 | Female | 12 | Chinese civet cat | Yes | Complete immunity |
| 4 | Male | 9 | American Shorthair | Yes | Complete immunity |
| 5 | Female | 8 | British Shorthair | Yes | Complete immunity |
| 6 | Male | 6 | Chinese civet cat | Yes | Complete immunity |
| 7 | Male | 21 | Maine Coon | Yes | Complete immunity |
| Primer Name | Primer Sequence 5′-3′ | Fragment Size, bp |
|---|---|---|
| FPV-NS1-F | TGTACTTTGCGGGACTTGGT | 401 |
| FPV-NS1-R | ACATTACCCACAGCTTGTGCT | |
| FHV-gB-F | GACGTGGTGAATTATCAGC | 288 |
| FHV-gB-R | CAACTAGATTTCCACCAGGA | |
| FCV-VP1-F | CAARGGAGAAAATTCDGACGA | 321 |
| FCV-VP1-R | GTATTTWAGCACGTTAGCGCAGGT | |
| FIPV-N-F | GGCAACCCGATGTTTAAAACTGG | 214 |
| FIPV-N-R | CACTAGATCCAGACGTTAGCTC | |
| FPV-VP2-JDF | GGAACTAGTGGCACACCAACA | 386 |
| FPV-VP2-JDR | GGCCCTTGTGTAGACGCTT | |
| FPV-VP2-F | GTATACCATATAACAAACCTTC | 1903 |
| FPV-VP2-R | ATGAGTGATGGAGCAGTTCAAC | |
| Q-FPV-VP2-F | GTATACCATATAACAAACCTTC | 130 |
| Q-FPV-VP2-R | TCATAGCTGCTGGAGTAAATGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.-Y.; Zhao, S.-B.; Wang, S.-Y.; Du, M.-H.; Ming, S.-L.; Zeng, L. Feline Panleukopenia Virus ZZ202303 Strain: Molecular Characterization and Structural Implications of the VP2 Gene Phylogenetic Divergence. Int. J. Mol. Sci. 2025, 26, 4573. https://doi.org/10.3390/ijms26104573
Wang M-Y, Zhao S-B, Wang S-Y, Du M-H, Ming S-L, Zeng L. Feline Panleukopenia Virus ZZ202303 Strain: Molecular Characterization and Structural Implications of the VP2 Gene Phylogenetic Divergence. International Journal of Molecular Sciences. 2025; 26(10):4573. https://doi.org/10.3390/ijms26104573
Chicago/Turabian StyleWang, Ming-Yang, Shi-Bo Zhao, Shu-Yi Wang, Meng-Hua Du, Sheng-Li Ming, and Lei Zeng. 2025. "Feline Panleukopenia Virus ZZ202303 Strain: Molecular Characterization and Structural Implications of the VP2 Gene Phylogenetic Divergence" International Journal of Molecular Sciences 26, no. 10: 4573. https://doi.org/10.3390/ijms26104573
APA StyleWang, M.-Y., Zhao, S.-B., Wang, S.-Y., Du, M.-H., Ming, S.-L., & Zeng, L. (2025). Feline Panleukopenia Virus ZZ202303 Strain: Molecular Characterization and Structural Implications of the VP2 Gene Phylogenetic Divergence. International Journal of Molecular Sciences, 26(10), 4573. https://doi.org/10.3390/ijms26104573





