Liensinine Prevents Acute Myocardial Ischemic Injury via Inhibiting the Inflammation Response Mediated by the Wnt/β-Catenin Signaling Pathway
Abstract
1. Introduction
2. Results
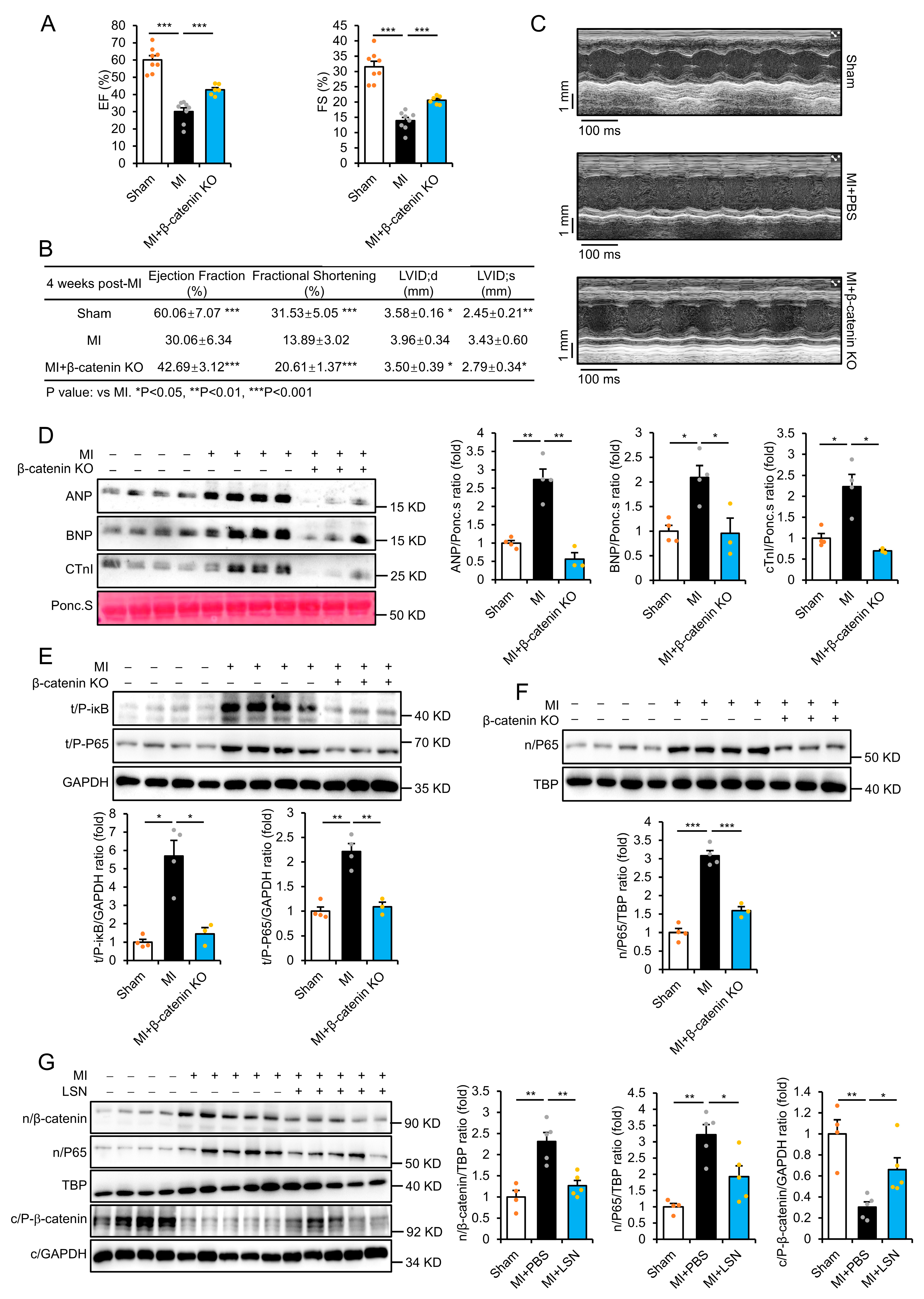
2.1. LSN Protects Against MI-Induced Cardiac Ischemic Injury in Mice
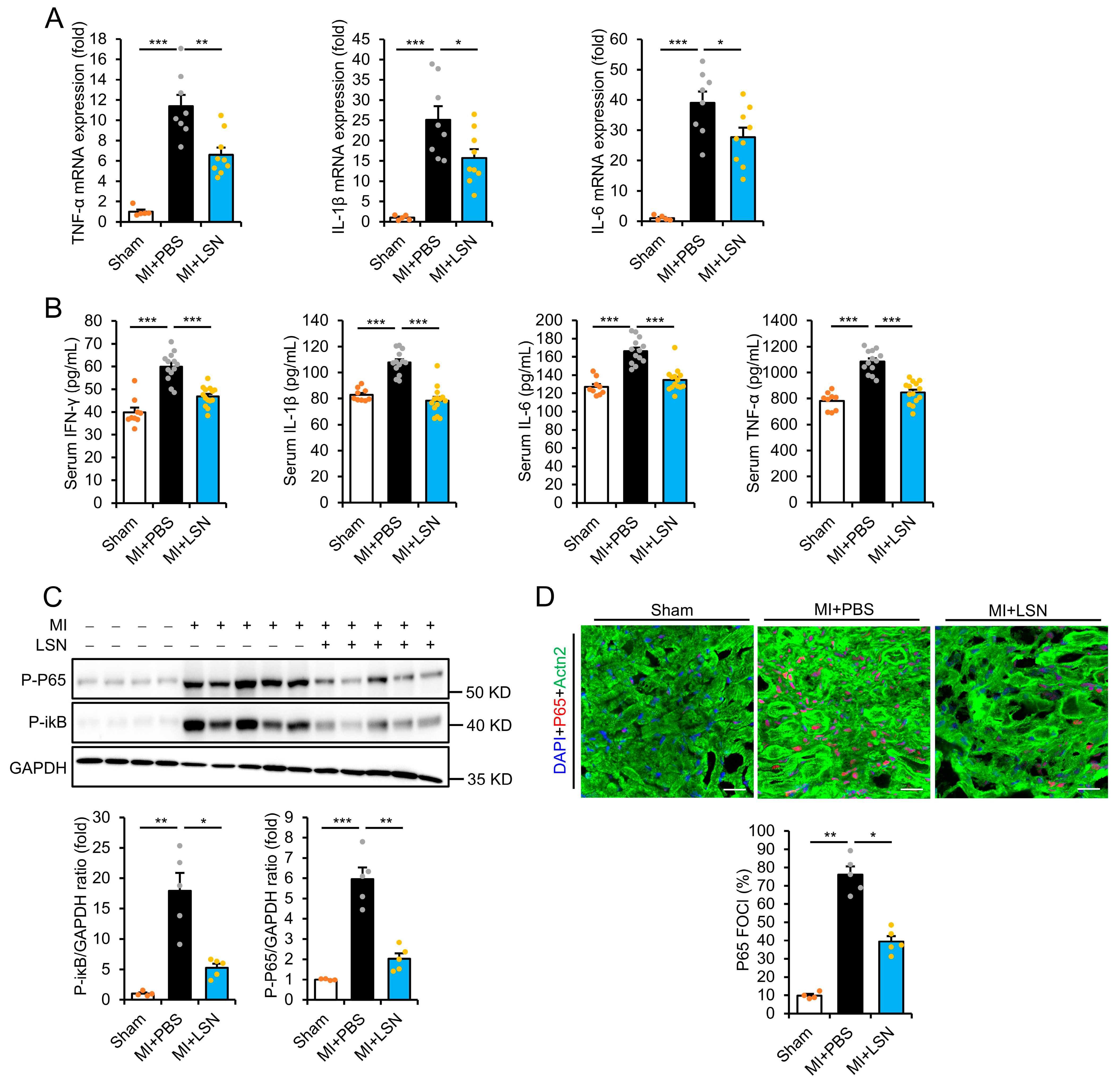
2.2. LSN Prevents MI-Induced Early Cardiac Inflammatory Response
2.3. β-Catenin Deletion Prevents MI-Induced Cardiac Injury and Inflammatory Response
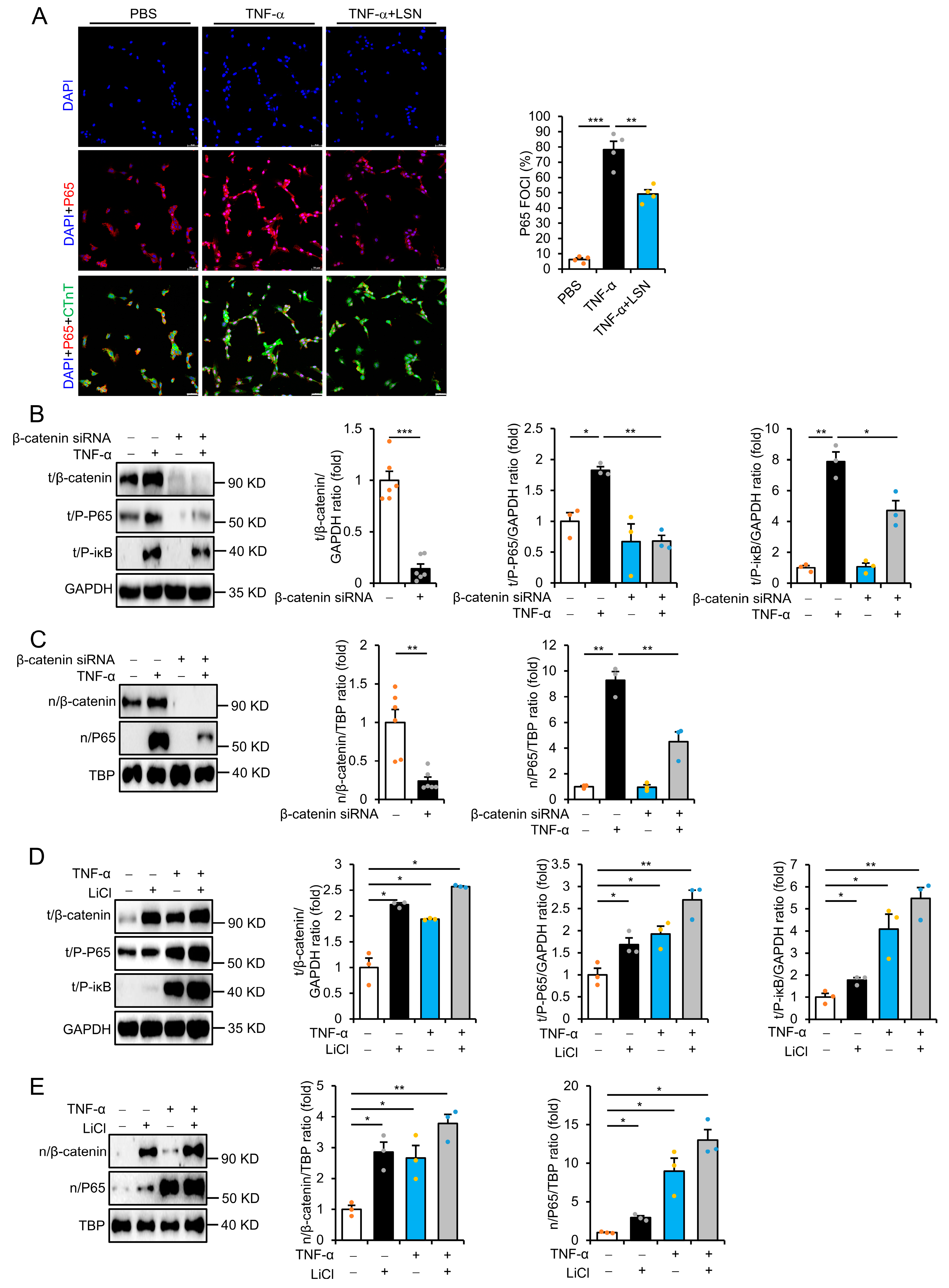
2.4. LSN Prevents Inflammatory Response via Inhibiting Wnt/β-Catenin Signaling
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Two-Dimensional Echocardiography
4.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. Cell Culture and siRNA Knockdown
4.5. Histology
4.6. Immunofluorescence
4.7. Western Blot Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| β-MHC | β myosin heavy chain |
| ANP | Atrial natriuretic peptide |
| BNP | Brain natriuretic peptide |
| CTnI | Cardiac troponin I |
| Col-I | Collagen-I |
| Col-III | Collagen-III |
| EF | Ejection fraction |
| FS | Fractional shortening |
| LSN | Liensinine |
| MI | Myocardial infarction |
| TNF-α | Tumor necrosis factor α |
References
- Wu, X.; Reboll, M.R.; Korf-Klingebiel, M.; Wollert, K.C. Angiogenesis after acute myocardial infarction. Cardiovasc. Res. 2021, 117, 1257–1273. [Google Scholar] [CrossRef]
- Neri, M.; Fineschi, V.; Di Paolo, M.; Pomara, C.; Riezzo, I.; Turillazzi, E.; Cerretani, D. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr. Vasc. Pharmacol. 2015, 13, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.; Irei, J.; Lozano-Gerona, J.; Vanapruks, S.; Bishop, T.; Boisvert, W.A. Macrophages in cardiac remodelling after myocardial infarction. Nat. Rev. Cardiol. 2023, 20, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, L.; Wang, S.; Cheng, H.; Xu, L.; Pei, G.; Wang, Y.; Fu, C.; Jiang, Y.; He, C.; et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct. Target. Ther. 2022, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Barandon, L.; Casassus, F.; Leroux, L.; Moreau, C.; Allières, C.; Lamazière, J.M.; Dufourcq, P.; Couffinhal, T.; Duplàa, C. Secreted frizzled-related protein-1 improves postinfarction scar formation through a modulation of inflammatory response. Arter. Thromb. Vasc. Biol. 2011, 31, e80–e87. [Google Scholar] [CrossRef]
- Meyer, I.S.; Jungmann, A.; Dieterich, C.; Zhang, M.; Lasitschka, F.; Werkmeister, S.; Haas, J.; Müller, O.J.; Boutros, M.; Nahrendorf, M.; et al. The cardiac microenvironment uses non-canonical WNT signaling to activate monocytes after myocardial infarction. EMBO Mol. Med. 2017, 9, 1279–1293. [Google Scholar] [CrossRef]
- Lin, J.C.; Chang, R.L.; Chen, Y.F.; Yang, J.J.; Baskaran, R.; Chung, L.C.; Chen, R.J.; Day, C.H.; Padma, V.V.; Huang, C.-Y. β-Catenin overexpression causes an increase in inflammatory cytokines and NF-κB activation in cardiomyocytes. Cell. Mol. Biol. 2016, 63, 17–22. [Google Scholar] [CrossRef]
- Meyer, I.S.; Li, X.; Meyer, C.; Voloshanenko, O.; Pohl, S.; Boutros, M.; Katus, H.A.; Frey, N.; Leuschner, F. Blockade of Wnt Secretion Attenuates Myocardial Ischemia-Reperfusion Injury by Modulating the Inflammatory Response. Int. J. Mol. Sci. 2022, 23, 12252. [Google Scholar] [CrossRef]
- Fan, J.; Qiu, L.; Shu, H.; Ma, B.; Hagenmueller, M.; Riffel, J.H.; Meryer, S.; Zhang, M.; Hardt, S.E.; Wang, L.; et al. Recombinant frizzled1 protein attenuated cardiac hypertrophy after myocardial infarction via the canonical Wnt signaling pathway. Onctarget 2018, 9, 3069–3080. [Google Scholar] [CrossRef]
- Yang, D.; Fu, W.; Li, L.; Xia, X.; Liao, Q.; Yue, R.; Chen, H.; Chen, X.; An, S.; Zeng, C.; et al. Therapeutic effect of a novel Wnt pathway inhibitor on cardiac regeneration after myocardial infarction. Clin. Sci. 2017, 131, 2919–2932. [Google Scholar] [CrossRef]
- Kirsch, N.; Chang, L.S.; Koch, S.; Glinka, A.; Dolde, C.; Colozza, G.; Benitez, M.D.J.; de Robertis, E.M.; Niehrs, C. Angiopoietin-like 4 Is a Wnt Signaling Antagonist that Promotes LRP6 Turnover. Dev. Cell 2017, 43, 71–82.E6. [Google Scholar] [CrossRef] [PubMed]
- Naito, A.T.; Sumida, T.; Nomura, S.; Liu, M.L.; Higo, T.; Nakagawa, A.; Okada, K.; Sakai, T.; Hashimoto, A.; Hara, Y.; et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell 2012, 149, 1298–1313. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.; Zhang, T.; Gao, X.; Tao, B.; Xing, H.; Zhuang, Z.; Dardik, A.; Kyriakides, T.R.; Goodwin, J.E. Loss of endothelial glucocorticoid receptor promotes angiogenesis via upregulation of Wnt/β-catenin pathway. Angiogenesis 2021, 24, 631–645. [Google Scholar] [CrossRef]
- Naito, A.T.; Shiojima, I.; Akazawa, H.; Hidaka, K.; Morisaki, T.; Kikuchi, A.; Komuro, I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19812–19817. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.B.; Wang, W.E.; Zeng, C.Y. Wnt signaling pathways in myocardial infarction and the therapeutic effects of Wnt pathway inhibitors. Acta Pharmacol. Sin. 2019, 40, 9–12. [Google Scholar] [CrossRef]
- Meyer, I.S.; Leuschner, F. The role of Wnt signaling in the healing myocardium: A focus on cell specificity. Basic Res. Cardiol. 2018, 113, 44. [Google Scholar] [CrossRef]
- Wo, D.; Peng, J.; Ren, D.N.; Qiu, L.; Chen, J.; Zhu, Y.; Yan, Y.; Yan, H.; Wu, J.; Ma, E.; et al. Opposing Roles of Wnt Inhibitors IGFBP-4 and Dkk1 in Cardiac Ischemia by Differential Targeting of LRP5/6 and β-catenin. Circulation 2016, 134, 1991–2007. [Google Scholar] [CrossRef]
- Matteucci, M.; Casieri, V.; Gabisonia, K.; Aquaro, G.D.; Agostini, S.; Pollio, G.; Diamanti, D.; Rossi, M.; Travagli, M.; Porcari, V.; et al. Magnetic resonance imaging of infarct-induced canonical wingless/integrated (Wnt)/β-catenin/T-cell factor pathway activation, in vivo. Cardiovasc. Res. 2016, 112, 645–655. [Google Scholar] [CrossRef][Green Version]
- He, J.; Wo, D.; Ma, E.; Wang, Q.; Chen, J.; Gao, Q.; Zhao, Q.; Shen, F.; Peng, J.; Zhu, W.; et al. Huoxin pill prevents excessive inflammation and cardiac dysfunction following myocardial infarction by inhibiting adverse Wnt/β-catenin signaling activation. Phytomedicine 2022, 104, 154293. [Google Scholar] [CrossRef]
- Wang, C.; Zou, K.; Diao, Y.; Zhou, C.; Zhou, J.; Yang, Y.; Zeng, Z. Liensinine alleviates LPS-induced acute lung injury by blocking autophagic flux via PI3K/AKT/mTOR signaling pathway. Biomed. Pharmacother. 2023, 168, 115813. [Google Scholar] [CrossRef]
- Wang, G.; Ma, F.; Xie, K.; Li, X.; Tan, X.; Xia, Y.; Wang, Y.; Dong, J. Liensinine alleviates mouse intestinal injury induced by sepsis through inhibition of oxidative stress, inflammation, and cell apoptosis. Int. Immunopharmacol. 2024, 127, 111335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, H.; Xu, Z.; Zhang, X.; Tan, X.; He, N.; Shen, J.; Dong, J. Liensinine pretreatment reduces inflammation, oxidative stress, apoptosis, and autophagy to alleviate sepsis acute kidney injury. Int. Immunopharmacol. 2023, 122, 110563. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Nian, M.; Lee, P.; Khaper, N.; Liu, P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 2004, 94, 1543–1553. [Google Scholar] [CrossRef]
- Jones, W.K.; Brown, M.; Wilhide, M.; He, S.; Ren, X. NF-kappaB in cardiovascular disease: Diverse and specific effects of a “general” transcription factor? Cardiovasc. Toxicol. 2005, 5, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Bartekova, M.; Radosinska, J.; Jelemensky, M.; Dhalla, N.S. Role of cytokines and inflammation in heart function during health and disease. Heart Fail. Rev. 2018, 23, 733–758. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The immune system and cardiac repair. Pharmacol. Res. 2008, 58, 88–111. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Liu, K.; Han, H. The Role of NF-κB in Myocardial Ischemia/Reperfusion Injury. Curr. Protein Pept. Sci. 2022, 23, 535–547. [Google Scholar]
- Shen, F.; Wu, C.; Zhong, X.; Ma, E.; Peng, J.; Zhu, W.; Wo, D.; Ren, D. Liensinine prevents ischemic injury following myocardial infarction via inhibition of Wnt/β-catenin signaling activation. Biomed. Pharmacother. 2023, 162, 114675. [Google Scholar] [CrossRef]
- Yang, M.; Wu, H.; Qian, H.; Li, D.; Xu, H.; Chen, J.; Zhong, J.; Wu, W.; Yang, H.; Chen, X.; et al. Linggui Zhugan decoction delays ventricular remodeling in rats with chronic heart failure after myocardial infarction through the Wnt/β-catenin signaling pathway. Phytomedicine 2023, 120, 155026. [Google Scholar] [CrossRef]
- Wang, L.; Du, A.; Lu, Y.; Zhao, Y.; Qiu, M.; Su, Z.; Shu, H.; Shen, H.; Sun, W.; Kong, X. Peptidase Inhibitor 16 Attenuates Left Ventricular Injury and Remodeling After Myocardial Infarction by Inhibiting the HDAC1-Wnt3a-β-Catenin Signaling Axis. J. Am. Heart Assoc. 2023, 12, e028866. [Google Scholar] [CrossRef] [PubMed]
- Haybar, H.; Khodadi, E.; Shahrabi, S. Wnt/β-catenin in ischemic myocardium: Interactions and signaling pathways as a therapeutic target. Heart Fail. Rev. 2019, 24, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Wen, L.; Li, J.; Wang, Y.; Ni, B.; Wang, R.; Wang, X.; Sun, M.; Fan, H.; Mao, X. LiCl inhibits PRRSV infection by enhancing Wnt/β-catenin pathway and suppressing inflammatory responses. Antivir. Res. 2015, 117, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, A.; Manganelli, V.; Riitano, G.; Caissutti, D.; Longo, A.; Garofalo, T.; Sorice, M.; Misasi, R. Advances in the Pathophysiology of Thrombosis in Antiphospholipid Syndrome: Molecular Mechanisms and Signaling through Lipid Rafts. J. Clin. Med. 2023, 12, 891. [Google Scholar] [CrossRef]
- Hart, N.R. A theoretical model of dietary lipid variance as the origin of primary ciliary dysfunction in preeclampsia. Front. Mol. Biosci. 2023, 10, 1173030. [Google Scholar] [CrossRef]
- Riitano, G.; Manganelli, V.; Capozzi, A.; Matti, V.; Recalchi, S.; Martellucci, S.; Longo, A.; Misasi, R.; Garofalo, T.; Sorice, M. LRP6 mediated signal transduction pathway triggered by tissue plasminogen activator acts through lipid rafts in neuroblastoma cells. J. Cell Commun. Signal. 2020, 14, 315–323. [Google Scholar] [CrossRef]
- Hong, S.; Feng, L.; Yang, Y.; Jiang, H.; Hou, X.; Guo, P.; Marlow, F.L.; Stanley, P.; Wu, P. In Situ Fucosylation of the Wnt Co-receptor LRP6 Increases Its Endocytosis and Reduces Wnt/β-Catenin Signaling. Cell Chem. Biol. 2020, 27, 1140–1150. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, E.; Zhang, J.; Tang, Y.; Fang, X.; Wang, C.; Wu, C.; Zhu, W.; Wo, D.; Ren, D.-n. Liensinine Prevents Acute Myocardial Ischemic Injury via Inhibiting the Inflammation Response Mediated by the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 4566. https://doi.org/10.3390/ijms26104566
Ma E, Zhang J, Tang Y, Fang X, Wang C, Wu C, Zhu W, Wo D, Ren D-n. Liensinine Prevents Acute Myocardial Ischemic Injury via Inhibiting the Inflammation Response Mediated by the Wnt/β-Catenin Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(10):4566. https://doi.org/10.3390/ijms26104566
Chicago/Turabian StyleMa, En, Jingwei Zhang, Yirong Tang, Xue Fang, Canran Wang, Celiang Wu, Weidong Zhu, Da Wo, and Dan-ni Ren. 2025. "Liensinine Prevents Acute Myocardial Ischemic Injury via Inhibiting the Inflammation Response Mediated by the Wnt/β-Catenin Signaling Pathway" International Journal of Molecular Sciences 26, no. 10: 4566. https://doi.org/10.3390/ijms26104566
APA StyleMa, E., Zhang, J., Tang, Y., Fang, X., Wang, C., Wu, C., Zhu, W., Wo, D., & Ren, D.-n. (2025). Liensinine Prevents Acute Myocardial Ischemic Injury via Inhibiting the Inflammation Response Mediated by the Wnt/β-Catenin Signaling Pathway. International Journal of Molecular Sciences, 26(10), 4566. https://doi.org/10.3390/ijms26104566




