Effects of Inorganic Salts on Phase Separation in Aqueous Solutions of Poly(ethylene glycol)
Abstract
1. Introduction
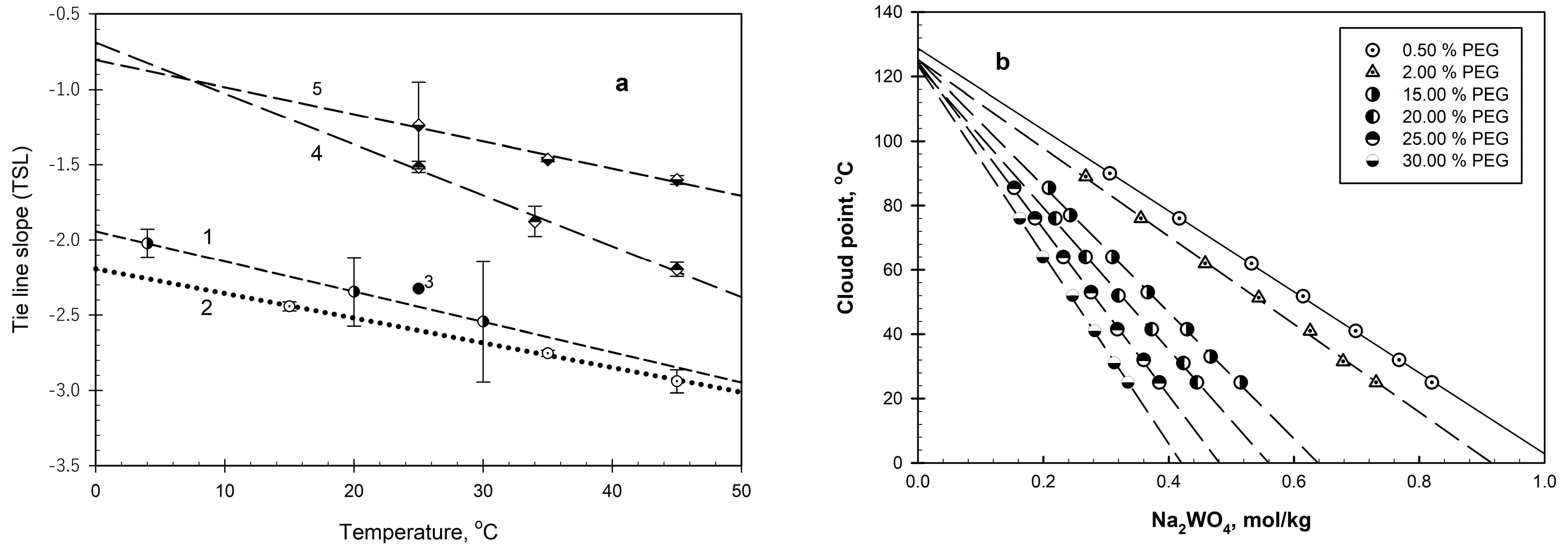
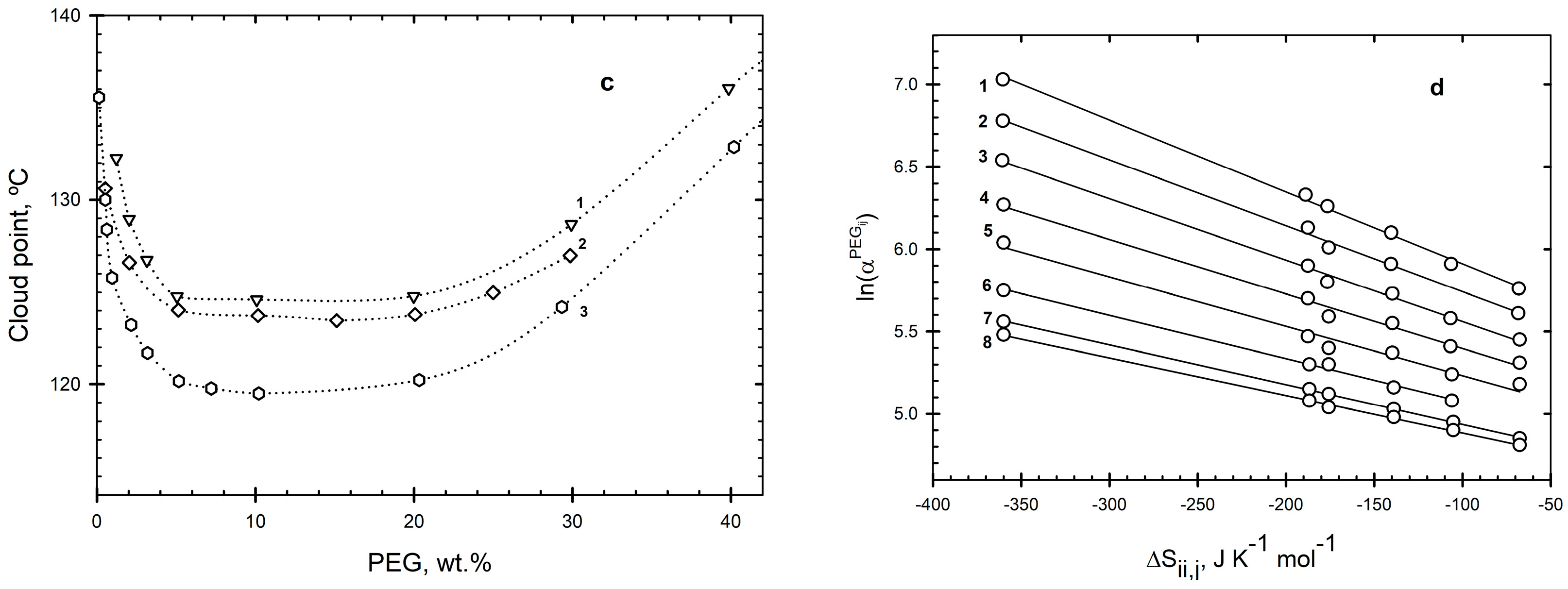
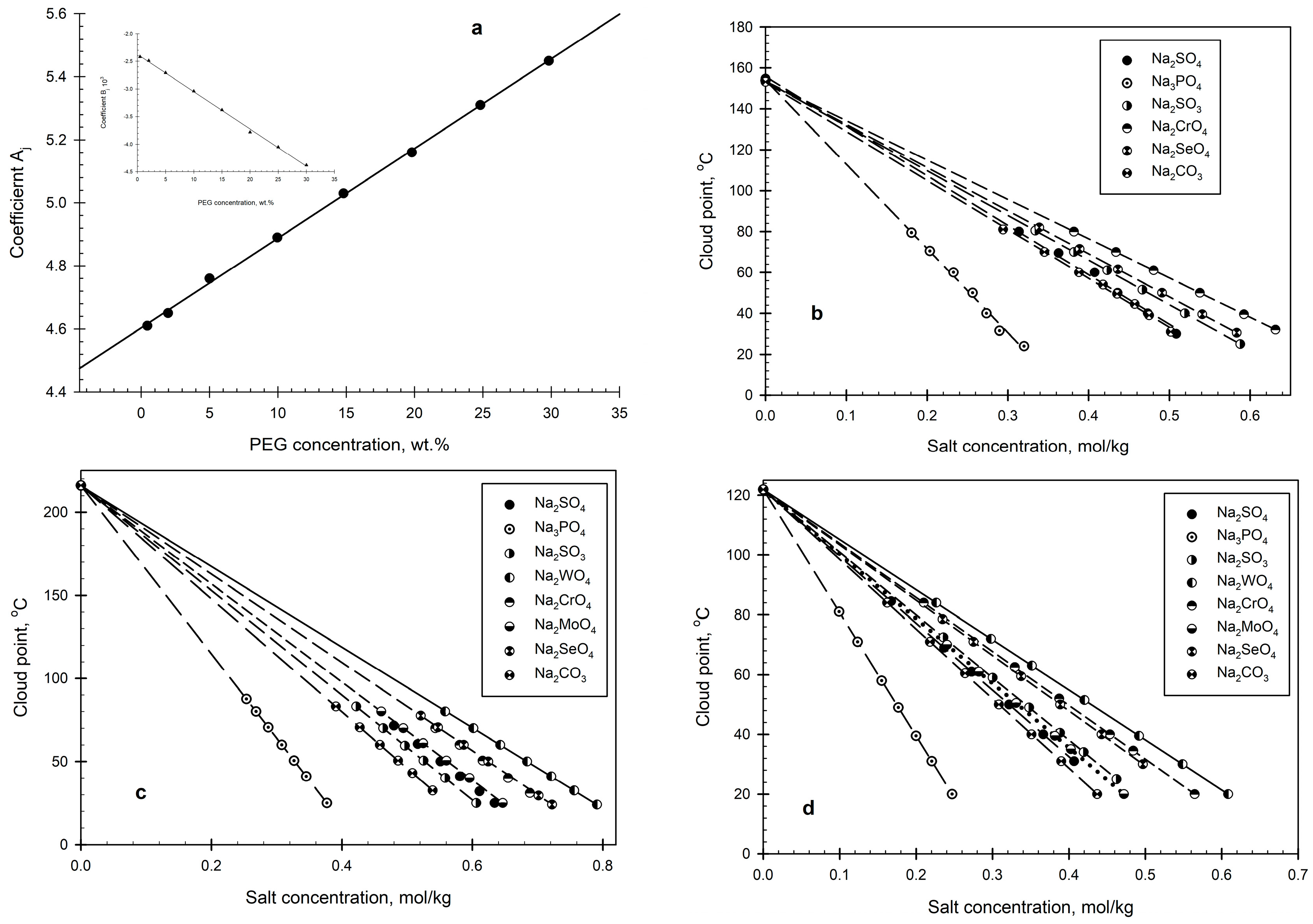
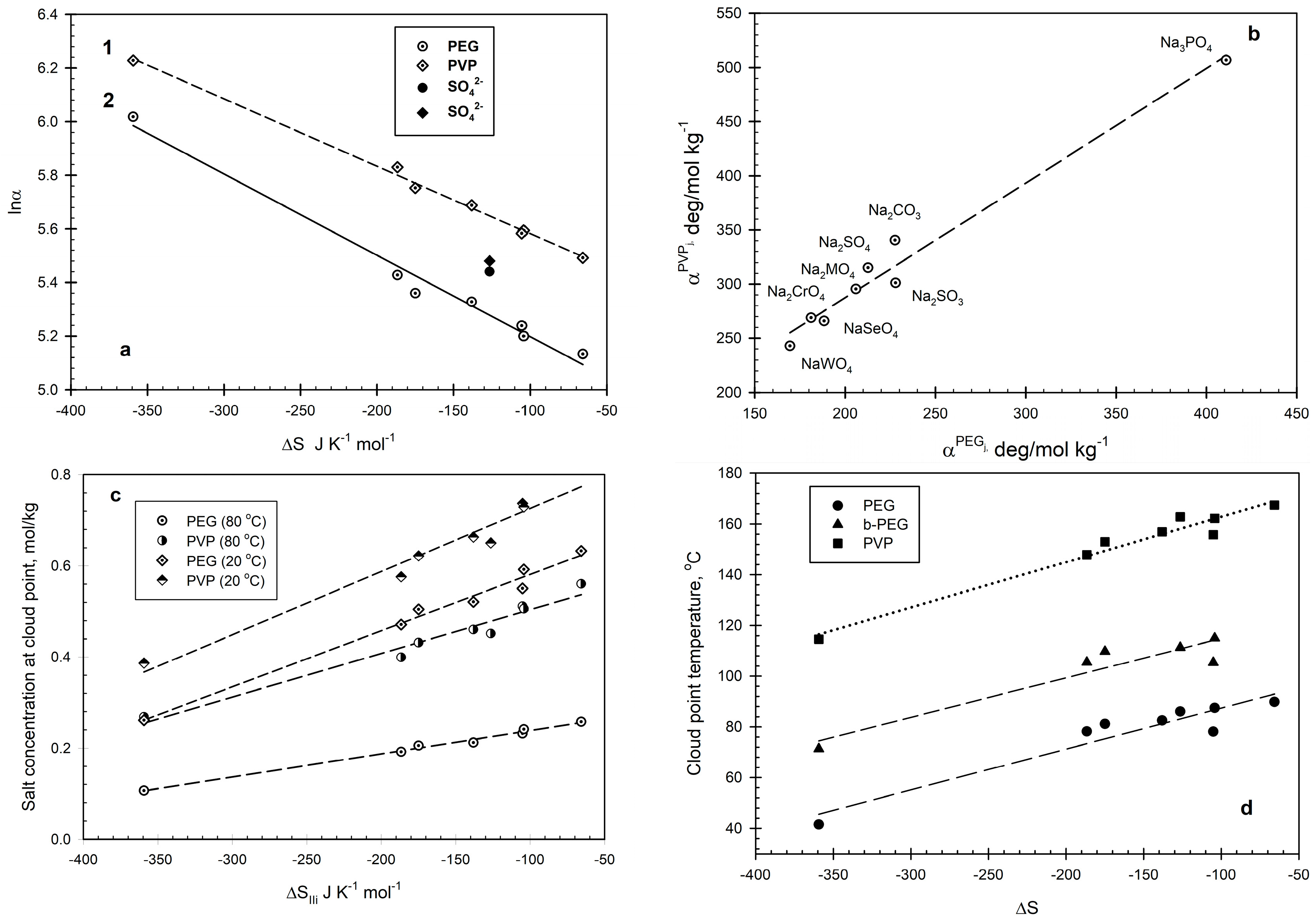
2. Results and Discussion
2.1. PEG–Salt–Water Systems
2.2. PVP–Salt–Water and Branched-PEG–Salt–Water Systems
3. Materials and Methods
3.1. Materials
3.2. Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Titus, A.R.; Madeira, P.P.; Uversky, V.N.; Zaslavsky, B.Y. Correlation of Solvent Interaction Analysis Signatures with Thermodynamic Properties and In Silico Calculations of the Structural Effects of Point Mutations in Two Proteins. Int. J. Mol. Sci. 2024, 25, 9652. [Google Scholar] [CrossRef]
- Uversky, V.N.; Madeira, P.P.; Zaslavsky, B.Y. What Can Be Learned from the Partitioning Behavior of Proteins in Aqueous Two-Phase Systems? Int. J. Mol. Sci. 2024, 25, 6339. [Google Scholar] [CrossRef] [PubMed]
- Zaslavsky, B.Y.; Uversky, V.N.; Chait, A. Analytical applications of partitioning in aqueous two-phase systems: Exploring protein structural changes and protein-partner interactions in vitro and in vivo by solvent interaction analysis method. Biochim. Biophys. Acta 2016, 1864, 622–644. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Chait, A.; Hafron, J.M.; Kernen, K.M.; Manickam, K.; Stephenson, A.J.; Wagner, M.; Zhu, H.; Kestranek, A.; Zaslavsky, B.; et al. The Single-parameter, Structure-based IsoPSA Assay Demonstrates Improved Diagnostic Accuracy for Detection of Any Prostate Cancer and High-grade Prostate Cancer Compared to a Concentration-based Assay of Total Prostate-specific Antigen: A Preliminary Report. Eur. Urol. 2017, 72, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Ananthapadmanabhan, K.; Goddard, E. Aqueous biphase formation in polyethylene oxide-inorganic salt systems. Langmuir 1987, 3, 25–31. [Google Scholar] [CrossRef]
- Mishima, K.; Nakatani, K.; Nomiyama, T.; Matsuyama, K.; Nagatani, M.; Nishikawa, H. Liquid-liquid equilibria of aqueous two-phase systems containing polyethylene glycol and dipotassium hydrogenphosphate. Fluid Phase Equilibria 1995, 107, 269–276. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Sadeghi, R. Liquid–liquid equilibria of aqueous two-phase systems containing polyethylene glycol and sodium dihydrogen phosphate or disodium hydrogen phosphate: Experiment and correlation. Fluid Phase Equilibria 2001, 181, 95–112. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Sadeghi, R.; Hamidi, A.A. Liquid–liquid equilibria of an aqueous two-phase system containing polyethylene glycol and sodium citrate: Experiment and correlation. Fluid Phase Equilibria 2004, 219, 149–155. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Sadeghi, R. Measurement and correlation of liquid–liquid equilibria of the aqueous two-phase system polyvinylpyrrolidone–sodium dihydrogen phosphate. Fluid Phase Equilibria 2002, 203, 177–191. [Google Scholar] [CrossRef]
- Perumalsamy, M.; Batcha, M.I. Synergistic extraction of bovine serum albumin using polyethylene glycol based aqueous biphasic system. Process Biochem. 2011, 46, 494–497. [Google Scholar] [CrossRef]
- Rogers, R.D.; Bond, A.H.; Bauer, C.B.; Zhang, J.; Griffin, S.T. Metal ion separations in polyethylene glycol-based aqueous biphasic systems: Correlation of partitioning behavior with available thermodynamic hydration data. J. Chromatogr. B Biomed. Appl. 1996, 680, 221–229. [Google Scholar] [CrossRef]
- An, B.; Zhang, W.; Han, J.; Wang, Y.; Ni, L. The Cloud Point Behavior and Liquid–Liquid Equilibrium of Poly (Ethylene Glycol)–Block-Poly (Propylene Glycol)–Block-Poly (Ethylene Glycol) with Five Salting-Out Salts (K2SO4, K2CO3, KCl, KNO3, KBr) at 283.15 K. J. Solut. Chem. 2016, 45, 1811–1825. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Seifi-Aghjekohal, P. Liquid–liquid equilibria of aqueous two-phase systems containing polyvinylpyrrolidone and tripotassium phosphate or dipotassium hydrogen phosphate: Experiment and correlation. Calphad 2007, 31, 553–559. [Google Scholar] [CrossRef]
- Cao, Q.; Li, S.; He, C.; Li, K.; Liu, F. Extraction and determination of papaverin in pericarpium papaveris using aqueous two-phase system of poly (ethylene glycol)–(NH4)2SO4 coupled with high-performance liquid chromatography. Anal. Chim. Acta 2007, 590, 187–194. [Google Scholar] [CrossRef]
- Gonsalves, R.T.; Farias, F.O.; Mafra, M.R.; Igarashi-Mafra, L. Aqueous biphasic systems as a suitable route to remove and concentrate parabens from aqueous media. Ind. Eng. Chem. Res. 2020, 59, 21882–21893. [Google Scholar] [CrossRef]
- Jorge, A.M.; Pereira, J.F. Aqueous two-phase systems–versatile and advanced (bio) process engineering tools. Chem. Commun. 2024, 60, 12144–12168. [Google Scholar] [CrossRef] [PubMed]
- Bonnassieux, S.; Pandya, R.; Skiba, D.A.; Degoulange, D.; Petit, D.; Seem, P.; Cowburn, R.P.; Gallant, B.M.; Grimaud, A. Revisiting the driving force inducing phase separation in PEG–phosphate aqueous biphasic systems. Faraday Discuss. 2024, 253, 181–192. [Google Scholar] [CrossRef]
- Peng, Q.; Li, Z.; Li, Y. Thermodynamics of potassium hydrogen phosphate-potassium dihydrogen phosphate-polyethylene glycol aqueous two-phase systems. Fluid Phase Equilibria 1994, 95, 341–357. [Google Scholar] [CrossRef]
- Großmann, C.; Tintinger, R.; Zhu, J.; Maurer, G. Aqueous Two-Phase Systems of Poly (ethylene glycol) and Di-Potassium Hydrogen Phosphate with and without partitioning Biomolecules–Experimental Results and Modeling of Thermodynamic Properties. Berichte Bunsenges. Phys. Chem. 1995, 99, 700–712. [Google Scholar] [CrossRef]
- Amaresh, S.P.; Murugesan, S.; Regupathi, I.; Murugesan, T. Liquid–Liquid Equilibrium of Poly (ethylene glycol) 4000+ Diammonium Hydrogen Phosphate+ Water at Different Temperatures. J. Chem. Eng. Data 2008, 53, 1574–1578. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Tolouei, S. Liquid-liquid equilibria of aqueous two-phase systems containing polyethylene glycol 4000 and di-potassium sodium tartrate, or di-potassium oxalate. Experiment and correlation. Calphad 2008, 32, 655–660. [Google Scholar] [CrossRef]
- Regupathi, I.; Murugesan, S.; Amaresh, S.P.; Govindarajan, R.; Thanabalan, M. Densities and viscosities of poly (ethylene glycol) 4000+ diammonium hydrogen phosphate+ water systems. J. Chem. Eng. Data 2009, 54, 1100–1106. [Google Scholar] [CrossRef]
- Sadeghi, R.; Jamehbozorg, B. Effect of temperature on the salting-out effect and phase separation in aqueous solutions of sodium di-hydrogen phosphate and poly (propylene glycol). Fluid. Phase Equilibria 2008, 271, 13–18. [Google Scholar] [CrossRef]
- Xie, X.; Han, J.; Wang, Y.; Yan, Y.; Yin, G.; Guan, W. Measurement and correlation of the phase diagram data for PPG400+(K3PO4, K2CO3, and K2HPO4)+ H2O aqueous two-phase systems at T = 298.15 K. J. Chem. Eng. Data 2010, 55, 4741–4745. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Hosseinpour-Hashemi, V. (Liquid+ liquid) equilibrium of the ternary aqueous system containing poly ethylene glycol dimethyl ether 2000 and tri-potassium citrate at different temperatures. J. Chem. Thermodyn. 2012, 48, 75–83. [Google Scholar] [CrossRef]
- Duraiayya, R.; Arumugam, S.; Settu, S. Equilibrium phase behavior of poly (ethylene glycol) 4000 and biodegradable salts at various temperatures [(20, 30, and 40) C]. J. Chem. Eng. Data 2012, 57, 1112–1117. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Nikjoo, D. Phase Diagrams for Liquid–Liquid and Liquid− Solid Equilibrium of the Ternary Poly (ethylene glycol) Dimethyl Ether 2000+ Sodium Carbonate+ Water System. J. Chem. Eng. Data 2009, 54, 2918–2922. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Nikjoo, D. Liquid–Liquid and Liquid–Liquid–Solid Equilibrium of the Poly (ethylene glycol) Dimethyl Ether 2000+ Sodium Sulfate+ Water System. J. Chem. Eng. Data 2008, 53, 2666–2670. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Nemati-Kande, E. Thermodynamic studies on the complete phase diagram of aqueous two phase system containing polyethylene glycol dimethyl ether 2000 and di-potassium hydrogen phosphate at different temperatures. Calphad 2011, 35, 165–172. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Hosseinpour-Hashemi, V. Effect of temperature on the aqueous two-phase system containing poly (ethylene glycol) dimethyl ether 2000 and dipotassium oxalate. J. Chem. Eng. Data 2012, 57, 532–540. [Google Scholar] [CrossRef]
- Xie, X.; Yan, Y.; Han, J.; Wang, Y.; Yin, G.; Guan, W. Liquid− liquid equilibrium of aqueous two-phase systems of PPG400 and biodegradable salts at temperatures of (298.15, 308.15, and 318.15) K. J. Chem. Eng. Data 2010, 55, 2857–2861. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Emamian, S.; Hamzehzadeh, S. Effect of temperature on the phase equilibrium of the aqueous two-phase poly (propylene glycol)+ tripotassium citrate system. J. Chem. Eng. Data 2008, 53, 456–461. [Google Scholar] [CrossRef]
- Sadeghi, R.; Jamehbozorg, B. The salting out effect and phase separation in aqueous solutions of sodium phosphate salts and polypropylene glycol. Fluid Phase Equilibria 2009, 280, 68–75. [Google Scholar] [CrossRef]
- Jimenez, Y.P.; Galleguillos, H.R. (Liquid+ liquid) equilibrium of (NaNO3+ PEG 4000+ H2O) ternary system at different temperatures. J. Chem. Thermodyn. 2011, 43, 1573–1578. [Google Scholar] [CrossRef]
- Jimenez, Y.P.; Taboada, M.E.; Graber, T.A.; Galleguillos, H.R. Measurement and modeling of density and viscosity of the NaClO4+ H2O+ poly (ethylene glycol) system at various temperatures. Fluid Phase Equilibria 2012, 334, 22–29. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Coutinho, J.A.P. Aqueous Two-Phase Systems. In Liquid-Phase Extraction; Poole, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 157–182. [Google Scholar]
- Zaslavsky, B.Y. Aqueous Two-Phase Partitioning: Physical Chemistry and Bioanalytical Applications; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Melander, W.; Horváth, C. Salt effects on hydrophobic interactions in precipitation and chromatography of proteins: An interpretation of the lyotropic series. Arch. Biochem. Biophys. 1977, 183, 200–215. [Google Scholar] [CrossRef]
- Thiyagarajan, P.; Chaiko, D.; Hjelm Jr, R. A neutron scattering study of poly (ethylene glycol) in electrolyte solutions. Macromolecules 1995, 28, 7730–7736. [Google Scholar] [CrossRef]
- Bae, Y.; Lambert, S.; Soane, D.; Prausnitz, J.M. Cloud-point curves of polymer solutions from thermooptical measurements. Macromolecules 1991, 24, 4403–4407. [Google Scholar] [CrossRef]
- Krestov, G.A. Thermodynamics of Solvation; Ellis Horwood: New York, NY, USA, 1991. [Google Scholar]
- Güner, A.; Ataman, M. Effects of inorganic salts on the properties of aqueous poly (vinylpyrrolidone) solutions. Colloid Polym. Sci. 1994, 272, 175–180. [Google Scholar] [CrossRef]
- Karlström, G. A new model for upper and lower critical solution temperatures in poly (ethylene oxide) solutions. J. Phys. Chem. 1985, 89, 4962–4964. [Google Scholar] [CrossRef]
- Titus, A.R.; Madeira, P.P.; Ferreira, L.A.; Chernyak, V.Y.; Uversky, V.N.; Zaslavsky, B.Y. Mechanism of Phase Separation in Aqueous Two-Phase Systems. Int. J. Mol. Sci. 2022, 23, 14366. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.; Madeira, P.P.; Breydo, L.; Reichardt, C.; Uversky, V.N.; Zaslavsky, B.Y. Role of solvent properties of aqueous media in macromolecular crowding effects. J. Biomol. Struct. Dyn. 2016, 34, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Yoshioka, S.; Aso, Y.; Kojima, S. Water mobility in aqueous solutions of macromolecular pharmaceutical excipients measured by oxygen-17 nuclear magnetic resonance. Chem. Pharm. Bull. 1995, 43, 1221–1223. [Google Scholar] [CrossRef]
- Zaslavsky, B.Y.; Miheeva, L.M.; Rodnikova, M.N.; Spivak, G.V.; Harkin, V.S.; Mahmudov, A.U. Dielectric properties of water in the coexisting phases of aqueous polymeric two-phase systems. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 2857–2865. [Google Scholar]
- Ferreira, L.A.; Uversky, V.N.; Zaslavsky, B.Y. Effects of the Hofmeister series of sodium salts on the solvent properties of water. Phys. Chem. Chem. Phys. 2017, 19, 5254–5261. [Google Scholar] [CrossRef]
- Freire, M.G.; Claudio, A.F.M.; Araujo, J.M.; Coutinho, J.A.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madeira, P.P.; Uversky, V.N.; Zaslavsky, B.Y. Effects of Inorganic Salts on Phase Separation in Aqueous Solutions of Poly(ethylene glycol). Int. J. Mol. Sci. 2025, 26, 4545. https://doi.org/10.3390/ijms26104545
Madeira PP, Uversky VN, Zaslavsky BY. Effects of Inorganic Salts on Phase Separation in Aqueous Solutions of Poly(ethylene glycol). International Journal of Molecular Sciences. 2025; 26(10):4545. https://doi.org/10.3390/ijms26104545
Chicago/Turabian StyleMadeira, Pedro P., Vladimir N. Uversky, and Boris Y. Zaslavsky. 2025. "Effects of Inorganic Salts on Phase Separation in Aqueous Solutions of Poly(ethylene glycol)" International Journal of Molecular Sciences 26, no. 10: 4545. https://doi.org/10.3390/ijms26104545
APA StyleMadeira, P. P., Uversky, V. N., & Zaslavsky, B. Y. (2025). Effects of Inorganic Salts on Phase Separation in Aqueous Solutions of Poly(ethylene glycol). International Journal of Molecular Sciences, 26(10), 4545. https://doi.org/10.3390/ijms26104545





