Perinatal Vitamin D Deficiency Enhances Brown Adipose Tissue Thermogenesis in Weanling Rats
Abstract
1. Introduction
2. Results
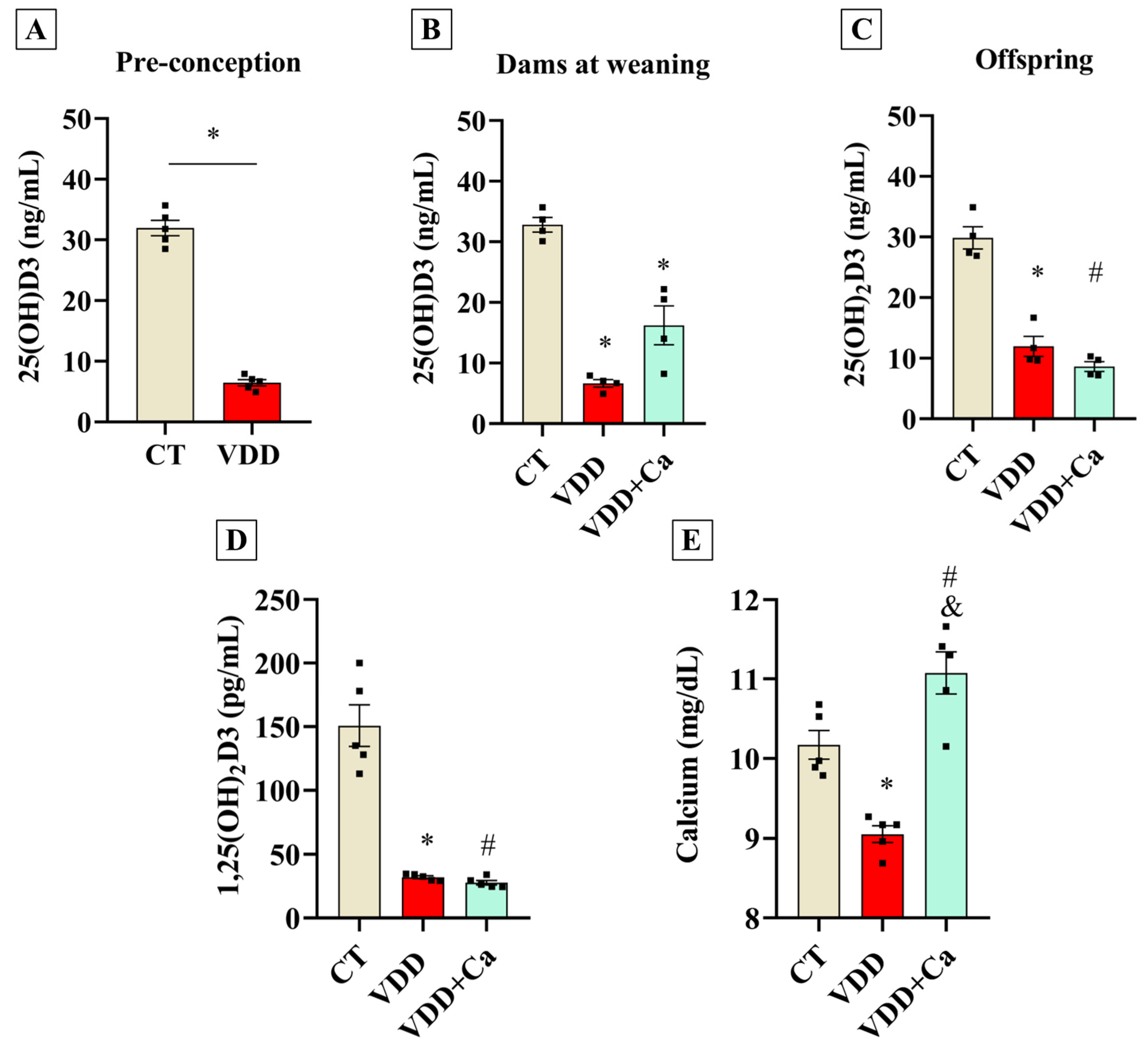
2.1. Maternal Vit. D Deficiency During Gestation and Lactation Affects Perinatal Vit. D Status in the Offspring
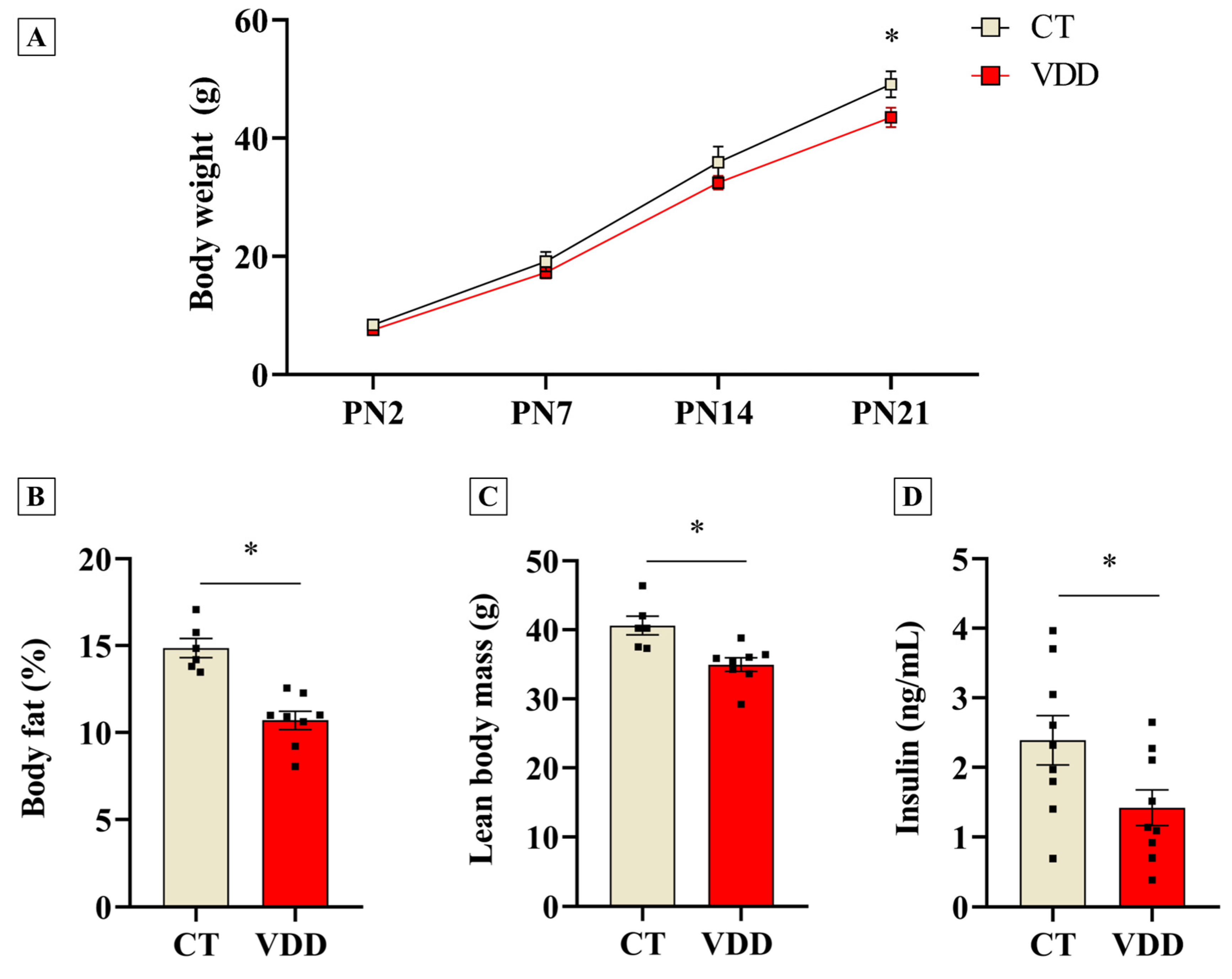
2.2. Vit. D Deficiency Impairs Postnatal Offspring Growth and Reduces Body Fat Percentage and Lean Mass at Weaning
2.3. Perinatal Vit. D Deficiency Increases Respiratory Capacity and Induces Thermogenic Program in BAT in Newly-Weaned Rats
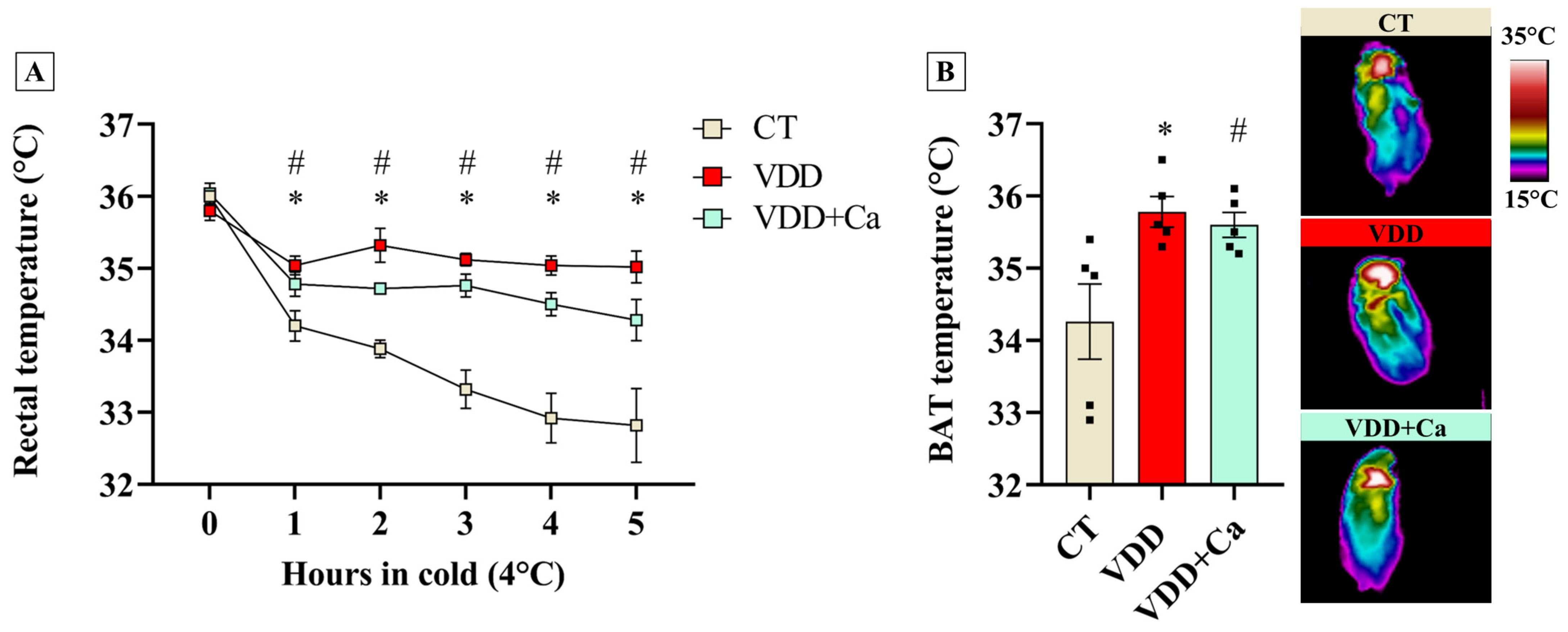
2.4. Vit. D Deficiency Increases BAT Recruitment and Cold Tolerance in the Offspring
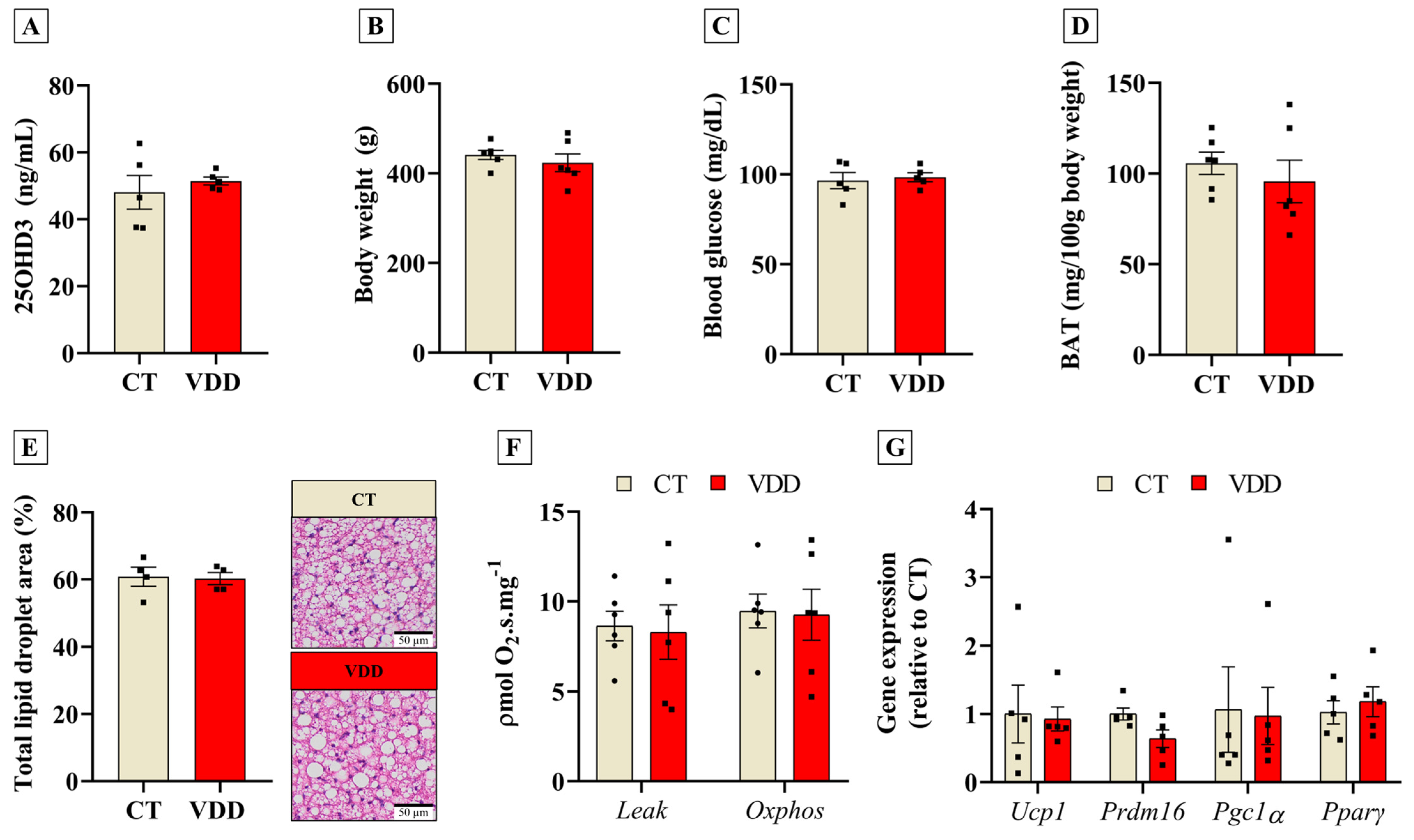
2.5. Recovery of Vit. D Status in Adulthood Abolishes Early BAT Adaptations
3. Discussion
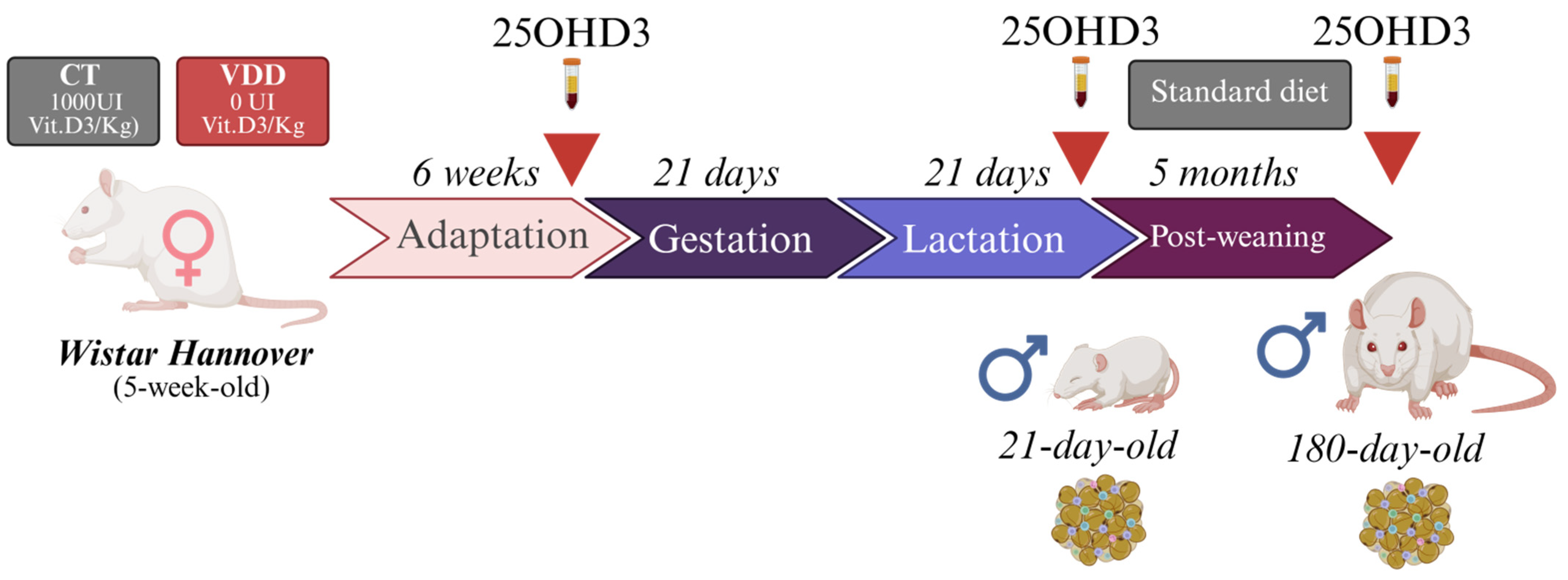
4. Materials and Methods
4.1. Animals
4.2. Body Composition Analysis
4.3. Cold Tolerance Test
4.4. Tissue and Blood Collection
4.5. Hormonal and Metabolites Measurements
4.6. High-Resolution Respirometry
4.7. Histological Analysis
4.8. Norepinephrine Measurements
4.9. Quantitative PCR
4.10. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Nutrient (g/kg) | AIN93G (CT) | AIN93G Without Vit. D (VDD) | AIN93G Without Vit. D + Ca2+ (VDD + Ca) |
|---|---|---|---|
| Corn starch | 397.50 | 397.50 | 173 |
| Casein | 200.00 | 200.00 | 200.00 |
| Starch dextrinated | 132.00 | 132.00 | 100 |
| Sucrose | 100.00 | 100.00 | 100.00 |
| Soy bean oil | 70 | 70 | 70 |
| L-Cystine | 3.00 | 3.00 | 3.00 |
| Choline Bitartrate | 2.50 | 2.50 | 2.50 |
| Butylated Hydroxytoluene | 0.014 | 0.014 | 0.014 |
| Mineral mix | 35.00 | 35.00 | 35.00 |
| Vitamin mix | 10.00 | 10.00 | 10.00 |
| Fibre | 50.00 | 50.00 | 50.00 |
| Vit. D3 | 25 × 10−6 | 0.00 | 0.00 |
| Calcium carbonate | 0 | 0 | 35.00 |
| Sodium phosphate dibasic anhydrous | 0 | 0 | 21.50 |
| Lactose | 0 | 0 | 200.00 |
References
- Aiello, G.; Lombardo, M.; Baldelli, S. Exploring Vitamin D Synthesis and Function in Cardiovascular Health: A Narrative Review. Appl. Sci. 2024, 14, 4339. [Google Scholar] [CrossRef]
- Castro, L.C.G.D. O sistema endocrinológico vitamina D. Arq. Bras. Endocrinol. Metabol. 2011, 55, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Januário Silva, I.C.; Lazaretti-Castro, M. Vitamin D metabolism and extraskeletal outcomes: An update. Arch. Endocrinol. Metab. 2022, 66, 748–755. [Google Scholar] [CrossRef]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef]
- Bikle, D.; Christakis, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Haussler, M.R.; Haussler, C.A.; Whitfield, G.K.; Hsieh, J.C.; Thompson, P.D.; Barthel, T.K.; Bartik, L.; Egan, J.B.; Wu, Y.; Jurutka, P.W.; et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J. Steroid Biochem. Mol. Biol. 2010, 121, 88–97. [Google Scholar] [CrossRef]
- Neme, A.; Seuter, S.; Carlberg, C. Selective regulation of biological processes by vitamin D based on the spatio-temporal cistrome of its receptor. BBA Gene Regul. Mech. 2017, 1860, 952–961. [Google Scholar] [CrossRef]
- Areco, V.A.; Kohan, R.; Talamoni, G.; Tolosa de Talamoni, N.G.; Peralta López, M.E. Intestinal Ca2+ absorption revisited: A molecular and clinical approach. World J. Gastroenterol. 2020, 26, 3344–3364. [Google Scholar] [CrossRef]
- Naveh-Many, T.; Silver, J. Regulation of parathyroid hormone gene expression by hypocalcemia, hypercalcemia, and vitamin D in the rat. J. Clin. Investig. 1990, 86, 1313–1319. [Google Scholar] [CrossRef]
- Eisman, J.A.; Bouillon, R. Vitamin D: Direct effects of vitamin D metabolites on bone: Lessons from genetically modified mice. Bonekey Rep. 2014, 3, 499. [Google Scholar] [CrossRef]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Anani Sarab, A. Immunomodulatory actions of vitamin D in various immune-related disorders: A comprehensive review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef] [PubMed]
- Callejo, M.; Morales-Cano, D.; Olivencia, M.A.; Mondejar-Parreño, G.; Barreira, B.; Tura-Ceide, O.; Martínez, V.G.; Serrano-Navarro, A.; Moreno, L.; Perez-Vizcaino, F.; et al. Vitamin D receptor and its antiproliferative effect in human pulmonary arterial hypertension. Sci. Rep. 2024, 14, 27445. [Google Scholar] [CrossRef] [PubMed]
- Taneera, J.; Yaseen, D.; Youssef, M.; Khalique, A.; Al Shehadat, O.S.; Mohammed, A.K.; Bustanji, Y.; Madkour, M.I.; El-Huneidi, W. Vitamin D augments insulin secretion via calcium influx and upregulation of voltage calcium channels: Findings from ins-1 cells and human islets. Mol. Cell. Endocrinol. 2025, 599, 112472. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Sahay, M.; Sahay, R. Rickets–vitamin D deficiency and dependency. Indian J. Endocrinol. Metab. 2012, 16, 164–176. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Razs, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Yu, J.; Sharma, P.; Girgis, C.M.; Gunton, J.E. Vitamin D and Beta Cells in Type 1 Diabetes: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14434. [Google Scholar] [CrossRef]
- Jensen, N.S.; Wehland, M.; Wise, P.M.; Grimm, D. Latest Knowledge on the Role of Vitamin D in Hypertension. Int. J. Mol. Sci. 2023, 24, 4679. [Google Scholar] [CrossRef]
- Barker, D.J.; Gluckman, P.D.; Godfrey, K.M.; Harding, J.E.; Owens, J.A.; Robinson, J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 10, 938–941. [Google Scholar] [CrossRef]
- da Silveira, E.A.; Nogueira e Moura, L.A.; Castro, M.C.R.; Kac, G.; Hadler, M.C.C.M.; Silva Noll, P.R.E.; Noll, M.; Rezende, A.T.O.; Delpino, F.M.; Oliveira, C. Prevalence of Vitamin D and Calcium Deficiency and Insufficiency in Women of Childbearing Age and Associated Risk Factors: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4351. [Google Scholar] [CrossRef]
- Ideraabdullah, F.Y.; Belenchia, A.M.; Rosenfeld, C.S.; Kullman, S.W.; Knuth, M.; Mahapatra, D.; Bereman, M.; Levin, E.D.; Peterson, C.A. Maternal vitamin D deficiency and developmental origins of health and disease (DOHaD). J. Endocrinol. 2019, 241, R65–R80. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Rahmani, S.; Zanjani, M.A.; Sadeghi, O.; Hosseini, S.A.; Javid, A.Z. Circulating vitamin D and the risk of gestational diabetes: A systematic review and dose-response meta-analysis. Endocrine 2020, 70, 36–47. [Google Scholar]
- Tous, M.; Villalobos, M.; Iglesias, L.; Fernández-Barrés, S.; Arija, V. Vitamin D status during pregnancy and offspring outcomes: A systematic review and meta-analysis of observational studies. Eur. J. Clin. Nutr. 2020, 74, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Vinkhuyzen, A.A.E.; Eyles, D.W.; Burne, T.H.J.; Blanken, L.M.E.; Kruithof, C.J.; Verhulst, F.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational vitamin D deficiency and autism-related traits: The Generation R Study. Mol. Psychiatry 2018, 23, 240–246. [Google Scholar] [CrossRef]
- Reis, N.G.; Assis, A.P.; Lautherbach, N.; Gonçalves, D.A.; Silveira, W.A.; Morgan, H.J.N.; Valentim, R.R.; Almeida, L.F.; Heck, L.L.; Zanon, N.M.; et al. Maternal vitamin D deficiency affects the morphology and function of glycolytic muscle in adult offspring rats. J. Cachexia Sarcopenia Muscle 2022, 13, 2175–2187. [Google Scholar] [CrossRef]
- Schavinski, A.Z.; Reis, N.G.; Morgan, H.J.N.; Assis, A.P.; Moro, M.L.; Valentim, R.R.; Seni-Silva, A.C.; Ramos, E.S.; Kettelhut, I.C.; Navegantes, L.C.C. Maternal Vitamin D Deficiency Impairs the Development of β Cells in Offspring Rats in a Sex-Dependent Manner. Int. J. Mol. Sci. 2024, 25, 4136. [Google Scholar] [CrossRef]
- Hachemi, I.; U-Din, M. Brown Adipose Tissue: Activation and Metabolism in Humans. Endocrinol. Metab. 2023, 38, 214–222. [Google Scholar] [CrossRef]
- Heaton, G.M.; Wagenvoord, R.J.; Kemp, A.; Nicholls, D.G. Brown-Adipose-Tissue Mitochondria: Photoaffinity Labelling of the Regulatory Site of Energy Dissipation. Eur. J. Biochem. 1978, 82, 515–521. [Google Scholar] [CrossRef]
- Aquila, H.; Link, T.A.; Klingenberg, M. The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. EMBO J. 1985, 4, 2369–2376. [Google Scholar] [CrossRef]
- Locke, R.M.; Rial, E.; Scott, I.D.; Nicholls, D.G. Fatty Acids as Acute Regulators of the Proton Conductance of Hamster Brown-Fat Mitochondria. Eur. J. Biochem. 1982, 129, 373–380. [Google Scholar] [CrossRef]
- Hoffmann, L.S.; Etzrodt, J.E.; Willkomm, L.; Sanyal, A.; Scheja, L.; Fisher, A.W.C.; Stasch, J.P.; Bloch, W.; Friebe, A.; Heeren, J.; et al. Stimulation of soluble guanylyl cyclase protects against obesity by recruiting brown adipose tissue. Nat. Commun. 2015, 6, 7235. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.W.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.C.; Blondin, D.P.; Haman, F.; Richard, D. Brown Adipose Tissue—A Translational Perspective. Endrocrine Rev. 2022, 44, 143–192. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef]
- Cypess, A.M.; Williams, G.; Goldfine, A.B.; Tseng, Y.-H.; Kolodny, G.M. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergran, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.V.; Enerbäck, S.; et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef]
- van Marken Lichtenbelt, W.D.; Vanhommering, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schöder, H.; et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef]
- Wibmer, A.G.; Becher, T.; Eljalby, M.; Crane, A.; Andrieu, P.C.; Jiang, C.S.; Vaughan, R.; Schöder, H.; Cohen, P. Brown adipose tissue is associated with healthier body fat distribution and metabolic benefits independent of regional adiposity. Cell Rep. Med. 2021, 2, 100332. [Google Scholar] [CrossRef]
- Schulz, T.J.; Tseng, Y.H. Brown adipose tissue: Development, metabolism and beyond. Biochem. J. 2013, 453, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Daniel, K.W.; Robidoux, J.; Puigserver, P.; Medvedev, A.V.; Bai, X.; Floering, L.M.; Spiegelman, B.M.; Collins, S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 2004, 24, 3057–3067. [Google Scholar] [CrossRef]
- Fain, J.; Jacobs, M.; Clement-Cormier, Y. Interrelationship of cyclic AMP, lipolysis, and respiration in brown fat cells. Am. J. Physiol. 1973, 224, 346–351. [Google Scholar] [CrossRef]
- Ricciardi, C.J.; Bae, J.; Esposito, D.; Komarnytsky, S.; Hu, P.; Chen, J.; Zhao, L. 1,25-Dihydroxyvitamin D3/vitamin D receptor suppresses brown adipocyte differentiation and mitochondrial respiration. Eur. J. Nutr. 2015, 54, 1001–1012. [Google Scholar] [CrossRef]
- Xu, Y.; Lou, Y.; Kong, J. VDR regulates energy metabolism by modulating remodeling in adipose tissue. Eur. J. Pharmacol. 2019, 865, 172761. [Google Scholar] [CrossRef]
- Wong, K.E.; Kong, J.; Zhang, W.; Szeto, F.L.; Ye, H.; Deb, D.K.; Brady, M.J.; Li, Y.C. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J. Biol. Chem. 2011, 286, 33804–33810. [Google Scholar] [CrossRef]
- Saramäki, A.; Diermeier, S.; Kellner, R.; Laitinen, H.; Vaïsänen, S.; Carlberg, C. Cyclical chromatin looping and transcription factor association on the regulatory regions of the p21 (CDKN1A) gene in response to 1alpha,25-dihydroxyvitamin D3. J. Biol. Chem. 2009, 284, 8073–8082. [Google Scholar] [CrossRef]
- Jiang, F.; Li, P.; Fornace Jr, A.J.; Nicosia, S.V.; Bai, W. G2/M arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through the induction of GADD45 via an exonic enhancer. J. Biol. Chem. 2003, 278, 48030–48040. [Google Scholar] [CrossRef]
- Negron, S.G.; Ercan-Sencicek, G.; Freed, J.; Walters, M.; Lin, Z. Both proliferation and lipogenesis of brown adipocytes contribute to postnatal brown adipose tissue growth in mice. Sci. Rep. 2020, 10, 20335. [Google Scholar] [CrossRef]
- Garcia, L.A.; King, K.K.; Ferrini, M.G.; Norris, K.C.; Artaza, J.N. 1,25(OH)2Vitamin D3 Stimulates Myogenic Differentiation by Inhibiting Cell Proliferation and Modulating the Expression of Promyogenic Growth Factors and Myostatin in C2C12 Skeletal Muscle Cells. Endocrinology 2011, 152, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, K.R.; Carlessi, R.; Cruzat, V.; Newsholme, P. Effects of vitamin D on primary human skeletal muscle cell proliferation, differentiation, protein synthesis and bioenergetics. J. Steroid Biochem. Mol. Biol. 2019, 193, 105423. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, G.V.; Veena, S.R.; Winder, N.R.; Hill, J.C.; Noonan, K.; Boucher, B.J.; Karat, S.C.; Fall, C.H.D. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: The Mysore Parthenon Study. Am. J. Clin. Nutr. 2011, 93, 628–635. [Google Scholar] [CrossRef]
- Markussen, L.K.; Rondini, E.A.; Johansen, O.S.; Madsen, J.G.S.; Sustarsic, E.G.; Marcher, A.B.; Hansen, J.B.; Gerhart-Hines, Z.; Granneman, J.G.; Mandrup, S. Lipolysis regulates major transcriptional programs in brown adipocytes. Nat. Commun. 2022, 13, 3956. [Google Scholar] [CrossRef]
- Reis, N.G. Muscle Plasticity of Male and Female Offspring Rats in Response to Maternal Vitamin D Deficiency: From Atrophy to Compensatory Muscle Hypertrophy. Ph.D. Thesis, University of São Paulo, Ribeirão Preto, Brazil, 2022. [Google Scholar]
- Pramme-Steinwachs, I.; Jastroch, M.; Ussar, S. Extracellular calcium modulates brown adipocyte differentiation and identity. Sci. Rep. 2017, 7, 8888. [Google Scholar] [CrossRef]
- Kir, S.; Komaba, H.; Garcia, A.P.; Economopoulos, K.P.; Liu, W.; Lanske, B.; Hodin, R.A.; Spiegelman, B.M. PTH/PTHrP Receptor Mediates Cachexia in Models of Kidney Failure and Cancer. Cell Metab. 2016, 23, 315–323. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Abbasi, A.; Hejazian, S.S.; Hekmatshoar, Y.; Ardalan, M.; Farnood, F.; Vahed, S.Z. Combating chronic kidney disease-associated cachexia: A literature review of recent therapeutic approaches. BMC Nephrol. 2025, 26, 133. [Google Scholar]
- Dumortier, O.; Roger, E.; Pisani, D.F.; Casamento, V.; Gautier, N.; Lebrun, P.; Johnston, H.; Lopez, P.; Amri, E.Z.; Obberghen, E.V.; et al. Age-Dependent Control of Energy Homeostasis by Brown Adipose Tissue in Progeny Subjected to Maternal Diet-Induced Fetal Programming. Diabetes 2017, 66, 627–639. [Google Scholar] [CrossRef]
- Dechandt, C.R.P.; Couto-Lima, C.A.; Alberici, L.C. Triglyceride depletion of brown adipose tissue enables analysis of mitochondrial respiratory function in permeabilized biopsies. Anal. Biochem. 2016, 515, 55–60. [Google Scholar] [CrossRef]
- Garofalo, M.A.; Kettelhut, I.C.; Roselino, J.E.; Migliorini, R.H. Effect of acute cold exposure on norepinephrine turnover rates in rat white adipose tissue. J. Auton. Nerv. Syst. 1996, 60, 206–208. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Ucp1 | CCGGTGGATGTGGTAAAAAC | GTTTTTACCACATCCACCGG |
| Prdm16 | CAGCACGGTGAAGCCATTC | GCGTGCATCCGCTTTG |
| Pgc1α | GCTTGACTGGCGTCATTCA | ACAGAGTCTTGGCTGCACATGT |
| Pparγ | GTGCCAGTTTCGATCCGTAGA | GGCCAGCATCGTGTAGATGA |
| Rpl39 | TCCTGGCAAAGAAACAAAAGC | TAGACCCAGCTTCGTTCTCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moro, M.L.; Reis, N.G.; Schavinski, A.Z.; Neto, J.B.C.; Assis, A.P.; Santos, J.R.; Albericci, L.C.; Kettelhut, I.C.; Navegantes, L.C.C. Perinatal Vitamin D Deficiency Enhances Brown Adipose Tissue Thermogenesis in Weanling Rats. Int. J. Mol. Sci. 2025, 26, 4534. https://doi.org/10.3390/ijms26104534
Moro ML, Reis NG, Schavinski AZ, Neto JBC, Assis AP, Santos JR, Albericci LC, Kettelhut IC, Navegantes LCC. Perinatal Vitamin D Deficiency Enhances Brown Adipose Tissue Thermogenesis in Weanling Rats. International Journal of Molecular Sciences. 2025; 26(10):4534. https://doi.org/10.3390/ijms26104534
Chicago/Turabian StyleMoro, Matheus L., Natany G. Reis, Aline Z. Schavinski, João B. Camargo Neto, Ana Paula Assis, Jonathas R. Santos, Luciane C. Albericci, Isis C. Kettelhut, and Luiz C. C. Navegantes. 2025. "Perinatal Vitamin D Deficiency Enhances Brown Adipose Tissue Thermogenesis in Weanling Rats" International Journal of Molecular Sciences 26, no. 10: 4534. https://doi.org/10.3390/ijms26104534
APA StyleMoro, M. L., Reis, N. G., Schavinski, A. Z., Neto, J. B. C., Assis, A. P., Santos, J. R., Albericci, L. C., Kettelhut, I. C., & Navegantes, L. C. C. (2025). Perinatal Vitamin D Deficiency Enhances Brown Adipose Tissue Thermogenesis in Weanling Rats. International Journal of Molecular Sciences, 26(10), 4534. https://doi.org/10.3390/ijms26104534






