Different Endophytes Colonized in Various Lotus Root Varieties and Their Associated Mealy and Crunchy Properties
Abstract
1. Introduction
2. Results
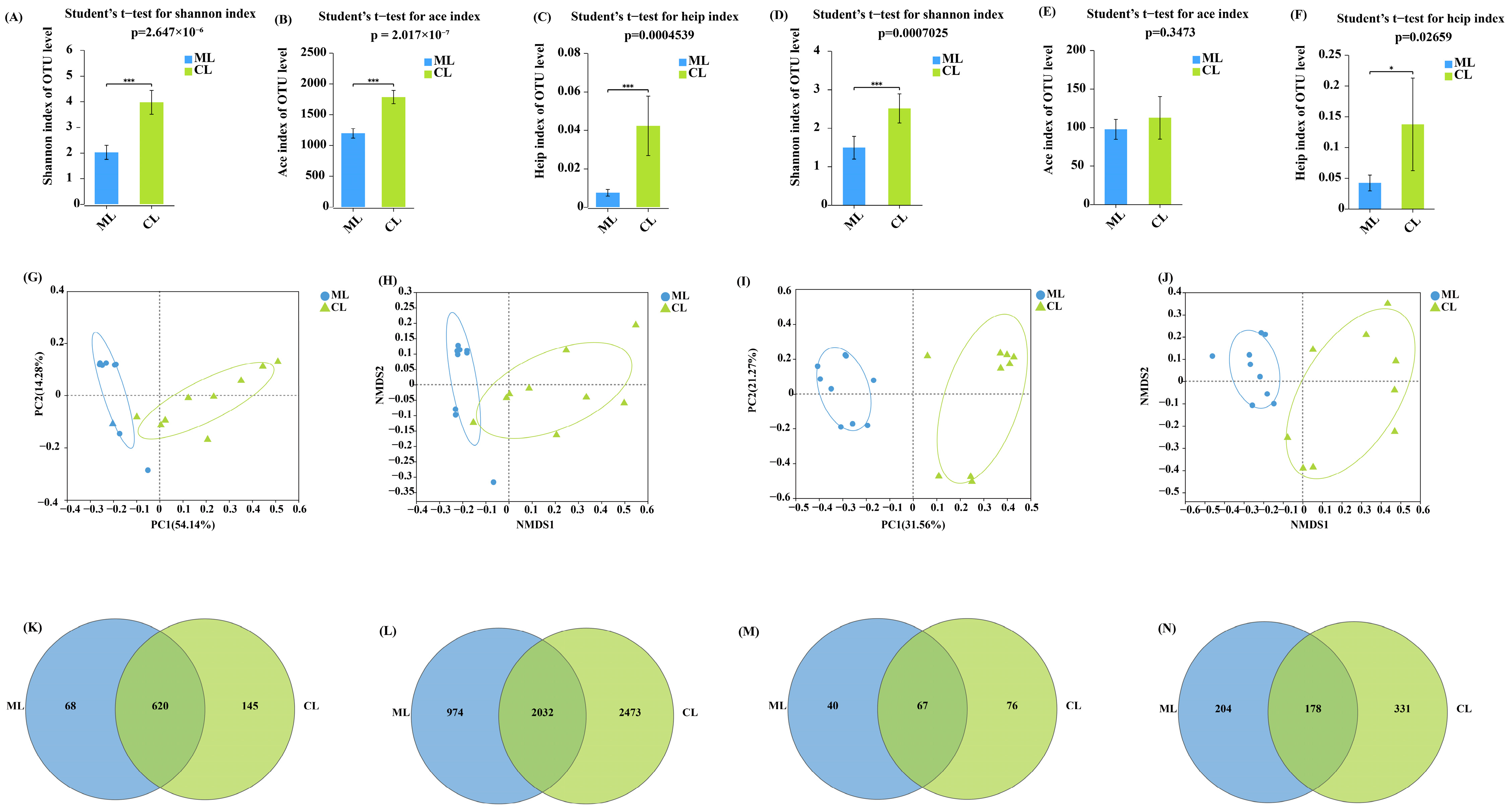
2.1. Diversity of Endophytic Microorganisms in Lotus Root
2.2. Composition of Endophytic Microorganisms in Lotus Root
2.3. Function Prediction of Endophytic Microorganisms in Lotus Roots
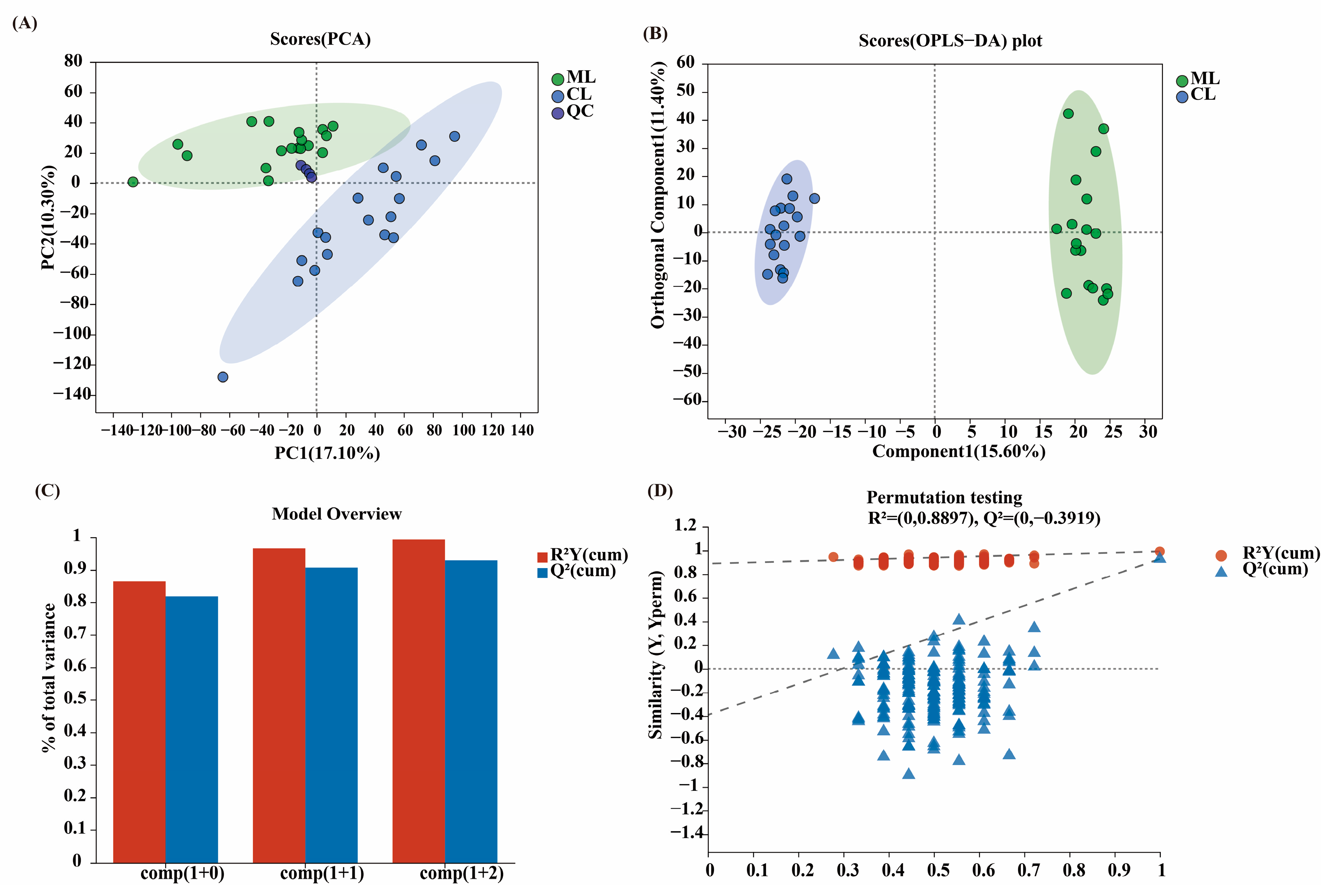
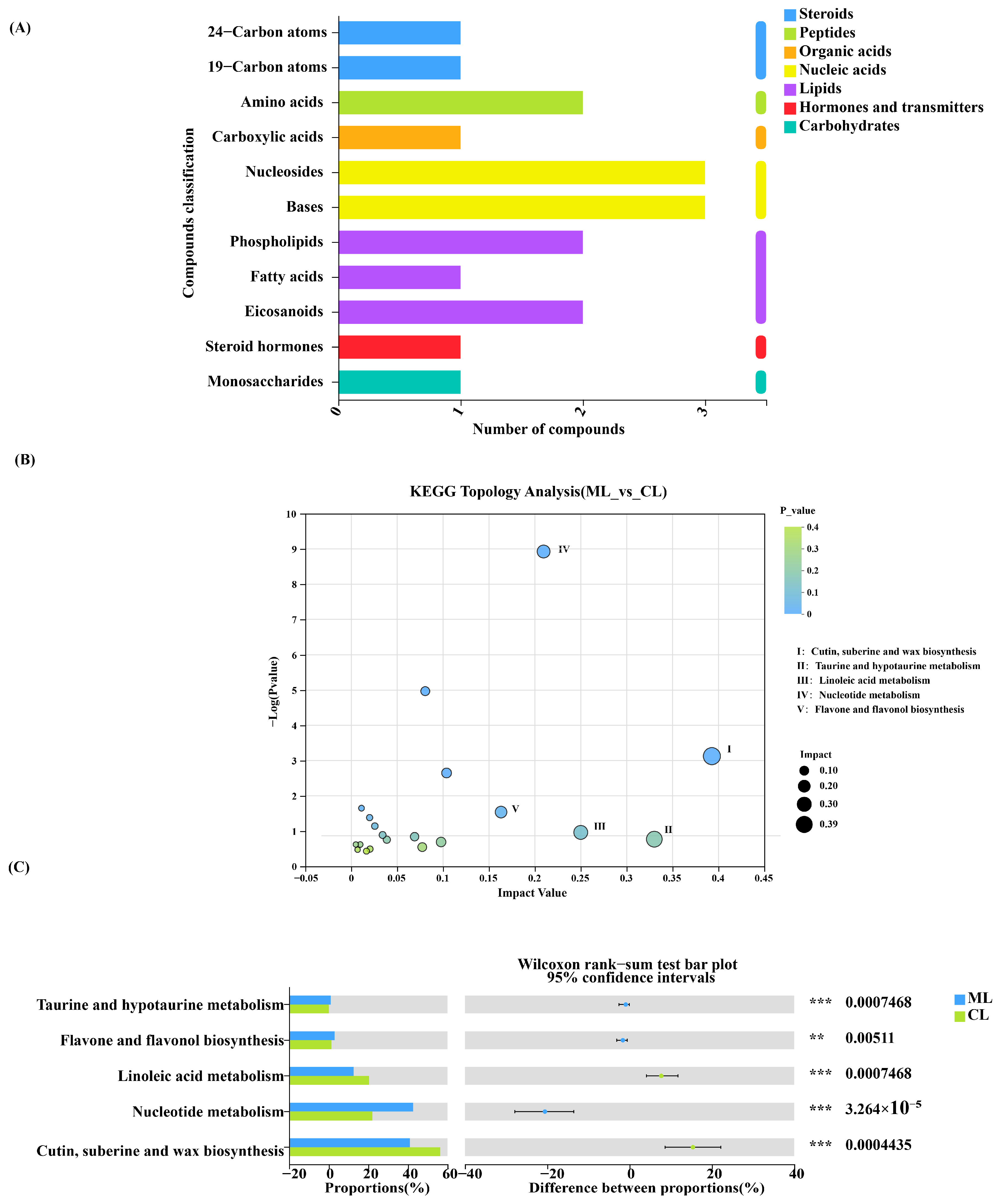
2.4. Composition of Metabolites in Lotus Root
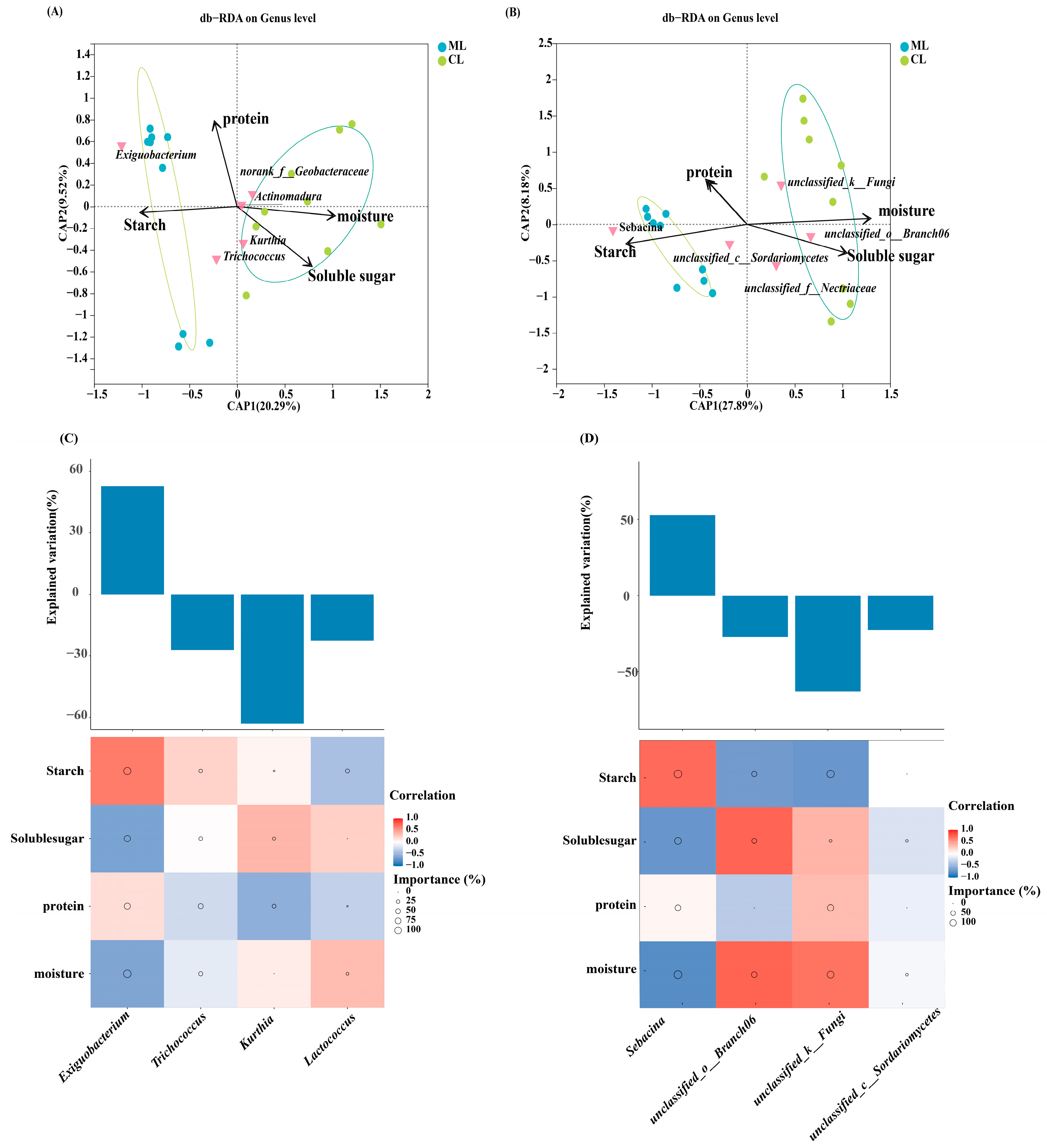
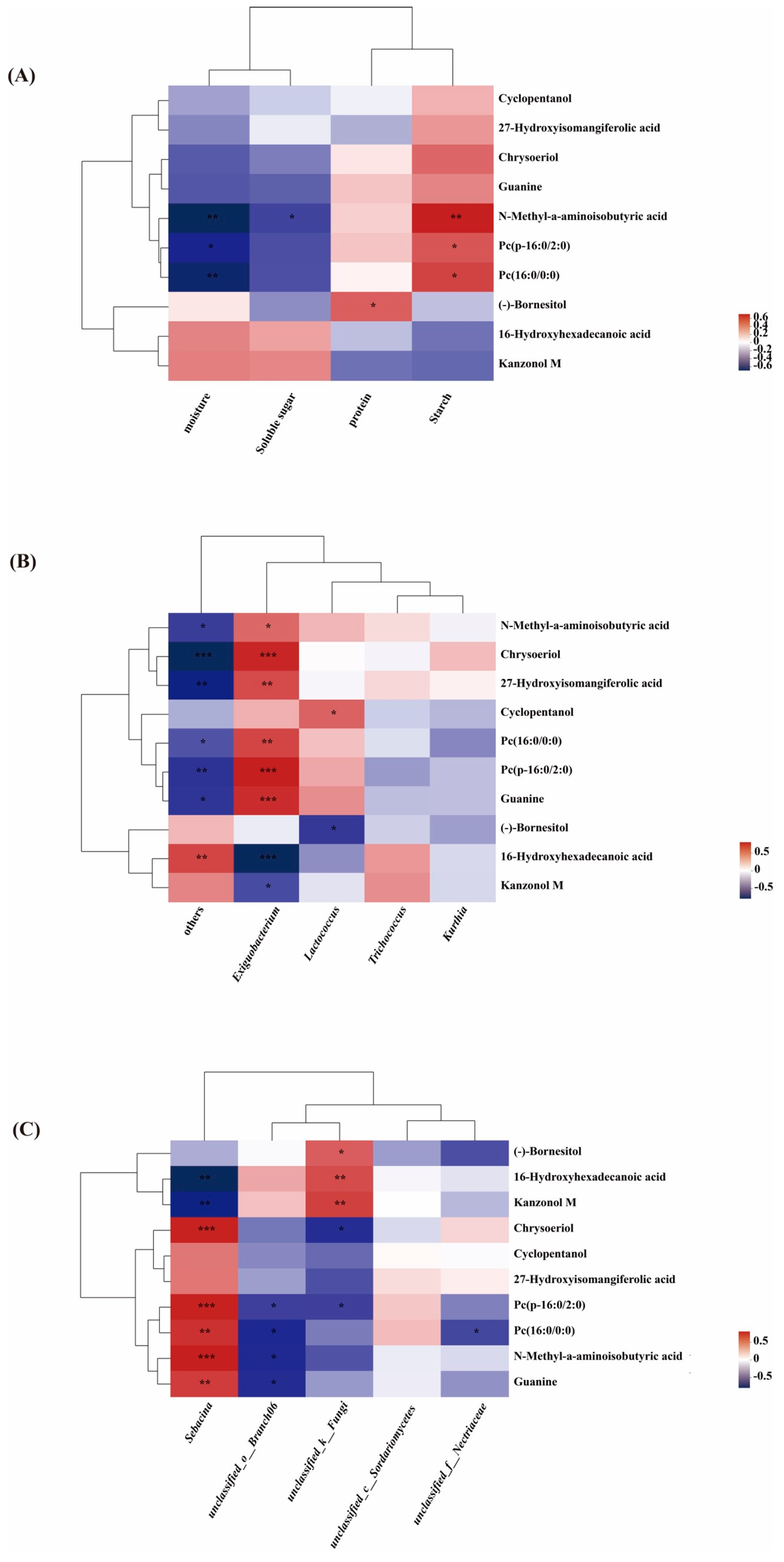
2.5. Comparison of Metabolites and Biological Characteristics of Endophytic Microbiota in Lotus Root
3. Discussion
4. Materials and Methods
4.1. Field Site Description and Experimental Designs
4.2. Test Methods
4.2.1. Analysis of Endophytic Microbial Diversity
4.2.2. Untargeted Metabolomic Assays and Analysis
4.2.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, Z.; Hu, X.; Zhang, Z.; Li, Z.; Lin, Q.; Yang, M.; Yang, P.; Ming, R.; Yu, Q.; Wang, K. Chromosome Nomenclature and Cytological Characterization of Sacred Lotus. Cytogenet. Genome Res. 2017, 153, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhu, M.; Guo, M. Research Advances in Traditional and Modern Use of Nelumbo nucifera: Phytochemicals, Health Promoting Activities and Beyond. Crit. Rev. Food Sci. Nutr. 2019, 59, S189–S209. [Google Scholar] [CrossRef]
- Yi, Y.; Sun, J.; Xie, J.; Min, T.; Wang, L.M.; Wang, H.X. Phenolic Profiles and Antioxidant Activity of Lotus Root Varieties. Molecules 2016, 21, 863. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Tang, H.S.; Sun, Y.; Xu, W.; Min, T.; Wang, H.X. Comprehensive Characterization of Lotus Root Polysaccharide-Phenol Complexes. Food Chem. 2022, 366, 130693. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Yi, Y.; Sun, J.; Lamikanra, O.; Min, T. Fingerprint Profiling of Polysaccharides from Different Parts of Lotus Root Varieties. RSC Adv. 2018, 8, 16574–16584. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Yi, Y.; Hou, W.; Wang, L.; Ai, Y.; Min, T. Varying Concentrations of Ethephon Induce Antioxidant Defences and Cell Wall Degradation to Regulate Storage Quality of Fresh-Cut Lotus Root. Food Biosci. 2024, 61, 104975. [Google Scholar] [CrossRef]
- Vu, V.V.; Hangasky, J.A.; Detomasi, T.C.; Henry, S.J.W.; Ngo, S.T.; Span, E.A.; Marletta, M.A. Substrate Selectivity in Starch Polysaccharide Monooxygenases. J. Biol. Chem. 2019, 294, 12157–12166. [Google Scholar] [CrossRef]
- Nie, W.; Zheng, X.; Feng, W.; Liu, Y.; Li, Y.; Liang, X. Characterization of Bacterial Cellulose Produced by Acetobacter pasteurianus MGC-N8819 Utilizing Lotus Rhizome. LWT 2022, 165, 113763. [Google Scholar] [CrossRef]
- Wei, X.; Huang, L.; Li, S.; Gao, S.; Jie, D.; Guo, Z.; Zheng, B. Fast Determination of Amylose Content in Lotus Seeds Based on Hyperspectral Imaging. Agronomy 2023, 13, 2104. [Google Scholar] [CrossRef]
- Salvi, P.; Mahawar, H.; Agarrwal, R.; Kajal; Gautam, V.; Deshmukh, R. Advancement in the Molecular Perspective of Plant-Endophytic Interaction to Mitigate Drought Stress in Plants. Front. Microbiol. 2022, 13, 981355. [Google Scholar] [CrossRef]
- Ogbe, A.A.; Gupta, S.; Stirk, W.A.; Finnie, J.F.; Van Staden, J. Growth-Promoting Characteristics of Fungal and Bacterial Endophytes Isolated from a Drought-Tolerant Mint Species Endostemon obtusifolius (E. Mey. Ex Benth.) N. E. Br. Plants 2023, 12, 638. [Google Scholar] [CrossRef]
- Oono, R.; Black, D.; Slessarev, E.; Sickler, B.; Strom, A.; Apigo, A. Species Diversity of Fungal Endophytes across a Stress Gradient for Plants. New Phytol. 2020, 228, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Haruna, E.; Zin, N.M.; Kerfahi, D.; Adams, J.M. Extensive Overlap of Tropical Rainforest Bacterial Endophytes between Soil, Plant Parts, and Plant Species. Microb. Ecol. 2017, 75, 88–103. [Google Scholar] [CrossRef]
- Silva, R.M.F.; Neto, W.P.P.; Oliveira, R.J.V.; Bezerra, J.D.P.; Bezerra, J.L.; De Lima, V.X.; Vieira, L.C.; Tabosa, J.N.; Souza-Motta, C.M.; Silva, G.A. Effect of Climate and Phenological Stage on Fungal Endophytes Community in Sorghum bicolor Leaves. Mycol. Prog. 2023, 22, 19. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, S.; Zhou, X.; Ding, D.; Song, J.; Yang, S. Endophytic Microorganisms in Tomato Roots, Changes in the Structure and Function of the Community at Different Growing Stages. Microorganisms 2024, 12, 1251. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, D.; Huang, R.-H.; Zheng, J.-Y.; Liu, Y.-Y.; Hu, B.-S.; Gu, Y.-Q.; Du, Q. A New Source of Diterpene Lactones From Andrographis paniculata (Burm. f.) Nees—Two Endophytic Fungi of Colletotrichum Sp. With Antibacterial and Antioxidant Activities. Front. Microbiol. 2022, 13, 819770. [Google Scholar] [CrossRef]
- Ogbe, A.A.; Gupta, S.; Stirk, W.A.; Finnie, J.F.; van Staden, J. Endophyte Inoculation Enhances Growth, Secondary Metabolites and Biological Activity of Endostemon obtusifolius Grown Under Drought Stress. J. Plant Growth Regul. 2023, 43, 1103–1117. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Tian, P.; Xiang, W.; Lu, X.; Huang, R.; Li, L. Fungal Endophytes from Medicinal Plant Bletilla striata (Thunb.) Reichb. F. Promote the Host Plant Growth and Phenolic Accumulation. S. Afr. J. Bot. 2021, 143, 25–32. [Google Scholar] [CrossRef]
- Ramírez, C.; Cardozo, M.; López Gastón, M.; Galdeano, E.; Collavino, M.M. Plant Growth Promoting Activities of Endophytic Bacteria from Melia azedarach (Meliaceae) and Their Influence on Plant Growth under Gnotobiotic Conditions. Heliyon 2024, 10, e35814. [Google Scholar] [CrossRef]
- Worarad, K.; Suzuki, T.; Norii, H.; Mochizuki, Y.; Ishii, T.; Shinohara, K.; Miyamoto, T.; Kuboyama, T.; Inoue, E. Transcriptome Profiling for Pericarp Browning during Long-Term Storage of Intact Lotus Root (Nelumbo nucifera). Plant Growth Regul. 2021, 95, 207–221. [Google Scholar] [CrossRef]
- Chen, X.; Huang, S.; Yan, S.; Li, J. Screening of the Compound Inhibitor of Blackening for Damaged Lotus Rhizome Epidermis and Evaluation of Its Fresh-Keeping Effect on Lotus Rhizome. J. Food Meas. Charact. 2024, 18, 3100–3112. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Yi, Y.; Hou, W.; Wang, L.; Ai, Y.; Wang, H.; Min, T. Regulations and Mechanisms of 1-Methylcyclopropene Treatment on Browning and Quality of Fresh-Cut Lotus (Nelumbo nucifera Gaertn.) Root Slices. Postharvest Biol. Technol. 2022, 185, 111782. [Google Scholar] [CrossRef]
- Chiang, P.; Luo, Y. Effects of Pressurized Cooking on the Nutritional and Functional Properties of Lotus Root (Nelumbo Nucifera Gaertn.). J. Sci. Food Agric. 2023, 103, 3159–3165. [Google Scholar] [CrossRef]
- Min, T.; Niu, L.-F.; Feng, X.; Yi, Y.; Wang, L.; Zhao, Y.; Wang, H. The Effects of Different Temperatures on the Storage Characteristics of Lotus (Nelumbo nucifera G.) Root. Food Chem. 2021, 348, 129109. [Google Scholar] [CrossRef]
- Cai, Z.; Jiang, Y.; Wang, F.; Liu, J.; Kan, J.; Zhang, M.; Qi, X.; Li, L.; Zhao, S.; Qian, C. Study on Quality and Starch Characteristics of Powdery and Crispy Lotus Roots. Foods 2024, 13, 3335. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y. Study on the Quality and Starch Characteristics of Crisp and Powdery Lotus Roots; Yangzhou University: Yangzhou, China, 2024. [Google Scholar] [CrossRef]
- Duan, R. Study on the Structural Differences and Rheological Properties of Alkali-Soluble Polysaccharides in the Cell Wall of Lotus Root with Crisp Texture; Huazhong Agricultural University: Wuhan, China, 2024. [Google Scholar] [CrossRef]
- Peng, J. Analysis of the Difference in Crisp Quality Between E-Lian 5 and E-Lian 6; Huazhong Agricultural University: Wuhan, China, 2024. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, A.; Kumar, R. A Cold-Active Cellulase Produced from Exiguobacterium sibiricum K1 for the Valorization of Agro-Residual Resources. Biomass Convers. Biorefin. 2022, 13, 14777–14787. [Google Scholar] [CrossRef]
- Karr, D.B.; Emerich, D.W. Protein Phosphorylation in Bradyrhizobium japonicum Bacteroids and Cultures. J. Bacteriol. 1989, 171, 3420–3426. [Google Scholar] [CrossRef] [PubMed]
- Thorn, R.G.; Banwell, A.; Pham, T.H.; Vidal, N.P.; Manful, C.F.; Nadeem, M.; Ivanov, A.G.; Szyszka Mroz, B.; Bonneville, M.B.; Hüner, N.P.A.; et al. Identification and Analyses of the Chemical Composition of a Naturally Occurring Albino Mutant Chanterelle. Sci. Rep. 2021, 11, 20590. [Google Scholar] [CrossRef]
- Zhu, N.; Yang, J.; Ji, L.; Liu, J.; Yang, Y.; Yuan, H. Metagenomic and Metaproteomic Analyses of a Corn Stover-Adapted Microbial Consortium EMSD5 Reveal Its Taxonomic and Enzymatic Basis for Degrading Lignocellulose. Biotechnol. Biofuels 2016, 9, 243. [Google Scholar] [CrossRef]
- Schwarz, W.H. The Cellulosome and Cellulose Degradation by Anaerobic Bacteria. Appl. Microbiol. Biotechnol. 2001, 56, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.N.; Mongkolthanaruk, W.; Riddech, N. Enhancing Soil Amendment for Salt Stress Using Pretreated Rice Straw and Cellulolytic Fungi. Sci. Rep. 2024, 14, 13903. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, D.N.; Finkina, E.I.; Bogdanov, I.V.; Tagaev, A.A.; Ovchinnikova, T.V. Features and Possible Applications of Plant Lipid-Binding and Transfer Proteins. Membranes 2022, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, Y.; Chen, L.; Peng, Y.; Wang, Q.; Shi, J. Hypotaurine Delays Senescence of Peach Fruit by Regulating Reactive Oxygen Species Metabolism. Sci. Hortic. 2019, 253, 295–302. [Google Scholar] [CrossRef]
- Tu, J.; Zhang, M.; Xu, B.; Liu, H. Effect of Physicochemical Properties on Freezing Suitability of Lotus (Nelumbo nucifera) Root. Int. J. Refrigeration 2015, 50, 1–9. [Google Scholar] [CrossRef]
- Salo-väänänen, P.P.; Koivistoinen, P.E. Determination of Protein in Foods: Comparison of Net Protein and Crude Protein (N × 6.25) Values. Food Chem. 1996, 57, 27–31. [Google Scholar] [CrossRef]
- Zhu, F.; Sun, H.; Wang, J.; Zheng, X.; Wang, T.; Diao, Y.; Hu, Z. Differential Expression Involved in Starch Synthesis Pathway Genes Reveal Various Starch Characteristics of Seed and Rhizome in Lotus (Nelumbo nucifera). J. Food Sci. 2022, 87, 4250–4263. [Google Scholar] [CrossRef]




| Group | Moisture (%) | Protein (%) | Starch (mg/g) | Souble Sugar (mg/g) |
|---|---|---|---|---|
| ML | 73.20 ± 2.65 b | 1.996 ± 0.359 a | 15.26 ± 2.22 a | 3.00 ± 1.21 a |
| CL | 82.26 ± 3.05 a | 1.943 ± 0.106 a | 8.54 ± 1.69 b | 4.16 ± 0.635 a |
| Metabolite | ML/CL | M/Z | Retention Time | FDR | Regulate | Mode |
|---|---|---|---|---|---|---|
| Phenylpropanoids and polyketides | ||||||
| 2-(4-Allyl-2,6-dimethoxyphenoxy)-1-(4-Hydroxy-3-methoxyphenyl)-1-Propanol | 1.39 | 397.16 | 12.07 | 0.0182 | up | ESI+ |
| Falcarindiol | 0.72 | 305.17 | 7.57 | 0.0188 | up | ESI− |
| Cavipetin C | 1.21 | 371.25 | 13.19 | 0.0330 | up | ESI+ |
| Organoheterocyclic compounds | ||||||
| Lucidenic acid J | 0.57 | 529.21 | 13.19 | 0.0197 | up | ESI+ |
| Fragransin C1 | 0.80 | 357.16 | 13.23 | 0.1027 | up | ESI+ |
| Withaferin A | 1.34 | 493.26 | 14.38 | 0.0135 | up | ESI+ |
| Lipids and lipid-like molecules | ||||||
| Persicachrome | 1.84 | 367.26 | 13.49 | 0.0000 | up | ESI+ |
| 9,10,13-Trihydroxystearic acid | 1.56 | 331.24 | 10.83 | 0.0001 | up | ESI− |
| Homovanillin | 1.60 | 167.07 | 13.23 | 0.0005 | up | ESI+ |
| Apo-12′-violaxanthal | 3.50 | 365.24 | 13.25 | 0.0186 | up | ESI+ |
| Ganoderol A | 2.16 | 921.70 | 14.42 | 0.0110 | up | ESI− |
| Aflatoxin P1 | 1.34 | 340.08 | 5.70 | 0.0000 | up | ESI+ |
| (3beta,22E,24R)-5,8-Epidioxy-23-methylergosta-6,22-dien-3-ol | 1.57 | 487.34 | 10.23 | 0.0018 | up | ESI− |
| (3alpha,20R,24Z)-3-Hydroxy-21-oxoeupha-8,24-dien-26-oic acid | 1.23 | 451.32 | 13.36 | 0.0008 | down | ESI− |
| Sativan | 1.30 | 287.12 | 12.50 | 0.0001 | up | ESI+ |
| Isofucosterol 3-O-[6-O-(9-Octadecenoyl)-b-D-glucopyranoside] | 1.28 | 873.64 | 14.05 | 0.0007 | up | ESI− |
| Mg(16:0/0:0/0:0) | 0.70 | 719.56 | 14.32 | 0.0055 | up | ESI− |
| (3beta,17alpha,23S)-17,23-Epoxy-3,28,29-trihydroxy-27-norlanost-8-en-24-one | 1.22 | 495.31 | 13.34 | 0.0081 | down | ESI− |
| 8-Isoprostaglandin F2a | 1.21 | 353.23 | 11.31 | 0.0004 | up | ESI− |
| 6-Ketoprostaglandin E1 | 0.71 | 333.20 | 8.59 | 0.0045 | up | ESI+ |
| Corosin | 1.51 | 499.30 | 10.35 | 0.0092 | up | ESI− |
| Bellidifolin | 1.43 | 275.05 | 5.95 | 0.0036 | down | ESI+ |
| Butyryl-L-carnitine | 1.40 | 232.15 | 2.48 | 0.0375 | up | ESI+ |
| 3,7-Dimethylocta-2,6-dien-1-ol | 1.21 | 307.26 | 13.84 | 0.0036 | up | ESI− |
| Dihomo-alpha-linolenic acid | 1.34 | 351.25 | 13.84 | 0.0202 | up | ESI− |
| 2-Phenylethyl 3-phenyl-2-propenoate | 1.28 | 270.15 | 7.11 | 0.0222 | down | ESI+ |
| Taurine | 1.21 | 124.01 | 0.61 | 0.0141 | up | ESI− |
| 2-Lysophosphatidylcholine | 1.44 | 546.36 | 13.30 | 0.0202 | up | ESI+ |
| Prostaglandin E1 | 1.56 | 353.23 | 9.36 | 0.0550 | up | ESI− |
| Cortexolone | 1.31 | 347.22 | 10.32 | 0.0519 | up | ESI+ |
| Neoisoastilbin | 1.29 | 449.11 | 3.82 | 0.0764 | up | ESI− |
| 4-oxo-Retinoic acid | 1.27 | 315.20 | 9.96 | 0.0985 | up | ESI+ |
| Lignans, neolignans and related compounds | ||||||
| 1-Dihydrocarveol | 1.87 | 350.31 | 14.36 | 0.0002 | up | ESI+ |
| Ethyl oleate | 1.86 | 309.28 | 14.11 | 0.0017 | up | ESI− |
| Benzenoids | ||||||
| Rutacridone epoxide | 1.42 | 304.10 | 6.72 | 0.0002 | down | ESI− |
| Sequencing Type | Prime Name | Prime Sequence | Length | Sequencing Platform |
|---|---|---|---|---|
| endopytic bacteria | 799F | 5′-AACMGGATTAGATACCCKG-3′ | 394 bp | MiseqPE250 |
| 1193R | 5′-ACGTCATCCCCACCTTCC-3′ | |||
| ITS | ITS1F | 5′-CTTGGTCATTTAGAGGAAGTAA-3′ | 350 bp | MiSeq PE300 |
| ITS2F | 5′-GCTGCGTTCTTCATCGATGC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Zhou, X.; Gao, M.; Ou, Y.; Hu, Y.; Jiang, W.; Jiang, H.; Yang, S. Different Endophytes Colonized in Various Lotus Root Varieties and Their Associated Mealy and Crunchy Properties. Int. J. Mol. Sci. 2025, 26, 4529. https://doi.org/10.3390/ijms26104529
Wei Y, Zhou X, Gao M, Ou Y, Hu Y, Jiang W, Jiang H, Yang S. Different Endophytes Colonized in Various Lotus Root Varieties and Their Associated Mealy and Crunchy Properties. International Journal of Molecular Sciences. 2025; 26(10):4529. https://doi.org/10.3390/ijms26104529
Chicago/Turabian StyleWei, Yufei, Xinyan Zhou, Meiping Gao, Yangxiu Ou, Yifeng Hu, Wen Jiang, Huiping Jiang, and Shangdong Yang. 2025. "Different Endophytes Colonized in Various Lotus Root Varieties and Their Associated Mealy and Crunchy Properties" International Journal of Molecular Sciences 26, no. 10: 4529. https://doi.org/10.3390/ijms26104529
APA StyleWei, Y., Zhou, X., Gao, M., Ou, Y., Hu, Y., Jiang, W., Jiang, H., & Yang, S. (2025). Different Endophytes Colonized in Various Lotus Root Varieties and Their Associated Mealy and Crunchy Properties. International Journal of Molecular Sciences, 26(10), 4529. https://doi.org/10.3390/ijms26104529






