Extraenteric Malignant Gastrointestinal Neuroectodermal Tumor of the Neck: A Diagnostic Challenge
Abstract
:1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MGNET | malignant gastrointestinal neuroectodermal tumor |
| CCS | clear cell sarcoma |
| GI | gastrointestinal |
| WHO | World Health Organization |
| IHC | immunohistochemistry |
| PAS | periodic acid–Schiff (staining) |
| FISH | fluorescence in situ hybridization |
| CNIO | Centro Nacional de Investigaciones Oncológicas (National Cancer Research Center, Spain) |
| PET-CT | positron emission tomography–computed tomography |
| HPF | high-power field (used in microscopy) |
| ANED | alive with no evidence of disease |
| AWD | alive with disease |
| E-MGNET | extraenteric malignant gastrointestinal neuroectodermal tumor |
References
- Alpers, C.E.; Beckstead, J.H. Malignant neuroendocrine tumor of the jejunum with osteoclast-like giant cells. Enzyme histochemistry distinguishes tumor cells from giant cells. Am. J. Surg. Pathol. 1985, 9, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Stockman, D.L.; Miettinen, M.; Suster, S.; Spagnolo, D.; Dominguez-Malagon, H.; Hornick, J.L.; Adsay, V.; Chou, P.M.; Amanuel, B.; Vantuinen, P.; et al. Malignant gastrointestinal neuroectodermal tumor: Clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am. J. Surg. Pathol. 2012, 36, 857–868. [Google Scholar] [CrossRef]
- Washimi, K.; Takagi, M.; Hisaoka, M.; Kawachi, K.; Takeyama, M.; Hiruma, T.; Narimatsu, H.; Yokose, T. Clear cell sarcoma-like tumor of the gastrointestinal tract: A clinicopathological review. Pathol. Int. 2017, 67, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, E.; Reyes-Mugica, M.; Franchi, A.; Rosai, J. Clear cell sarcoma-like tumor of the gastrointestinal tract: An immunohistochemical, ultrastructural, and molecular study of a distinctive GI neoplasm. Mod. Pathol. 2003, 16, 502–511. [Google Scholar]
- Antonescu, C.R.; Nafa, K.; Segal, N.H. Genomic hallmarks of GNET and its clinical implications. Clin. Cancer Res. 2016, 22, 4620–4628. [Google Scholar]
- Kuo, C.T.; Kao, Y.C.; Huang, H.Y.; Hsiao, C.H.; Lee, J.C. Malignant gastrointestinal neuroectodermal tumor in head and neck: Two challenging cases with diverse morphology and different considerations for differential diagnosis. Virchows Arch. 2022, 481, 131–136. [Google Scholar] [CrossRef] [PubMed]
- French, C.A. EWS-ATF1 in malignant clear cell tumors. Oncogene 2010, 29, 5043–5051. [Google Scholar]
- Fletcher, C.D.; Unni, K.K.; Mertens, F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone; IARC Press: Lyon, France, 2002. [Google Scholar]
- Breton, S.; Dubois, M.; Geay, J.F.; Gillebert, Q.; Tordjman, M.; Guinebretière, J.M.; Denoux, Y. Clear cell sarcoma or gastrointestinal neuroectodermal tumor (GNET) of the tongue? Case report and review of the literature of an extremely rare tumor localization. Ann. Pathol. 2019, 39, 167–171. [Google Scholar] [CrossRef]
- Sbaraglia, M.; Zanatta, L.; Toffolatti, L.; Spallanzani, A.; Bertolini, F.; Mattioli, F.; Lami, F.; Presutti, L.; Tos, A.P.D. Clear cell sarcoma-like/malignant gastrointestinal neuroectodermal tumor of the tongue: A clinicopathologic and molecular case report. Virchows Arch. 2021, 478, 1203–1207. [Google Scholar] [CrossRef]
- Allanson, B.M.; Weber, M.A.; Jackett, L.A.; Chan, C.; Lau, L.; Ziegler, D.S.; Warby, M.; Mayoh, C.; Cowley, M.J.; Tucker, K.M.; et al. Oral malignant gastrointestinal neuroectodermal tumour with junctional component mimicking mucosal melanoma. Pathology 2018, 50, 648–653. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Chen, S.; Han, A. Malignant gastrointestinal neuroectodermal tumour in soft tissue. Pathology 2021, 53, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Kraft, S.; Antonescu, C.R.; Rosenberg, A.E.; Deschler, D.G.; Nielsen, G.P. Primary clear cell sarcoma of the tongue. Arch. Pathol. Lab. Med. 2013, 137, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Baus, A.; Culie, D.; Duong, L.T.; Ben Lakhdar, A.; Schaff, J.B.; Janot, F.; Kolb, F. Primary clear cell sarcoma of the tongue and surgical reconstruction: About a rare case report. Ann. Chir. Plast. Esthet. 2019, 64, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Donzel, M.; Zidane-Marinnes, M.; Paindavoine, S.; Breheret, R.; de la Fouchardière, A. Clear cell sarcoma of the soft palate mimicking unclassified melanoma. Pathology 2019, 51, 331–334. [Google Scholar] [CrossRef]
- Argani, P.; Perez-Atayde, A.R.; Fletcher, C.D. Clear cell sarcoma of soft parts and gastrointestinal clear cell sarcoma-like tumor: Two distinct clinicopathologic entities. Am. J. Surg. Pathol. 2000, 24, 699–707. [Google Scholar]
- Chi, P.; D’Angelo, S.P.; Ryan, C.W. Gastrointestinal neuroectodermal tumor: A rare tumor with unique molecular characteristics. Cancer 2018, 124, 3460–3468. [Google Scholar]
- Çomunoglu, N.; Dervisoglu, S.; Elçin, B.; Tekant, G.; Apak, H. Malignant extragastrointestinal neuroectodermal tumor located at right cervical region. Open J. Pathol. 2015, 5, 125e128. [Google Scholar] [CrossRef]
- Ulici, V.; Hornick, J.L.; Davis, J.L.; Mehrotra, S.; Meis, J.M.; Halling, K.C.; Fletcher, C.D.M.; Kao, E.; Folpe, A.L. Extraenteric Malignant Gastrointestinal Neuroectodermal Tumor-A Clinicopathologic and Molecular Genetic Study of 11 Cases. Mod. Pathol. 2023, 36, 100160. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours, 5th ed.; WHO Classification of Tumours, Volume 3; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- van Diest, P.J.; van der Wall, E.; Baak, J.P. Prognostic value of proliferation in invasive breast cancer: A review. J. Clin. Pathol. 2004, 57, 675–681. [Google Scholar] [CrossRef]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Kalia, V.; Levine, P.; Ludwig, K. Malignant gastrointestinal neuroectodermal tumor: Treatment outcomes and review of the literature. J. Surg. Oncol. 2019, 120, 492–498. [Google Scholar]
- Schöffski, P.; Wozniak, A.; Stacchiotti, S.; Rutkowski, P.; Blay, J.Y.; Lindner, L.H.; Strauss, S.J.; Anthoney, A.; Duffaud, F.; Richter, S.; et al. Activity and safety of crizotinib in patients with advanced clear-cell sarcoma with MET alterations: European Organization for Research and Treatment of Cancer phase II trial 90101 ‘CREATE’. Ann. Oncol. 2017, 28, 3000–3008. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Holmes, O.; Gowen, K.; Spritz, D.; Amini, B.; Wang, W.L.; Schrock, A.B.; Meric-Bernstam, F.; Zinner, R.; Piha-Paul, S.; et al. Activity of c-Met/ALK inhibitor crizotinib and multi-kinase VEGF inhibitor pazopanib in metastatic gastrointestinal neuroectodermal tumor harboring EWSR1-CREB1 fusion. Oncology 2016, 91, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Cornillie, J.; van Cann, T.; Wozniak, A.; Hompes, D.; Schöffski, P. Biology and management of clear cell sarcoma: State of the art and future perspectives. Expert Rev. Anticancer. Ther. 2016, 16, 839–845. [Google Scholar] [CrossRef] [PubMed]
- El-Salam, M.A.A.; Troulis, M.J.; Pan, C.-X.; Rao, R.A. Unlocking the potential of organoids in cancer treatment and translational research: An application of cytologic techniques. Cancer Cytopathol. 2024, 132, 96–102. [Google Scholar] [CrossRef]
- Rivera, C.M.; Faquin, W.C.; Thierauf, J.; Afrogheh, A.H.; Jaquinet, A.; Iafrate, A.J.; Rivera, M.N.; Troulis, M.J. Detection of EWSR1 fusions in CCOC by targeted RNA-seq. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 240–244. [Google Scholar] [CrossRef]
- Xhori, O.; Deol, N.; Rivera, C.M.; Zavras, J.; Weil, S.; Zafari, H.; Thierauf, J.H.; Faquin, W.C.; Choy, E.; Rivera, M.N.; et al. A comparison of Clear Cell Sarcoma to Jaw and Salivary Tumors bearing EWS Fusions. Head. Neck Pathol. 2024, 18, 25. [Google Scholar] [CrossRef]
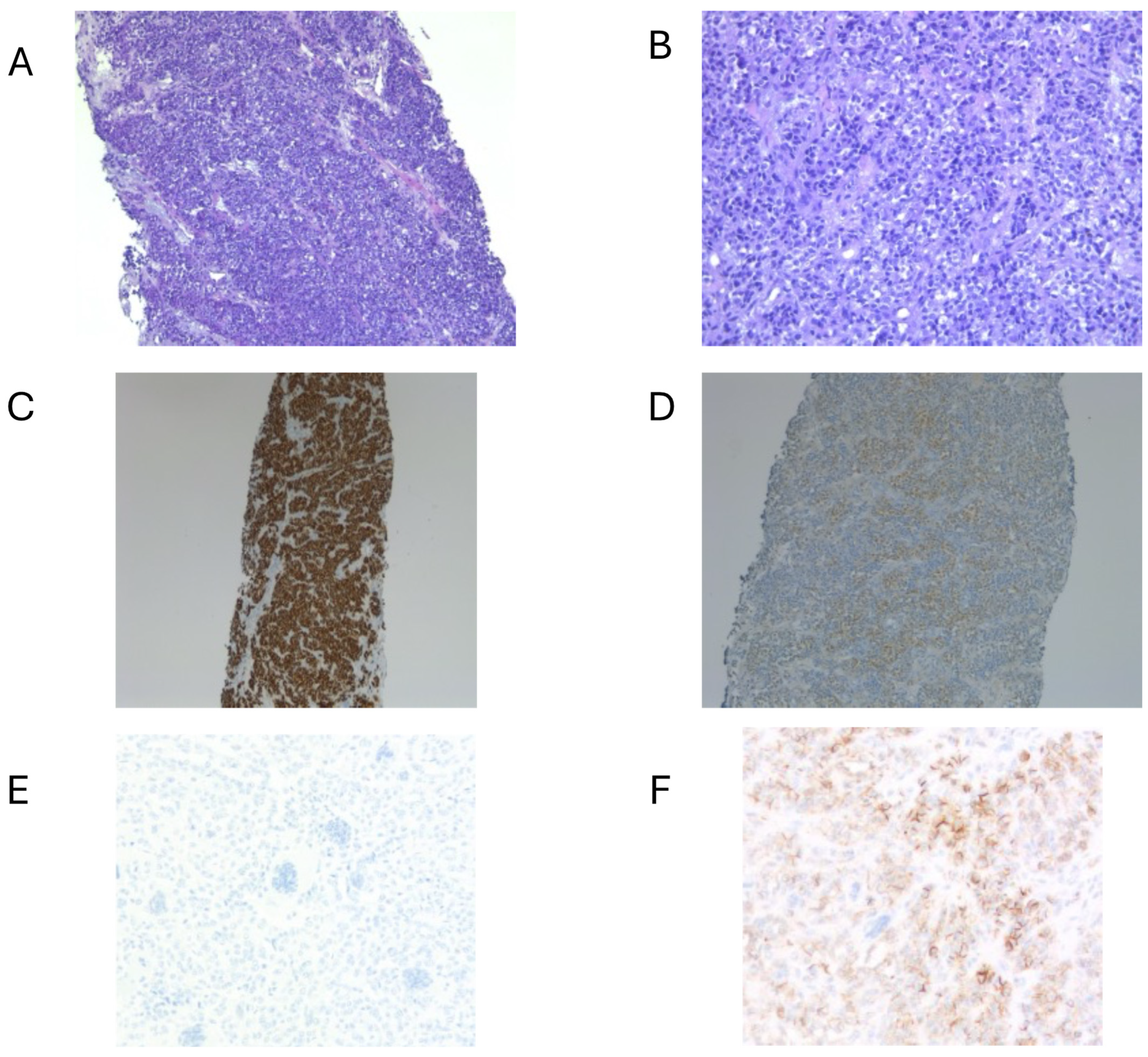
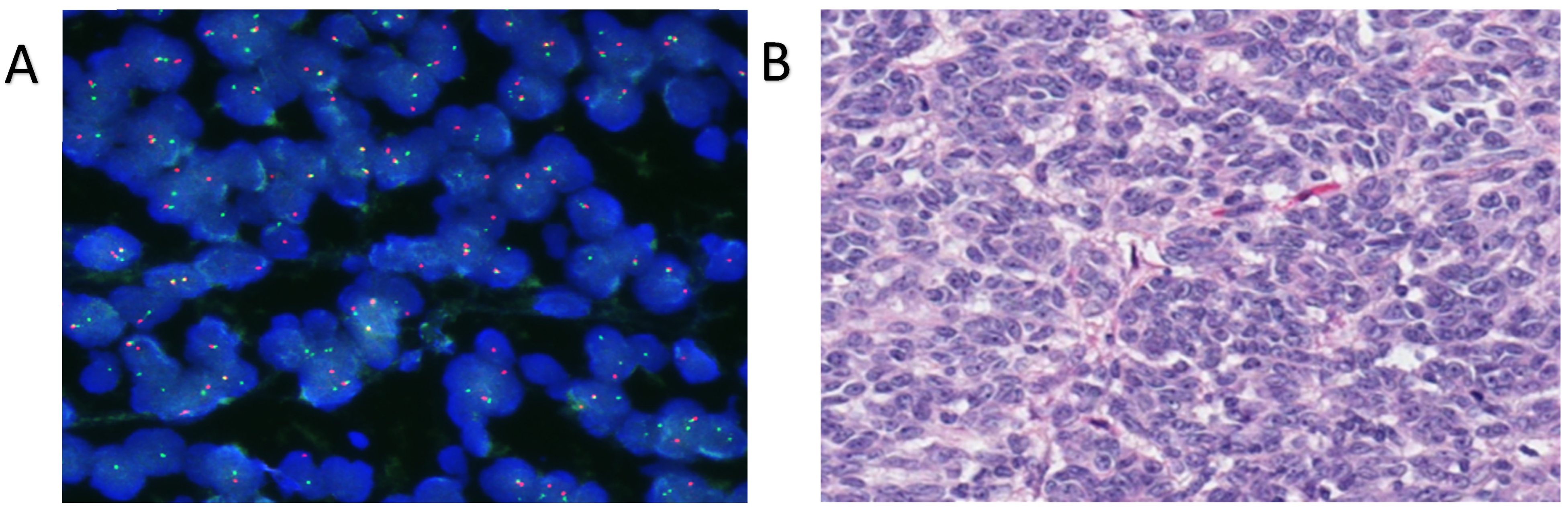
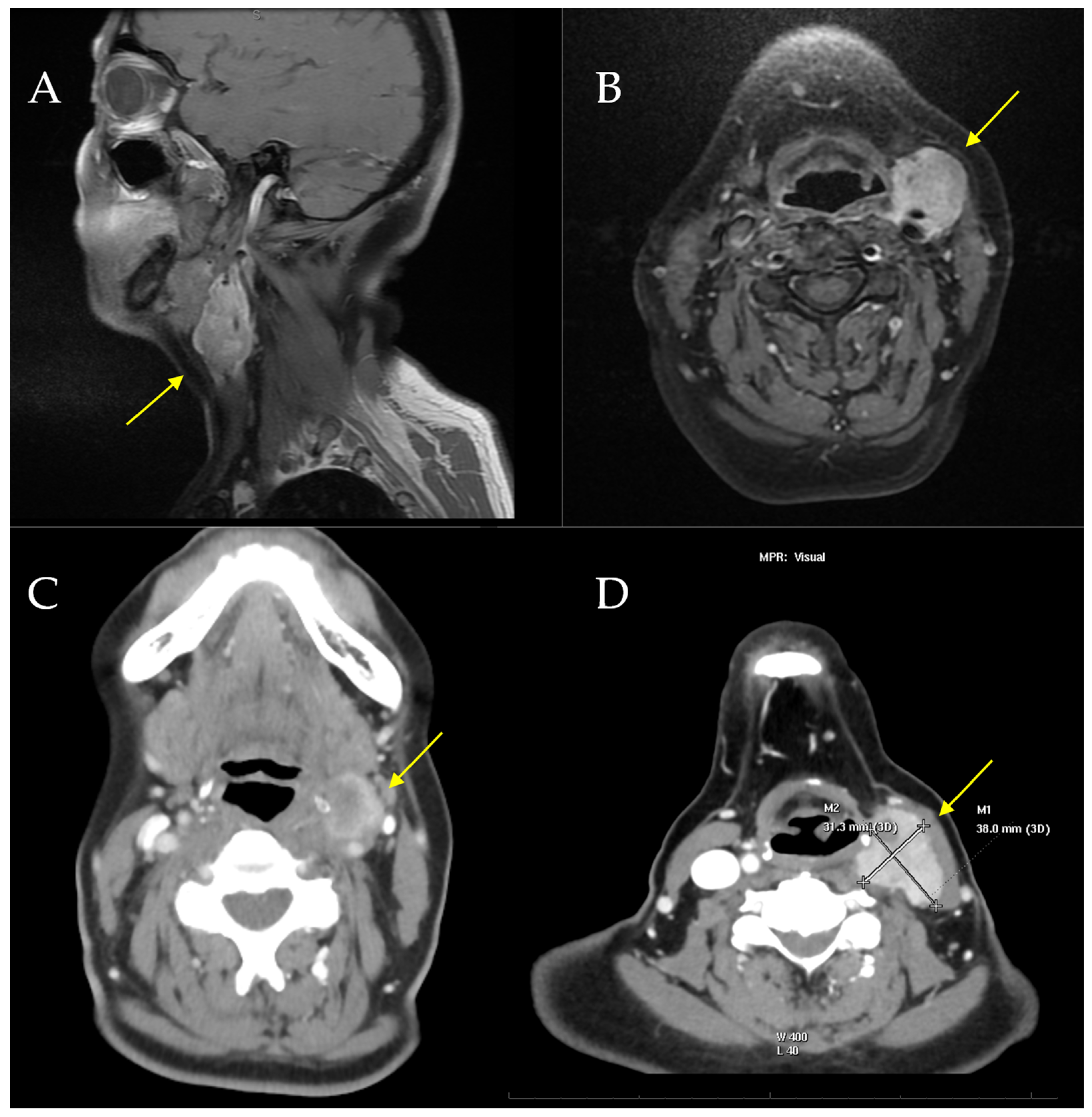
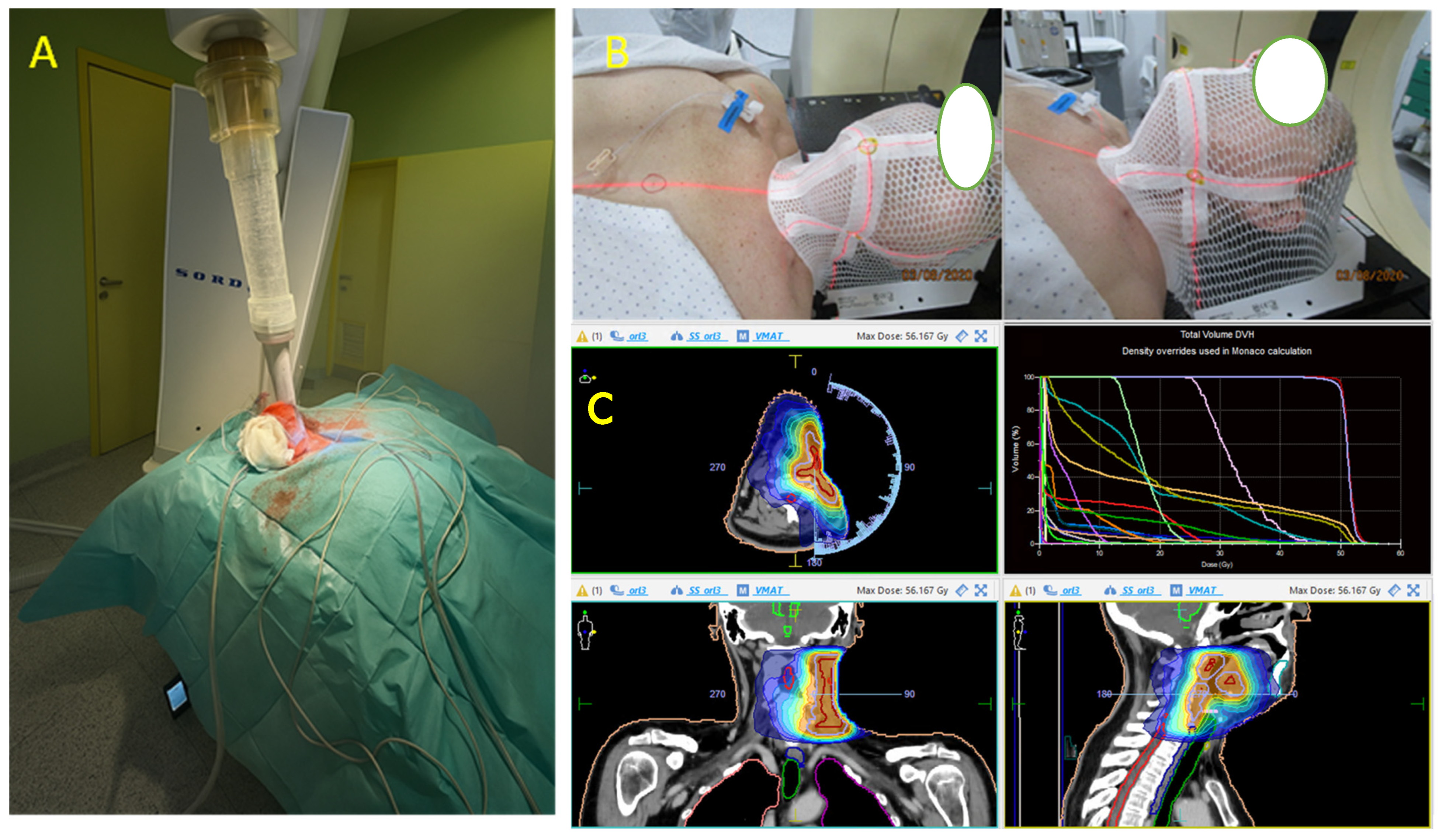

| Case | Age/Sex | Location/Size | IHC Results | Molecular Results | Therapy | Local Recurrence | Metastasis | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 [18] | 9/M | Right neck/5.5 cm | Positive: S100 protein, SOX10, synaptophysin, CD56. Negative: HMB45, Melan A, MART-1, TTF1, EMA, pancytokeratin, cytokeratin AE1/AE3, CAM5.2, CK5/6, p63, CD31, SMA, desmin, synaptophysin, chromogranin, GFAP, CD68 | EWSR1 gene locus was attempted on the specimen but was technically unsatisfactory | Chemotherapy | No | No | ANED at 12 mo |
| 2 [19] | 14/M | Right neck/3.7 cm | Positive: S100 protein, SOX10, synaptophysin, CD56; Negative: Keratins, HMB-45, Melan-A, MiTF, chromogranin A, CD117, DOG1, ALK | EWSR1 exon 8::ATF1 exon 4 | Resection with + margin and radiotherapy | No: persistent local disease, undergoing radiotherapy | No | AWD at 11 mo |
| 3 [19] | 30/M | Right neck/NA | Positive: S100 protein, SOX10, synaptophysin, CD56; Negative: Keratins, HMB-45, Melan-A, MiTF, chromogranin A, CD117 | EWSR1 exon 8::ATF1 exon 4 | No radical resection and radiotherapy | Yes: 57 mo | No | ANED at 70 mo |
| 4 [19] | 48/F | Right neck/5.5 cm | Positive: S100 protein, SOX10; Negative: Keratins, synaptophysin, MiTF, HMB-45, Melan-A, chromogranin A, CD117, ALK | EWSR1 exon 7::ATF1 exon 5 | No radical resection | No | No | ANED at 10 mo |
| 5 [our case] | 58/F | Left neck/4 cm | Positive: S100, SOX10, CD99 (weak/focal), Fli-1, and synaptophysin (focal). Negative: Melan-A, HMB-45, CKIT, AE1/AE3 | EWSR1 gene translocation | Surgery (radical resection) and radiotherapy | Yes: 48 mo | Yes: 36 mo | Died: 54 mo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tousidonis, M.; Troulis, M.J.; Agra, C.; Alijo, F.; Alvarez-Gonzalez, A.; Navarro-Cuellar, C.; Khayat, S.; Ruiz-de-Leon, G.; Lopez-Lopez, A.M.; Salmeron, J.I.; et al. Extraenteric Malignant Gastrointestinal Neuroectodermal Tumor of the Neck: A Diagnostic Challenge. Int. J. Mol. Sci. 2025, 26, 4517. https://doi.org/10.3390/ijms26104517
Tousidonis M, Troulis MJ, Agra C, Alijo F, Alvarez-Gonzalez A, Navarro-Cuellar C, Khayat S, Ruiz-de-Leon G, Lopez-Lopez AM, Salmeron JI, et al. Extraenteric Malignant Gastrointestinal Neuroectodermal Tumor of the Neck: A Diagnostic Challenge. International Journal of Molecular Sciences. 2025; 26(10):4517. https://doi.org/10.3390/ijms26104517
Chicago/Turabian StyleTousidonis, Manuel, Maria J. Troulis, Carolina Agra, Francisco Alijo, Ana Alvarez-Gonzalez, Carlos Navarro-Cuellar, Saad Khayat, Gonzalo Ruiz-de-Leon, Ana Maria Lopez-Lopez, Jose Ignacio Salmeron, and et al. 2025. "Extraenteric Malignant Gastrointestinal Neuroectodermal Tumor of the Neck: A Diagnostic Challenge" International Journal of Molecular Sciences 26, no. 10: 4517. https://doi.org/10.3390/ijms26104517
APA StyleTousidonis, M., Troulis, M. J., Agra, C., Alijo, F., Alvarez-Gonzalez, A., Navarro-Cuellar, C., Khayat, S., Ruiz-de-Leon, G., Lopez-Lopez, A. M., Salmeron, J. I., & Ochandiano, S. (2025). Extraenteric Malignant Gastrointestinal Neuroectodermal Tumor of the Neck: A Diagnostic Challenge. International Journal of Molecular Sciences, 26(10), 4517. https://doi.org/10.3390/ijms26104517









