Unveiling the Multifaceted Role of HP6: A Critical Regulator of Humoral Immunity in Antheraea pernyi (Lepidoptera: Saturniidae)
Abstract
1. Introduction
2. Results
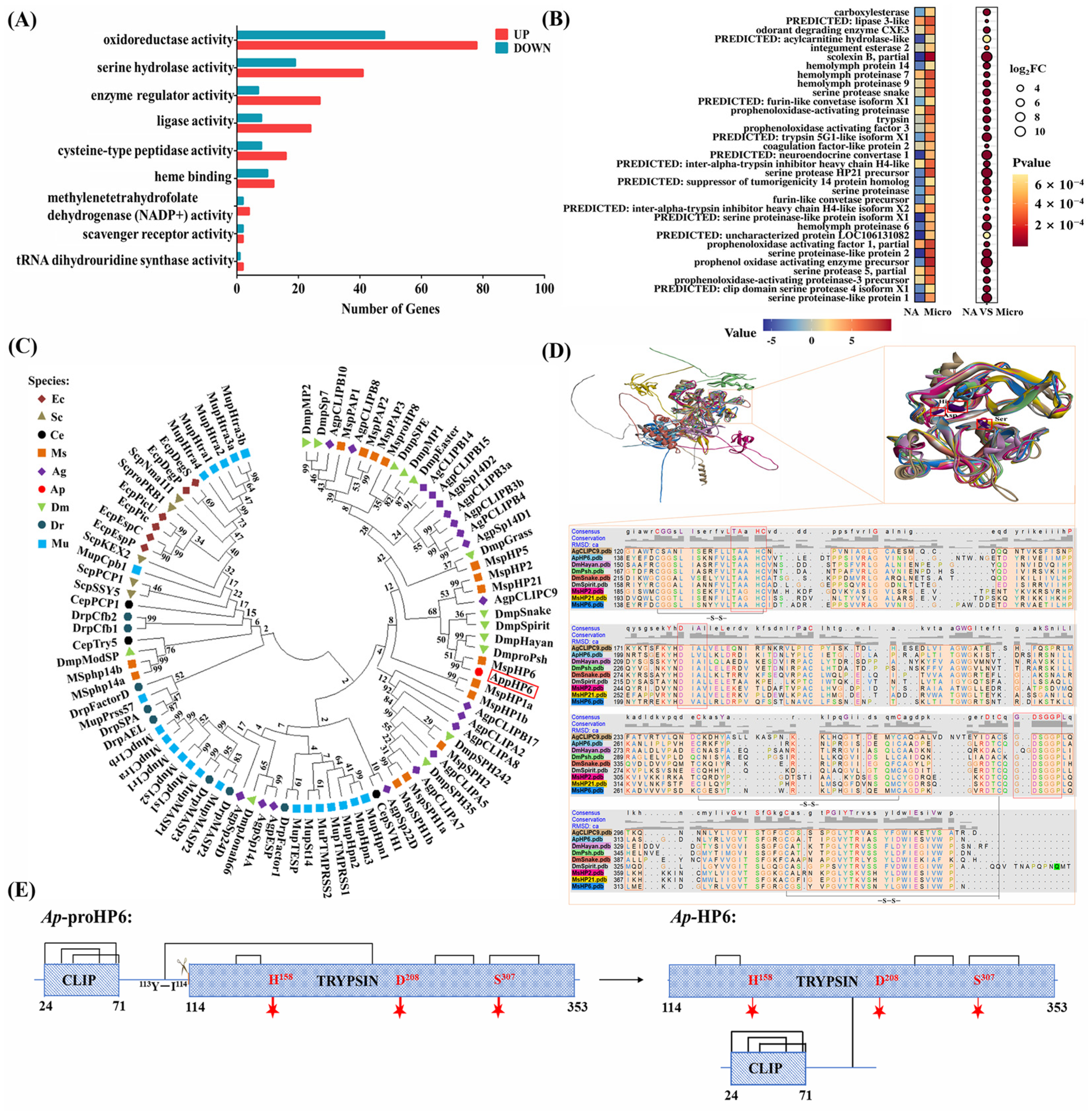
2.1. Ap-HP6 Is Involved in the Immune Response of A. pernyi
2.2. Expression Profiles of Ap-HP6 in Response to Immune Challenge
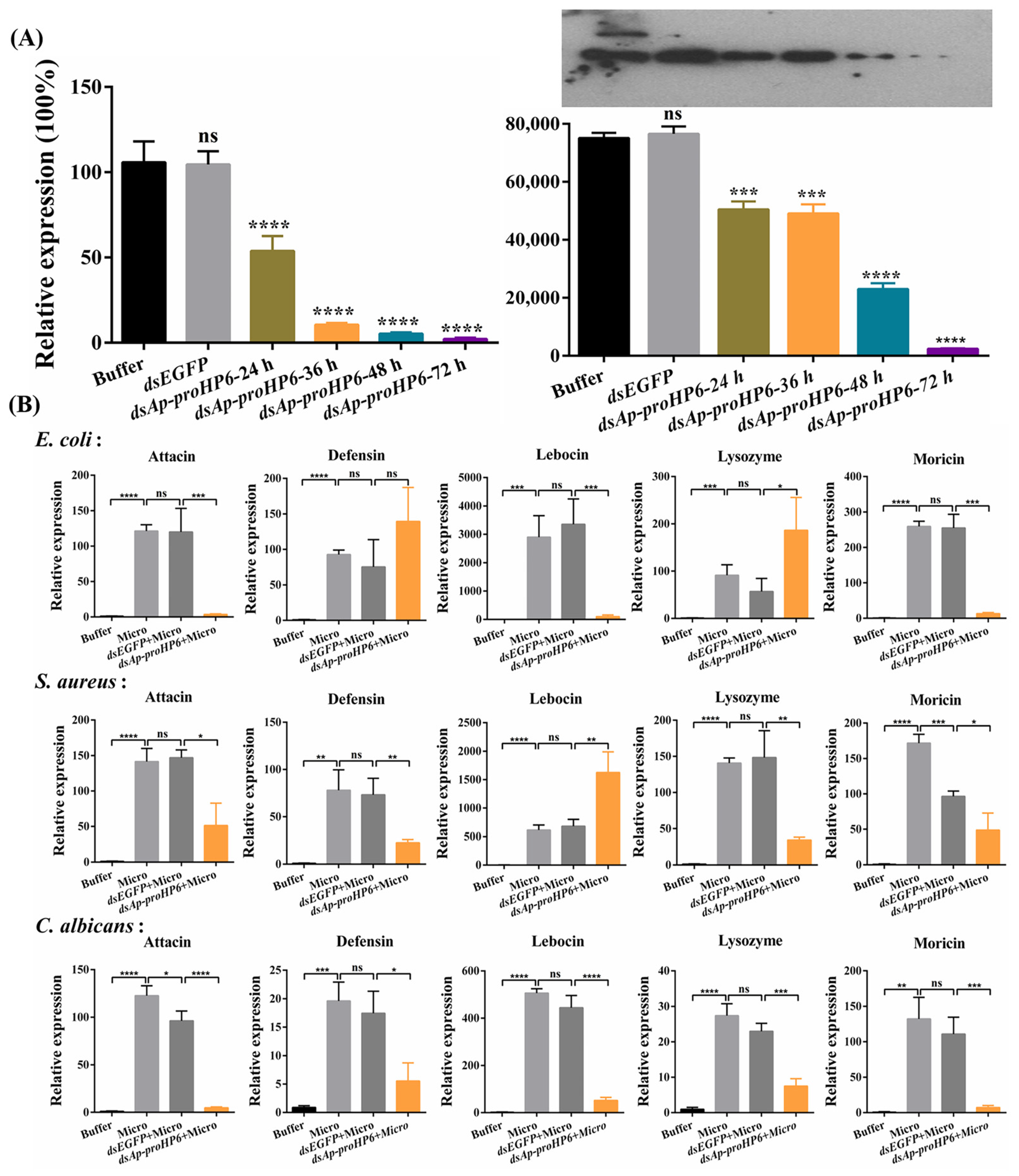
2.3. Involvement of Ap-HP6 in AMPs Production
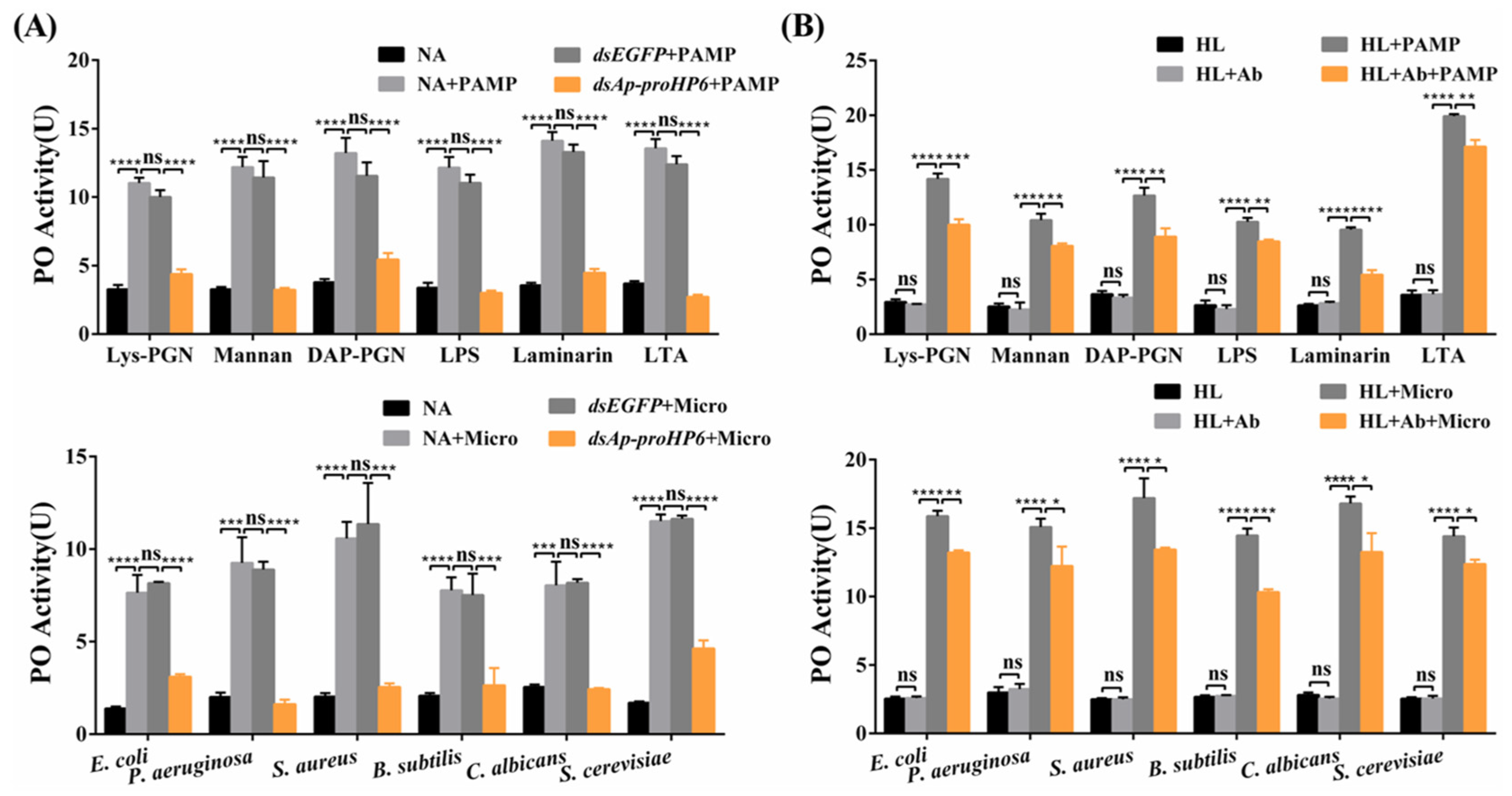
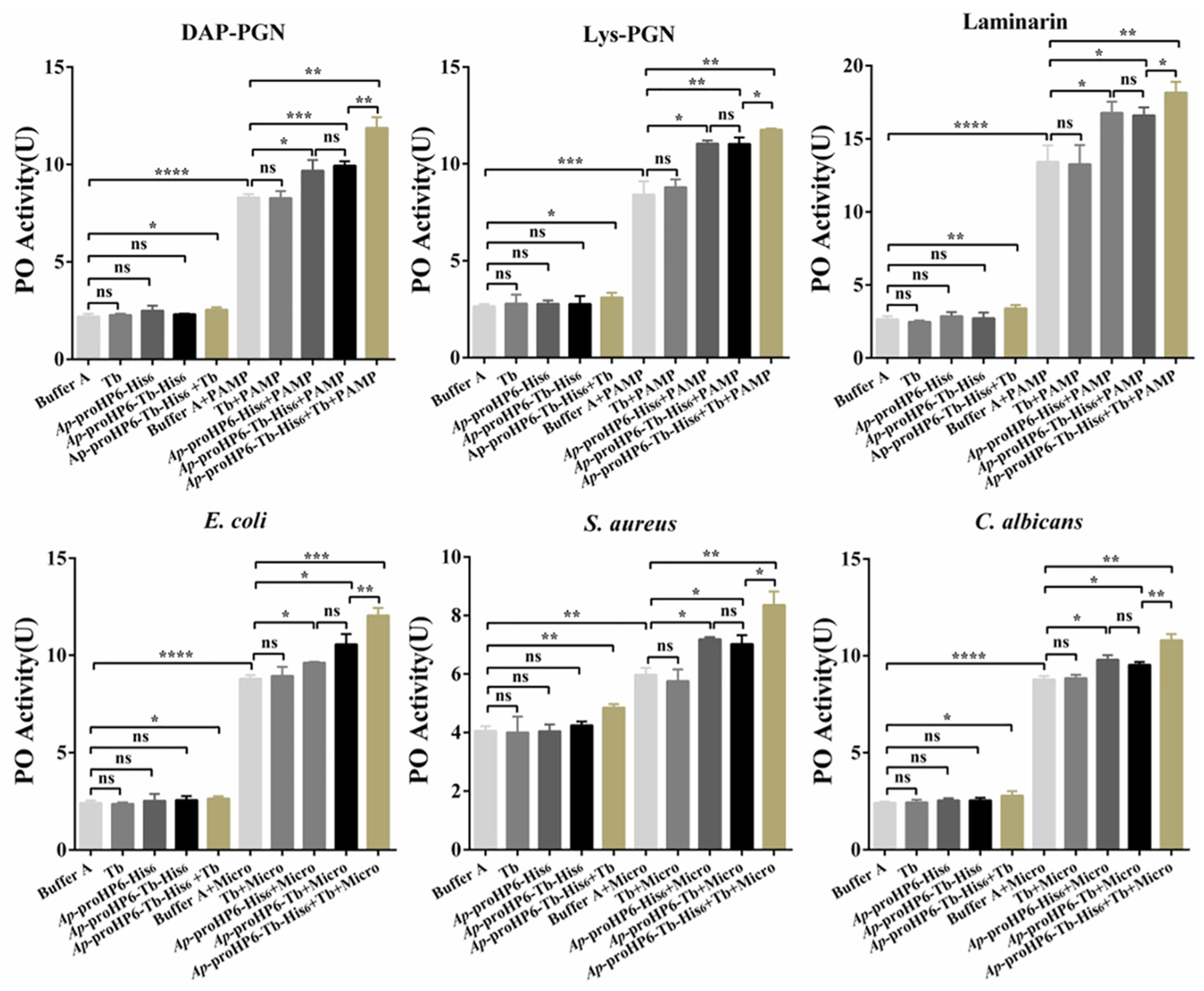
2.4. Contribution of Ap-HP6 to the PPO Activation System in A. pernyi
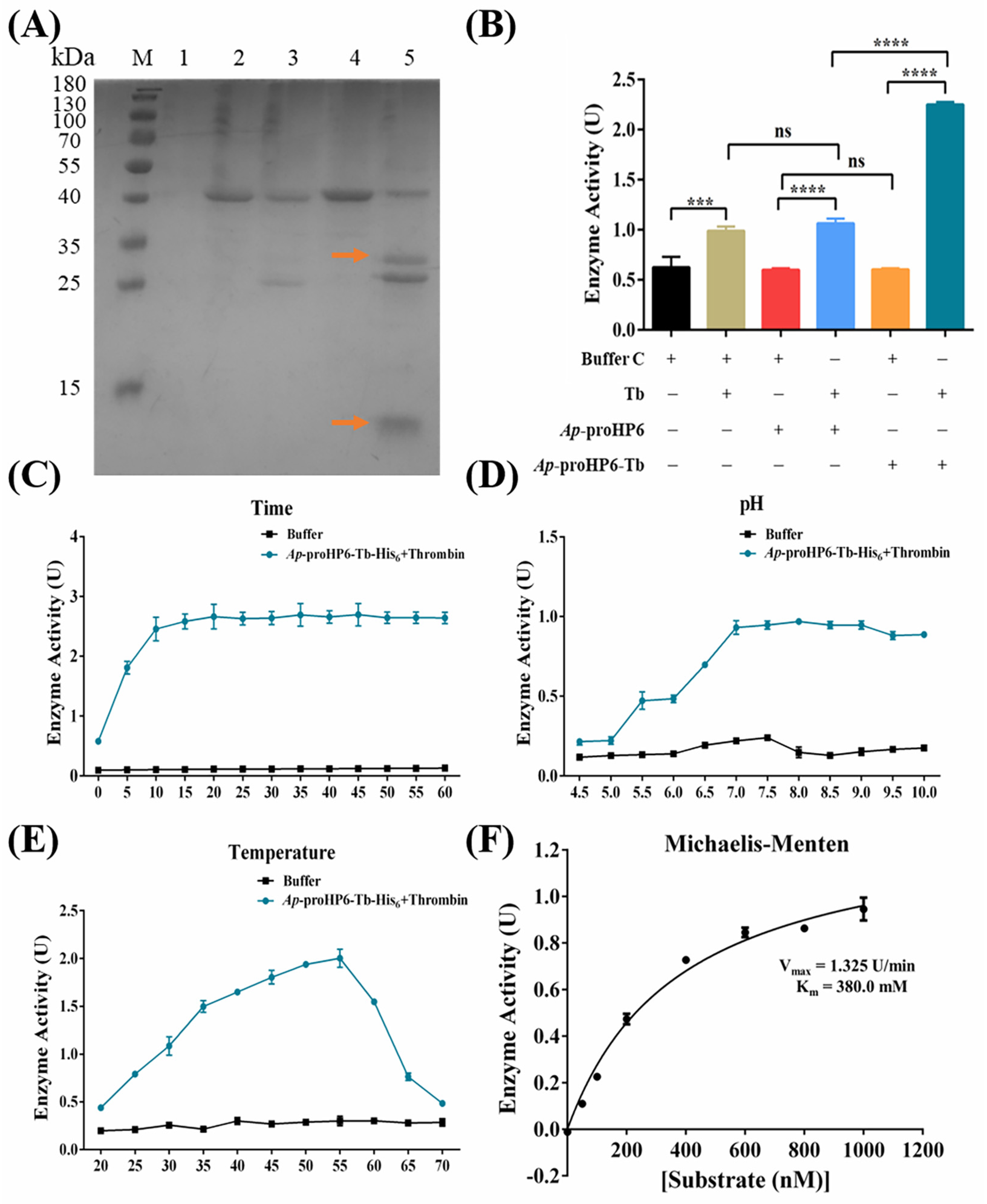
2.5. Serine Protease Enzymatic Activity Assay of rAp-HP6
2.6. The Effect of Exogenous rAp-HP6 on PP0-AS
3. Discussion
4. Materials and Methods
4.1. Insects, Microorganisms, and PAMPs
4.2. RNA Isolation and RNA-seq
4.3. Sequence Analysis and Alignment
4.4. Isolation and Purification of Recombinant Protein and Preparation of the Polyclonal Antibody
4.5. Expression Properties in Response to Pathogen Injection
4.6. Detection of RNAi Efficiency
4.7. Transcription Level of AMPs in Response to Ap-HP6 Depletion
4.8. PO Activity Assay
4.9. Determined Enzymatic Catalysis Conditions
4.9.1. Determination of the Catalytic Activity of rAp-HP6
4.9.2. Effect of Time on the Enzyme Activity of rAp-HP6
4.9.3. Effect of pH on the Enzyme Activity of rAp-HP6
4.9.4. Effect of Temperature on the Enzyme Activity of rAp-HP6
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPO | Prophenoloxidase |
| PO | Phenoloxidase |
| PPO-AS | Prophenoloxidase activating system |
| AMP | Antimicrobial peptide |
| Ap-HP6 | Hemolymph protease 6 of Antheraea pernyi |
| RNAi | RNA interference |
| PAMPs | Pathogen-associated molecular patterns |
| SPH | Serine protease homolog |
| Tb | Thrombin |
| PVDF | Polyvinylidene difluoride |
| EGFP | Enhanced green fluorescent protein |
References
- Zhu, L.; Wang, N.; Guo, G.; Fan, Z.; Shi, X.; Ji, X. Male zooid extracts of Antheraea pernyi ameliorates non-alcoholic fatty liver disease and intestinal dysbacteriosis in mice induced by a high-fat diet. Front. Cell. Infect. Microbiol. 2022, 12, 1059647. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Yang, Z.; Zhang, X.; Li, M.; Han, N.; Yang, X.; He, J.; Yu, G.; Meng, X.; Jia, Q.; et al. Fermented cordyceps powder alleviates silica-induced pulmonary inflammation and fibrosis in rats by regulating the Th immune response. Chin. Med. 2023, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Jang, H.-J.; Yang, K.E.; Jang, M.S.; Huh, Y.H.; Yoo, H.-S.; Park, J.S.; Jang, I.-S.; Park, S.J. Cordyceps militaris induces apoptosis in ovarian cancer cells through TNF-α/TNFR1-mediated inhibition of NF-κB phosphorylation. BMC Complement. Med. Ther. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.C.M.C.; Ramos, I.B.; Travassos, L.H.; Mendez, A.P.G.; Gomes, F.M. Evolution of innate immunity: Lessons from mammalian models shaping our current view of insect immunity. J. Comp. Physiol. B 2024, 194, 105–119. [Google Scholar] [CrossRef]
- Aggarwal, C.; Paul, S.; Nain, V.; Tripathi, V.; Paul, B.; Aslam Khan, M. Comparative response of Spodoptera litura challenged per os with Serratia marcescens strains differing in virulence. J. Invertebr. Pathol. 2021, 183, 107562. [Google Scholar] [CrossRef]
- Yu, S.; Luo, F.; Xu, Y.; Zhang, Y.; Jin, L.H. Drosophila Innate Immunity Involves Multiple Signaling Pathways and Coordinated Communication Between Different Tissues. Front. Immunol. 2022, 13, 905370. [Google Scholar] [CrossRef]
- Aalto, A.L.; Luukkonen, V.; Meinander, A. Ubiquitin signalling in Drosophila innate immune responses. FEBS J. 2023, 291, 4397–4413. [Google Scholar] [CrossRef]
- Singh, S.R.; Zeng, X.; Zhao, J.; Liu, Y.; Hou, G.; Liu, H.; Hou, S.X. The lipolysis pathway sustains normal and transformed stem cells in adult Drosophila. Nature 2016, 538, 109–113. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Q.; Shen, W.; Jiang, Z.; Gu, X.; Li, F.; Li, B.; Wei, J. The Toll/IMD pathways mediate host protection against dipteran parasitoids. J. Insect Physiol. 2024, 153, 104614. [Google Scholar] [CrossRef]
- Muhammad, A.; Sun, C.; Shao, Y. The humoral immune response of the lepidopteran model insect, silkworm Bombyx mori L., to microbial pathogens. Curr. Res. Insect Sci. 2024, 6, 100097. [Google Scholar] [CrossRef]
- Lu, Y.; Su, F.; Li, Q.; Zhang, J.; Li, Y.; Tang, T.; Hu, Q.; Yu, X.-Q. Pattern recognition receptors in Drosophila immune responses. Dev. Comp. Immunol. 2020, 102, 103468. [Google Scholar] [CrossRef] [PubMed]
- Kwankaew, P.; Madsari, N.; Thongsoi, R.; Utarabhand, P.; Runsaeng, P. Effects of the interaction between a clip domain serine protease and a white spot syndrome virus protein on phenoloxidase activity. Dev. Comp. Immunol. 2022, 130, 104360. [Google Scholar] [CrossRef]
- Gonias, S.L. Plasminogen activator receptor assemblies in cell signaling, innate immunity, and inflammation. Am. J. Physiol.-Cell Physiol. 2021, 321, C721–C734. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, C.; Huang, M. A general strategy to inhibit serine protease by targeting its autolysis loop. FASEB J. 2021, 35, e21259. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Sousa, G.L.; Bishnoi, R.; Baxter, R.H.G.; Povelones, M. The CLIP-domain serine protease CLIPC9 regulates melanization downstream of SPCLIP1, CLIPA8, and CLIPA28 in the malaria vector Anopheles gambiae. PLoS Pathog. 2020, 16, e1008985. [Google Scholar] [CrossRef]
- Si, Q.; Huang, Y.; Mao, W.-l.; Wang, T.-w.; Qin, W.; Cai, B.-b.; Ren, Q. Characterization of a serine protease homolog from Macrobrachium nipponense and its involvement in AMP synthesis and proPO activation. Fish Shellfish Immunol. 2025, 158, 110177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, G.H.; Dong, Z.M.; Duan, J.; Xu, P.Z.; Cheng, T.C.; Xiang, Z.H.; Xia, Q.Y. Genome-wide identification and expression analysis of serine proteases and homologs in the silkworm Bombyx mori. BMC Genom. 2010, 11, 405. [Google Scholar] [CrossRef]
- Li, W.; Dou, W.; Wang, J.J. BdcSP10 is a prophenoloxidase-activating protease in Bactrocera dorsalis. Dev. Comp. Immunol. 2023, 138, 104558. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Xiao, K.R.; Wang, L.Z.; Wang, J.; Song, Q.S.; Stanley, D.; Wei, S.J.; Zhu, J.Y. Identification and expression profiling of serine protease-related genes in Tenebrio molitor. Arch. Insect Biochem. Physiol. 2022, 111, e21963. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, H. Building a platform for predicting functions of serine protease-related proteins in Drosophila melanogaster and other insects. Insect Biochem. Mol. Biol. 2018, 103, 53–69. [Google Scholar] [CrossRef]
- Alvarenga, P.H.; Alves e Silva, T.L.; Suzuki, M.; Nardone, G.; Cecilio, P.; Vega-Rodriguez, J.; Ribeiro, J.M.C.; Andersen, J.F. Comprehensive Proteomics Analysis of the Hemolymph Composition of Sugar-Fed Aedes aegypti Female and Male Mosquitoes. J. Proteome Res. 2024, 23, 1471–1487. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Heng, J.; Wang, L.; Tang, X.; Guo, P.; Li, Y.; Xia, Q.; Zhao, P. Identification, characterization, and expression analysis of clip-domain serine protease genes in the silkworm, Bombyx mori. Dev. Comp. Immunol. 2020, 105, 103584. [Google Scholar] [CrossRef]
- Keshavarz, M.; Jo, Y.H.; Edosa, T.T.; Bae, Y.M.; Han, Y.S. TmPGRP-SA regulates Antimicrobial Response to Bacteria and Fungi in the Fat Body and Gut of Tenebrio molitor. Int. J. Mol. Sci. 2020, 21, 2113. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.A.; Kojour, M.A.M.; Patnaik, B.B.; Han, Y.S.; Jo, Y.H. Current Status of Immune Deficiency Pathway in Tenebrio molitor Innate Immunity. Front. Immunol. 2022, 13, 906192. [Google Scholar] [CrossRef]
- Bae, Y.M.; Jo, Y.H.; Patnaik, B.B.; Kim, B.B.; Park, K.B.; Edosa, T.T.; Keshavarz, M.; Kojour, M.A.M.; Lee, Y.S.; Han, Y.S. Tenebrio molitor Spätzle 1b Is Required to Confer Antibacterial Defense Against Gram-Negative Bacteria by Regulation of Antimicrobial Peptides. Front. Physiol. 2021, 12, 758859. [Google Scholar] [CrossRef]
- Mollah, M.M.I. Spätzle processing enzyme is required to activate dorsal switch protein 1 induced Toll immune signalling pathway in Tenebrio molitor. PLoS ONE 2023, 18, e0291976. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Hu, Y.; Jiang, H. Manduca sexta hemolymph protease-2 (HP2) activated by HP14 generates prophenoloxidase-activating protease-2 (PAP2) in wandering larvae and pupae. Insect Biochem. Mol. Biol. 2018, 101, 57–65. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cao, X.; Zhang, S.; Rogers, J.; Hartson, S.; Jiang, H. Changes in the Plasma Proteome of Manduca sexta Larvae in Relation to the Transcriptome Variations after an Immune Challenge: Evidence for High Molecular Weight Immune Complex Formation. Mol. Cell. Proteom. 2016, 15, 1176–1187. [Google Scholar] [CrossRef]
- Wang, Q.; Yin, M.; Yuan, C.; Liu, X.; Jiang, H.; Wang, M.; Zou, Z.; Hu, Z. The Micrococcus luteus infection activates a novel melanization pathway of cSP10, cSP4, and cSP8 in Helicoverpa armigera. Insect Biochem. Mol. Biol. 2022, 147, 103775. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Sumathipala, N.; Cao, X.; Kanost, M.R.; Jiang, H. Manduca sexta serpin-12 controls the prophenoloxidase activation system in larval hemolymph. Insect Biochem. Mol. Biol. 2018, 99, 27–36. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Cao, X.; Zou, Z.; Lu, Z.; Kanost, M.R.; Jiang, H. Hemolymph protease-5 links the melanization and Toll immune pathways in the tobacco hornworm, Manduca sexta. Proc. Natl. Acad. Sci. USA 2020, 117, 23581–23587. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Yang, F.; Jiang, H. Manduca sexta hemolymph protease-1, activated by an unconventional non-proteolytic mechanism, mediates immune responses. Insect Biochem. Mol. Biol. 2017, 84, 23–31. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Ishibashi, J.; Ragan, E.J.; Jiang, H.; Kanost, M.R. Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways. J. Biol. Chem. 2009, 284, 19716–19726. [Google Scholar] [CrossRef]
- Shan, T.; Wang, Y.; Bhattarai, K.; Jiang, H. An evolutionarily conserved serine protease network mediates melanization and Toll activation in Drosophila. Sci. Adv. 2023, 9, eadk2756. [Google Scholar] [CrossRef]
- Evans, C.J.; Liu, T.; Girard, J.R.; Banerjee, U. Injury-induced inflammatory signaling and hematopoiesis in Drosophila. Proc. Natl. Acad. Sci. USA 2022, 119, e2119109119. [Google Scholar] [CrossRef]
- Contreras, E.G.; Glavic, Á.; Brand, A.H.; Sierralta, J.A. The Serine Protease Homolog, Scarface, Is Sensitive to Nutrient Availability and Modulates the Development of the Drosophila Blood–Brain Barrier. J. Neurosci. 2021, 41, 6430–6448. [Google Scholar] [CrossRef] [PubMed]
- Perrimon, N.; Nakano, S.; Kashio, S.; Nishimura, K.; Takeishi, A.; Kosakamoto, H.; Obata, F.; Kuranaga, E.; Chihara, T.; Yamauchi, Y.; et al. Damage sensing mediated by serine proteases Hayan and Persephone for Toll pathway activation in apoptosis-deficient flies. PLoS Genet. 2023, 19, e1010761. [Google Scholar] [CrossRef]
- Dudzic, J.P.; Hanson, M.A.; Iatsenko, I.; Kondo, S.; Lemaitre, B. More Than Black or White: Melanization and Toll Share Regulatory Serine Proteases in Drosophila. Cell Rep. 2019, 27, 1050–1061.e3. [Google Scholar] [CrossRef]
- Gorman, M.J.; Paskewitz, S.M. Serine proteases as mediators of mosquito immune responses. Insect Biochem. Mol. Biol. 2001, 31, 257–262. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; He, Y.; Jiang, H. In search of a function of Manduca sexta hemolymph protease-1 in the innate immune system. Insect Biochem. Mol. Biol. 2016, 76, 1–10. [Google Scholar] [CrossRef]
- Zakhia, R.; Osta, M.A. CLIPA7 Exhibits Pleiotropic Roles in the Anopheles gambiae Immune Response. J. Innate Immun. 2023, 15, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Wang, Q.H.; Bhowmick, B.; Li, Y.X.; Han, Q. Functional characterization of two clip domain serine proteases in innate immune responses of Aedes aegypti. Parasites Vectors 2021, 14, 584. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.E.; Park, K.B.; Ko, H.J.; Keshavarz, M.; Bae, Y.M.; Kim, B.; Patnaik, B.B.; Jang, H.A.; Lee, Y.S.; Han, Y.S.; et al. Aedes albopictus Autophagy-Related Gene 8 (AaAtg8) Is Required to Confer Anti-Bacterial Gut Immunity. Int. J. Mol. Sci. 2020, 21, 2944. [Google Scholar] [CrossRef]
- Xie, Y.; Hua, H.; Zhou, P. Magnolol as a potent antifungal agent inhibits Candida albicans virulence factors via the PKC and Cek1 MAPK signaling pathways. Front. Cell. Infect. Microbiol. 2022, 12, 935322. [Google Scholar] [CrossRef]
- Qiu, C.; Yuan, Z.; He, Z.; Chen, H.; Liao, Y.; Li, S.; Zhou, W.; Song, Z. Lipopolysaccharide Preparation Derived from Porphyromonas gingivalis Induces a Weaker Immuno-Inflammatory Response in BV-2 Microglial Cells Than Escherichia coli by Differentially Activating TLR2/4-Mediated NF-κB/STAT3 Signaling Pathways. Front. Cell. Infect. Microbiol. 2021, 11, 606986. [Google Scholar] [CrossRef] [PubMed]
- Hixson, B.; Huot, L.; Morejon, B.; Yang, X.; Nagy, P.; Michel, K.; Buchon, N. The transcriptional response in mosquitoes distinguishes between fungi and bacteria but not Gram types. BMC Genom. 2024, 25, 353. [Google Scholar] [CrossRef]
- He, X.; Zhou, T.; Cai, Y.; Liu, Y.; Zhao, S.; Zhang, J.; Wang, X.; Zhang, R. A Versatile Hemolin with Pattern Recognitional Contributions to the Humoral Immune Responses of the Chinese Oak Silkworm Antheraea pernyi. Front. Immunol. 2022, 13, 904862. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, B.Y.; Choo, Y.M.; Jin, B.R. Dual role of the serine protease homolog BmSPH-1 in the development and immunity of the silkworm Bombyx mori. Dev. Comp. Immunol. 2018, 85, 170–176. [Google Scholar] [CrossRef]
- Vadivel, K.; Schmidt, A.E.; Cascio, D.; Padmanabhan, K.; Krishnaswamy, S.; Brandstetter, H.; Bajaj, S.P. Structure of human factor VIIa–soluble tissue factor with calcium, magnesium and rubidium. Acta Crystallogr. Sect. D Struct. Biol. 2021, 77, 809–819. [Google Scholar] [CrossRef]
- Duan, X.; Fu, T.; Liu, C.; Wang, F.; Liu, C.; Zhao, L.; Yu, J.; Wang, X.; Zhang, R. The role of a novel secretory peptidoglycan recognition protein with antibacterial ability from the Chinese Oak Silkworm Antheraea pernyi in humoral immunity. Insect Biochem. Mol. Biol. 2024, 171, 104151. [Google Scholar] [CrossRef]
- Qie, X.; Yan, X.; Wang, W.; Liu, Y.; Zhang, L.; Hao, C.; Lu, Z. Serpin-4 Negatively Regulates Prophenoloxidase Activation and Antimicrobial Peptide Synthesis in the Silkworm, Bombyx mori. Int. J. Mol. Sci. 2023, 25, 313. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.R.; Sazonova, I.Y.; Erdem, S.S.; Hara, T.; Thompson, B.D.; Patel, P.; Botnaru, I.; Lin, C.P.; Reed, G.L.; Weissleder, R.; et al. Multifunctional nanoagent for thrombus-targeted fibrinolytic therapy. Nanomedicine 2012, 7, 1017–1028. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Yu, J.; Fu, T.; He, X.; Zhao, L.; Wang, X.; Zhang, R. Unveiling the Multifaceted Role of HP6: A Critical Regulator of Humoral Immunity in Antheraea pernyi (Lepidoptera: Saturniidae). Int. J. Mol. Sci. 2025, 26, 4514. https://doi.org/10.3390/ijms26104514
Liu C, Yu J, Fu T, He X, Zhao L, Wang X, Zhang R. Unveiling the Multifaceted Role of HP6: A Critical Regulator of Humoral Immunity in Antheraea pernyi (Lepidoptera: Saturniidae). International Journal of Molecular Sciences. 2025; 26(10):4514. https://doi.org/10.3390/ijms26104514
Chicago/Turabian StyleLiu, Chengbao, Jinzhu Yu, Ting Fu, Xueshan He, Lin Zhao, Xialu Wang, and Rong Zhang. 2025. "Unveiling the Multifaceted Role of HP6: A Critical Regulator of Humoral Immunity in Antheraea pernyi (Lepidoptera: Saturniidae)" International Journal of Molecular Sciences 26, no. 10: 4514. https://doi.org/10.3390/ijms26104514
APA StyleLiu, C., Yu, J., Fu, T., He, X., Zhao, L., Wang, X., & Zhang, R. (2025). Unveiling the Multifaceted Role of HP6: A Critical Regulator of Humoral Immunity in Antheraea pernyi (Lepidoptera: Saturniidae). International Journal of Molecular Sciences, 26(10), 4514. https://doi.org/10.3390/ijms26104514




