Exploring Microbial Ecosystem Services for Environmental Stress Amelioration: A Review
Abstract
1. Introduction
2. Abiotic Stress and Its Impact on Plant Productivity and Soil Health
3. Role of Microbes in Abiotic Stress Management
4. Mechanism Involved in Microbe-Mediated Abiotic Stress Mitigation
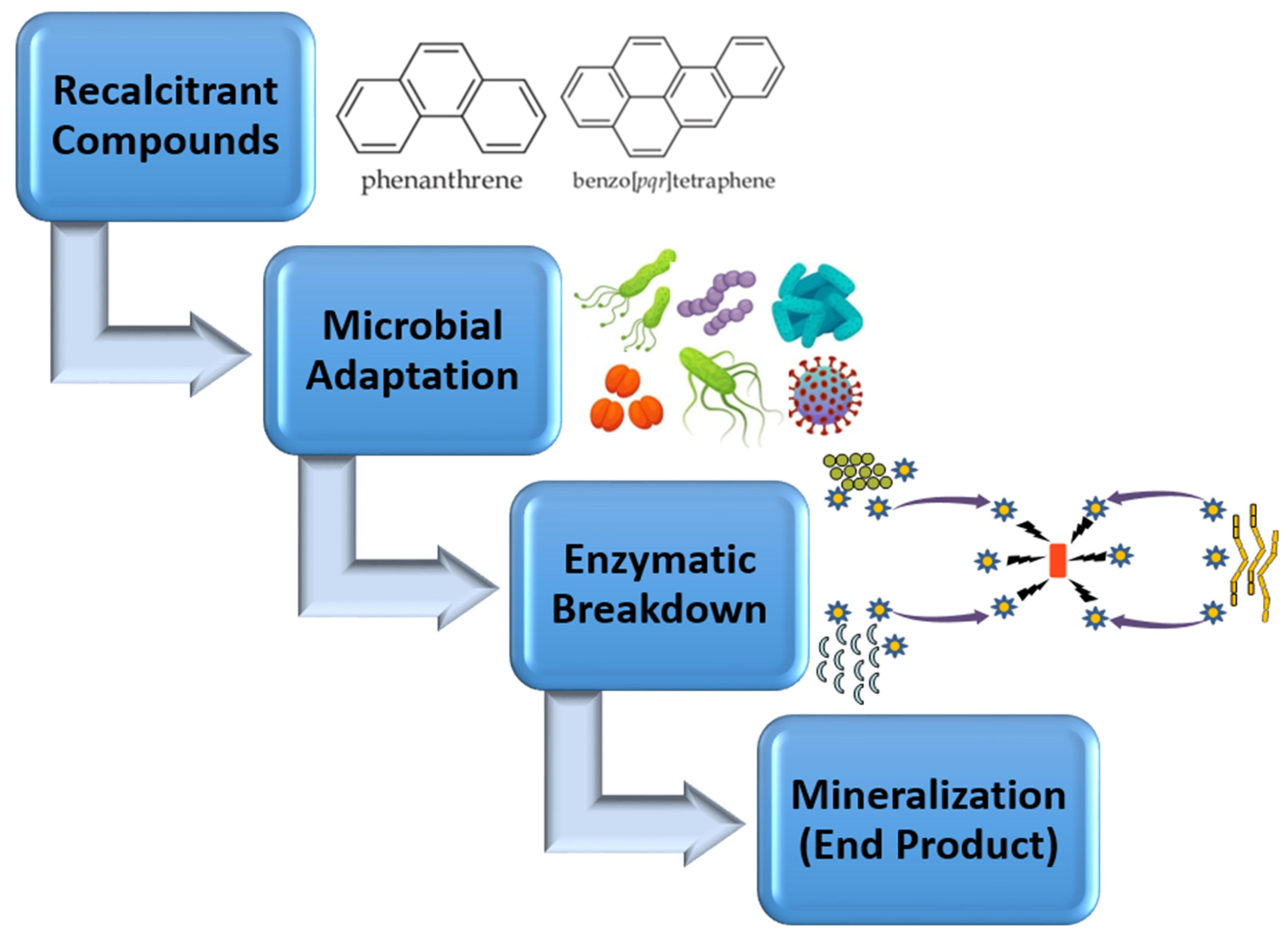
5. Biodegradation of Recalcitrant Compounds
6. Biodegradation of Xenobiotics
7. Biodegradation of Environmental Pollutants
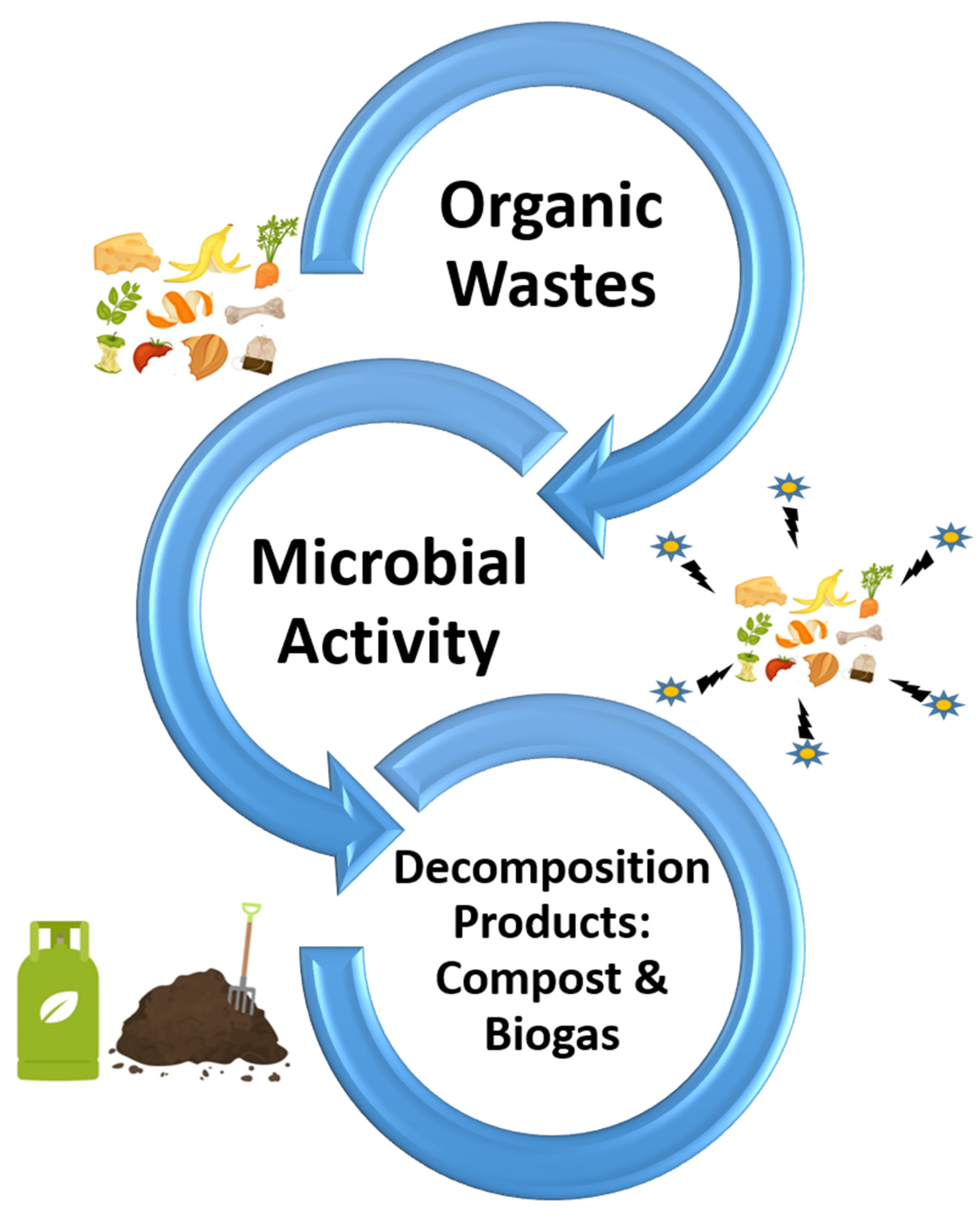
8. Microbial Management of Environmental Wastes
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, R.; Misra, S. Biostimulants: An eco-friendly regulator of plant stress tolerance and sustainable solution to future agriculture. Proc. Indian Natl. Sci. Acad. 2024, 1–8. [Google Scholar] [CrossRef]
- Dixit, R.; Bisht, N.; Misra, S.; Gupta, S.C.; Chauhan, P.S. Bacillus Consortia Modulate Transcriptional and Metabolic Machinery of Arabidopsis Plants for Salt Tolerance. Curr. Microbiol. 2023, 80, 77. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, D.; Sihi, D.; Bhowmik, A.; Verma, B.C.; Munda, S.; Dari, B. A review on effective soil health bio-indicators for ecosystem restoration and sustainability. Front. Microbiol. 2022, 13, 938481. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, M.; Ran, J.; Hu, W.; Irshad, M.K.; Dong, L.; Akram, M.A.; Eldesoky, G.E.; Aljuwayid, A.M.; Chuah, L.F.; Deng, J. Plant-soil-microbe interactions in maintaining ecosystem stability and coordinated turnover under changing environmental conditions. Chemosphere 2023, 318, 137924. [Google Scholar] [CrossRef]
- Majhi, B.; Semwal, P.; Mishra, S.K.; Chauhan, P.S. Strategies for microbes-mediated arsenic bioremediation: Impact of quorum sensing in the rhizosphere. Sci. Total Environ. 2024, 956, 177321. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant growth-promoting rhizobacteria for sustainable agricultural production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef]
- Bhagat, N.; Raghav, M.; Dubey, S.; Bedi, N. Bacterial exopolysaccharides: Insight into their role in plant abiotic stress tolerance. J. Microbiol. Biotechnol. 2021, 31, 1045. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, U.B.; Sahu, P.K.; Paul, S.; Kumar, A.; Malviya, D.; Singh, S.; Kuppusamy, P.; Singh, P.; Paul, D.; et al. Linking soil microbial diversity to modern agriculture practices: A review. Int. J. Environ. Res. Public Health 2022, 19, 3141. [Google Scholar] [CrossRef]
- Semwal, P.; Mishra, S.K.; Majhi, B.; Mishra, A.; Joshi, H.; Misra, S.; Misra, A.; Srivastava, S.; Chauhan, P.S. Bacillus australimaris protect Gloriosa superba L. against Alternaria alternata infestation. World J. Microbiol. Biotechnol. 2024, 40, 354. [Google Scholar] [CrossRef]
- Majhi, B.; Semwal, P.; Mishra, S.K.; Misra, S.; Chauhan, P.S. Arsenic stress management through arsenite and arsenate-tolerant growth-promoting bacteria in rice. Int. Microbiol. 2023, 1–15. [Google Scholar] [CrossRef]
- Ali, J.; Mukarram, M.; Ojo, J.; Dawam, N.; Riyazuddin, R.; Ghramh, H.A.; Khan, K.A.; Chen, R.; Kurjak, D.; Bayram, A. Harnessing phytohormones: Advancing plant growth and defense strategies for sustainable agriculture. Physiol. Plant. 2024, 176, 14307. [Google Scholar] [CrossRef]
- Ansari, M.M.; Singh, T.; Majhi, B.; Misra, S.; Chauhan, P.S. Biosurfactant producing plant growth promoting bacteria: Eco-friendly approaches for charcoal rot management. In Macrophomina Phaseolina; Academic Press: Cambridge, MA, USA, 2023; pp. 313–321. [Google Scholar]
- Umrao, V.; Yadav, S.; Semwal, P.; Misra, S.; Mishra, S.K.; Chauhan, P.S.; Shirke, P.A. Endophytic bacilli from Cyamopsis tetragonoloba (L.) Taub. induces plant growth and drought tolerance. Int. Microbiol. 2024, 27, 1541–1556. [Google Scholar] [CrossRef] [PubMed]
- Babangida, A.A.; Uddin, A.; Stephen, K.T.; Yusuf, B.A.; Zhang, L.; Ge, D. A Roadmap from Functional Materials to Plant Health Monitoring (PHM). Macromol. Biosci. 2024, 24, 2300283. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Gupta, S.; Misra, S.; Kumar, M.; Mishra, S.K.; Tiwari, S.; Narayan, S.; Agrawal, L.; Chauhan, P.S. Enhancement of Drought Tolerance in Transgenic Arabidopsis thaliana Plants Overexpressing Chickpea Ca14-3-3 Gene. J. Plant Growth Regul. 2023, 42, 1544–1557. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative water content, proline, and antioxidant enzymes in leaves of long shelf-life tomatoes under drought stress and rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Mondal, S.; Karmakar, S.; Panda, D.; Pramanik, K.; Bose, B.; Singhal, R.K. Crucial plant processes under heat stress and tolerance through heat shock proteins. Plant Stress 2023, 10, 100227. [Google Scholar] [CrossRef]
- El Haddad, N.; Choukri, H.; Ghanem, M.E.; Smouni, A.; Mentag, R.; Rajendran, K.; Hejjaoui, K.; Maalouf, F.; Kumar, S. High-temperature and drought stress effects on growth, yield and nutritional quality with transpiration response to vapor pressure deficit in lentil. Plants 2021, 11, 95. [Google Scholar] [CrossRef]
- Shabbir, R.; Javed, T.; Hussain, S.; Ahmar, S.; Naz, M.; Zafar, H.; Pandey, S.; Chauhan, J.; Siddiqui, M.H.; Pinghua, C. Calcium homeostasis and potential roles in combatting environmental stresses in plants. S. Afr. J. Bot. 2022, 148, 683–693. [Google Scholar] [CrossRef]
- Nie, Z.; Xiao, C.; Wang, Y.; Li, R.; Zhao, F. Heat shock proteins (HSPs) in non-alcoholic fatty liver disease (NAFLD): From molecular mechanisms to therapeutic avenues. Biomark. Res. 2024, 12, 120. [Google Scholar] [CrossRef]
- Misra, S.; Prasad, P.; Semwal, P.; Mishra, S.K.; Asif, M.H.; Chauhan, P.S. Genomic characterization of the salt-tolerant Bacillus and Jeotgalicoccus strains reveals a diverse metabolism relevant to plant growth promotion and salt stress tolerance. 3 Biotech 2024, 14, 316. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, R.; Venkidasamy, B.; Easwaran, M.; Chi, H.Y.; Thiruvengadam, M.; Kim, S.H. Dynamic interplay of reactive oxygen and nitrogen species (ROS and RNS) in plant resilience: Unveiling the signaling pathways and metabolic responses to biotic and abiotic stresses. Plant Cell Rep. 2024, 43, 198. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.; Bhadra, P.; Yadav, A.N.; Palai, J.B.; Jena, J.; Shankar, T. The omics strategies for abiotic stress responses and microbe-mediated mitigation in plants. In Soil Microbiomes for Sustainable Agriculture; Springer: Cham, Switzerland, 2021; pp. 315–377. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of low temperature stress on photosynthesis and allied traits: A review. In Physiological Processes in Plants Under Low Temperature Stress; Springer: Singapore, 2022; pp. 199–297. [Google Scholar]
- Majhi, B.; Semwal, P.; Mishra, S.K.; Srivastava, V.; Singh, R.P.; Chauhan, P.S. Metalliferous Soil Remediation Through Heavy Metal-Resistant Plant Growth-Promoting Bacteria: Prospects and Paradigms. In Heavy Metal Toxicity: Environmental Concerns, Remediation and Opportunities; Springer Nature: Singapore, 2023; pp. 225–243. [Google Scholar]
- Shahid, M.; Javed, M.T.; Mushtaq, A.; Akram, M.S.; Mahmood, F.; Ahmed, T.; Azeem, M. Microbe-mediated mitigation of cadmium toxicity in plants. In Cadmium Toxicity and Tolerance in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 427–449. [Google Scholar]
- Jaiswal, P.; Kar, S.; Misra, S.; Dixit, V.; Mishra, S.K.; Chauhan, P.S. Novel trends in endophytic applications for plant disease management. In Biocontrol Mechanisms of Endophytic Microorganisms; Academic Press: Cambridge, MA, USA, 2022; pp. 167–180. [Google Scholar]
- Kar, S.; Mishra, S.K.; Misra, S.; Agarwal, R.; Kumar, S.; Chauhan, P.S. Endophytic Alkalotolerant Plant Growth-Promoting Bacteria Render Maize (Zea mays L.) Growth Under Alkaline Stress. Curr. Microbiol. 2024, 81, 43. [Google Scholar] [CrossRef]
- Mishra, S.K.; Misra, S.; Dixit, V.K.; Kar, S.; Chauhan, P.S. Ochrobactrum sp. NBRISH6 Inoculation Enhances Zea mays Productivity, Mitigating Soil Alkalinity and Plant Immune Response. Curr. Microbiol. 2023, 80, 328. [Google Scholar] [CrossRef]
- Misra, S.; Dixit, V.K.; Mishra, S.K.; Chauhan, P.S. Demonstrating the potential of abiotic stress-tolerant Jeotgalicoccus huakuii NBRI 13E for plant growth promotion and salt stress amelioration. Ann. Microbiol. 2019, 69, 419–434. [Google Scholar] [CrossRef]
- Dixit, V.K.; Misra, S.; Mishra, S.K.; Joshi, N.; Chauhan, P.S. Rhizobacteria-Mediated Bioremediation: Insights and Future Perspectives. In Soil Bioremediation: An Approach Towards Sustainable Technology; Parray, J.A., Mahmoud, A.H.A.E., Sayyed, R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 193–211. [Google Scholar]
- Uzma, M.; Iqbal, A.; Hasnain, S. Drought tolerance induction and growth promotion by indole acetic acid producing Pseudomonas aeruginosa in Vigna radiata. PLoS ONE 2022, 17, e0262932. [Google Scholar] [CrossRef] [PubMed]
- Ebbisa, A. Arbuscular mycorrhizal fungi (AMF) in optimizing nutrient bioavailability and reducing agrochemicals for maintaining sustainable agroecosystems. In Arbuscular Mycorrhizal Fungi in Agriculture-New Insights; IntechOpen: London, UK, 2022. [Google Scholar]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Belabess, Z.; Jiang, Y.; Mokrini, F.; Tahiri, A.; Peng, G. Bacillus spp.-Mediated drought stress tolerance in plants: Current and future prospects. In Bacilli in Agrobiotechnology: Plant Stress Tolerance, Bioremediation, and Bioprospecting; Springer International Publishing: Cham, Switzerland, 2022; pp. 487–518. [Google Scholar]
- Bharti, C.; Fatima, T.; Mishra, P.; Verma, P.; Bhattacharya, A.; Alaylar, B.; Arora, N.K. Salt-tolerant endophytic Bacillus altitudinis NKA32 with ACC deaminase activity modulates physiochemical mechanisms in rice for adaptation in saline ecosystem. Environ. Sustain. 2024, 7, 231–249. [Google Scholar] [CrossRef]
- Devi, P.; Kumar, P.; Dey, S.R.; Banik, D.; Kumar, G.; Mehta, C.M. Integrated omics approaches for nutrient stress management in plants. In Current Omics Advancement in Plant Abiotic Stress Biology; Academic Press: Cambridge, MA, USA, 2024; pp. 93–117. [Google Scholar]
- Misra, S.; Semwal, P.; Pandey, D.D.; Mishra, S.K.; Chauhan, P.S. Siderophore-producing Spinacia oleracea bacterial endophytes enhance nutrient status and vegetative growth under iron-deficit conditions. J. Plant Growth Regul. 2024, 43, 1317–1330. [Google Scholar] [CrossRef]
- Semwal, P.; Misra, S.; Misra, A.; Kar, S.; Majhi, B.; Mishra, S.K.; Srivastava, S.; Chauhan, P.S. Endophytic Bacillus strains enhance biomass and bioactive metabolites of Gloriosa superba. Ind. Crops Prod. 2023, 204, 117296. [Google Scholar] [CrossRef]
- Meena, M.; Mehta, T.; Nagda, A.; Yadav, G.; Sonigra, P. PGPR-mediated synthesis and alteration of different secondary metabolites during plant-microbe interactions. In Plant-Microbe Interaction-Recent Advances in Molecular and Biochemical Approaches; Academic Press: Cambridge, MA, USA, 2023; pp. 229–255. [Google Scholar]
- Thakur, M.; Khushboo; Shah, S.; Kumari, P.; Kumar, M.; Vibhuti, R.K.; Pramanik, A.; Yadav, V.; Raina, M.; Negi, N.P.; et al. Unlocking the Secrets of Rhizosphere Microbes: A New Dimension for Agriculture. Symbiosis 2024, 92, 305–322. [Google Scholar] [CrossRef]
- Zhao, J.; Li, F.; Cao, Y.; Zhang, X.; Chen, T.; Song, H.; Wang, Z. Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms. Biotechnol. Adv. 2021, 53, 107682. [Google Scholar] [CrossRef] [PubMed]
- Semwal, P.; Majhi, B.; Pandey, D.D.; Misra, S.; Mishra, S.K.; Chauhan, P.S. Agriwaste burning management through microbial intervention. In Recent Trends in Solid Waste Management; Elsevier: Amsterdam, The Netherlands, 2023; pp. 333–347. [Google Scholar]
- Janes-Bassett, V.; Blackwell, M.S.; Blair, G.; Davies, J.; Haygarth, P.M.; Mezeli, M.M.; Stewart, G. A meta-analysis of phosphatase activity in agricultural settings in response to phosphorus deficiency. Soil Biol. Biochem. 2022, 165, 108537. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant growth-promoting bacteria of soil: Designing of consortia beneficial for crop production. Microorganisms 2023, 11, 2864. [Google Scholar] [CrossRef]
- Ajinde, A.O.; Dayo-Olagbende, O.G.; Akpor, O.B. Direct and indirect mechanisms of growth promotion by plant growth promoting rhizobacteria. In Proceedings of the 2024 International Conference on Science, Engineering, and Business for Driving Sustainable Development Goals (SEB4SDG), Omu-Aran, Nigeria, 2–4 April 2024; pp. 1–16. [Google Scholar]
- Fu, S.F.; Balasubramanian, V.K.; Chen, C.L.; Tran, T.T.; Muthuramalingam, J.B.; Chou, J.Y. The phosphate-solubilising fungi in sustainable agriculture: Unleashing the potential of fungal biofertilisers for plant growth. Folia. Microbiol. 2024, 69, 697–712. [Google Scholar] [CrossRef]
- Yaghoubi, K.M.; Strafella, S.; Filannino, P.; Minervini, F.; Crecchio, C. Importance of Lactic Acid Bacteria as an Emerging Group of Plant Growth-Promoting Rhizobacteria in Sustainable Agroecosystems. Appl. Sci. 2024, 14, 1798. [Google Scholar] [CrossRef]
- Misra, S.; Chauhan, P.S. ACC deaminase-producing rhizosphere competent Bacillus spp. mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech 2020, 10, 119. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.K.; Zhou, Y.; Wang, J.; Jiang, Y.; Shen, N.; Wang, Y.; Yang, L.; Jiang, M. Unlocking the strength of plant growth promoting Pseudomonas in improving crop productivity in normal and challenging environments: A review. J. Plant Interact. 2022, 17, 220–238. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Yang, S.; Imran Ortas, I. Impact of mycorrhiza on plant nutrition and food security. J. Plant Nutr. 2023, 46, 3247–3272. [Google Scholar] [CrossRef]
- Misra, S. Exploration of soil operative resistance factors: Modulators of plant Eco physiological responses. Proc. Indian Natl. Sci. Acad. 2024, 1–4. [Google Scholar] [CrossRef]
- Raza, A.; Tabassum, J.; Fakhar, A.Z.; Sharif, R.; Chen, H.; Zhang, C.; Ju, L.; Fotopoulos, V.; Siddique, K.H.; Singh, R.K.; et al. Smart reprograming of plants against salinity stress using modern biotechnological tools. Crit. Rev. Biotechnol. 2023, 43, 1035–1062. [Google Scholar] [CrossRef] [PubMed]
- Muratova, A.; Golubev, S.; Romanova, V.; Sungurtseva, I.; Nurzhanova, A. Effect of heavy-metal-resistant PGPR inoculants on growth, rhizosphere microbiome and remediation potential of miscanthus× giganteus in Zinc-contaminated Soil. Microorganisms 2023, 11, 1516. [Google Scholar] [CrossRef]
- Gaur, N.; Dutta, D.; Singh, A.; Dubey, R.; Kamboj, D.V. Recent advances in the elimination of persistent organic pollutants by photocatalysis. Front. Environ. Sci. 2022, 10, 872514. [Google Scholar] [CrossRef]
- Akhtar, A.B.T.; Naseem, S.; Yasar, A.; Naseem, Z. Persistent organic pollutants (POPs): Sources, types, impacts, and their remediation. In Environmental Pollution and Remediation; Springer: Singapore, 2021; pp. 213–246. [Google Scholar]
- Anand, U.; Adelodun, B.; Cabreros, C.; Kumar, P.; Suresh, S.; Dey, A.; Ballesteros, F.; Bontempi, E. Occurrence, transformation, bioaccumulation, risk and analysis of pharmaceutical and personal care products from wastewater: A review. Environ. Chem. Lett. 2022, 20, 3883–3904. [Google Scholar] [CrossRef]
- Wacławek, S.; Krawczyk, K.; Silvestri, D.; Padil, V.V.; Řezanka, M.; Černík, M.; Jaroniec, M. Cyclodextrin-based strategies for removal of persistent organic pollutants. Adv. Colloid. Interface. Sci. 2022, 310, 102807. [Google Scholar] [CrossRef]
- Kumar, L.; Chhogyel, N.; Gopalakrishnan, T.; Hasan, M.K.; Jayasinghe, S.L.; Kariyawasam, C.S.; Kogo, B.K.; Ratnayake, S. Climate change and future of agri-food production. In Future Foods; Academic Press: Cambridge, MA, USA, 2022; pp. 49–79. [Google Scholar]
- Cao, Z.; Yan, W.; Ding, M.; Yuan, Y. Construction of microbial consortia for microbial degradation of complex compounds. Front. Bioeng. Biotechnol. 2022, 10, 1051233. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.Q.; He, Z.Q.; Ntakirutimana, S.; Liu, Z.H.; Li, B.Z.; Yuan, Y.J. Artificial mixed microbial system for polycyclic aromatic hydrocarbons degradation. Front. Microbiol. 2023, 14, 1207196. [Google Scholar] [CrossRef]
- Guerrero Ramírez, J.R.; Ibarra Muñoz, L.A.; Balagurusamy, N.; Frías Ramírez, J.E.; Alfaro Hernández, L.; Carrillo Campos, J. Microbiology and Biochemistry of Pesticides Biodegradation. Int. J. Mol. Sci. 2023, 24, 15969. [Google Scholar] [CrossRef]
- Bher, A.; Mayekar, P.C.; Auras, R.A.; Schvezov, C.E. Biodegradation of Biodegradable Polymers in Mesophilic Aerobic Environments. Int. J. Mol. Sci. 2022, 23, 12165. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Khalifa, H.O.; Yoon, H.J.; Ki, M.R.; Pack, S.P. Microbial Immobilized Enzyme Biocatalysts for Multipollutant Mitigation: Harnessing Nature’s Toolkit for Environmental Sustainability. Int. J. Mol. Sci. 2024, 25, 8616. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Babalola, O.O. Bioremediation of environmental wastes: The role of microorganisms. Front. Agron. 2023, 5, 1183691. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.; Do, H.; Loan Trinh, K.; Lee, N. Metagenomics: An Effective Approach for Exploring Microbial Diversity and Functions. Foods 2023, 12, 2140. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Das, A.; Das, S.; Bhatawadekar, V.; Pandey, P.; Choure, K.; Damare, S.; Pandey, P. Petroleum Hydrocarbon Catabolic Pathways as Targets for Metabolic Engineering Strategies for Enhanced Bioremediation of Crude-Oil-Contaminated Environments. Fermentation 2023, 9, 196. [Google Scholar] [CrossRef]
- Davletgildeeva, A.T.; Kuznetsov, N.A. Bioremediation of Polycyclic Aromatic Hydrocarbons by Means of Bacteria and Bacterial Enzymes. Microorganisms 2024, 12, 1814. [Google Scholar] [CrossRef]
- Rafeeq, H.; Afsheen, N.; Rafique, S.; Arshad, A.; Intisar, M.; Hussain, A.; Bilal, M.; Iqbal, H.M.N. Genetically engineered microorganisms for environmental remediation. Chemosphere 2023, 310, 136751. [Google Scholar] [CrossRef]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial Interventions in Bioremediation of Heavy Metal Contaminants in Agroecosystem. Front. Microbiol. 2022, 13, 824084. [Google Scholar] [CrossRef]
- Bharti, S. Xenobiotic Remediation for Sustainable Urban Development and Ecosystem Resilience. Water Air Soil Pollut. 2024, 235, 580. [Google Scholar] [CrossRef]
- Štefanac, T.; Grgas, D.; Landeka Dragičević, T. Xenobiotics—Division and Methods of Detection: A Review. J. Xenobiot. 2021, 11, 130–141. [Google Scholar] [CrossRef]
- Piwowarska, D.; Kiedrzyńska, E. Xenobiotics as a contemporary threat to surface waters. Ecohydrol Hydrobiol. 2022, 22, 337–354. [Google Scholar] [CrossRef]
- Wang, X.; Sial, M.U.; Bashir, M.A.; Bilal, M.; Raza, Q.U.A.; Ali Raza, H.M.; Rehim, A.; Geng, Y. Pesticides Xenobiotics in Soil Ecosystem and Their Remediation Approaches. Sustainability 2022, 14, 3353. [Google Scholar] [CrossRef]
- Bera, K.; Bhattacharya, D.; Mukhopadhyay, M. Leveraging bacterial laccases to facilitate the decomposition of xenobiotic compounds: A review. 3 Biotech 2024, 14, 317. [Google Scholar] [CrossRef] [PubMed]
- Miglani, R.; Parveen, N.; Kumar, A.; Ansari, M.A.; Khanna, S.; Rawat, G.; Panda, A.K.; Bisht, S.S.; Upadhyay, J.; Ansari, M.N. Degradation of Xenobiotic Pollutants: An Environmentally Sustainable Approach. Metabolites 2022, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Muruganandam, M.; Kumar, A.; Senthilkumar, N.; Shkir, M.; Pandit, B.; Imran, M.; Prakash, C.; Ubaidullah, M. Recent advances in microbial-assisted degradation and remediation of xenobiotic contaminants; challenges and future prospects. J. Water. Process. Eng. 2024, 60, 105106. [Google Scholar] [CrossRef]
- Malla, M.A.; Dubey, A.; Yadav, S.; Kumar, A.; Hashem, A.; Abd Allah, E.F. Understanding and Designing the Strategies for the Microbe-Mediated Remediation of Environmental Contaminants Using Omics Approaches. Front. Microbiol. 2018, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, E.; Barra Caracciolo, A.; Rolando, L. Recent Advances in Bacterial Degradation of Hydrocarbons. Water 2023, 15, 375. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef]
- Matturro, B.; Di Franca, M.L.; Tonanzi, B.; Cruz Viggi, C.; Aulenta, F.; Di Leo, M.; Giandomenico, S.; Rossetti, S. Enrichment of Aerobic and Anaerobic Hydrocarbon-Degrading Bacteria from Multicontaminated Marine Sediment in Mar Piccolo Site (Taranto, Italy). Microorganisms 2023, 11, 2782. [Google Scholar] [CrossRef]
- Khalid, F.; Hashmi, M.Z.; Jamil, N.; Qadir, A.; Ali, M.I. Microbial and enzymatic degradation of PCBs from e-waste-contaminated sites: A review. Environ. Sci. Pollut. Res. 2021, 28, 10474–10487. [Google Scholar] [CrossRef]
- Suenaga, H.; Yamazoe, A.; Hosoyama, A.; Kimura, N.; Hirose, J.; Watanabe, T.; Fujihara, H.; Futagami, T.; Goto, M.; Furukawa, K. Complete Genome Sequence of the Polychlorinated Biphenyl-Degrading Bacterium Pseudomonas putida KF715 (NBRC 110667) Isolated from Biphenyl-Contaminated Soil. Genome Announc. 2017, 5, e01624-16. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Ma, F.; Yang, J. Characterisation of an efficient atrazine-degrading bacterium, Arthrobacter sp. ZXY-2: An attempt to lay the foundation for potential bioaugmentation applications. Biotechnol. Biofuels 2018, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Mou, T.; Wang, J.; Su, J.; Yan, Y.; Zhang, Y.Q. Characterization of three rapidly growing novel Mycobacterium species with significant polycyclic aromatic hydrocarbon bioremediation potential. Front. Microbiol. 2023, 14, 1225746. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Doty, S.L. Cometabolic degradation of trichloroethylene by Burkholderia cepacia G4 with poplar leaf homogenate. Can. J. Microbiol. 2014, 60, 487–490. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Li, T.; Peng, X.; Tang, Y.; Jia, X. Biodegradation of phenol by Candida tropicalis sp.: Kinetics, identification of putative genes and reconstruction of catabolic pathways by genomic and transcriptomic characteristics. Chemosphere 2022, 308, 136443. [Google Scholar] [CrossRef]
- Maqsood, Q.; Hussain, N.; Sumrin, A.; Ali, S.W.; Tariq, M.R.; Mahnoor, M. Monitoring and abatement of synthetic pollutants using engineered microbial systems. Dis. Life 2024, 54, 9. [Google Scholar] [CrossRef]
- Muter, O. Current Trends in Bioaugmentation Tools for Bioremediation: A Critical Review of Advances and Knowledge Gaps. Microorganisms 2023, 11, 710. [Google Scholar] [CrossRef] [PubMed]
- Pashirova, T.; Salah-Tazdaït, R.; Tazdaït, D.; Masson, P. Applications of Microbial Organophosphate-Degrading Enzymes to Detoxification of Organophosphorous Compounds for Medical Countermeasures against Poisoning and Environmental Remediation. Int. J. Mol. Sci. 2024, 25, 7822. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Sabreena Hassan, S.; Kumar, V.; Bhat, S.A.; Ganai, B.A. Unraveling microbes as potential proxies for remediation of heavy metal and pesticide contamination: A state-of-the art review. Int. J. Environ. Res. 2023, 17, 55. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, H.; Du, D.; Li, G.; Alam, O.; Cheng, Z.; Liu, X.; Jiang, S.; Li, J. Remediation of heavy metals polluted soil environment: A critical review on biological approaches. Ecotoxicol. Environ. Saf. 2024, 284, 116883. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Shah, G.; Singh, H.; Bhatt, U.; Singhal, K.; Soni, V. Advancements in Natural Remediation Management Techniques for Oil Spills: Challenges, Innovations, and Future Directions. Environ. Pollut. Manag. 2024, 1, 128–146. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum Hydrocarbon-Degrading Bacteria for the Remediation of Oil Pollution Under Aerobic Conditions: A Perspective Analysis. Front Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef]
- Mitra, S.; Saran, R.K.; Srivastava, S.; Rensing, C. Pesticides in the environment: Degradation rout;s, pesticide transformation products and ecotoxicological considerations. Sci. Total Environ. 2024, 934, 173026. [Google Scholar] [CrossRef]
- Ethiraj, S.; Samuel, M.S.; Indumathi, S.M. A comprehensive review of the challenges and opportunities in microalgae-based wastewater treatment for eliminating organic, inorganic, and emerging pollutants. Biocatal. Agric. Biotechnol. 2024, 60, 103316. [Google Scholar] [CrossRef]
- Palaniveloo, K.; Amran, M.A.; Norhashim, N.A.; Mohamad-Fauzi, N.; Peng-Hui, F.; Hui-Wen, L.; Kai-Lin, Y.; Jiale, L.; Chian-Yee, M.G.; Jing-Yi, L.; et al. Food Waste Composting and Microbial Community Structure Profiling. Processes 2020, 8, 723. [Google Scholar] [CrossRef]
- Gyadi, T.; Bharti, A.; Basack, S.; Kumar, P.; Lucchi, E. Influential factors in anaerobic digestion of rice-derived food waste and animal manure: A comprehensive review. Bioresour. Technol. 2024, 413, 131398. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Noman, M.; Qi, Y.; Shahid, M.; Hussain, S.; Masood, H.A.; Xu, L.; Ali, H.M.; Negm, S.; El-Kott, A.F.; et al. Fertilization of Microbial Composts: A Technology for Improving Stress Resilience in Plants. Plants 2023, 12, 3550. [Google Scholar] [CrossRef]
- Martín-González, D.; De La Fuente Tagarro, C.; De Lucas, A.; Bordel, S.; Santos-Beneit, F. Genetic Modifications in Bacteria for the Degradation of Synthetic Polymers: A Review. Int. J. Mol. Sci. 2024, 25, 5536. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H. Microbial Consortia Are Needed to Degrade Soil Pollutants. Microorganisms 2022, 10, 261. [Google Scholar] [CrossRef]
- Kumba, H.; Makepa, D.C.; Charamba, A.N.; Olanrewaju, O.A. Towards Circular Economy: Integrating Waste Management for Renewable Energy Optimization in Zimbabwe. Sustainability 2024, 16, 5014. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, P.; Wu, Y.; Wu, X.; Ni, H.; Lu, Q.; Zang, S. Long-term surface composts application enhances saline-alkali soil carbon sequestration and increases bacterial community stability and complexity. Environ. Res. 2024, 240, 117425. [Google Scholar] [CrossRef] [PubMed]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef] [PubMed]

| S. No. | Xenobiotics | Microbes | Mechanism | Influencing Factors | Degradation Pathways | References |
|---|---|---|---|---|---|---|
| 1 | Polychlorinated Biphenyls (PCBs) | Pseodomonas putida | Reductive dichlorination and oxidation | pH, temperature | Anaerobic | [84,85] |
| 2 | Atrazine | Anthrobacter sp. | Hydrolysis followed by ring cleavage | Soil moisture, nitrogen content, organic matter | Aerobic | [86] |
| 3 | Polycyclic aromatic hydrocarbons (PAHs) | Mycobacterium sp. | Oxidation via deoxygenases enzymes | Oxygen levels, nutrient availability | Aerobic and anaerobic | [87] |
| 4 | Trichloroethylene (TCE) | Burkholderia cepacia | Reductive dichlorination through cometabolism | Microbial community structure | Anaerobic | [88] |
| 5 | Phenol | Candida tropicalis | Oxidative ring cleavage | pH, temperature, dissolved oxygen | Aerobic | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semwal, P.; Dave, A.; Israr, J.; Misra, S.; Kumar, M.; Paul, D. Exploring Microbial Ecosystem Services for Environmental Stress Amelioration: A Review. Int. J. Mol. Sci. 2025, 26, 4515. https://doi.org/10.3390/ijms26104515
Semwal P, Dave A, Israr J, Misra S, Kumar M, Paul D. Exploring Microbial Ecosystem Services for Environmental Stress Amelioration: A Review. International Journal of Molecular Sciences. 2025; 26(10):4515. https://doi.org/10.3390/ijms26104515
Chicago/Turabian StyleSemwal, Pradeep, Anand Dave, Juveriya Israr, Sankalp Misra, Manish Kumar, and Diby Paul. 2025. "Exploring Microbial Ecosystem Services for Environmental Stress Amelioration: A Review" International Journal of Molecular Sciences 26, no. 10: 4515. https://doi.org/10.3390/ijms26104515
APA StyleSemwal, P., Dave, A., Israr, J., Misra, S., Kumar, M., & Paul, D. (2025). Exploring Microbial Ecosystem Services for Environmental Stress Amelioration: A Review. International Journal of Molecular Sciences, 26(10), 4515. https://doi.org/10.3390/ijms26104515







