Lycium ruthenicum Murray Anthocyanins Alleviate Aging Through SIRT1/P53 Signaling Pathway
Abstract
1. Introduction
2. Results
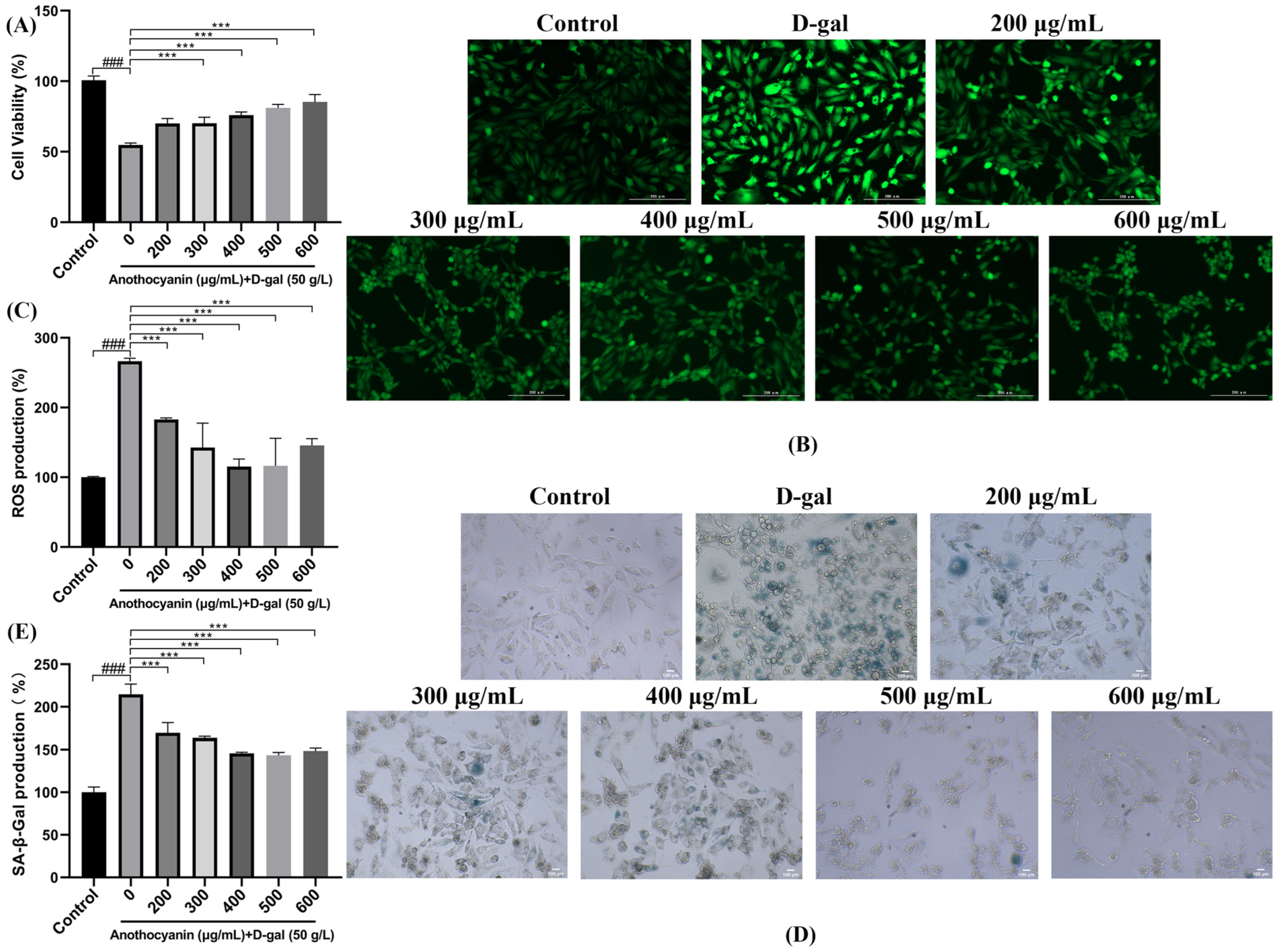
2.1. LRAs Attenuate D-gal-Induced Cellular Senescence
2.2. LRAs Scavenge ROS and Alleviate Cell Aging
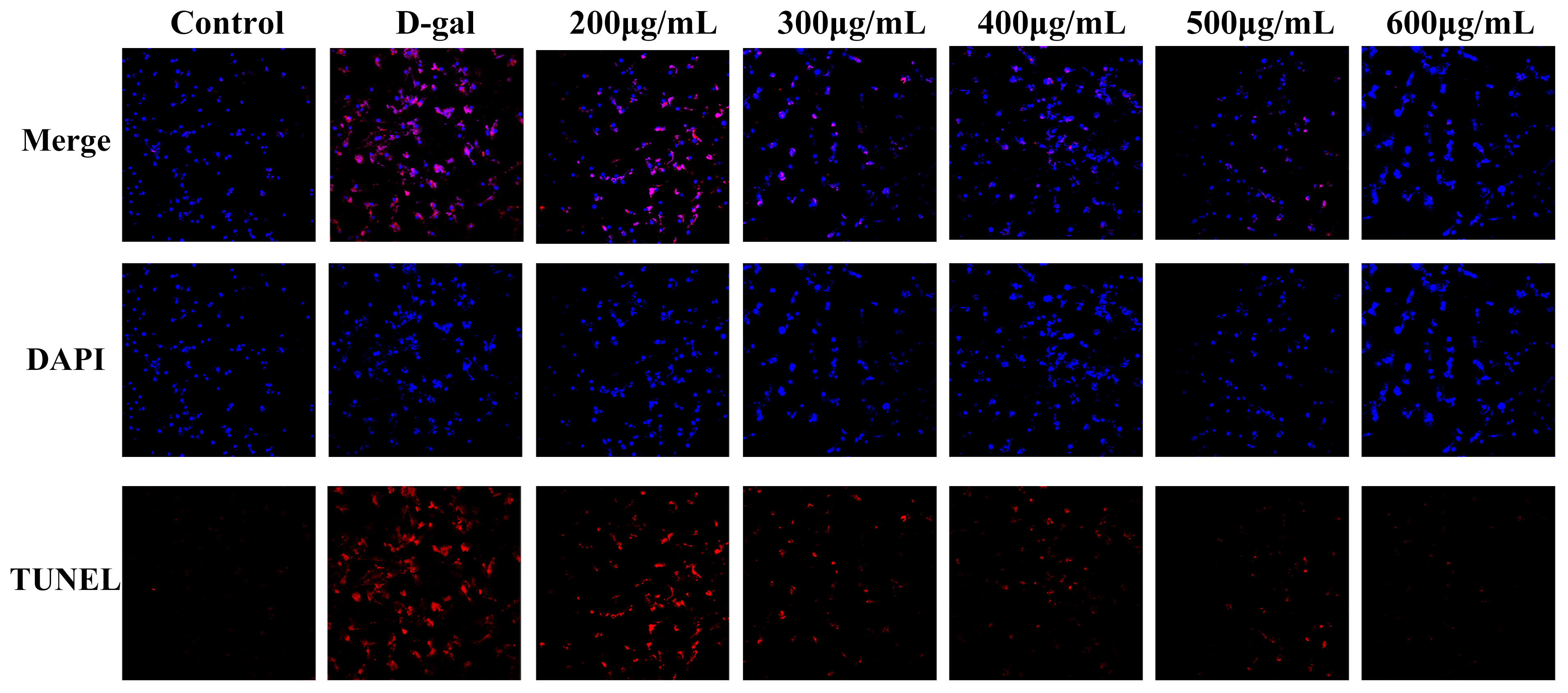
2.3. LRAs Alleviate D-gal-Induced Apoptosis
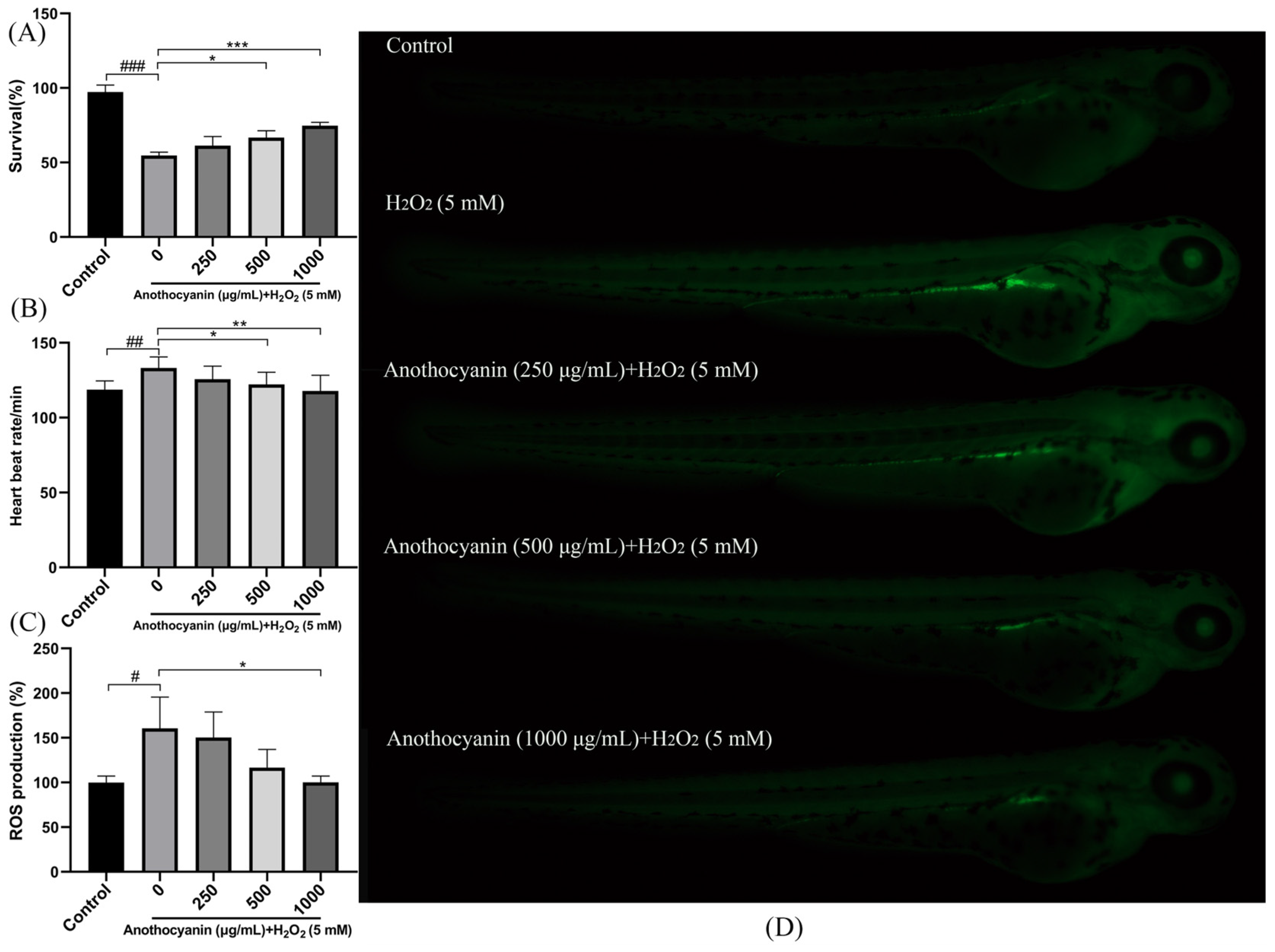
2.4. Effects of LRAs on Survival Rate and Heartbeat in Zebrafish
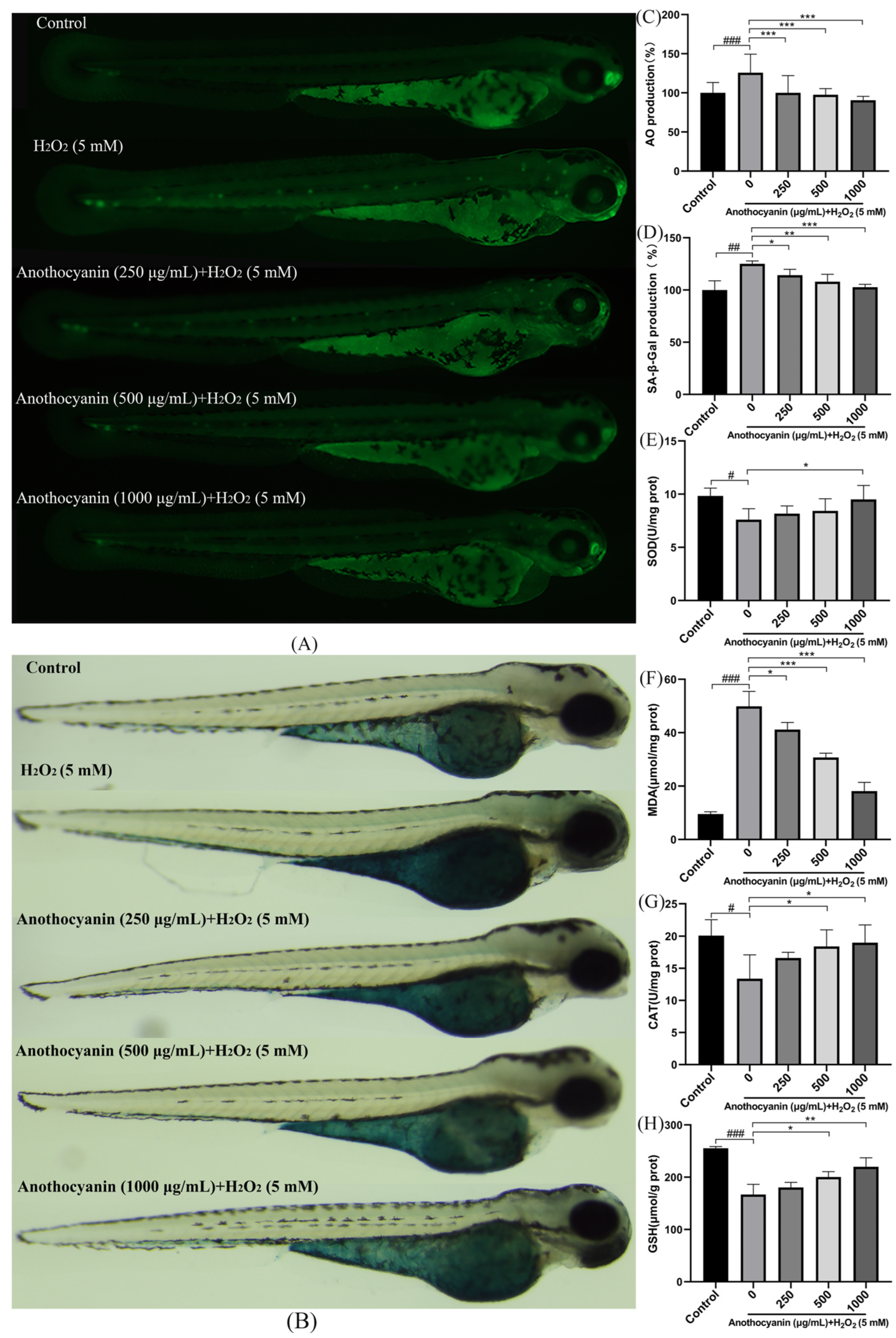
2.5. Protective Effect of LRAs on H2O2-Induced ROS Generation in Zebrafish
2.6. Protective Effect of LRAs on H2O2-Induced Zebrafish Cell Senescence
2.7. Effect of LRAs on H2O2 Induced Oxidative Stress Factors in Zebrafish Larvae
2.8. Effect of LRAs on H2O2 Induced Genes Related to Zebrafish Larvae
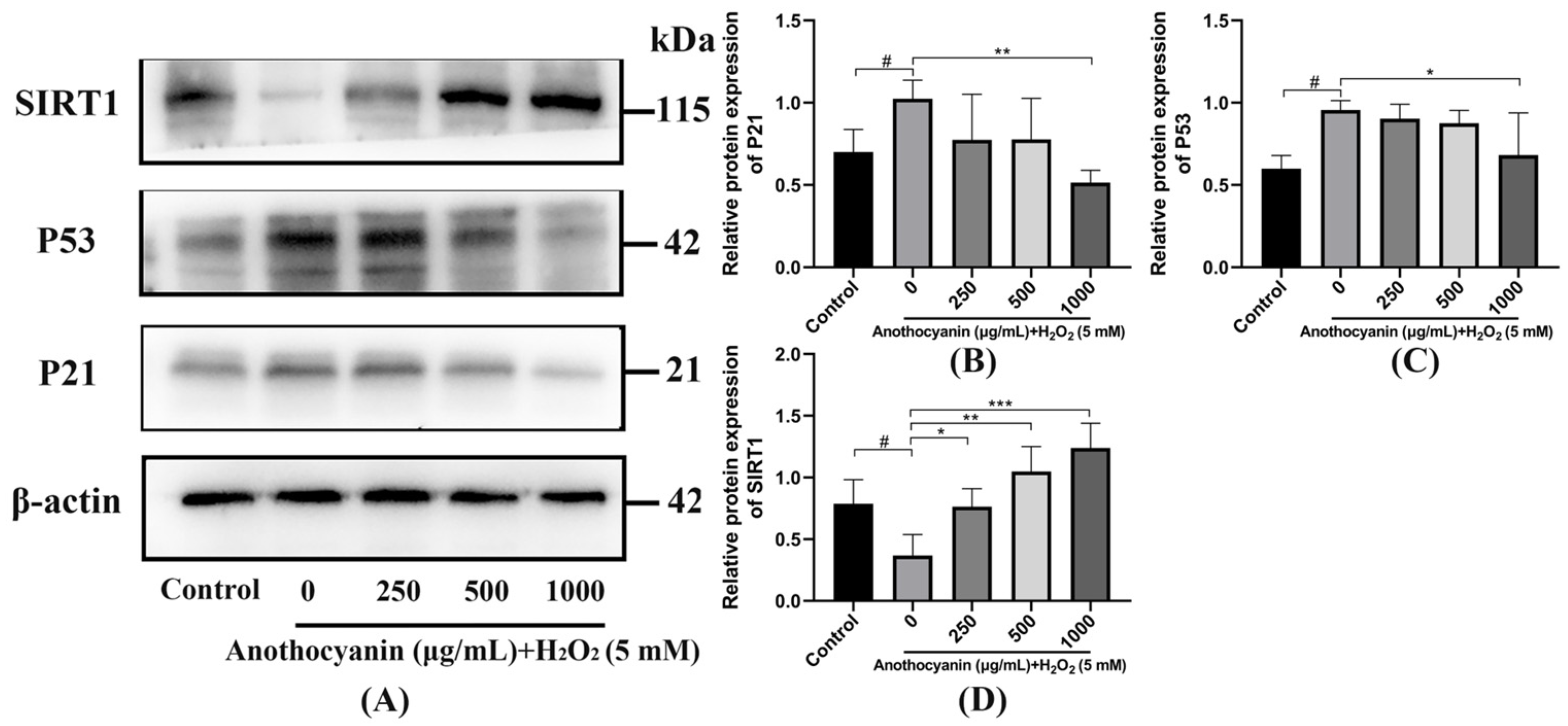
2.9. Effect of LRAs on SIRT1/P53 Signaling Pathway
2.10. Transcriptome Sequencing
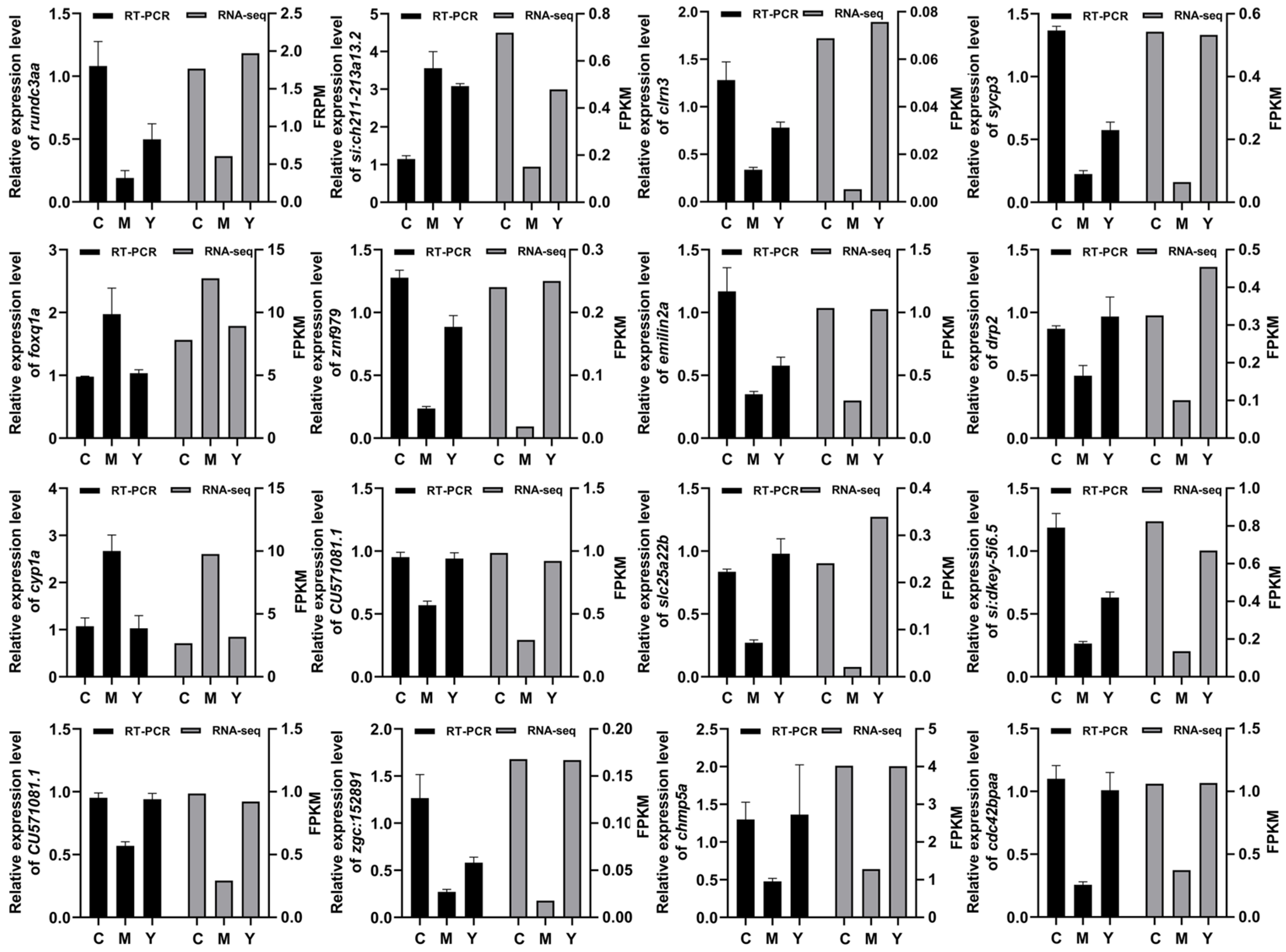
2.11. RT-PCR Results Correlated Strongly with RNA-seq Data
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Cell Cultures
4.3. Assessment of Cell Viability
4.4. TUNEL Assay for Detecting Apoptosis in H9c2 Cells
4.5. Maintenance and Treatment of Zebrafish
4.6. Determination of ROS and Apoptosis Levels
4.7. Determination of SA-β-gal Content
4.8. Biochemical Indicator Detection
4.9. RNA-Seq and Data Analysis
4.10. Real-Time Quantitative PCR (RT-qPCR) Analysis
4.11. Western Blotting
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| List of Abbreviations | |||
| LRA | Lycium ruthenicum Murray | H2O2 | Hydrogen Peroxide |
| D-gal | D-galactose | GSH | Glutathione |
| ROS | Reactive Oxygen Species | DAPI | 4′,6-diamidino-2-phenylindole |
| SA-β-gal | Senescence-Associated β-Galactosidase | CCK-8 | Cell Counting Kit-8 |
| TUNEL | Terminal Deoxynucleotidyl Transferase-mediated dUTP-biotin Nick End Labeling | ECL | Enhanced Chemiluminescence |
| RT-PCR | Reverse Transcription-Polymerase Chain Reaction | TBST | Tris-Borate-Sodium Tween-20 |
| WB | Western Blotting | RNE-seq | RNA sequencing |
| AO | Acridine Orange | KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MDA | Malondialdehyde | GO | Gene Ontology |
| CAT | Catalase | DEGs | Differential expressed genes |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis | PVDF | Polyvinylidene Fluoride |
References
- Liu, S.; Hu, Z.; Guo, Y.; Zhou, F.; Li, S.; Xu, H. Association of sleep quality and nap duration with cognitive frailty among older adults living in nursing homes. Front. Public Health 2022, 10, 963105. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Ding, Z.; Luo, Q.; Ju, Y.; Song, G. Exogenous NAD+ Postpones the D-Gal-Induced Senescence of Bone Marrow-Derived Mesenchymal Stem Cells via Sirt1 Signaling. Antioxidants 2021, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Chhunchha, B.; Singh, P.; Stamer, W.D.; Singh, D.P. Prdx6 retards senescence and restores trabecular meshwork cell health by regulating reactive oxygen species. Cell Death Discov. 2017, 3, 17060. [Google Scholar] [CrossRef]
- Singh, S.P.; Chhunchha, B.; Fatma, N.; Kubo, E.; Singh, S.P.; Singh, D.P. Delivery of a protein transduction domain-mediated Prdx6 protein ameliorates oxidative stress-induced injury in human and mouse neuronal cells. Am. J. Physiol. Physiol. 2016, 310, C1–C16. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Pinazo-Durán, M.; Gallego-Pinazo, R.; Medina, J.J.G.; Zanón-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martinez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramírez, C.; López-Gálvez, M.I.; et al. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef]
- Brennan, L.A.; Kantorow, M. Mitochondrial function and redox control in the aging eye: Role of MsrA and other repair systems in cataract and macular degenerations. Exp. Eye Res. 2009, 88, 195–203. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Vishnyakova, K.S.; Yegorov, Y.E. Oxidative damage impact on aging and age-related diseases: Drug targeting of telomere attrition and dynamic telomerase activity flirting with imidazole-containing dipeptides. Recent Patents Drug Deliv. Formul. 2014, 8, 163–192. [Google Scholar] [CrossRef]
- Pacifici, F.; Della-Morte, D.; Piermarini, F.; Arriga, R.; Scioli, M.G.; Capuani, B.; Pastore, D.; Coppola, A.; Rea, S.; Donadel, G.; et al. Prdx6 Plays a Main Role in the Crosstalk Between Aging and Metabolic Sarcopenia. Antioxidants 2020, 9, 329. [Google Scholar] [CrossRef]
- Fernández-Ortiz, M.; Sayed, R.K.A.; Fernández-Martínez, J.; Cionfrini, A.; Aranda-Martínez, P.; Escames, G.; de Haro, T.; Acuña-Castroviejo, D. Melatonin/Nrf2/NLRP3 Connection in Mouse Heart Mitochondria during Aging. Antioxidants 2020, 9, 1187. [Google Scholar] [CrossRef]
- Chhunchha, B.; Fatma, N.; Kubo, E.; Rai, P.; Singh, S.P.; Singh, D.P. Curcumin abates hypoxia-induced oxidative stress based-ER stress-mediated cell death in mouse hippocampal cells (HT22) by controlling Prdx6 and NF-κB regulation. Am. J. Physiol. Physiol. 2013, 304, C636–C655. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Kondo-Okamoto, N.; Ohsumi, Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev. Cell 2009, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.; Li, T.Y.; Mottis, A.; Auwerx, J. Pleiotropic effects of mitochondria in aging. Nat. Aging 2022, 2, 199–213. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular mechanisms of aging and anti-aging strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, F.; Zang, Z.; Jiang, C.; Xu, Y.; Huang, X. Effect of ultrasonic far-infrared synergistic drying on the characteristics and qualities of wolfberry (Lycium barbarum L.). J. Ultrason. Sonochem. 2022, 89, 106134. [Google Scholar] [CrossRef]
- Tang, J.; Yan, Y.; Ran, L.; Mi, J.; Sun, Y.; Lu, L.; Gao, Y.; Zeng, X.; Cao, Y. Isolation, antioxidant property and protective effect on PC12 cell of the main anthocyanin in fruit of Lycium ruthenicum Murray. J. Funct. Foods 2017, 30, 97–107. [Google Scholar] [CrossRef]
- Yin, J.; Wu, T. Anthocyanins from black wolfberry (Lycium ruthenicum Murr.) prevent inflammation and increase fecal fatty acid in diet-induced obese rats. RSC Adv. 2017, 7, 47848–47853. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, S.; Zhou, W.; Meng, J.; Deng, K.; Zhou, H.; Hu, N.; Suo, Y. Anthocyanin composition of fruit extracts from Lycium ruthenicum and their protective effect for gouty arthritis. Ind. Crops Prod. 2019, 129, 414–423. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, Y.; Wang, X.; Wang, W. Lycium ruthenicum extract alleviates high-fat diet-induced nonalcoholic fatty liver disease via enhancing the AMPK signaling pathway. Mol. Med. Rep. 2015, 12, 3835–3840. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, L.; Fang, Z.; Nisar, T.; Zou, L.; Li, D.; Guo, Y. Lycium ruthenicum Murray anthocyanins effectively inhibit α-glucosidase activity and alleviate insulin resistance. Food Biosci. 2021, 41, 100949. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, W.; Xu, W.; Peng, Y.; Yan, Y.; Lu, L.; Mi, J.; Zeng, X.; Cao, Y. The Main Anthocyanin Monomer from Lycium ruthenicum Murray Fruit Mediates Obesity via Modulating the Gut Microbiota and Improving the Intestinal Barrier. Foods 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, Y.; Wan, P.; Dong, W.; Huang, K.; Ran, L.; Mi, J.; Lu, L.; Zeng, X.; Cao, Y. Effects of long-term intake of anthocyanins from Lycium ruthenicum Murray on the organism health and gut microbiota in vivo. Food Res. Int. 2020, 130, 108952. [Google Scholar] [CrossRef]
- Yan, Y.L.; Xu, D.L. Effects of Anthocyanins on Malondialdehyde, Superoxide Dismutase, and Total Antioxidant Capacity in Mice with Acute Kidney Injury. Chin. Community Dr. 2021, 37, 4–5. [Google Scholar]
- Sadeghi, N.; Behzad, M.; Jannat, B.; Oveisi, M.R.; Hajimahmoodi, M.; Kashanipour, A.H. Total phenolic compounds content and antioxidant activity in packed and bulk milk in different regions of Tehran, Iran. J. Food Safe Hyg. 2019, 4, 8–12. [Google Scholar]
- Wu, Q.; Xue, C.H.; Wang, E.J. Exploring the effect of anthocyanins from Lycium ruthenicum on autophagy in mouse macrophage RAW264.7 cells based on the AMPK-mTOR signaling pathway. Chin. J. Vet. Sci. 2021, 41, 2176–2181. [Google Scholar]
- Wei, L. Study on the Effects of Anthocyanins from Qaidam Lycium ruthenicum Murray on Senescence and p53, p21 in UVB-Irradiated Human Skin Fibroblasts in Vitro. Master’s Thesis, Qinghai University, Xining, China, 2022. [Google Scholar]
- Yisilam, G.; Wang, C.-X.; Xia, M.-Q.; Comes, H.P.; Li, P.; Li, J.; Tian, X.-M. Phylogeography and Population Genetics Analyses Reveal Evolutionary History of the Desert Resource Plant Lycium ruthenicum (Solanaceae). Front. Plant Sci. 2022, 13, 915526. [Google Scholar] [CrossRef]
- Singal, P.; Deally, C.; Weinberg, L. Subcellular effects of adriamycin in the heart: A concise review. J. Mol. Cell. Cardiol. 1987, 19, 817–828. [Google Scholar] [CrossRef]
- Nithipongvanitch, R.; Ittarat, W.; Cole, M.P.; Tangpong, J.; Clair, D.K.S.; Oberley, T.D. Mitochondrial and nuclear p53 localization in cardiomyocytes: Redox modulation by doxorubicin (Adriamycin)? Antioxid. Redox Signal. 2007, 9, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Štěrba, M.; Popelová, O.; Vávrová, A.; Jirkovský, E.; Kovaříková, P.; Geršl, V.; Šimůnek, T. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid. Redox Signal. 2013, 18, 899–929. [Google Scholar] [CrossRef] [PubMed]
- Mosteiro, L.; Pantoja, C.; de Martino, A.; Serrano, M. Senescence promotes in vivo reprogramming through p16INK4a and IL-6. Aging Cell 2018, 17, e12711. [Google Scholar] [CrossRef]
- Antonangeli, F.; Soriani, A.; Ricci, B.; Ponzetta, A.; Benigni, G.; Morrone, S.; Bernardini, G.; Santoni, A. Natural killer cell recognition of in vivo drug-induced senescent multiple myeloma cells. OncoImmunology 2016, 5, e1218105. [Google Scholar] [CrossRef]
- Barinaga, M. Zebrafish: Swimming into the development mainstream. Science 1990, 250, 34–35. [Google Scholar] [CrossRef]
- Barros, T.P.; Alderton, W.K.; Reynolds, H.M.; Roach, A.G.; Berghmans, S. Zebrafish: An emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br. J. Pharmacol. 2008, 154, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Vascotto, S.G.; Beckham, Y.; Kelly, G.M. The zebrafish’s swim to fame as an experimental model in biology. Biochem. Cell Biol. 1997, 75, 479–485. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Le, H.D.; Kim, T.N.T.; The, H.P.; Nguyen, T.M.; Cornet, V.; Lambert, J.; Kestemont, P. Anti-Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum Cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish. Antioxidants 2020, 9, 192. [Google Scholar] [CrossRef]
- Souza, J.P.; Mansano, A.S.; Venturini, F.P.; Santos, F.; Zucolotto, V. Antioxidant metabolism of zebrafish after sub-lethal exposure to graphene oxide and recovery. Fish Physiol. Biochem. 2019, 45, 1289–1297. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.; Hou, Y.; Zhou, H.; Wang, X.; Mai, K.; He, G. Differential Apoptotic and Mitogenic Effects of Lectins in Zebrafish. Front. Endocrinol. 2019, 10, 356. [Google Scholar] [CrossRef]
- Wei, Y.-H.; Lee, H.-C. Oxidative stress, mitochondrial dna mutation, and impairment of antioxidant enzymes in aging. Exp. Biol. Med. 2002, 227, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Lu, K.; Lin, P.; Che, H.; Li, H.; Song, L.; Yang, X.; Xie, W. Saccharina japonica Ethanol Extract Ameliorates Depression/Anxiety-Like Behavior by Inhibiting Inflammation, Oxidative Stress, and Apoptosis in Dextran Sodium Sulfate Induced Ulcerative Colitis Mice. Front. Nutr. 2021, 8, 784532. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Jeong, E.S.; Jang, G.; Na, J.R.; Park, S.; Kang, W.S.; Kim, E.; Choi, H.; Kim, J.S.; Kim, S. Unripe Rubus coreanus Miquel Extract Containing Ellagic Acid Regulates AMPK, SREBP-2, HMGCR, and INSIG-1 Signaling and Cholesterol Metabolism In Vitro and In Vivo. Nutrients 2020, 12, 610. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Huang, Q.; Li, S.; Zhou, Y.; Li, Z. Cardioprotection of CAPE-oNO2 against myocardial ischemia/reperfusion induced ROS generation via regulating the SIRT1/eNOS/NF-κB pathway in vivo and in vitro. Redox Biol. 2018, 15, 62–73. [Google Scholar] [CrossRef]
- Hong, T.; Ge, Z.; Meng, R.; Wang, H.; Zhang, P.; Tang, S.; Lu, J.; Gu, T.; Zhu, D.; Bi, Y. Erythropoietin alleviates hepatic steatosis by activating SIRT1-mediated autophagy. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 595–603. [Google Scholar] [CrossRef]
- Sun, W.; Qiao, W.; Zhou, B.; Hu, Z.; Yan, Q.; Wu, J.; Wang, R.; Zhang, Q.; Miao, D. Overexpression of Sirt1 in mesenchymal stem cells protects against bone loss in mice by FOXO3a deacetylation and oxidative stress inhibition. Metabolism 2018, 88, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.S.; Choi, Y.; Lee, J.W. Cellular localization of NRF2 determines the self-renewal and osteogenic differentiation potential of human MSCs via the P53–SIRT1 axis. Cell Death Dis. 2016, 7, e2093. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Luo, L.; Chen, Z.; Guo, Y.; Guo, L. Significance of PRO2000/ANCCA expression, a novel proliferation-associated protein in hepatocellular carcinoma. Cancer Cell Int. 2014, 14, 33. [Google Scholar] [CrossRef][Green Version]
- Oh, S.; Rhee, D.-Y.; Batsukh, S.; Son, K.H.; Byun, K. High-Intensity Focused Ultrasound Increases Collagen and Elastin Fiber Synthesis by Modulating Caveolin-1 in Aging Skin. Cells 2023, 12, 2275. [Google Scholar] [CrossRef]
- Mohan, M.; Kumar, V.; Lackner, A.A.; Alvarez, X. Dysregulated miR-34a–SIRT1–Acetyl p65 axis is a potential mediator of immune activation in the colon during chronic simian immunodeficiency virus infection of rhesus macaques. J. Immunol. 2015, 194, 291–306. [Google Scholar] [CrossRef]
- Tran, D.; Bergholz, J.; Zhang, H.; He, H.; Wang, Y.; Zhang, Y.; Li, Q.; Kirkland, J.L.; Xiao, Z.X. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell 2014, 13, 669–678. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Bax | CGGCATGGCGACAGGGATG | CATAGCAGGAGACGGTGGTGATG |
| Bcl-2 | CTCCTTCTCATACTTCAGCCTCCAC | ACCTTCAATGCCTCCTCCATCTTAC |
| NF-κB | CCTGTCTGTCTGTCTGTCTGTCTG | TCGTGGTGTCGTTGCTCTTCTC |
| MAPK | GTCCTACAGCAGCACAACTTCTAC | TCAACCCACAACGAAACACTCAG |
| P21 | CCAGAGACGACACCGTTTATT | GGAAGACTGAGGAATGGATCTTT |
| P53 | CGAGCCACTGCCATCTATAA | CTGATTGCCCTCCACTCTTATC |
| SIRT1 | CGCAAAGACATCAACACGTTAG | CAGGAATCCCACAGGAAACA |
| TNF-α | AGGAGAGTTGCCTTTACCGC | AATGGATGGCAGCCTTGGAA |
| foxq1a | ACTCCATCGCTACGCCTCCTTC | CATACGGCAGCACAGTGTCCAC |
| cyp1a | CAGTGTCGTGTGGCTCTTCGTAG | TCGGTCTTCGCAGTGGTTGATAAG |
| rundc3aa | AGAGTTGCTGACATTGCGGAGAAG | TGGACGGTGGGTGGGTTATGG |
| sycp3 | GCGGATCTGACGAAGACACGAG | AAACATCCCGACAGCACACAAGG |
| zgc:152891 | AGACATGCGGTACAGGTGAATTGG | GCCCTTCCAGCCACAACTCATC |
| chmp5a | AAACAAGCGGCGTCTCCCAAC | AACACTCAGCATCCACAGCACATC |
| slc25a22b | ACTTCTGCCCTTTGCCCTTTGTC | TCTGCTTGCTTGTCTGGAACTCTC |
| emilin2a | AGAAGGACAGGAGAACGGCTACG | TTGGCGGTTTGTGGGCATGAG |
| si:ch211-213a13.2 | GAACACGAGGTCAGGCAGATTCAG | GACTTCCCGATGCCAGCAACTC |
| BX936382.1 | CAATGGTATCGGCTGGCGGAAC | GACGAGGAACGGAGTTTCTGGATG |
| clrn3 | TTTCGGGAAGAGAGTGGGTGAGG | GAGGCTGCTGTTGACTGACTGAC |
| drp2 | ATATTCGGCGGTTCAAGGCAAGG | TTGGCTGTCTCGTCTGTCGTTTG |
| si:dkey-5i16.5 | ACTCCTTCCTGTACGGCTAACTGG | ATGCTCGCTCGCTGGAACATAAC |
| cdc42bpaa | CACCAGCACCAGGGACATAACAC | TGAGGATGAAGGAAGCAACAGCAG |
| znf979 | ACTCCATAACCTCTGCTCCTCTGC | ACCTCCCTACCTCTCCTCCTCAC |
| CU571081.1 | CCGCCGCAGAAGCTCAATGG | CGAGGGTCCACGAGAAGTTGTTTG |
| β-actin | TCGAGCAGGAGATGGGAACC | CTCGTGGATACCGCAAGATTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Ga, Z.; Wu, J.; Wang, Y.; Dongzhu, N.; Qieyang, R.; Li, P.; Huaqian, S. Lycium ruthenicum Murray Anthocyanins Alleviate Aging Through SIRT1/P53 Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 4510. https://doi.org/10.3390/ijms26104510
Liang J, Ga Z, Wu J, Wang Y, Dongzhu N, Qieyang R, Li P, Huaqian S. Lycium ruthenicum Murray Anthocyanins Alleviate Aging Through SIRT1/P53 Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(10):4510. https://doi.org/10.3390/ijms26104510
Chicago/Turabian StyleLiang, Jialin, Zang Ga, Jiaqin Wu, Yingjie Wang, Nanjia Dongzhu, Rangzhong Qieyang, Ping Li, and Sangduo Huaqian. 2025. "Lycium ruthenicum Murray Anthocyanins Alleviate Aging Through SIRT1/P53 Signaling Pathway" International Journal of Molecular Sciences 26, no. 10: 4510. https://doi.org/10.3390/ijms26104510
APA StyleLiang, J., Ga, Z., Wu, J., Wang, Y., Dongzhu, N., Qieyang, R., Li, P., & Huaqian, S. (2025). Lycium ruthenicum Murray Anthocyanins Alleviate Aging Through SIRT1/P53 Signaling Pathway. International Journal of Molecular Sciences, 26(10), 4510. https://doi.org/10.3390/ijms26104510





