Validation Study of Analytical Methods for Multiparameter Flow Cytometry-Based Measurable Residual Disease Assessment in Acute Myeloid Leukemia
Abstract
1. Introduction
2. Results
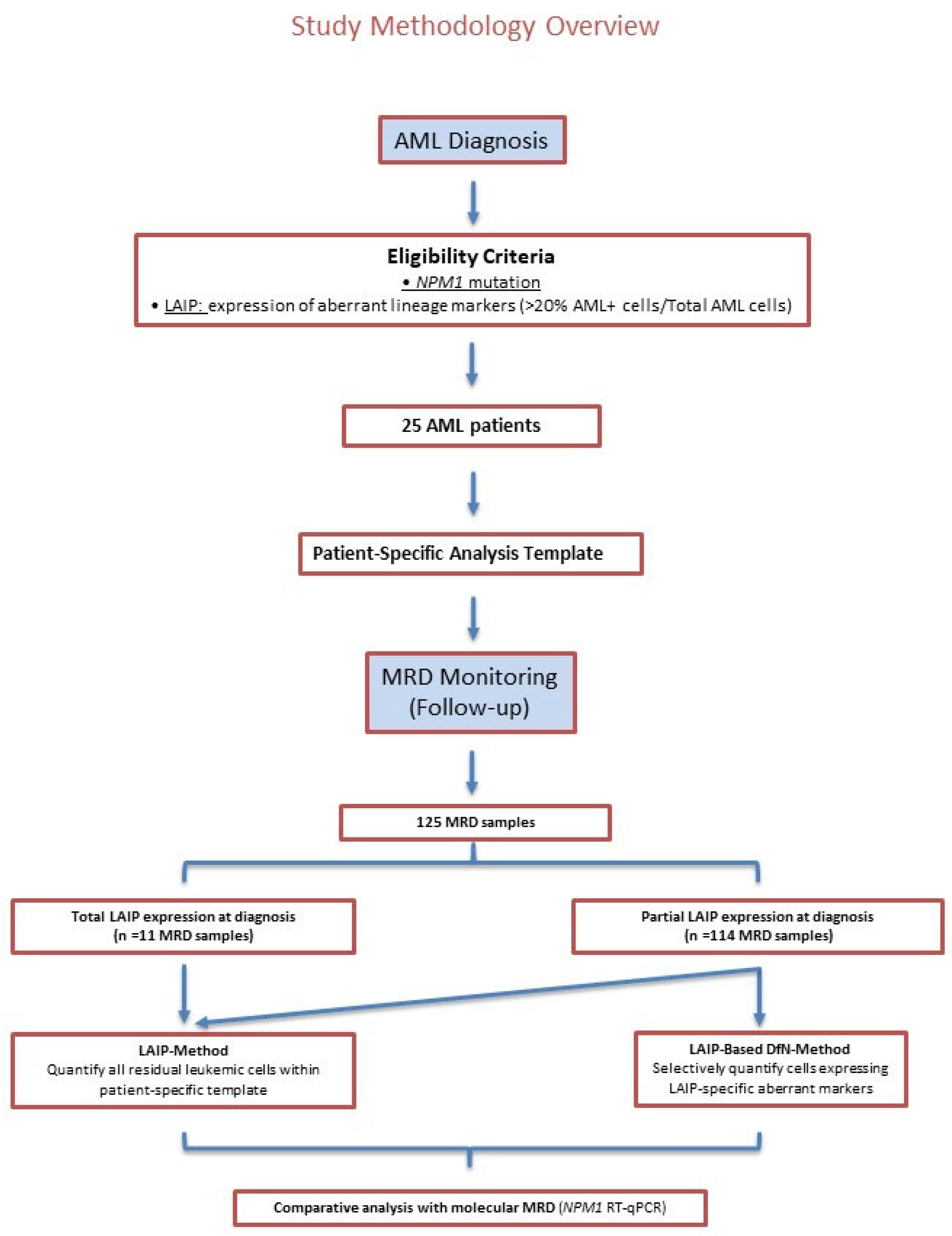
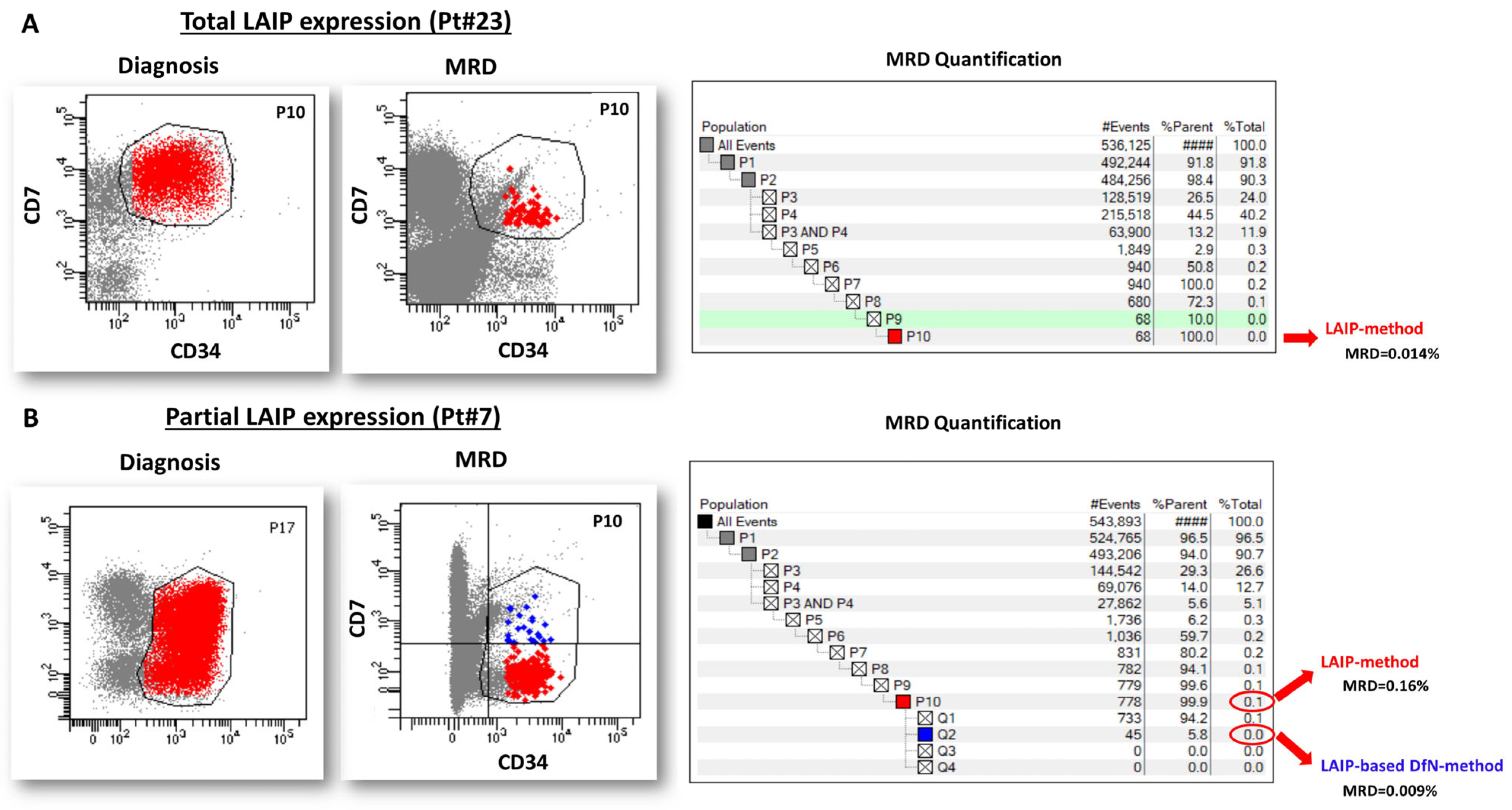
2.1. Study Design and MRD Analysis Overview
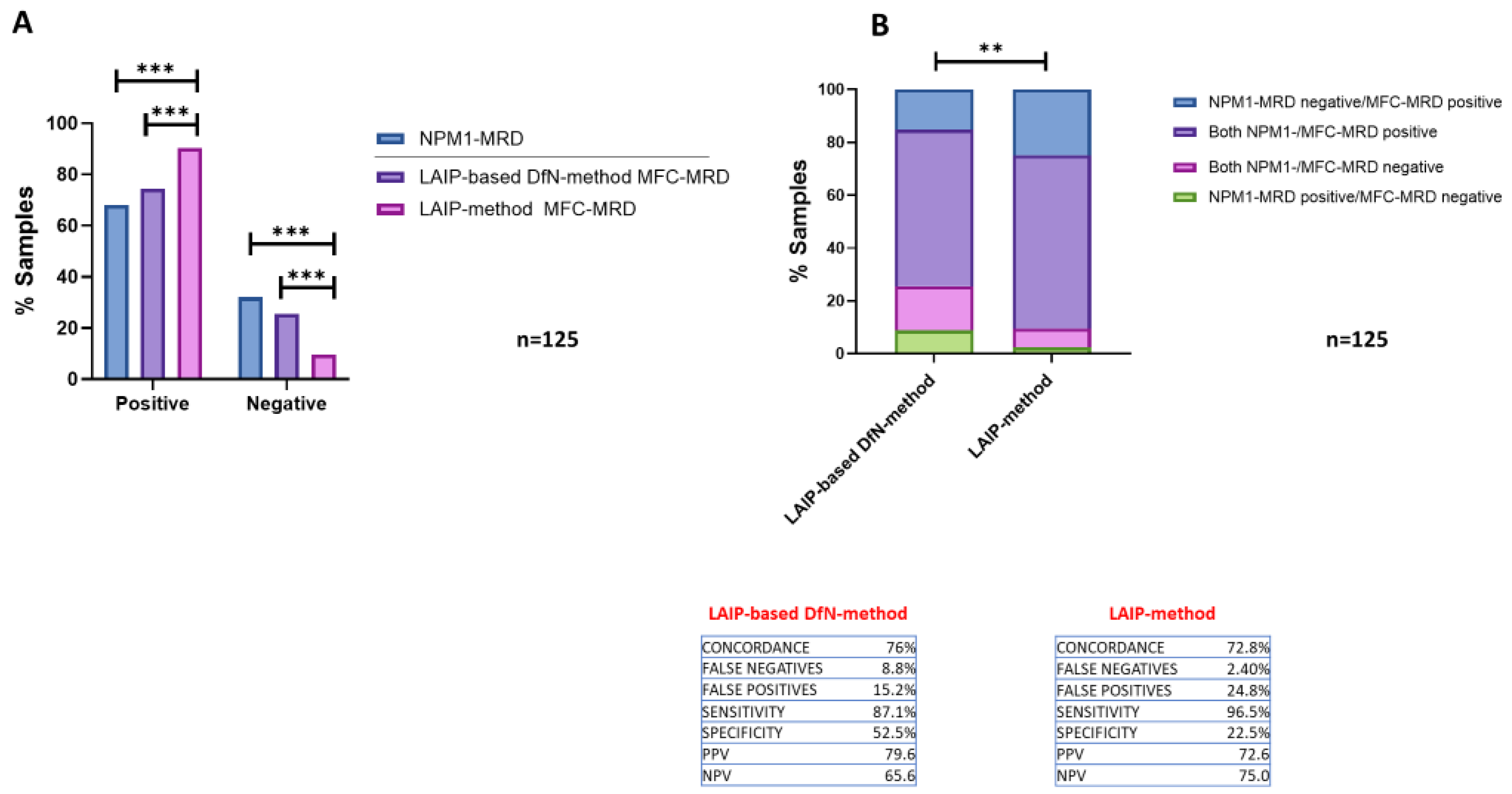
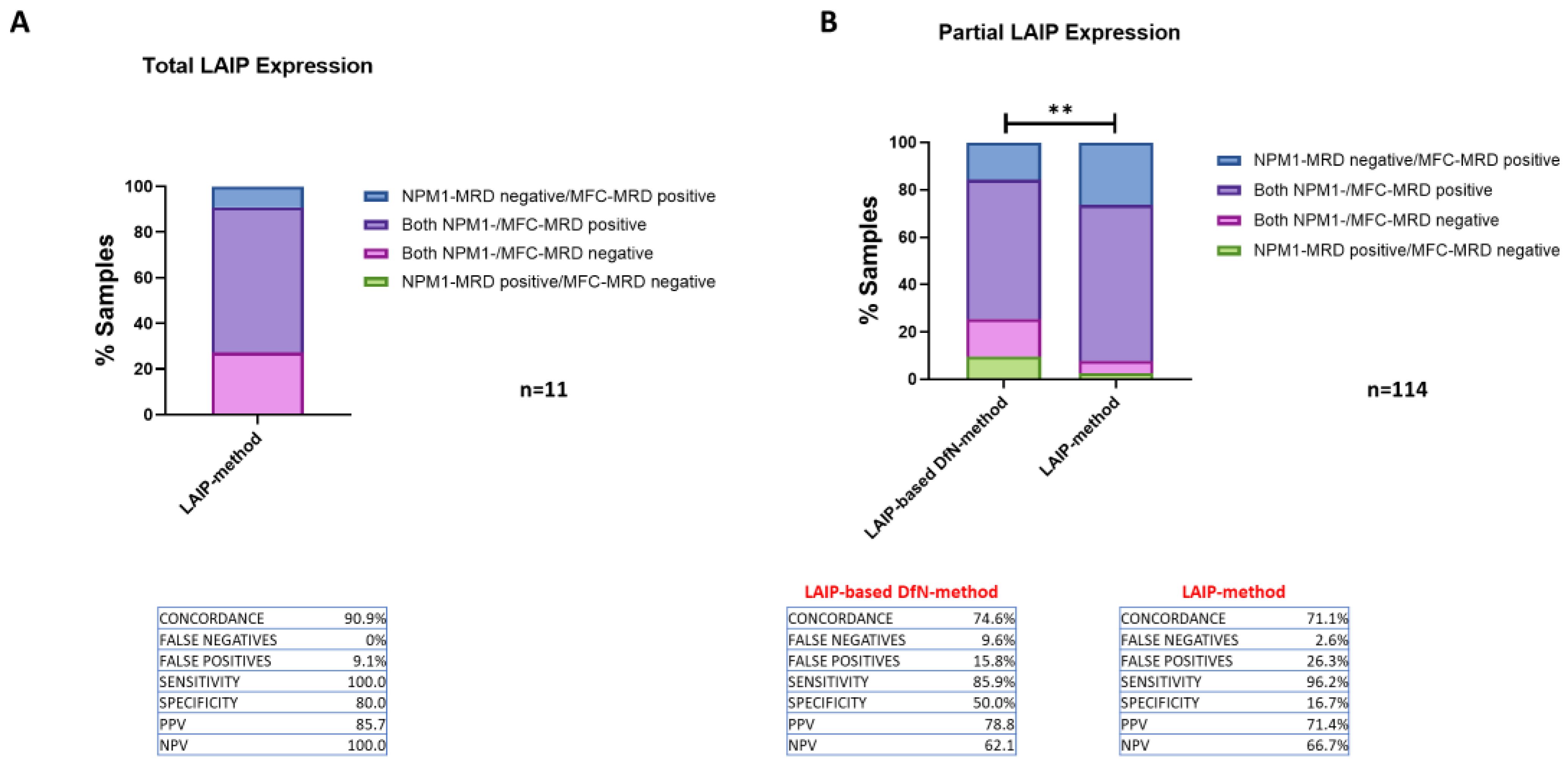
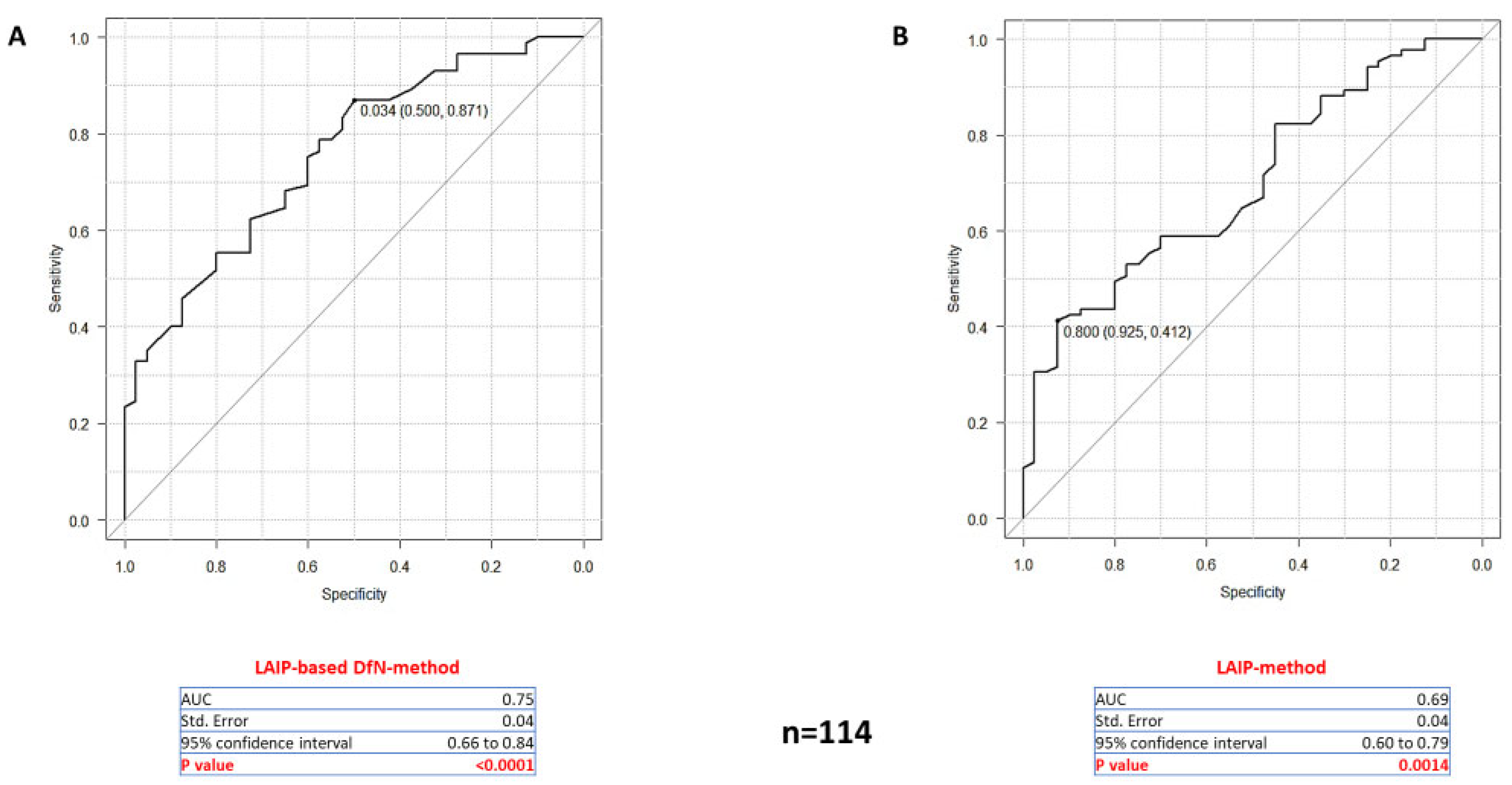
2.2. Comparison of the Concordance of the Two MFC Analytical Methods Results with NPM1-MRD Outcome
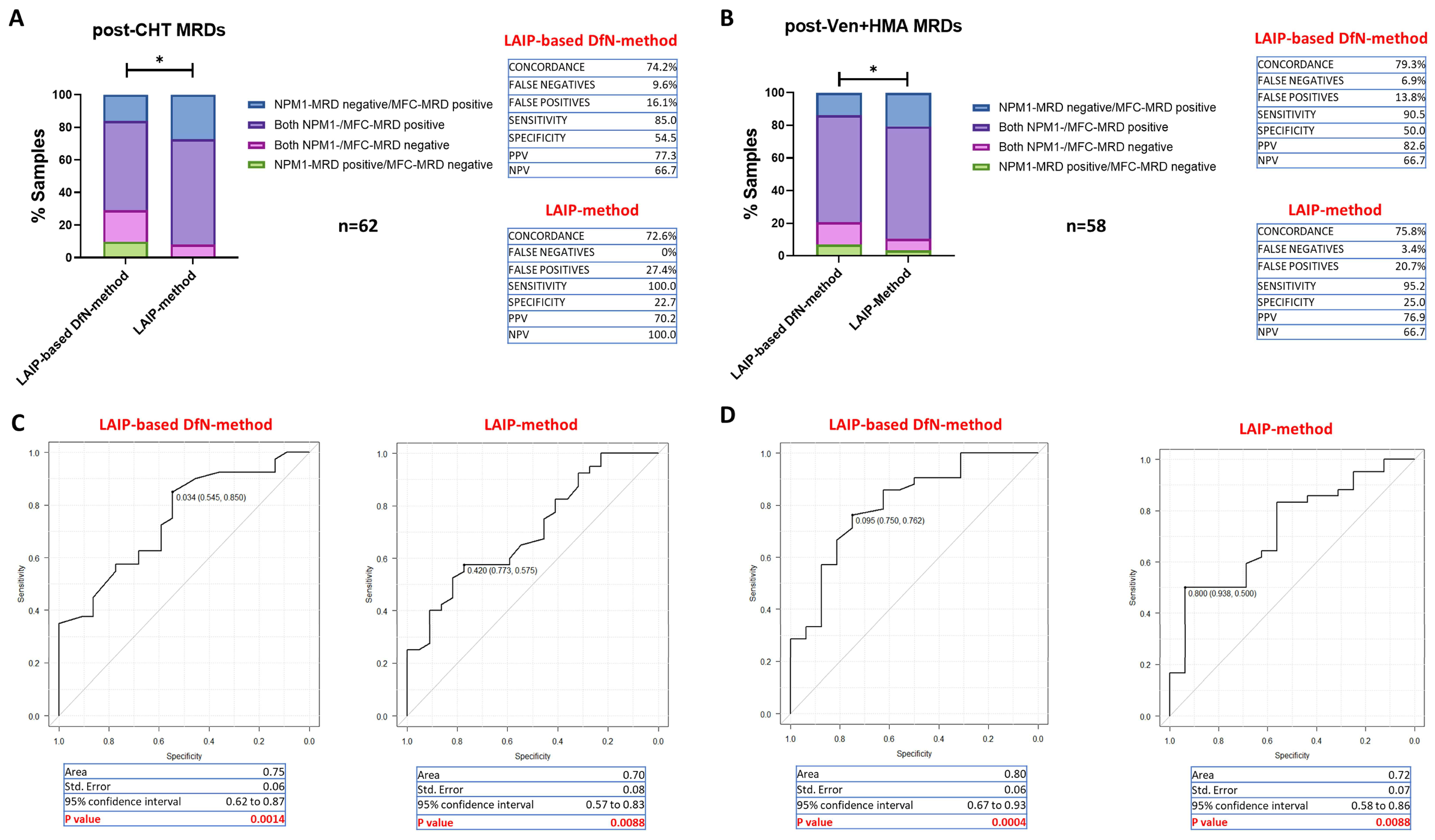
2.3. Comparing the Accuracy of Two MFC Approaches for MRD Quantification
2.4. Accuracy of MFC-MRD Assessment According to Therapy
2.5. Evaluation of the Reliability of Distinct LAIP-Specific Aberrant Lineage Markers in MFC-MRD Monitoring
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Immunophenotyping of AML at Diagnosis
4.3. Flow Cytometric Evaluation of MRD
4.4. Patterns and Ranges in Normal and Regenerating Bone Marrow Samples
4.5. Molecular Evaluation of MRD
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.C.; et al. 2021 Update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef] [PubMed]
- Chea, M.; Rigolot, L.; Canali, A.; Vergez, F. Minimal Residual Disease in Acute Myeloid Leukemia: Old and New Concepts. Int. J. Mol. Sci. 2024, 25, 2150. [Google Scholar] [CrossRef]
- Moritz, J.; Schwab, A.; Reinisch, A.; Zebisch, A.; Sill, H.; Wölfler, A. Measurable Residual Disease Detection in Acute Myeloid Leukemia: Current Challenges and Future Directions. Biomedicines 2024, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Loken, M.R.; Alonzo, T.A.; Pardo, L.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Ho, P.A.; Franklin, J.; Cooper, T.M.; Gamis, A.S.; et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: A report from Children’s Oncology Group. Blood 2012, 120, 1581–1588. [Google Scholar] [CrossRef]
- Ivey, A.; Hills, R.K.; Simpson, M.A.; Jovanovic, J.V.; Gilkes, A.; Grech, A.; Patel, Y.; Bhudia, N.; Farah, H.; Mason, J.; et al. Assessment of Minimal Residual Disease in Standard-Risk AML. N. Engl. J. Med. 2016, 374, 422–433. [Google Scholar] [CrossRef]
- Grimwade, D.; Freeman, S.D. Defining minimal residual disease in acute myeloid leukemia: Which platforms are ready for “prime time”? Hematol. Am. Soc. Hematol. Educ. Program 2014, 2014, 222–233. [Google Scholar] [CrossRef]
- Zhou, Y.; Moon, A.; Hoyle, E.; Fromm, J.R.; Chen, X.; Soma, L.; Salipante, S.J.; Wood, B.L.; Wu, D. Pattern associated leukemia immunophenotypes and measurable disease detection in acute myeloid leukemia or myelodysplastic syndrome with mutated NPM1. Cytom. Part B Clin. Cytom. 2019, 96, 67–72. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Rossi, G.; Giambra, V.; Minervini, M.M.; De Waure, C.; Mancinelli, S.; Ciavarella, M.; Sinisi, N.P.; Scalzulli, P.R.; Carella, A.M.; Cascavilla, N. Leukemia-associated immunophenotypes subdivided in “categories of specificity” improve the sensitivity of minimal residual disease in predicting relapse in acute myeloid leukemia. Cytom. Part B Clin. Cytom. 2020, 98, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Tettero, J.M.; Freeman, S.; Buecklein, V.; Venditti, A.; Maurillo, L.; Kern, W.; Walter, R.B.; Wood, B.L.; Roumier, C.; Philippé, J.; et al. Technical Aspects of Flow Cytometry-based Measurable Residual Disease Quantification in Acute Myeloid Leukemia: Experience of the European LeukemiaNet MRD Working Party. Hemasphere 2022, 6, e676. [Google Scholar] [CrossRef] [PubMed]
- Al-Mawali, A.; Gillis, D.; Hissaria, P.; Lewis, I. Incidence, sensitivity, and specificity of leukemia-associated phenotypes in acute myeloid leukemia using specific five-color multiparameter flow cytometry. Am. J. Clin. Pathol. 2008, 129, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Feller, N.; van der Velden, V.H.; Brooimans, R.A.; Boeckx, N.; Preijers, F.; Kelder, A.; de Greef, I.; Westra, G.; Te Marvelde, J.G.; Aerts, P.; et al. Defining consensus leukemia-associated immunophenotypes for detection of minimal residual disease in acute myeloid leukemia in a multicenter setting. Blood Cancer J. 2013, 3, e129. [Google Scholar] [CrossRef]
- Olaru, D.; Campos, L.; Flandrin, P.; Nadal, N.; Duval, A.; Chautard, S.; Guyotat, D. Multiparametric analysis of normal and postchemotherapy bone marrow: Implication for the detection of leukemia-associated immunophenotypes. Cytom. Part B Clin. Cytom. 2008, 74, 17–24. [Google Scholar] [CrossRef]
- Venditti, A.; Maurillo, L.; Buccisano, F.; Del Poeta, G.; Mazzone, C.; Tamburini, A.; Del Principe, M.I.; Consalvo, M.I.; De Fabritiis, P.; Cudillo, L.; et al. Pretransplant minimal residual disease level predicts clinical outcome in patients with acute myeloid leukemia receiving high-dose chemotherapy and autologous stem cell transplantation. Leukemia 2003, 17, 2178–2182. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, D.; Cunningham, M.T.; Tilzer, L. Leukemia-associated aberrant immunophenotype in patients with acute myeloid leukemia: Changes at refractory disease or first relapse and clinicopathological findings. Int. J. Lab. Hematol. 2014, 36, 636–649. [Google Scholar] [CrossRef]
- San Miguel, J.F.; Vidriales, M.B.; López-Berges, C.; Díaz-Mediavilla, J.; Gutiérrez, N.; Cañizo, C.; Ramos, F.; Calmuntia, M.J.; Pérez, J.J.; González, M.; et al. Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood 2001, 98, 1746–1751. [Google Scholar] [CrossRef]
- Terwijn, M.; van Putten, W.L.; Kelder, A.; van der Velden, V.H.; Brooimans, R.A.; Pabst, T.; Maertens, J.; Boeckx, N.; de Greef, G.E.; Valk, P.J.; et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: Data from the HOVON/SAKK AML 42A study. J. Clin. Oncol. 2013, 31, 3889–3897. [Google Scholar] [CrossRef]
- Cocciardi, S.; Dolnik, A.; Kapp-Schwoerer, S.; Rücker, F.G.; Lux, S.; Blätte, T.J.; Skambraks, S.; Krönke, J.; Heidel, F.H.; Schnöder, T.M.; et al. Clonal evolution patterns in acute myeloid leukemia with NPM1 mutation. Nat. Commun. 2019, 10, 2031. [Google Scholar] [CrossRef]
- Höllein, A.; Meggendorfer, M.; Dicker, F.; Jeromin, S.; Nadarajah, N.; Kern, W.; Haferlach, C.; Haferlach, T. NPM1 mutated AML can relapse with wild-type. Blood Adv. 2018, 2, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Marumo, A.; Wakita, S.; Morita, K.; Oh, I.; Kako, S.; Toya, T.; Najima, Y.; Doki, N.; Kanda, J.; Kuroda, J.; et al. NPM1-mutation-based measurable residual disease assessment after completion of two courses of post-remission therapy is a valuable clinical predictor of the prognosis of acute myeloid leukemia. Int. J. Hematol. 2022, 116, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Forghieri, F.; Comoli, P.; Marasca, R.; Potenza, L.; Luppi, M. Minimal/Measurable Residual Disease Monitoring in. Int. J. Mol. Sci. 2018, 19, 3492. [Google Scholar] [CrossRef]
- Kalina, T.; Flores-Montero, J.; van der Velden, V.H.; Martin-Ayuso, M.; Böttcher, S.; Ritgen, M.; Almeida, J.; Lhermitte, L.; Asnafi, V.; Mendonça, A.; et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia 2012, 26, 1986–2010. [Google Scholar] [CrossRef]
- Krönke, J.; Schlenk, R.F.; Jensen, K.O.; Tschürtz, F.; Corbacioglu, A.; Gaidzik, V.I.; Paschka, P.; Onken, S.; Eiwen, K.; Habdank, M.; et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: A study from the German-Austrian acute myeloid leukemia study group. J. Clin. Oncol. 2011, 29, 2709–2716. [Google Scholar] [CrossRef]
- Schnittger, S.; Weisser, M.; Schoch, C.; Hiddemann, W.; Haferlach, T.; Kern, W. New score predicting for prognosis in PML-RARA+, AML1-ETO+, or CBFBMYH11+ acute myeloid leukemia based on quantification of fusion transcripts. Blood 2003, 102, 2746–2755. [Google Scholar] [CrossRef]
- Yin, J.A.; O’Brien, M.A.; Hills, R.K.; Daly, S.B.; Wheatley, K.; Burnett, A.K. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: Results of the United Kingdom MRC AML-15 trial. Blood 2012, 120, 2826–2835. [Google Scholar] [CrossRef]
- Buckley, S.A.; Wood, B.L.; Othus, M.; Hourigan, C.S.; Ustun, C.; Linden, M.A.; DeFor, T.E.; Malagola, M.; Anthias, C.; Valkova, V.; et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: A meta-analysis. Haematologica 2017, 102, 865–873. [Google Scholar] [CrossRef]
- Paiva, B.; Vidriales, M.B.; Sempere, A.; Tarín, F.; Colado, E.; Benavente, C.; Cedena, M.T.; Sánchez, J.; Caballero-Velazquez, T.; Cordón, L.; et al. Impact of measurable residual disease by decentralized flow cytometry: A PETHEMA real-world study in 1076 patients with acute myeloid leukemia. Leukemia 2021, 35, 2358–2370. [Google Scholar] [CrossRef]
- Venditti, A.; Buccisano, F.; Del Poeta, G.; Maurillo, L.; Tamburini, A.; Cox, C.; Battaglia, A.; Catalano, G.; Del Moro, B.; Cudillo, L.; et al. Level of minimal residual disease after consolidation therapy predicts outcome in acute myeloid leukemia. Blood 2000, 96, 3948–3952. [Google Scholar] [CrossRef]
- Venditti, A.; Piciocchi, A.; Candoni, A.; Melillo, L.; Calafiore, V.; Cairoli, R.; de Fabritiis, P.; Storti, G.; Salutari, P.; Lanza, F.; et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood 2019, 134, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Buccisano, F.; Maurillo, L.; Gattei, V.; Del Poeta, G.; Del Principe, M.I.; Cox, M.C.; Panetta, P.; Consalvo, M.I.; Mazzone, C.; Neri, B.; et al. The kinetics of reduction of minimal residual disease impacts on duration of response and survival of patients with acute myeloid leukemia. Leukemia 2006, 20, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Ofran, Y.; Wierzbowska, A.; Ravandi, F.; Hourigan, C.S.; Ngai, L.L.; Venditti, A.; Buccisano, F.; Ossenkoppele, G.J.; Roboz, G.J. Measurable residual disease as a biomarker in acute myeloid leukemia: Theoretical and practical considerations. Leukemia 2021, 35, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Minervini, M.M.; Carella, A.M.; de Waure, C.; di Nardo, F.; Melillo, L.; D’Arena, G.; Zini, G.; Cascavilla, N. Comparison between multiparameter flow cytometry and WT1-RNA quantification in monitoring minimal residual disease in acute myeloid leukemia without specific molecular targets. Leuk. Res. 2012, 36, 401–406. [Google Scholar] [CrossRef]
- Plesa, A.; Dumezy, F.; Mathis, S.; Lhoumeau, A.-C.; Bardet, V.; Saada, V.; Arnoux, I.; Badaoui, B.; Cornet, E.; Osman, J.; et al. AML MRD By Multiparameter Flow Cytometry Using Laip/Dfn and LSC: Methodological Aspects in a Multicentric Study of the French-Flow MRD AML ALFA Network. Blood 2022, 140, 6279–6281. [Google Scholar] [CrossRef]
- Plesa, A.; Mathis, S.; Dumezy, F.; Lhoumeau, A.-C.; Saada, V.; Arnoux, I.; Osman, J.; Chapuis, N.; Geneviève, F.; Roussel, M.; et al. Flow MRD Monitoring Combining Laip/Dfn and CD34+CD38- LSCs Is a Strong Predictor of Outcome in Adult AML Independently of the ELN-2022 Risk: First Results from the Multicentric Acute Leukemia French Intergroup MRD Flow Network. Blood 2024, 144, 226. [Google Scholar] [CrossRef]
- Ngai, L.L.; Hanekamp, D.; Kelder, A.; Scholten, W.; Carbaat-Ham, J.; Fayed, M.M.; Snel, A.N.; Bachas, C.; Tettero, J.M.; Mocking, T.R.; et al. The Laip-Based-Dfn Approach Is Superior in Terms of Useful MRD Results As Compared to the Laip Approach after Cycle II in Acute Myeloid Leukemia. Blood 2023, 142, 1572. [Google Scholar] [CrossRef]
- Tettero, J.M.; Dakappagari, N.; Heidinga, M.E.; Oussoren-Brockhoff, Y.; Hanekamp, D.; Pahuja, A.; Burns, K.; Kaur, P.; Alfonso, Z.; van der Velden, V.H.J.; et al. Analytical assay validation for acute myeloid leukemia measurable residual disease assessment by multiparametric flow cytometry. Cytom. Part B Clin. Cytom. 2023, 104, 426–439. [Google Scholar] [CrossRef]
- Wang, S.A.; Jorgensen, J.L.; Hu, S.; Jia, F.; Li, S.; Loghavi, S.; Ok, C.Y.; Thakral, B.; Xu, J.; Medeiros, L.J.; et al. Validation of a 12-color flow cytometry assay for acute myeloid leukemia minimal/measurable residual disease detection. Cytom. Part B Clin. Cytom. 2023, 104, 356–366. [Google Scholar] [CrossRef]
- Hanekamp, D.; Tettero, J.M.; Ossenkoppele, G.J.; Kelder, A.; Cloos, J.; Schuurhuis, G.J. AML/Normal Progenitor Balance Instead of Total Tumor Load (MRD) Accounts for Prognostic Impact of Flowcytometric Residual Disease in AML. Cancers 2021, 13, 2597. [Google Scholar] [CrossRef]
- Simoes, C.; Paiva, B.; Martínez-Cuadrón, D.; Bergua, J.M.; Vives, S.; Algarra, L.; Tormo, M.; Martinez, P.; Serrano, J.; Herrera, P.; et al. Measurable residual disease in elderly acute myeloid leukemia: Results from the PETHEMA-FLUGAZA phase 3 clinical trial. Blood Adv. 2021, 5, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Kalina, T.; Lundsten, K.; Engel, P. Relevance of Antibody Validation for Flow Cytometry. Cytom. Part A 2020, 97, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Hubmann, M.; Köhnke, T.; Hoster, E.; Schneider, S.; Dufour, A.; Zellmeier, E.; Fiegl, M.; Braess, J.; Bohlander, S.K.; Subklewe, M.; et al. Molecular response assessment by quantitative real-time polymerase chain reaction after induction therapy in NPM1-mutated patients identifies those at high risk of relapse. Haematologica 2014, 99, 1317–1325. [Google Scholar] [CrossRef] [PubMed]

| AML Patient | n° Post-CHT MRDs | n° Post-Ven+HMA MRDs | n° Post-Allo-SCT MRDs | n° Total MRDs | n° LAIP | % AML Cells at Diagnosis | CD34 | CD117 | CD33 | CD13 | HLA-DR | CD4 | CD7 | CD56 | CD25 | CD15 | CD123 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 2 | 2 | 0 | 4 | 2 | 47% | 10% | 100% | 100% | 100% | 100% | 35% | 0% | 58% | 0% | 38% | 100% |
| #2 | 8 | 0 | 1 | 9 | 2 | 27% | 100% | 100% | 100% | 77% | 100% | 85% | 0% | 20% | 0% | 0% | 0% |
| #3 | 2 | 0 | 1 | 3 | 2 | 36% | 0% | 100% | 100% | 100% | 100% | 32% | 0% | 50% | 0% | 0% | 0% |
| #4 | 3 | 2 | 1 | 6 | 2 | 30% | 5% | 100% | 100% | 85% | 100% | 60% | 0% | 24% | 0% | 0% | 100% |
| #5 | 2 | 5 | 0 | 7 | 3 | 57% | 2% | 100% | 100% | 100% | 100% | 10% | 64% | 38% | 53% | 50% | 0% |
| #6 | 3 | 1 | 0 | 4 | 2 | 14% | 50% | 100% | 100% | 100% | 100% | 55% | 0% | 25% | 0% | 0% | 0% |
| #7 | 6 | 0 | 1 | 7 | 2 | 77% | 100% | 80% | 100% | 100% | 100% | 35% | 75% | 0% | 0% | 0% | 0% |
| #8 | 3 | 3 | 0 | 6 | 3 | 67% | 78% | 100% | 100% | 100% | 70% | 54% | 63% | 0% | 67% | 0% | 80% |
| #9 | 0 | 5 | 0 | 5 | 2 | 14% | 0% | 100% | 100% | 100% | 100% | 68% | 30% | 0% | 0% | 0% | 0% |
| #10 | 7 | 0 | 0 | 7 | 2 | 50% | 1% | 100% | 100% | 75% | 90% | 78% | 2% | 21% | 1% | 50% | 0% |
| #11 | 0 | 6 | 0 | 6 | 2 | 44% | 0% | 100% | 100% | 78% | 100% | 67% | 22% | 0% | 0% | 0% | 0% |
| #12 | 3 | 1 | 0 | 4 | 2 | 25% | 0% | 100% | 100% | 100% | 100% | 32% | 46% | 0% | 0% | 0% | 0% |
| #13 | 8 | 0 | 0 | 8 | 2 | 20% | 2% | 100% | 100% | 67% | 100% | 61% | 0% | 30% | 0% | 33% | 100% |
| #14 | 0 | 7 | 0 | 7 | 2 | 60% | 10% | 100% | 100% | 60% | 100% | 66% | 22% | 0% | 0% | 28% | 80% |
| #15 | 0 | 2 | 0 | 2 | 2 | 63% | 100% | 100% | 100% | 100% | 100% | 50% | 100% | 0% | 0% | 0% | 0% |
| #16 | 0 | 5 | 0 | 5 | 2 | 35% | 100% | 100% | 100% | 100% | 100% | 40% | 50% | 0% | 0% | 0% | 0% |
| #17 | 4 | 3 | 0 | 7 | 2 | 20% | 0% | 100% | 100% | 42% | 100% | 75% | 10% | 20% | 0% | 6% | 100% |
| #18 | 3 | 3 | 0 | 6 | 3 | 62% | 0% | 100% | 100% | 100% | 100% | 32% | 48% | 21% | 0% | 0% | 0% |
| #19 | 2 | 0 | 0 | 2 | 2 | 82% | 100% | 100% | 60% | 85% | 100% | 11% | 20% | 50% | 0% | 0% | 0% |
| #20 | 0 | 4 | 0 | 4 | 2 | 20% | 100% | 100% | 100% | 100% | 100% | 20% | 30% | 0% | 0% | 0% | 0% |
| #21 | 2 | 2 | 0 | 4 | 2 | 20% | 5% | 100% | 100% | 100% | 100% | 15% | 58% | 0% | 98% | 70% | 100% |
| #22 | 0 | 2 | 0 | 2 | 2 | 21% | 8% | 100% | 100% | 100% | 100% | 27% | 8% | 37% | 0% | 0% | 0% |
| #23 | 0 | 2 | 0 | 2 | 2 | 81% | 100% | 100% | 100% | 100% | 100% | 32% | 100% | 0% | 0% | 0% | 0% |
| #24 | 0 | 3 | 0 | 3 | 3 | 73% | 5% | 100% | 100% | 100% | 100% | 60% | 33% | 0% | 95% | 80% | 0% |
| #25 | 4 | 0 | 1 | 5 | 2 | 50% | 43% | 100% | 100% | 52% | 58% | 48% | 6% | 0% | 56% | 25% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, M.; Patuelli, A.; Dicataldo, M.; Irno Consalvo, M.; Chirumbolo, G.; Bandini, L.; Atzeni, G.; Forte, D.; Cristiano, G.; Ottaviani, E.; et al. Validation Study of Analytical Methods for Multiparameter Flow Cytometry-Based Measurable Residual Disease Assessment in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2025, 26, 4506. https://doi.org/10.3390/ijms26104506
Barone M, Patuelli A, Dicataldo M, Irno Consalvo M, Chirumbolo G, Bandini L, Atzeni G, Forte D, Cristiano G, Ottaviani E, et al. Validation Study of Analytical Methods for Multiparameter Flow Cytometry-Based Measurable Residual Disease Assessment in Acute Myeloid Leukemia. International Journal of Molecular Sciences. 2025; 26(10):4506. https://doi.org/10.3390/ijms26104506
Chicago/Turabian StyleBarone, Martina, Agnese Patuelli, Michele Dicataldo, Maria Irno Consalvo, Gabriella Chirumbolo, Lorenza Bandini, Giulia Atzeni, Dorian Forte, Gianluca Cristiano, Emanuela Ottaviani, and et al. 2025. "Validation Study of Analytical Methods for Multiparameter Flow Cytometry-Based Measurable Residual Disease Assessment in Acute Myeloid Leukemia" International Journal of Molecular Sciences 26, no. 10: 4506. https://doi.org/10.3390/ijms26104506
APA StyleBarone, M., Patuelli, A., Dicataldo, M., Irno Consalvo, M., Chirumbolo, G., Bandini, L., Atzeni, G., Forte, D., Cristiano, G., Ottaviani, E., Curti, A., Buccisano, F., Catani, L., & Arpinati, M. (2025). Validation Study of Analytical Methods for Multiparameter Flow Cytometry-Based Measurable Residual Disease Assessment in Acute Myeloid Leukemia. International Journal of Molecular Sciences, 26(10), 4506. https://doi.org/10.3390/ijms26104506








