Cannabidiol Treatment in a Predator-Based Animal Model of PTSD: Assessing Oxidative Stress and Memory Performance
Abstract
1. Introduction
2. Results
2.1. Behavioral Tests
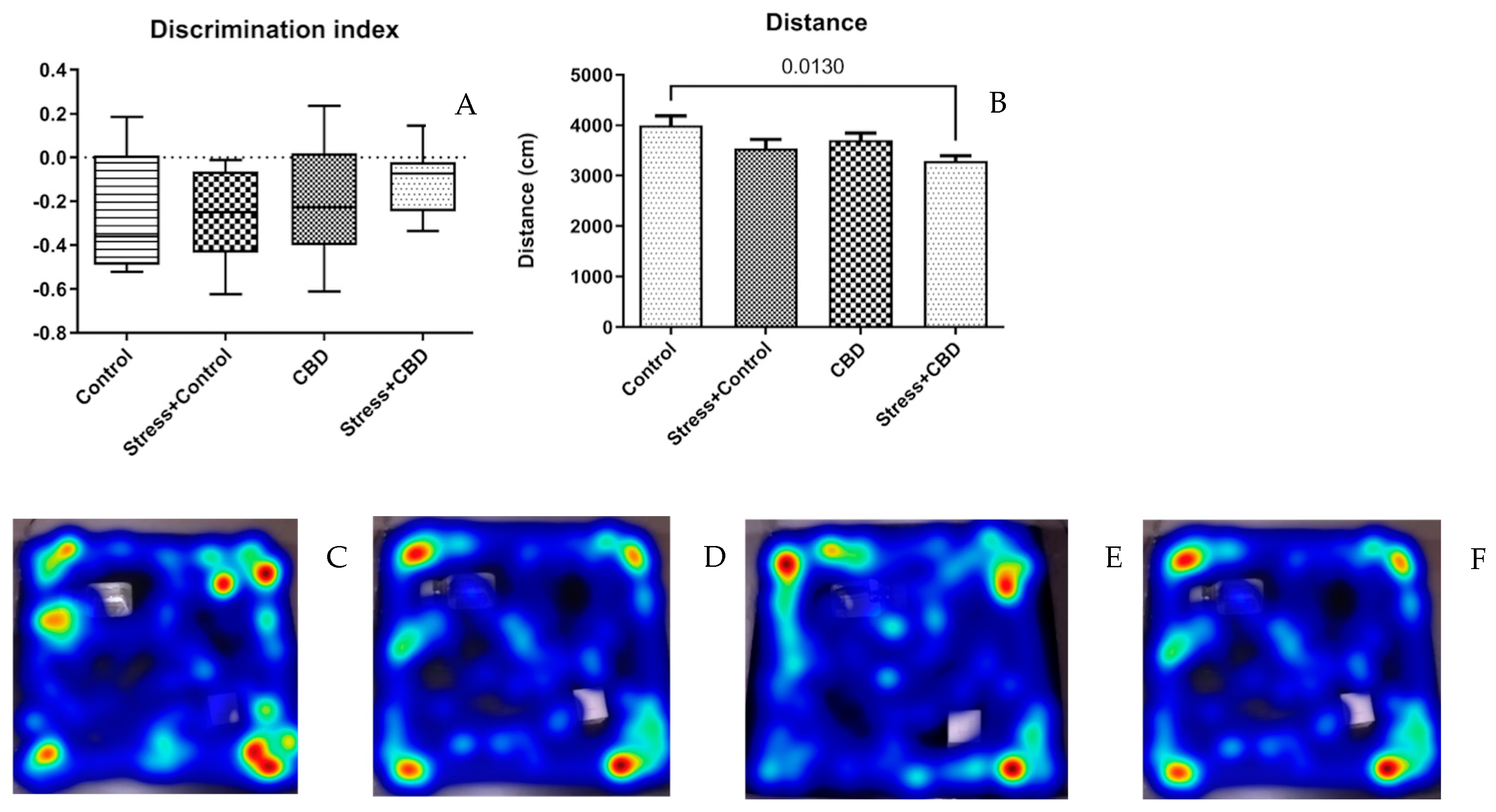
2.1.1. Open Field Test (OF)
2.1.2. Novel Object Recognition (NOR)
2.1.3. Elevated Plus Maze Test (EPM)
2.1.4. Morris Water Maze (MWM)
2.2. Plasma and Brain Sampling
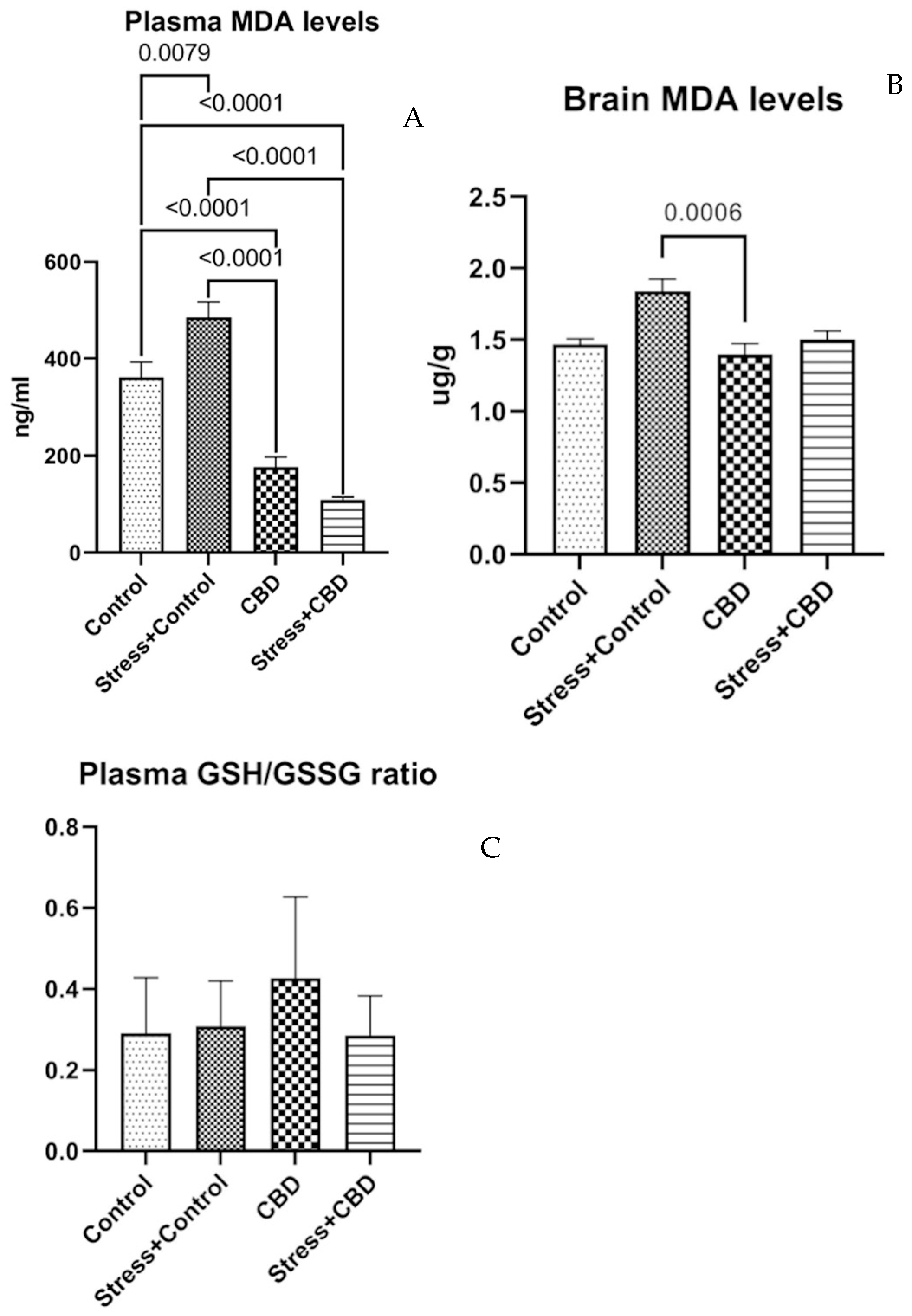
2.2.1. Plasma and Brain MDA, Plasma GSH/GSSG Ratio
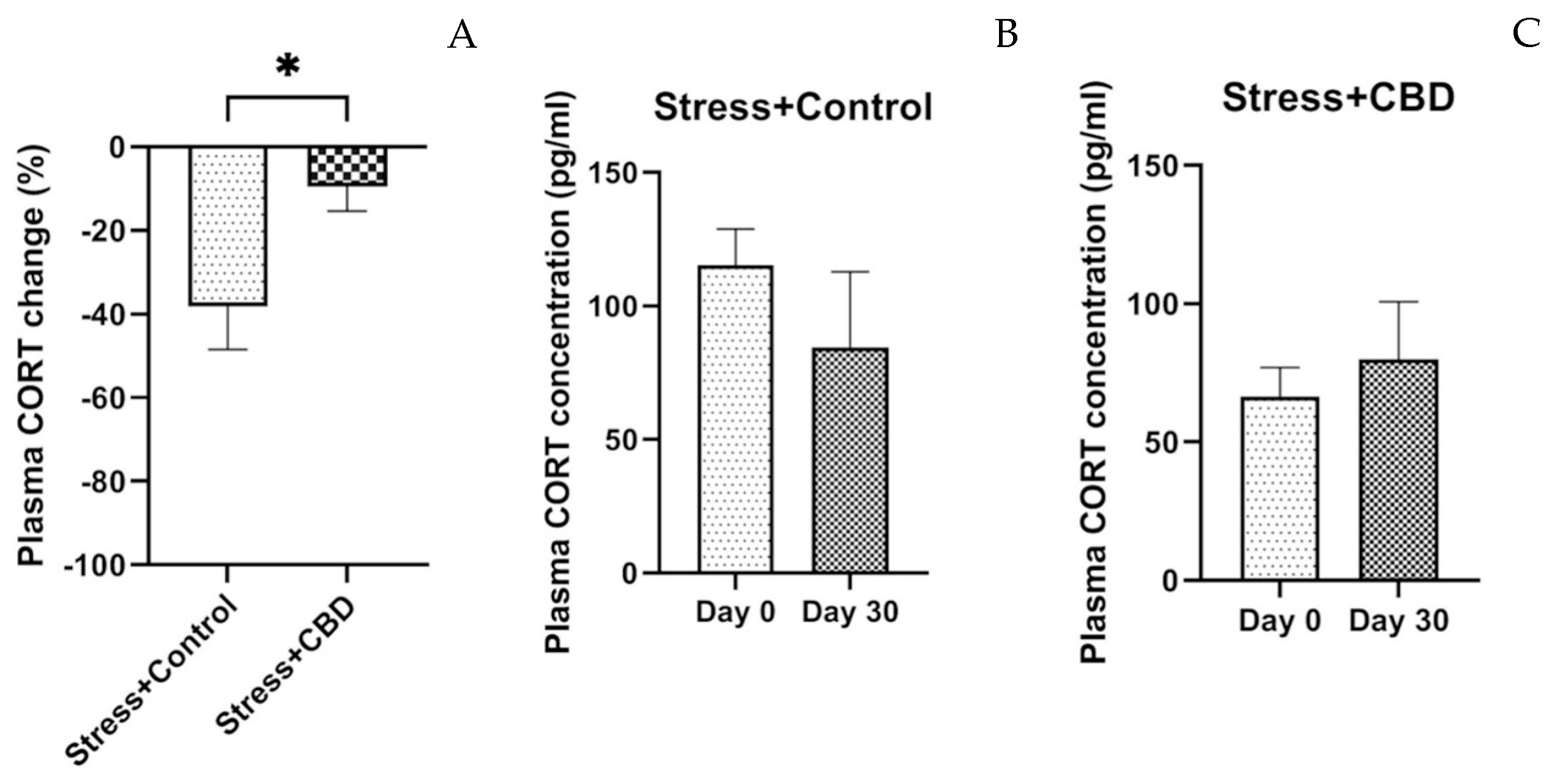
2.2.2. Cortisol Level
3. Discussion
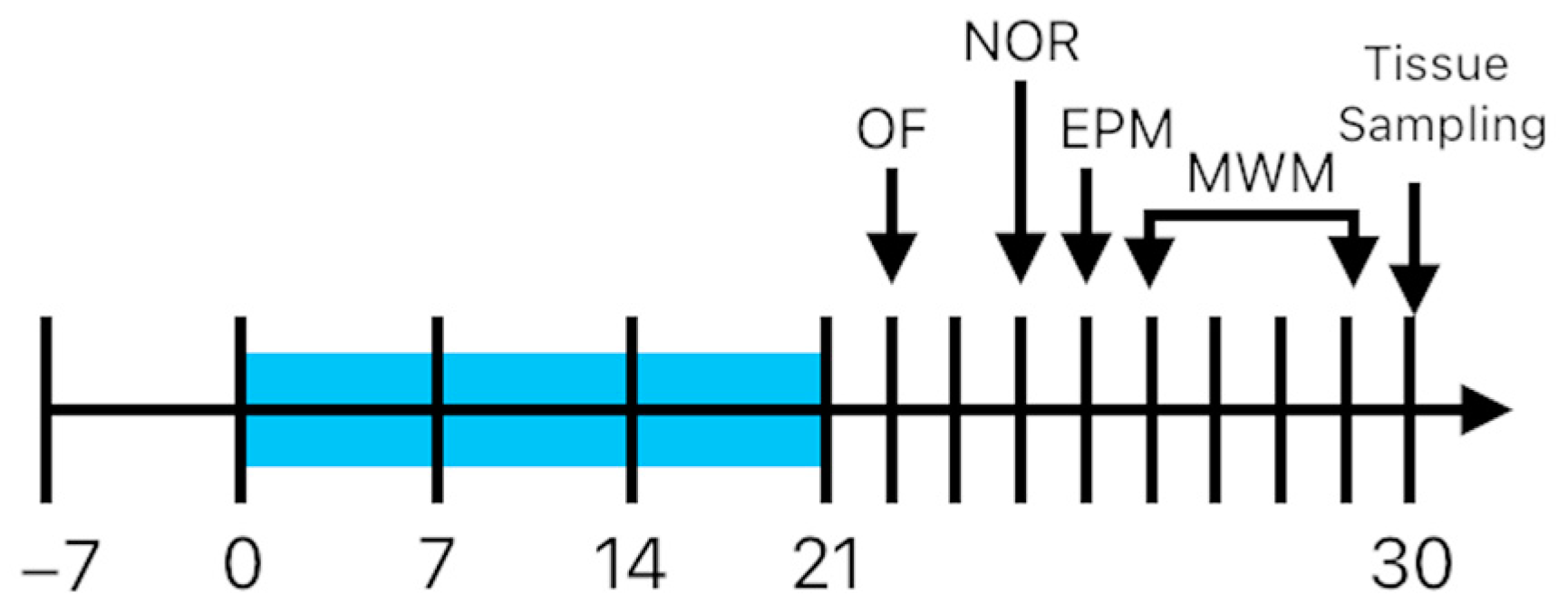
4. Materials and Methods
4.1. Animals
4.2. Drugs and Reagents
4.3. Behavioral Tests
4.3.1. Open Field Test (OF)
4.3.2. Novel Object Recognition (NOR)
4.3.3. Elevated Plus Maze Test (EPM)
4.3.4. Morris Water Maze (MWM)
4.4. Plasma and Brain Sampling
4.4.1. Oxidative Stress Parameters
4.4.2. Cortisol Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT1A | Receptor 5-HT1A for serotonin |
| CB1 | Receptor CB1 for CBD |
| CBD | Cannabidiol |
| EPM | Elevated plus maze |
| GABAA | GABA A receptor |
| GPR55 | G protein coupled receptor 55 |
| GSH/GSSG | Ratio between reduced glutathione and oxidized glutathione |
| HIF-1α | Hypoxia-inducible factor-1 alpha |
| MDA | Malondialdehyde |
| MWM | Morris water maze |
| NOR | Novel object recognition |
| OF | Open field |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PTSD | Post-traumatic stress disorder |
| TRPV1 | Transient receptor potential for vanilloid |
References
- Schöner, J.; Heinz, A.; Endres, M.; Gertz, K.; Kronenberg, G. Post-traumatic stress disorder and beyond: An overview of rodent stress models. J. Cell. Mol. Med. 2017, 21, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- Atli, A.; Bulut, M.; Bez, Y.; Kaplan, İ.; Özdemir, P.G.; Uysal, C.; Selçuk, H.; Sir, A. Altered lipid peroxidation markers are related to post-traumatic stress disorder (PTSD) and not trauma itself in earthquake survivors. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Borovac Štefanović, L.; Kalinić, D.; Mimica, N.; Beer Ljubić, B.; Aladrović, J.; Mandelsamen Perica, M.; Curić, M.; Grošić, P.F.; Delaš, I. Oxidative status and the severity of clinical symptoms in patients with post-traumatic stress disorder. Ann. Clin. Biochem. 2015, 52, 95–104. [Google Scholar] [CrossRef]
- Bulut, M.; Selek, S.; Bez, Y.; Karababa, I.F.; Kaya, M.C.; Gunes, M.; Emhan, A.; Aksoy, N.; Sir, A. Reduced PON1 enzymatic activity and increased lipid hydroperoxide levels that point out oxidative stress in generalized anxiety disorder. J. Affect. Disord. 2013, 150, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, O.; Selvi, Y.; Ozkol, H.; Tuluce, Y.; Besiroglu, L.; Aydin, A. Comparison of superoxide dismutase, glutathione peroxidase and adenosine deaminase activities between respiratory and nocturnal subtypes of patients with panic disorder. Neuropsychobiology 2012, 66, 244–251. [Google Scholar] [CrossRef]
- Mao, H.; Fang, X.; Floyd, K.M.; Polcz, J.E.; Zhang, P.; Liu, B. Induction of microglial reactive oxygen species production by the organochlorinated pesticide dieldrin. Brain Res. 2007, 1186, 267–274. [Google Scholar] [CrossRef]
- Roy, A.; Jana, A.; Yatish, K.; Freidt, M.B.; Fung, Y.K.; Martinson, J.A.; Pahan, K. Reactive oxygen species up-regulate CD11b in microglia via nitric oxide: Implications for neurodegenerative diseases. Free Radic. Biol. Med. 2008, 45, 686–699. [Google Scholar] [CrossRef]
- Fan, Z.; Yang, C.; Qu, X.; Zhang, J.; Wu, H.; Yang, Y.; Huang, Y.; Zeng, P.; Xiang, Z.; Yang, J. Association of Oxidative Stress on Cognitive Function: A Bidirectional Mendelian Randomisation Study. Mol. Neurobiol. 2024, 61, 10551–10560. [Google Scholar] [CrossRef]
- Lee, H.S.; Min, D.; Baik, S.Y.; Kwon, A.; Jin, M.J.; Lee, S.H. Association between Dissociative Symptoms and Morning Cortisol Levels in Patients with Post-traumatic Stress Disorder. Clin. Psychopharmacol. Neurosci. 2022, 20, 292–299. [Google Scholar] [CrossRef]
- Steudte-Schmiedgen, S.; Kirschbaum, C.; Alexander, N.; Stalder, T. An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neurosci. Biobehav. Rev. 2016, 69, 124–135. [Google Scholar] [CrossRef]
- Klaassens, E.R.; Giltay, E.J.; Cuijpers, P.; van Veen, T.; Zitman, F.G. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: A meta-analysis. Psychoneuroendocrinology 2012, 37, 317–331. [Google Scholar] [CrossRef]
- Schumacher, S.; Niemeyer, H.; Engel, S.; Cwik, J.C.; Laufer, S.; Klusmann, H.; Knaevelsrud, C. HPA axis regulation in posttraumatic stress disorder: A meta-analysis focusing on potential moderators. Neurosci. Biobehav. Rev. 2019, 100, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, Z.; Wu, X.; Wen, S.W.; Liu, A. Salivary cortisol in post-traumatic stress disorder: A systematic review and meta-analysis. BMC Psychiatry 2018, 18, 324. [Google Scholar] [CrossRef]
- Schier, A.R.; Ribeiro, N.P.; Silva, A.C.; Hallak, J.E.; Crippa, J.A.; Nardi, A.E.; Zuardi, A.W. Cannabidiol, a Cannabis sativa constituent, as an anxiolytic drug. Braz. J. Psychiatry. 2012, 34 (Suppl. 1), S104–S110. [Google Scholar] [CrossRef] [PubMed]
- de Mello Schier, A.R.; de Oliveira Ribeiro, N.P.; Coutinho, D.S.; Machado, S.; Arias-Carrión, O.; Crippa, J.A.; Zuardi, A.W.; Nardi, A.E.; Silva, A.C. Antidepressant-like and anxiolytic-like effects of cannabidiol: A chemical compound of Cannabis sativa. CNS Neurol. Disord. Drug Targets 2014, 13, 953–960. [Google Scholar] [CrossRef]
- Micale, V.; Di Marzo, V.; Sulcova, A.; Wotjak, C.T.; Drago, F. Endocannabinoid system and mood disorders: Priming a target for new therapies. Pharmacol. Ther. 2013, 138, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Spriggs, S.; Alishayev, J.; Winkel, G.; Gurgov, K.; Kudrich, C.; Oprescu, A.M.; Salsitz, E. Cannabidiol for the Reduction of Cue-Induced Craving and Anxiety in Drug-Abstinent Individuals With Heroin Use Disorder: A Double-Blind Randomized Placebo-Controlled Trial. Am. J. Psychiatry 2019, 176, 911–922. [Google Scholar] [CrossRef]
- Freeman, T.P.; Hindocha, C.; Baio, G.; Shaban, N.D.C.; Thomas, E.M.; Astbury, D.; Freeman, A.M.; Lees, R.; Craft, S.; Morrison, P.D.; et al. Cannabidiol for the treatment of cannabis use disorder: A phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry 2020, 7, 865–874. [Google Scholar] [CrossRef]
- Han, K.; Wang, J.Y.; Wang, P.Y.; Peng, Y.C. Therapeutic potential of cannabidiol (CBD) in anxiety disorders: A systematic review and meta-analysis. Psychiatry Res. 2024, 339, 116049. [Google Scholar] [CrossRef]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (−)Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 14. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Trovato, A.E.; Comelli, F.; Giagnoni, G.; Colleoni, M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 2007, 556, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.D.; Vitetta, L.; Quezada, M.; Hall, S. Enhancing Endocannabinoid Control of Stress with Cannabidiol. J. Clin. Med. 2021, 10, 5852. [Google Scholar] [CrossRef]
- Glodosky, N.C.; Cuttler, C.; McLaughlin, R.J. A review of the effects of acute and chronic cannabinoid exposure on the stress response. Front. Neuroendocrinol. 2021, 63, 100945. [Google Scholar] [CrossRef]
- Zoladz, P.R.; D’Alessio, P.A.; Seeley, S.L.; Kasler, C.D.; Goodman, C.S.; Mucher, K.E.; Allison, A.S.; Smith, I.F.; Dodson, J.L.; Stoops, T.S.; et al. A predator-based psychosocial stress animal model of PTSD in females: Influence of estrous phase and ovarian hormones. Horm. Behav. 2019, 115, 104564. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Diamond, D.M. Predator-based psychosocial stress animal model of PTSD: Preclinical assessment of traumatic stress at cognitive, hormonal, pharmacological, cardiovascular and epigenetic levels of analysis. Exp. Neurol. 2016, 284 Pt B, 211–219. [Google Scholar] [CrossRef]
- Lisboa, S.F.; Stern, C.A.J.; Gazarini, L.; Bertoglio, L.J. Cannabidiol effects on fear processing and implications for PTSD: Evidence from rodent and human studies. Int. Rev. Neurobiol. 2024, 177, 235–250. [Google Scholar] [CrossRef]
- Zlatanova-Tenisheva, H.; Georgieva-Kotetarova, M.; Vilmosh, N.; Kandilarov, I.; Delev, D.; Dermendzhiev, T.; Kostadinov, I.D. Exploring the Anxiolytic, Antidepressant, and Immunomodulatory Effects of Cannabidiol in Acute Stress Rat Models. Appl. Biosci. 2025, 4, 4. [Google Scholar] [CrossRef]
- ElBatsh, M.M.; Assareh, N.; Marsden, C.A.; Kendall, D.A. Anxiogenic-like effects of chronic cannabidiol administration in rats. Psychopharmacology 2012, 221, 239–247. [Google Scholar] [CrossRef]
- Song, C.; Stevenson, C.W.; Guimaraes, F.S.; Lee, J.L. Bidirectional Effects of Cannabidiol on Contextual Fear Memory Extinction. Front. Pharmacol. 2016, 7, 493. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Conrad, C.D.; Fleshner, M.; Diamond, D.M. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress 2008, 11, 259–281. [Google Scholar] [CrossRef]
- Lee, J.L.C.; Bertoglio, L.J.; Guimarães, F.S.; Stevenson, C.W. Cannabidiol regulation of emotion and emotional memory processing: Relevance for treating anxiety-related and substance abuse disorders. Br. J. Pharmacol. 2017, 174, 3242–3256. [Google Scholar] [CrossRef]
- Edwards, S.; Baynes, B.B.; Carmichael, C.Y.; Zamora-Martinez, E.R.; Barrus, M.; Koob, G.F.; Gilpin, N.W. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl. Psychiatry 2013, 3, e296. [Google Scholar] [CrossRef]
- Robinson, J.; Sareen, J.; Cox, B.J.; Bolton, J. Self-medication of anxiety disorders with alcohol and drugs: Results from a nationally representative sample. J. Anxiety Disord. 2009, 23, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.B.; Li, H.; Liu, X.Q.; Sun, C.Y.; Cheng, S.R.; Zhang, M.H.; Liu, S.C.; Wang, W.X. Chronic multiple stress enhances learning and memory capability in rats. Sheng Li Xue Bao 2004, 56, 615–619, In Chinese. [Google Scholar] [PubMed]
- Han, X.; Song, X.; Song, D.; Xie, G.; Guo, H.; Wu, N.; Li, J. Comparison between cannabidiol and sertraline for the modulation of post-traumatic stress disorder-like behaviors and fear memory in mice. Psychopharmacology 2022, 239, 1605–1620. [Google Scholar] [CrossRef] [PubMed]
- Shallcross, J.; Hámor, P.; Bechard, A.R.; Romano, M.; Knackstedt, L.; Schwendt, M. The Divergent Effects of CDPPB and Cannabidiol on Fear Extinction and Anxiety in a Predator Scent Stress Model of PTSD in Rats. Front. Behav. Neurosci. 2019, 13, 91. [Google Scholar] [CrossRef]
- Trofimiuk, E.; Walesiuk, A.; Braszko, J.J. St John’s wort (Hypericum perforatum) diminishes cognitive impairment caused by the chronic restraint stress in rats. Pharmacol. Res. 2005, 51, 239–246. [Google Scholar] [CrossRef]
- Walesiuk, A.; Trofimiuk, E.; Braszko, J.J. Gingko biloba extract diminishes stress-induced memory deficits in rats. Pharmacol. Rep. 2005, 57, 176–187. [Google Scholar] [PubMed]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell Signal 2011, 23, 317–323. [Google Scholar] [CrossRef]
- Peralta, D.; Bronowska, A.; Morgan, B.; Dóka, É.; Van Laer, K.; Nagy, P.; Gräter, F.; Dick, T.P. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat. Chem. Biol. 2015, 11, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.; Knuesting, J.; Berndt, C.; Morgan, B.; Scheibe, R. Cytosolic thiol switches regulating basic cellular functions: GAPDH as an information hub? Biol. Chem. 2015, 396, 523–537. [Google Scholar] [CrossRef]
- Schiavon, A.P.; Soares, L.M.; Bonato, J.M.; Milani, H.; Guimarães, F.S.; Weffort de Oliveira, R.M. Protective effects of cannabidiol against hippocampal cell death and cognitive impairment induced by bilateral common carotid artery occlusion in mice. Neurotox. Res. 2014, 26, 307–316. [Google Scholar] [CrossRef]
- Schleicher, E.M.; Ott, F.W.; Müller, M.; Silcher, B.; Sichler, M.E.; Löw, M.J.; Wagner, J.M.; Bouter, Y. Prolonged Cannabidiol Treatment Lacks on Detrimental Effects on Memory, Motor Performance and Anxiety in C57BL/6J Mice. Front. Behav. Neurosci. 2019, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaça, M.V.; Scarante, F.F.; Joca, S.R.L.; Sales, A.J.; Gomes, F.V.; Sonego, A.B.; Rodrigues, N.S.; Galve-Roperh, I.; Guimarães, F.S. Plastic and Neuroprotective Mechanisms Involved in the Therapeutic Effects of Cannabidiol in Psychiatric Disorders. Front. Pharmacol. 2017, 8, 269. [Google Scholar] [CrossRef]
- Martín-Moreno, A.M.; Brera, B.; Spuch, C.; Carro, E.; García-García, L.; Delgado, M.; Pozo, M.A.; Innamorato, N.G.; Cuadrado, A.; de Ceballos, M.L. Prolonged oral cannabinoid administration prevents neuroinflammation, lowers β-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J. Neuroinflamm. 2012, 9, 8. [Google Scholar] [CrossRef]
- Uhernik, A.L.; Montoya, Z.T.; Balkissoon, C.D.; Smith, J.P. Learning and memory is modulated by cannabidiol when administered during trace fear-conditioning. Neurobiol. Learn. Mem. 2018, 149, 68–76. [Google Scholar] [CrossRef]
- Cheng, D.; Spiro, A.S.; Jenner, A.M.; Garner, B.; Karl, T. Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer’s disease transgenic mice. J. Alzheimers Dis. 2014, 42, 1383–1396. [Google Scholar] [CrossRef]
- Kletkiewicz, H.; Wojciechowski, M.S.; Rogalska, J. Cannabidiol effectively prevents oxidative stress and stabilizes hypoxia-inducible factor-1 alpha (HIF-1α) in an animal model of global hypoxia. Sci. Rep. 2014, 14, 15952. [Google Scholar] [CrossRef]
- Kajero, J.A.; Seedat, S.; Ohaeri, J.; Akindele, A.; Aina, O. Effects of cannabidiol on vacuous chewing movements, plasma glucose and oxidative stress indices in rats administered high dose risperidone. Sci. Rep. 2022, 12, 19718. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Joyera, N.; Félix, J.; Díaz-Del Cerro, E.; Linillos-Pradillo, B.; Rancan, L.; Tresguerres, J.A.F. Cannabidiol, a Strategy in Aging to Improve Redox State and Immunity in Male Rats. Int. J. Mol. Sci. 2024, 25, 12288. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Kaur, S.; Rishi, A.K.; Boire, B.; Aare, M.; Singh, M. Cannabidiol and Beta-Caryophyllene Combination Attenuates Diabetic Neuropathy by Inhibiting NLRP3 Inflammasome/NFκB through the AMPK/sirT3/Nrf2 Axis. Biomedicines 2024, 12, 1442. [Google Scholar] [CrossRef] [PubMed]
- Preeti, K.; Sood, A.; Fernandes, V.; Khan, I.; Khatri, D.K.; Singh, S.B. Experimental Type 2 diabetes and lipotoxicity-associated neuroinflammation involve mitochondrial DNA-mediated cGAS/STING axis: Implication of Type-1 interferon response in cognitive impairment. Mol. Neurobiol. 2024, 61, 6217–6244. [Google Scholar] [CrossRef]
- Strekalova, T.; Spanagel, R.; Bartsch, D.; Henn, F.A.; Gass, P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 2004, 29, 2007–2017. [Google Scholar] [CrossRef]
- Kuehne, A.; Emmert, H.; Soehle, J.; Winnefeld, M.; Fischer, F.; Wenck, H.; Gallinat, S.; Terstegen, L.; Lucius, R.; Hildebrand, J.; et al. Acute Activation of Oxidative Pentose Phosphate Pathway as First-Line Response to Oxidative Stress in Human Skin Cells. Mol Cell. 2015, 59, 359–371. [Google Scholar] [CrossRef]
- Rancan, L.; Linillos-Pradillo, B.; Centeno, J.; Paredes, S.D.; Vara, E.; Tresguerres, J.A.F. Protective Actions of Cannabidiol on Aging-Related Inflammation, Oxidative Stress and Apoptosis Alterations in Liver and Lung of Long Evans Rats. Antioxidants 2023, 12, 1837. [Google Scholar] [CrossRef]
- Meewisse, M.L.; Reitsma, J.B.; de Vries, G.J.; Gersons, B.P.; Olff, M. Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2007, 191, 387–392. [Google Scholar] [CrossRef]
- Traslaviña, G.A.A.; Torres, F.P.; de Barcelos Filho, P.C.G.; Lucio-Oliveira, F.; Franci, C.R. Hypothalamic-pituitary-adrenal axis responsivity to an acute novel stress in female rats subjected to the chronic mild stress paradigm. Brain Res. 2019, 1723, 146402. [Google Scholar] [CrossRef]
- Bremner, J.D.; Vythilingam, M.; Vermetten, E.; Adil, J.; Khan, S.; Nazeer, A.; Afzal, N.; McGlashan, T.; Elzinga, B.; Anderson, G.M.; et al. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology 2003, 28, 733–750. [Google Scholar] [CrossRef]
- Conrad, C.D.; LeDoux, J.E.; Magariños, A.M.; McEwen, B.S. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 1999, 113, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.L.; Conrad, C.D. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress 2005, 8, 151–154. [Google Scholar] [CrossRef]
- Wilson, C.B.; Ebenezer, P.J.; McLaughlin, L.D.; Francis, J. Predator exposure/psychosocial stress animal model of post-traumatic stress disorder modulates neurotransmitters in the rat hippocampus and prefrontal cortex. PLoS ONE 2014, 9, e89104. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.B.; McLaughlin, L.D.; Nair, A.; Ebenezer, P.J.; Dange, R.; Francis, J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS ONE 2013, 8, e76146. [Google Scholar] [CrossRef]
- Wilson, C.B.; McLaughlin, L.D.; Ebenezer, P.J.; Nair, A.R.; Francis, J. Valproic acid effects in the hippocampus and prefrontal cortex in an animal model of post-traumatic stress disorder. Behav. Brain Res. 2014, 268, 72–80. [Google Scholar] [CrossRef]
- Wilson, C.B.; McLaughlin, L.D.; Ebenezer, P.J.; Nair, A.R.; Dange, R.; Harre, J.G.; Shaak, T.L.; Diamond, D.M.; Francis, J. Differential effects of sertraline in a predator exposure animal model of post-traumatic stress disorder. Front. Behav. Neurosci. 2014, 8, 256. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress and psychological disorders. Curr. Neuropharmacol. 2014, 12, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, N.M.; Randjelović, P.J.; Pavlović, D.; Stojiljković, N.I.; Jovanović, I.; Sokolović, D.; Radulović, N.S. An Impact of Psychological Stress on the Interplay between Salivary Oxidative Stress and the Classic Psychological Stress-Related Parameters. Oxid. Med. Cell. Longev. 2021, 2021, 6635310. [Google Scholar] [CrossRef]
- Kim, E.; Zhao, Z.; Rzasa, J.R.; Glassman, M.; Bentley, W.E.; Chen, S.; Kelly, D.L.; Payne, G.F. Association of acute psychosocial stress with oxidative stress: Evidence from serum analysis. Redox Biol. 2021, 47, 102138. [Google Scholar] [CrossRef]
- Gáll, Z.; Farkas, S.; Albert, Á.; Ferencz, E.; Vancea, S.; Urkon, M.; Kolcsár, M. Effects of Chronic Cannabidiol Treatment in the Rat Chronic Unpredictable Mild Stress Model of Depression. Biomolecules 2020, 10, 801. [Google Scholar] [CrossRef]
- Fogarasi, E.; Croitoru, M.D.; Fülöp, I.; Nemes-Nagy, E.; Tripon, R.G.; Simon-Szabo, Z.; Muntean, D.L. Malondialdehyde levels can be measured in serum and saliva by using a fast HPLC method with visible detection/Determinarea printr-o metodă HPLC-VIS rapidă a concentraţiilor serice şi salivare ale malondialdehidei. Rev. Romana Med. Laborator. 2016, 24, 319–326. [Google Scholar] [CrossRef]
- Jîtcă, G.; Fogarasi, E.; Ősz, B.E.; Vari, C.E.; Tero-Vescan, A.; Miklos, A.; Bătrînu, M.G.; Rusz, C.M.; Croitoru, M.D.; Dogaru, M.T. A Simple HPLC/DAD Method Validation for the Quantification of Malondialdehyde in Rodent’s Brain. Molecules 2021, 26, 5066. [Google Scholar] [CrossRef] [PubMed]
- Jîtcă, G.; Fogarasi, E.; Ősz, B.E.; Vari, C.E.; Fülöp, I.; Croitoru, M.D.; Rusz, C.M.; Dogaru, M.T. Profiling the Concentration of Reduced and Oxidized Glutathione in Rat Brain Using HPLC/DAD Chromatographic System. Molecules 2021, 26, 6590. [Google Scholar] [CrossRef]
- Jîtcă, G.; Gáll, Z.; Jîtcă, C.M.; Buț, M.G.; Májai, E. Drug Repurposing of Metformin for the Treatment of Haloperidol-Related Behavior Disorders and Oxidative Stress: A Preliminary Study. Pharmaceutics 2024, 16, 403. [Google Scholar] [CrossRef]
- Gáll, Z.; Csüdör, Á.; Sável, I.G.; Kelemen, K.; Kolcsár, M. Cholecalciferol Supplementation Impacts Behavior and Hippocampal Neuroglial Reorganization in Vitamin D-Deficient Rats. Nutrients 2024, 16, 2326. [Google Scholar] [CrossRef] [PubMed]




| Model | Advantages | Disadvantages |
|---|---|---|
| Predator-based stress | Simulates natural threats, persistent effects, activates relevant neural circuits | High variability, difficult to standardize, ethical implications |
| Uncontrollable electric shock | Very controllable, persistent symptoms | Physical pain, less realistic for psychological PTSD |
| Social stress (e.g., dominance/isolation) | Relevant for social trauma-based PTSD, induces clear behavioral changes | Effects appear slower, less intense than in Predator based stress |
| Variable chronic stress | Simulates cumulative stress, applicable to PTSD from prolonged exposure | Requires time to induce symptoms, harder to standardize |
| Traumatic sound-based stress | Relevance for war-induced PTSD (e.g., exposure to explosions), non-invasive | Does not capture all aspects of PTSD, does not induce extreme fear like Predator based stress |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jîtcă, G.; Stoicescu, R.; Májai, E. Cannabidiol Treatment in a Predator-Based Animal Model of PTSD: Assessing Oxidative Stress and Memory Performance. Int. J. Mol. Sci. 2025, 26, 4491. https://doi.org/10.3390/ijms26104491
Jîtcă G, Stoicescu R, Májai E. Cannabidiol Treatment in a Predator-Based Animal Model of PTSD: Assessing Oxidative Stress and Memory Performance. International Journal of Molecular Sciences. 2025; 26(10):4491. https://doi.org/10.3390/ijms26104491
Chicago/Turabian StyleJîtcă, George, Robert Stoicescu, and Erzsébet Májai. 2025. "Cannabidiol Treatment in a Predator-Based Animal Model of PTSD: Assessing Oxidative Stress and Memory Performance" International Journal of Molecular Sciences 26, no. 10: 4491. https://doi.org/10.3390/ijms26104491
APA StyleJîtcă, G., Stoicescu, R., & Májai, E. (2025). Cannabidiol Treatment in a Predator-Based Animal Model of PTSD: Assessing Oxidative Stress and Memory Performance. International Journal of Molecular Sciences, 26(10), 4491. https://doi.org/10.3390/ijms26104491






