Olfactory Dysfunction in a Novel Model of Prodromal Parkinson’s Disease in Adult Zebrafish
Abstract
1. Introduction
2. Results
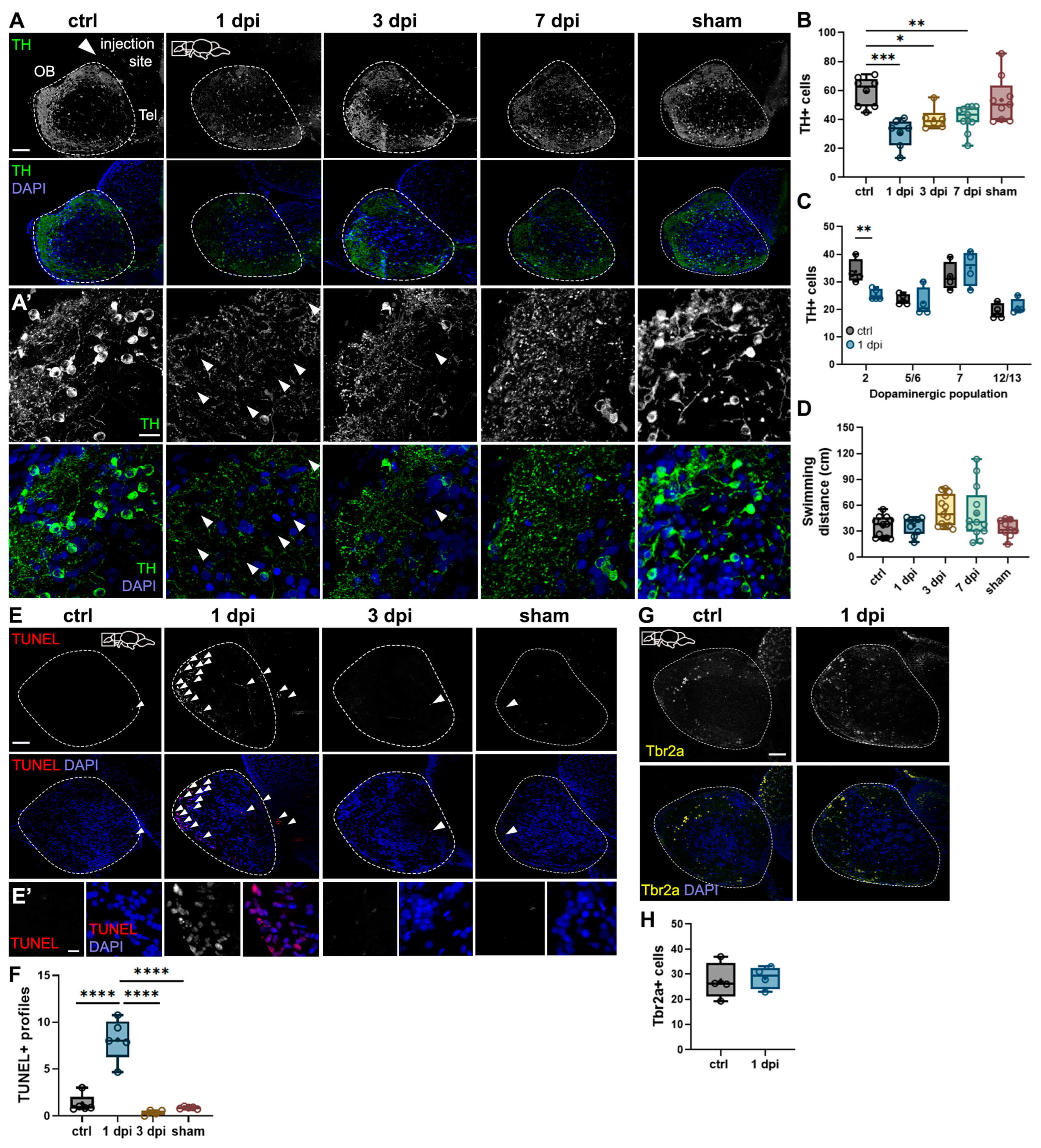
2.1. 6-OHDA Injections in the Dorsal Telencephalic Ventricle Target Dopaminergic Neurons in the OB Without Impairing Motor Function
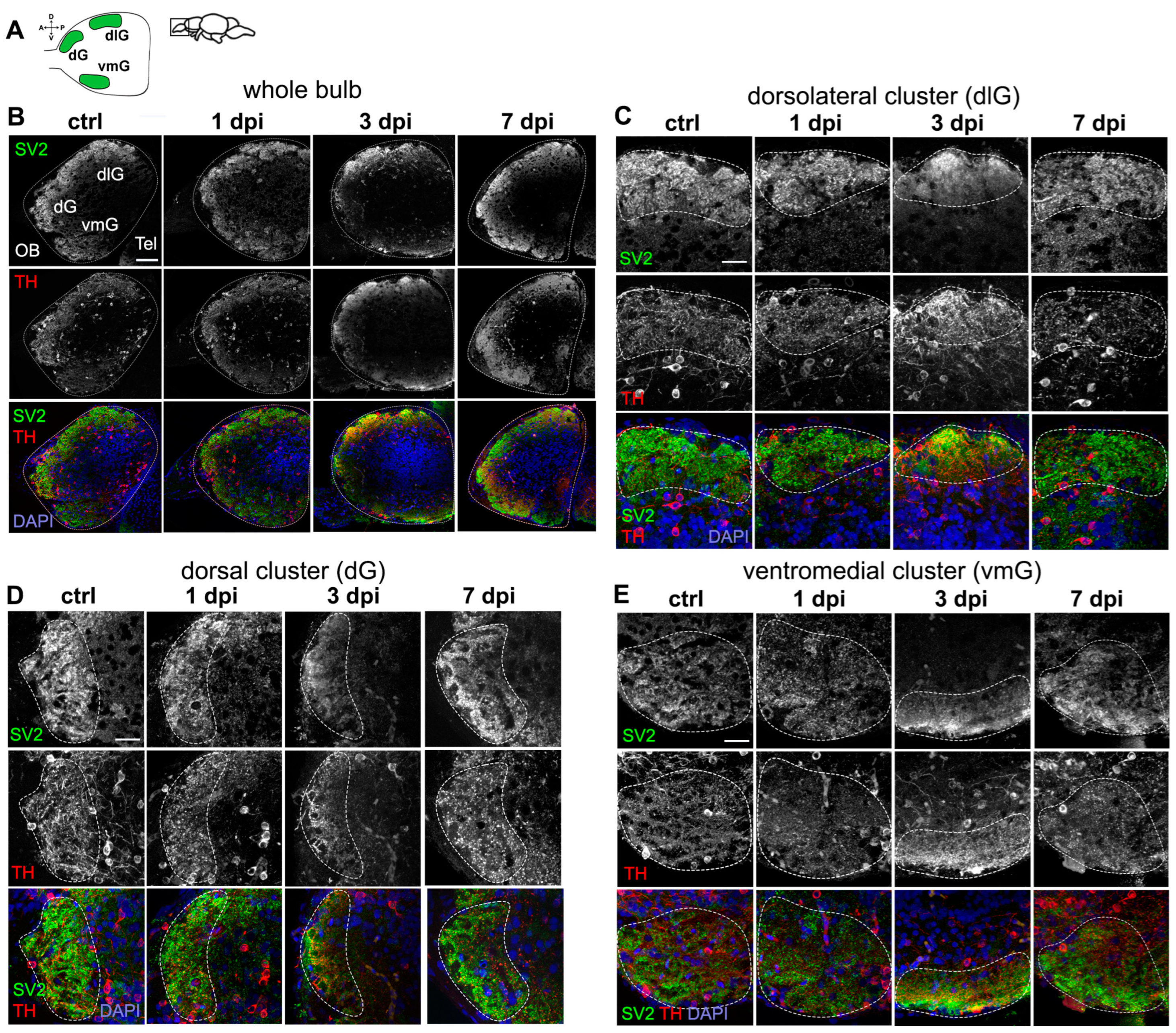
2.2. Periglomerular Cell Loss Is Associated with Severe Synaptic Disruption in the OB
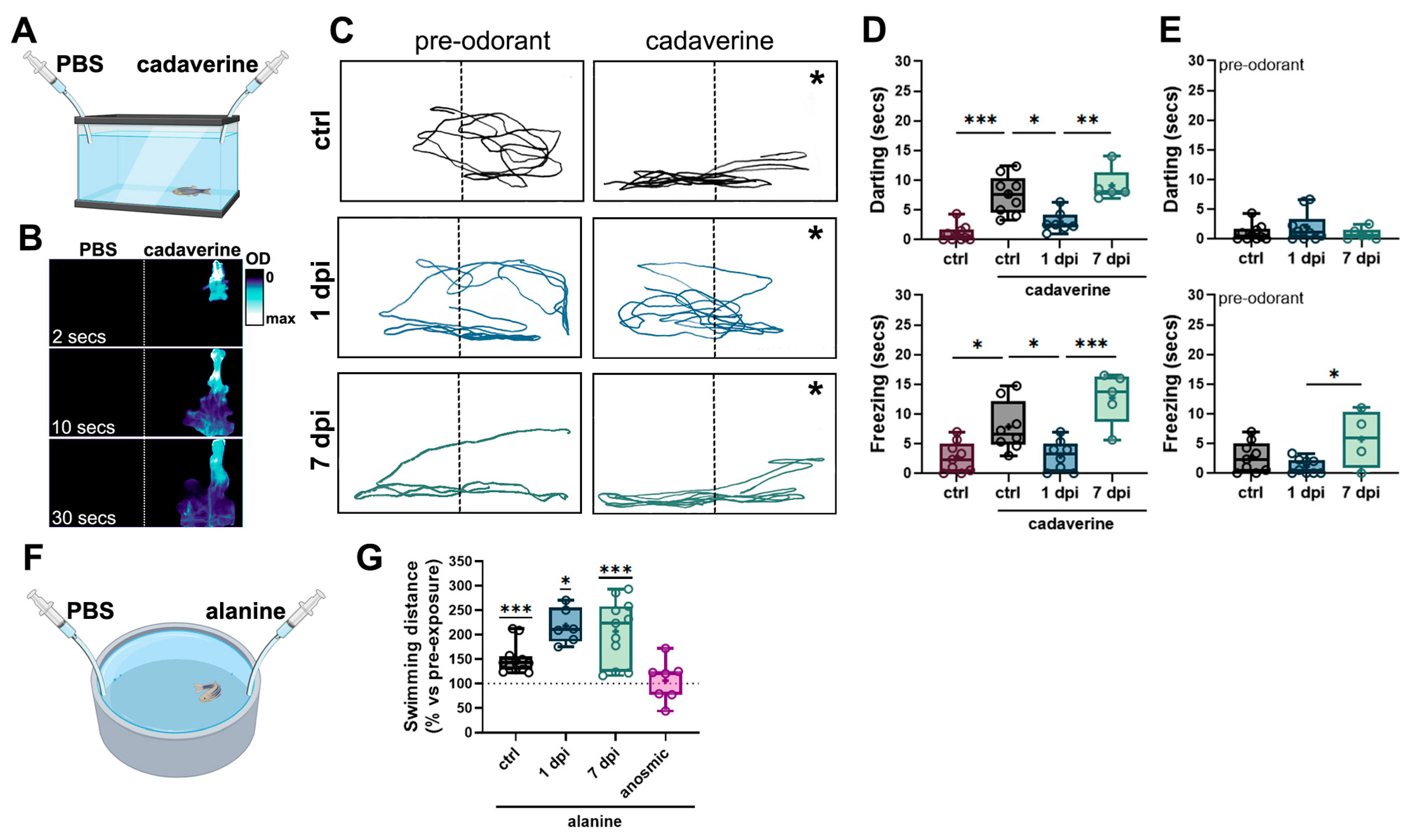
2.3. Periglomerular and Synaptic Degeneration in the OB Lead to Impaired Olfactory Responses to Selective Odorants Without Altering Locomotion
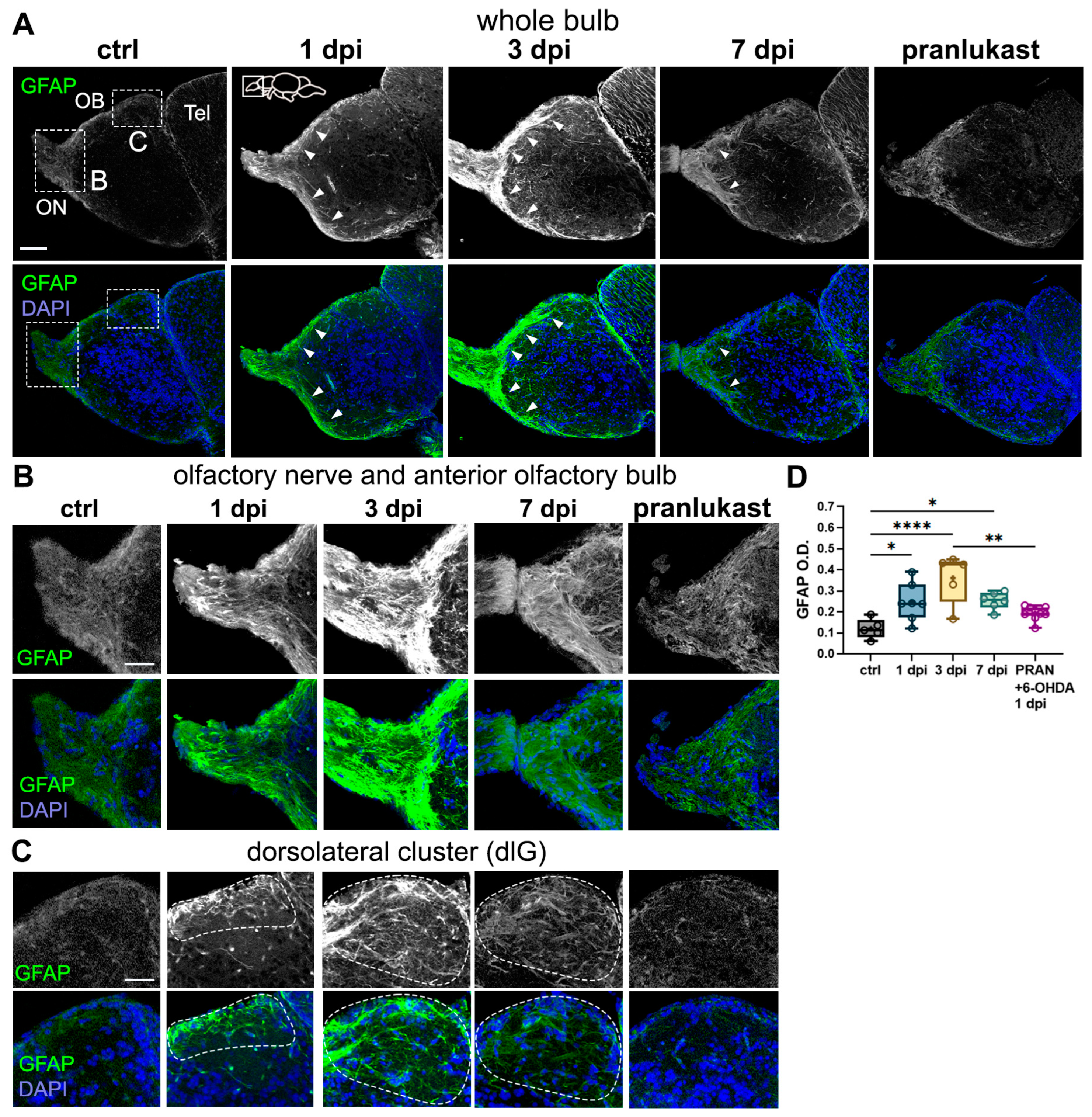
2.4. Astroglial Cells Are Activated in the OB Following 6-OHDA Injections
2.5. 6-OHDA Promotes Microglial Activation and Leukocyte Migration to the OB
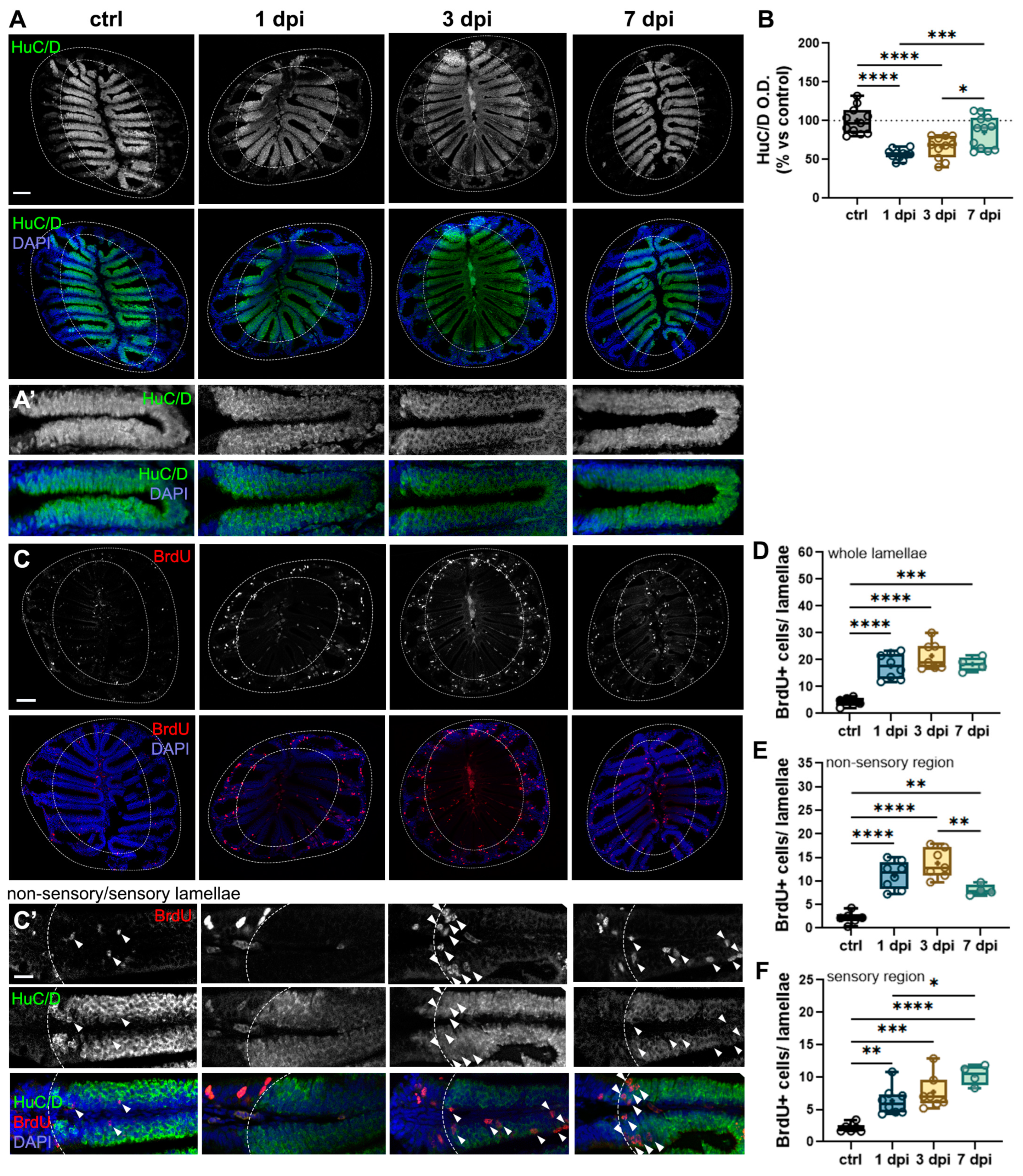
2.6. 6-OHDA-Mediated Changes in the OB Cause Retrograde Degeneration and Cell Proliferation in the OE
2.7. Peripheral Leukocytes Migrate to OE’s Sensory Epithelium Following 6-OHDA Injections
3. Discussion
3.1. 6-OHDA-Mediated Dopaminergic Loss in the OB Leads to Olfactory Dysfunction
3.2. 6-OHDA Injections in the Dorsal Telenchpehalic Ventricle Selectively Target Dopaminergic Neurons in the OB and Subpallium Without Affecting Motor Function
3.3. Neuroinflammatory Processes Parallel Synaptic Disruption and Recovery in the OB
3.4. 6-OHDA Injections Lead to Retrograde Degeneration and Neurogenesis in the Peripheral OE
4. Materials and Methods
4.1. Animals
4.2. Intracerebroventricular (i.c.v.) 6-OHDA Injections
4.3. Tissue Processing for Immunohistochemistry
4.4. Cell Proliferation Assays
4.5. Immunohistochemistry
4.6. Densitometry
4.7. Cell Quantification
4.8. Olfactory-Mediated Behavioral Assays
4.9. Video Analysis
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OB | Olfactory bulb |
| PD | Directory of open access journals |
| 6-OHDA | 6-hydroxydopamine |
| DA | Dopaminergic |
| OSN | Olfactory Sensory Neuron |
| OE | Olfactory Epithelium |
| dpi | Days post-injection |
| TH | Tyrosine hydroxylase |
| TUNEL | dUTP Nick-End Labeling |
| SV2 | Synaptic vesicle protein |
| dG | Dorsolateral glomerular cluster |
| dlG | Dorsolateral glomerular cluster |
| vmG | Ventromedial glomerular cluster |
| cad | Cadaverine |
| ON | Olfactory nerve |
| ONL | Olfactory nerve layer |
| PRAN | Pranlukast |
| Lcp1 | Lymphocyte cytosolic protein 1 |
| GBC | Globose basal cell |
| HBC | Horizontal basal cell |
| O.D. | Optical density |
| i.c.v. | Intracerebroventricular |
| PFA | Paraformaldehyde |
| IHC | Immunohistochemistry |
| NGS | Normal goat serum |
References
- Cacabelos, R. Parkinson’s Disease: From Pathogenesis to Pharmacogenomics. Int. J. Mol. Sci. 2017, 18, 551. [Google Scholar] [CrossRef]
- Galvan, A.; Wichmann, T. Pathophysiology of parkinsonism. Clin. Neurophysiol. 2008, 119, 1459–1474. [Google Scholar] [CrossRef]
- Double, K.L.; Rowe, D.B.; Hayes, M.; Chan, D.K.; Blackie, J.; Corbett, A.; Joffe, R.; Fung, V.S.; Morris, J.; Halliday, G.M. Identifying the pattern of olfactory deficits in Parkinson disease using the brief smell identification test. Arch. Neurol. 2003, 60, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Fullard, M.E.; Morley, J.F.; Duda, J.E. Olfactory Dysfunction as an Early Biomarker in Parkinson’s Disease. Neurosci. Bull. 2017, 33, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.; Jensen, M.B.; Stenager, E.; Andersen, A.D. The use of olfactory testing when diagnosing Parkinson’s disease—A systematic review. Dan. Med. J. 2018, 65, A5481. [Google Scholar] [PubMed]
- Dijkstra, A.A.; Ingrassia, A.; de Menezes, R.X.; van Kesteren, R.E.; Rozemuller, A.J.; Heutink, P.; van de Berg, W.D. Evidence for Immune Response, Axonal Dysfunction and Reduced Endocytosis in the Substantia Nigra in Early Stage Parkinson’s Disease. PLoS ONE 2015, 10, e0128651. [Google Scholar] [CrossRef]
- Michel, P.P.; Hirsch, E.C.; Hunot, S. Understanding Dopaminergic Cell Death Pathways in Parkinson Disease. Neuron 2016, 90, 675–691. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Alvarez-Fischer, D.; Arias-Carrión, O.; Djufri, M.; Windolph, A.; Keber, U.; Borta, A.; Ries, V.; Schwarting, R.K.; Scheller, D.; et al. A new dopaminergic nigro-olfactory projection. Acta Neuropathol. 2015, 130, 333–348. [Google Scholar] [CrossRef]
- Bundschuh, S.T.; Zhu, P.; Schärer, Y.-P.Z.; Friedrich, R.W. Dopaminergic modulation of mitral cells and odor responses in the zebrafish olfactory bulb. J. Neurosci. 2012, 32, 6830–6840. [Google Scholar] [CrossRef]
- Li, J.; Gu, C.Z.; Su, J.B.; Zhu, L.H.; Zhou, Y.; Huang, H.Y.; Liu, C.F. Changes in Olfactory Bulb Volume in Parkinson’s Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0149286. [Google Scholar] [CrossRef]
- Wang, J.; You, H.; Liu, J.F.; Ni, D.F.; Zhang, Z.X.; Guan, J. Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR Am. J. Neuroradiol. 2011, 32, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Postlethwait, J.H. The zebrafish genome in context: Ohnologs gone missing. J. Exp. Zool. Part B Mol. Dev. Evol. 2007, 308, 563–577. [Google Scholar] [CrossRef]
- Calvo-Ochoa, E.; Byrd-Jacobs, C.A.; Fuss, S.H. Diving into the streams and waves of constitutive and regenerative olfactory neurogenesis: Insights from zebrafish. Cell Tissue Res. 2021, 383, 227–253. [Google Scholar] [CrossRef]
- Doyle, J.M.; Croll, R.P. A critical review of zebrafish models of Parkinson’s disease. Front. Pharmacol. 2022, 13, 835827. [Google Scholar] [CrossRef]
- Facchinello, N. 17 Transgenic Zebrafish Reporter Lines for. In Zebrafish as a Model for Parkinson’s Disease; CRC Press: Boca Raton, FL, USA, 2024; p. 247. [Google Scholar]
- Bretaud, S.; Lee, S.; Guo, S. Sensitivity of zebrafish to environmental toxins implicated in Parkinson’s disease. Neurotoxicol Teratol. 2004, 26, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Callizot, N.; Combes, M.; Henriques, A.; Poindron, P. Necrosis, apoptosis, necroptosis, three modes of action of dopaminergic neuron neurotoxins. PLoS ONE 2019, 14, e0215277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Yang, J.; Wang, F.; Sima, Y.; Zhong, Z.M.; Wang, H.; Hu, L.F.; Liu, C.F. Parkinson’s disease-like motor and non-motor symptoms in rotenone-treated zebrafish. Neurotoxicology 2017, 58, 103–109. [Google Scholar] [CrossRef]
- Feng, C.W.; Wen, Z.H.; Huang, S.Y.; Hung, H.C.; Chen, C.H.; Yang, S.N.; Chen, N.F.; Wang, H.M.; Hsiao, C.D.; Chen, W.F. Effects of 6-hydroxydopamine exposure on motor activity and biochemical expression in zebrafish (Danio rerio) larvae. Zebrafish 2014, 11, 227–239. [Google Scholar] [CrossRef]
- Blandini, F.; Armentero, M.-T.; Martignoni, E. The 6-hydroxydopamine model: News from the past. Park. Relat. Disord. 2008, 14, S124–S129. [Google Scholar] [CrossRef]
- Vijayanathan, Y.; Lim, F.T.; Lim, S.M.; Long, C.M.; Tan, M.P.; Majeed, A.B.A.; Ramasamy, K. 6-OHDA-Lesioned Adult Zebrafish as a Useful Parkinson’s Disease Model for Dopaminergic Neuroregeneration. Neurotox. Res. 2017, 32, 496–508. [Google Scholar] [CrossRef]
- Caldwell, L.J.; Davies, N.O.; Cavone, L.; Mysiak, K.S.; Semenova, S.A.; Panula, P.; Armstrong, J.D.; Becker, C.G.; Becker, T. Regeneration of dopaminergic neurons in adult zebrafish depends on immune system activation and differs for distinct populations. J. Neurosci. 2019, 39, 4694–4713. [Google Scholar] [CrossRef] [PubMed]
- Byrd, C.A.; Brunjes, P.C. Organization of the olfactory system in the adult zebrafish: Histological, immunohistochemical, and quantitative analysis. J. Comp. Neurol. 1995, 358, 247–259. [Google Scholar] [CrossRef]
- Barrios, J.P.; Wang, W.C.; England, R.; Reifenberg, E.; Douglass, A.D. Hypothalamic Dopamine Neurons Control Sensorimotor Behavior by Modulating Brainstem Premotor Nuclei in Zebrafish. Curr. Biol. 2020, 30, 4606–4618. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A.D.; Barrios, J.P.; Luks-Morgan, S.J.; Manfredi, J.P.; Bonkowsky, J.L.; Douglass, A.D.; Dorsky, R.I. Motor Behavior Mediated by Continuously Generated Dopaminergic Neurons in the Zebrafish Hypothalamus Recovers after Cell Ablation. Curr. Biol. 2016, 26, 263–269. [Google Scholar] [CrossRef]
- Lambert, A.M.; Bonkowsky, J.L.; Masino, M.A. The conserved dopaminergic diencephalospinal tract mediates vertebrate locomotor development in zebrafish larvae. J. Neurosci. 2012, 32, 13488–13500. [Google Scholar] [CrossRef]
- Zuch, C.L.; Nordstroem, V.K.; Briedrick, L.A.; Hoernig, G.R.; Granholm, A.C.; Bickford, P.C. Time course of degenerative alterations in nigral dopaminergic neurons following a 6-hydroxydopamine lesion. J. Comp. Neurol. 2000, 427, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Ochoa, E.; Vorhees, N.W.; Lockett, T.P.; DeWitt, S.L.; Thomas, E.A.; Gray, A.B.; Miyasaka, N.; Yoshihara, Y.; Byrd-Jacobs, C.A. Structural regeneration and functional recovery of the olfactory system of zebrafish following brain injury. bioRxiv 2024. [Google Scholar] [CrossRef]
- Miyasaka, N.; Morimoto, K.; Tsubokawa, T.; Higashijima, S.; Okamoto, H.; Yoshihara, Y. From the olfactory bulb to higher brain centers: Genetic visualization of secondary olfactory pathways in zebrafish. J. Neurosci. 2009, 29, 4756–4767. [Google Scholar] [CrossRef]
- Fuller, C.L.; Yettaw, H.K.; Byrd, C.A. Mitral cells in the olfactory bulb of adult zebrafish (Danio rerio): Morphology and distribution. J. Comp. Neurol. 2006, 499, 218–230. [Google Scholar] [CrossRef]
- Braubach, O.R.; Fine, A.; Croll, R.P. Distribution and functional organization of glomeruli in the olfactory bulbs of zebrafish (Danio rerio). J. Comp. Neurol. 2012, 520, 2317–2339. [Google Scholar] [CrossRef]
- Koide, T.; Miyasaka, N.; Morimoto, K.; Asakawa, K.; Urasaki, A.; Kawakami, K.; Yoshihara, Y. Olfactory neural circuitry for attraction to amino acids revealed by transposon-mediated gene trap approach in zebrafish. Proc. Natl. Acad. Sci. USA 2009, 106, 9884–9889. [Google Scholar] [CrossRef]
- Jones, K. The palatability of amino acids and related compounds to rainbow trout, Salmo gairdneri Richardson. J. Fish Biol. 1989, 34, 149–160. [Google Scholar] [CrossRef]
- Arena, G.; Sharma, K.; Agyeah, G.; Krüger, R.; Grünewald, A.; Fitzgerald, J.C. Neurodegeneration and neuroinflammation in Parkinson’s disease: A self-sustained loop. Curr. Neurol. Neurosci. Rep. 2022, 22, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Booth, H.D.; Hirst, W.D.; Wade-Martins, R. The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Scheib, J.; Byrd-Jacobs, C. Zebrafish Astroglial Morphology in the Olfactory Bulb Is Altered with Repetitive Peripheral Damage. Front. Neuroanat. 2020, 14, 4. [Google Scholar] [CrossRef]
- Baumgart, E.V.; Barbosa, J.S.; Bally-Cuif, L.; Götz, M.; Ninkovic, J. Stab wound injury of the zebrafish telencephalon: A model for comparative analysis of reactive gliosis. Glia 2012, 60, 343–357. [Google Scholar] [CrossRef]
- Lazzari, M.; Bettini, S.; Franceschini, V. Immunocytochemical characterisation of olfactory ensheathing cells of zebrafish. J. Anat. 2014, 224, 192–206. [Google Scholar] [CrossRef]
- Peterson, L.J.; Flood, P.M. Oxidative stress and microglial cells in Parkinson′ s disease. Mediat. Inflamm. 2012, 2012, 401264. [Google Scholar] [CrossRef]
- Rovira, M.; Miserocchi, M.; Montanari, A.; Hammou, L.; Chomette, L.; Pozo, J.; Imbault, V.; Bisteau, X.; Wittamer, V. Zebrafish Galectin 3 binding protein is the target antigen of the microglial 4C4 monoclonal antibody. Dev. Dyn. 2023, 252, 400–414. [Google Scholar] [CrossRef]
- Var, S.R.; Byrd-Jacobs, C.A. Microglial response patterns following damage to the zebrafish olfactory bulb. IBRO Rep. 2019, 7, 70–79. [Google Scholar] [CrossRef]
- Doucette, J.R.; Kiernan, J.A.; Flumerfelt, B.A. Two different patterns of retrograde degeneration in the olfactory epithelium following transection of primary olfactory axons. J. Anat. 1983, 136 Pt 4, 673–689. [Google Scholar] [PubMed]
- Calvo-Ochoa, E.; Byrd-Jacobs, C.A. The Olfactory System of Zebrafish as a Model for the Study of Neurotoxicity and Injury: Implications for Neuroplasticity and Disease. Int. J. Mol. Sci. 2019, 20, 1639. [Google Scholar] [CrossRef] [PubMed]
- Kocagöz, Y.; Demirler, M.C.; Eski, S.E.; Güler, K.; Dokuzluoglu, Z.; Fuss, S.H. Disparate progenitor cell populations contribute to maintenance and repair neurogenesis in the zebrafish olfactory epithelium. Cell Tissue Res. 2022, 388, 331–358. [Google Scholar] [CrossRef]
- Palominos, M.F.; Calfún, C.; Nardocci, G.; Candia, D.; Torres-Paz, J.; Whitlock, K.E. The olfactory organ is a unique site for neutrophils in the brain. Front. Immunol. 2022, 13, 881702. [Google Scholar] [CrossRef]
- Sepahi, A.; Kraus, A.; Casadei, E.; Johnston, C.A.; Galindo-Villegas, J.; Kelly, C.; García-Moreno, D.; Muñoz, P.; Mulero, V.; Huertas, M.; et al. Olfactory sensory neurons mediate ultrarapid antiviral immune responses in a TrkA-dependent manner. Proc. Natl. Acad. Sci. USA 2019, 116, 12428–12436. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, K.E. The sense of scents: Olfactory behaviors in the zebrafish. Zebrafish 2006, 3, 203–213. [Google Scholar] [CrossRef]
- Hussain, A.; Saraiva, L.R.; Ferrero, D.M.; Ahuja, G.; Krishna, V.S.; Liberles, S.D.; Korsching, S.I. High-affinity olfactory receptor for the death-associated odor cadaverine. Proc. Natl. Acad. Sci. USA 2013, 110, 19579–19584. [Google Scholar] [CrossRef]
- Dieris, M.; Ahuja, G.; Krishna, V.; Korsching, S.I. A single identified glomerulus in the zebrafish olfactory bulb carries the high-affinity response to death-associated odor cadaverine. Sci. Rep. 2017, 7, 40892. [Google Scholar] [CrossRef]
- Sato, Y.; Miyasaka, N.; Yoshihara, Y. Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J. Neurosci. 2005, 25, 4889–4897. [Google Scholar] [CrossRef]
- Miyasaka, N.; Arganda-Carreras, I.; Wakisaka, N.; Masuda, M.; Sümbül, U.; Seung, H.S.; Yoshihara, Y. Olfactory projectome in the zebrafish forebrain revealed by genetic single-neuron labelling. Nat. Commun. 2014, 5, 3639. [Google Scholar] [CrossRef]
- Godoy, R.; Hua, K.; Kalyn, M.; Cusson, V.M.; Anisman, H.; Ekker, M. Dopaminergic neurons regenerate following chemogenetic ablation in the olfactory bulb of adult Zebrafish (Danio rerio). Sci. Rep. 2020, 10, 12825. [Google Scholar] [CrossRef]
- Alonso, M.; Ortega-Pérez, I.; Grubb, M.S.; Bourgeois, J.P.; Charneau, P.; Lledo, P.M. Turning astrocytes from the rostral migratory stream into neurons: A role for the olfactory sensory organ. J. Neurosci. 2008, 28, 11089–11102. [Google Scholar] [CrossRef]
- Kishimoto, N.; Alfaro-Cervello, C.; Shimizu, K.; Asakawa, K.; Urasaki, A.; Nonaka, S.; Kawakami, K.; Garcia-Verdugo, J.M.; Sawamoto, K. Migration of neuronal precursors from the telencephalic ventricular zone into the olfactory bulb in adult zebrafish. J. Comp. Neurol. 2011, 519, 3549–3565. [Google Scholar] [CrossRef]
- März, M.; Schmidt, R.; Rastegar, S.; Strähle, U. Regenerative response following stab injury in the adult zebrafish telencephalon. Dev. Dyn. 2011, 240, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Kyritsis, N.; Kizil, C.; Zocher, S.; Kroehne, V.; Kaslin, J.; Freudenreich, D.; Iltzsche, A.; Brand, M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 2012, 338, 1353–1356. [Google Scholar] [CrossRef]
- Grupp, L.; Wolburg, H.; Mack, A.F. Astroglial structures in the zebrafish brain. J. Comp. Neurol. 2010, 518, 4277–4287. [Google Scholar] [CrossRef]
- Kroehne, V.; Freudenreich, D.; Hans, S.; Kaslin, J.; Brand, M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 2011, 138, 4831–4841. [Google Scholar] [CrossRef] [PubMed]
- Jurisch-Yaksi, N.; Yaksi, E.; Kizil, C. Radial glia in the zebrafish brain: Functional, structural, and physiological comparison with the mammalian glia. Glia 2020, 68, 2451–2470. [Google Scholar] [CrossRef] [PubMed]
- Rieger, A.; Deitmer, J.W.; Lohr, C. Axon-glia communication evokes calcium signaling in olfactory ensheathing cells of the developing olfactory bulb. Glia 2007, 55, 352–359. [Google Scholar] [CrossRef]
- Nazareth, L.; Shelper, T.B.; Chacko, A.; Basu, S.; Delbaz, A.; Lee, J.Y.P.; Chen, M.; St John, J.A.; Ekberg, J.A.K. Key differences between olfactory ensheathing cells and Schwann cells regarding phagocytosis of necrotic cells: Implications for transplantation therapies. Sci. Rep. 2020, 10, 18936. [Google Scholar] [CrossRef]
- Denaro, S.; D’Aprile, S.; Alberghina, C.; Pavone, A.M.; Torrisi, F.; Giallongo, S.; Longhitano, L.; Mannino, G.; Lo Furno, D.; Zappalà, A.; et al. Neurotrophic and immunomodulatory effects of olfactory ensheathing cells as a strategy for neuroprotection and regeneration. Front. Immunol. 2022, 13, 1098212. [Google Scholar] [CrossRef] [PubMed]
- Witheford, M.; Westendorf, K.; Roskams, A.J. Olfactory ensheathing cells promote corticospinal axonal outgrowth by a L1 CAM-dependent mechanism. Glia 2013, 61, 1873–1889. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.K.; Kwan, J.S.; Wong, G.T.; Yi, Z.N.; Ma, A.C.; Chang, R.C. Leukocyte invasion of the brain after peripheral trauma in zebrafish (Danio rerio). Exp. Mol. Med. 2022, 54, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Cuoghi, B.; Mola, L. Macroglial cells of the teleost central nervous system: A survey of the main types. Cell Tissue Res. 2009, 338, 319–332. [Google Scholar] [CrossRef]
- Holcomb, J.D.; Mumm, J.S.; Calof, A.L. Apoptosis in the neuronal lineage of the mouse olfactory epithelium: Regulationin vivoandin vitro. Dev. Biol. 1995, 172, 307–323. [Google Scholar] [CrossRef]
- Rodriguez, A.; Zhang, H.; Klaminder, J.; Brodin, T.; Andersson, P.L.; Andersson, M. ToxTrac: A fast and robust software for tracking organisms. Methods Ecol. Evol. 2018, 9, 460–464. [Google Scholar] [CrossRef]


| Antibody | Species | Dilution | Source | Cat. Number |
|---|---|---|---|---|
| HuC/D | Mouse | 1:100 | ThermoFisher (Waltham, MA, USA) | A21271 |
| GFAP | Rabbit | 1:500 | DAKO (Glostrup, Denmark) | Z033429-2 |
| TH | Rabbit | 1:500 | Millipore (Burlington, MA, USA) | AB152 |
| BrdU | Rat | 1:100 | Abcam (Cambridge, UK) | AB6326-1001 |
| SV2 | Mouse | 1:1000 | DSHB (Iowa City, IA, USA) | N/A |
| LCP1 | Rabbit | 1:100 | Genetex (Irvine, CA, USA) | GTX124420 |
| Tbr2a | Rabbit | 1:500 | Gift from the Yoshihara lab | |
| Secondaries: anti-mouse, rabbit, and rat IgG | Goat | 1:200 | Invitrogen (Waltham, MA, USA) | A11001 A11005 A11008 A11012 XH346510 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorhees, N.W.; Groenwold, S.L.; Williams, M.T.; Putt, L.S.; Sanchez-Gama, N.; Stalions, G.A.; Taylor, G.M.; Van Dort, H.E.; Calvo-Ochoa, E. Olfactory Dysfunction in a Novel Model of Prodromal Parkinson’s Disease in Adult Zebrafish. Int. J. Mol. Sci. 2025, 26, 4474. https://doi.org/10.3390/ijms26104474
Vorhees NW, Groenwold SL, Williams MT, Putt LS, Sanchez-Gama N, Stalions GA, Taylor GM, Van Dort HE, Calvo-Ochoa E. Olfactory Dysfunction in a Novel Model of Prodromal Parkinson’s Disease in Adult Zebrafish. International Journal of Molecular Sciences. 2025; 26(10):4474. https://doi.org/10.3390/ijms26104474
Chicago/Turabian StyleVorhees, Nathaniel W., Samantha L. Groenwold, Mackenzie T. Williams, Lexus S. Putt, Nereyda Sanchez-Gama, Grace A. Stalions, Gabriella M. Taylor, Heather E. Van Dort, and Erika Calvo-Ochoa. 2025. "Olfactory Dysfunction in a Novel Model of Prodromal Parkinson’s Disease in Adult Zebrafish" International Journal of Molecular Sciences 26, no. 10: 4474. https://doi.org/10.3390/ijms26104474
APA StyleVorhees, N. W., Groenwold, S. L., Williams, M. T., Putt, L. S., Sanchez-Gama, N., Stalions, G. A., Taylor, G. M., Van Dort, H. E., & Calvo-Ochoa, E. (2025). Olfactory Dysfunction in a Novel Model of Prodromal Parkinson’s Disease in Adult Zebrafish. International Journal of Molecular Sciences, 26(10), 4474. https://doi.org/10.3390/ijms26104474







