Molecular Identification of Borreliella Species in Ixodes hexagonus Ticks Infesting Hedgehogs (Erinaceus europaeus and E. roumanicus) in North-Western Poland
Abstract
1. Introduction
2. Results
2.1. Morphological Identification of Ticks
2.2. Detection of Borreliaceae DNA and Idetification of Species
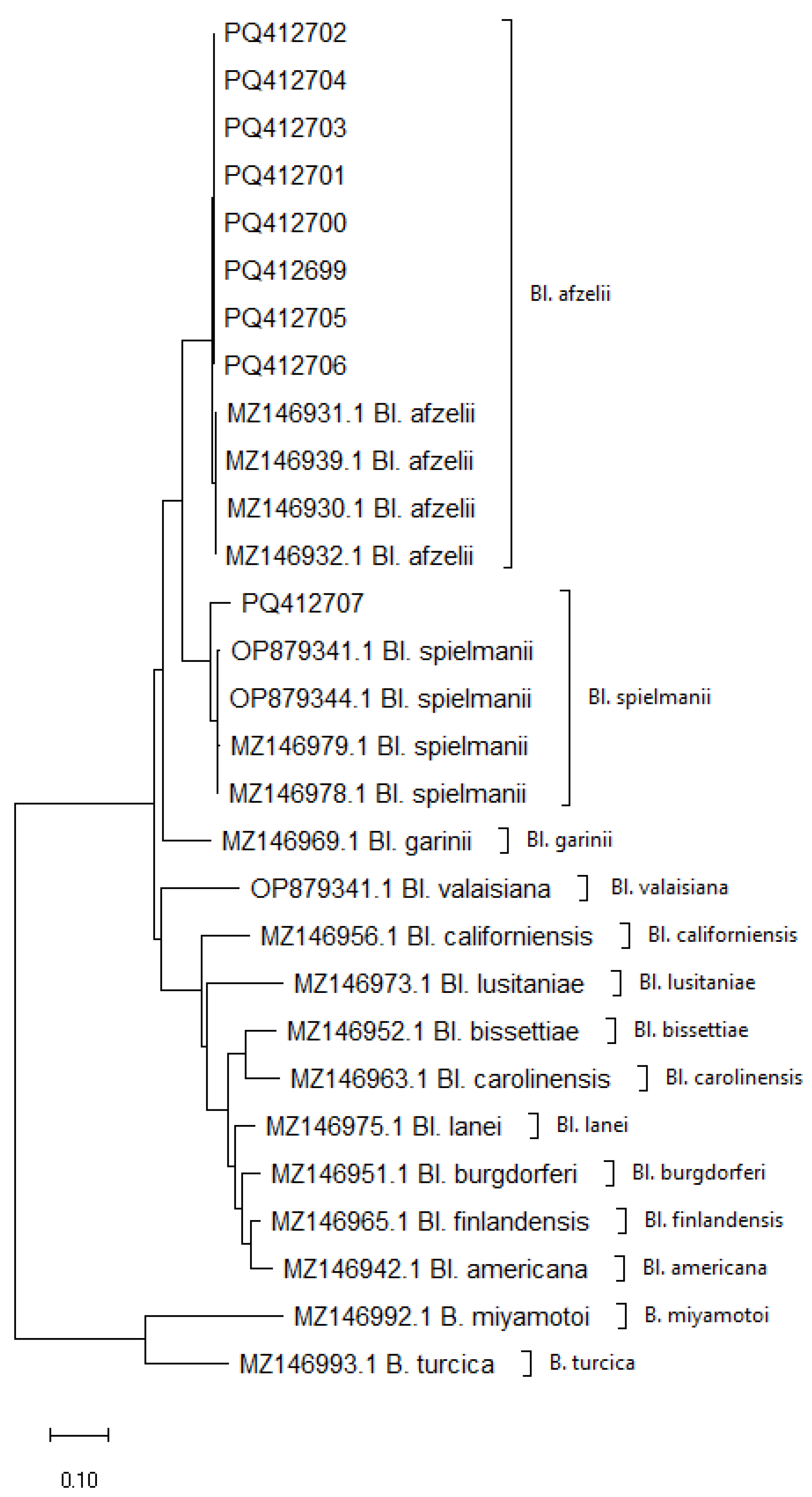
2.3. Analysis of the Genetic Variability of Borreliaceae Spirochetes
3. Discussion
4. Materials and Methods
4.1. Ticks and Hedgehogs Sampling
4.2. DNA Extraction
4.3. Detection of DNA of Borreliaceae Spirochetes and Identification of Species
4.4. DNA Sequencing of Borreliaceae Spirochetes and Genetic Variation Analysis
4.5. Statistical Analyses
4.6. Morphological Identification of Ticks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lurz, P.W.; Garson, P.J.; Wauters, L.A. Effects of temporal and spatial variations in food supply on the space and habitat use of red squirrels (Sciurus vulgaris L.). J. Zool. 2000, 251, 167–178. [Google Scholar] [CrossRef]
- Gaglio, G.; Allen, J.; Bowden, L.; Bryant, M.; Morgan, E.R. Parasites of European hedgehogs (Erinaceus europaeus) in Britain: Epidemiological study and coprological test evaluation. Eur. J. Wildl. Res. 2010, 56, 839–844. [Google Scholar] [CrossRef]
- Gern, L.; Rouvinez, E.; Toutoungi, L.N.; Godfroid, E. Transmission cycles of Borrelia burgdorferi sensu lato involving Ixodes ricinus and/or I. hexagonus ticks and the European hedgehog, Erinaceus europaeus, in suburban and urban areas in Switzerland. Folia Parasitol. 1997, 44, 309–314. [Google Scholar]
- Skuballa, J.; Petney, T.; Pfäffle, M.; Oehme, R.; Hartelt, K.; Fingerle, V.; Kimmig, P.; Taraschewski, H. Occurrence of different Borrelia burgdorferi sensu lato genospecies including B. afzelii, B. bavariensis, and B. spielmanii in hedgehogs (Erinaceus spp.) in Europe. Ticks Tick-Borne Dis. 2012, 3, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Adeolu, M.; Gupta, R.S. A phylogenomic and molecular marker-based proposal for the division of the genus Borrelia into two genera: The emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek 2014, 105, 1049–1072. [Google Scholar]
- Cutler, S.; Vayssier-Taussat, M.; Estrada-Peña, A.; Potkonjak, A.; Mihalca, A.D.; Zeller, H. A new Borrelia on the block: Borrelia miyamotoi—A human health risk? Euro Surveill. 2019, 24, 1800170. [Google Scholar] [CrossRef]
- Majerová, K.; Hönig, V.; Houd, M.; Papežík, P.; Fonville, M.; Sprong, H.; Rudenko, N.; Golovchenko, M.; Černá Bolfíková, B.; Hulva, P.; et al. Hedgehogs, squirrels, and blackbirds as sentinel hosts for active surveillance of Borrelia miyamotoi and Borrelia burgdorferi complex in urban and rural environments. Microorganisms 2020, 8, 1908. [Google Scholar] [CrossRef]
- Arahal, D.R.; Bull, C.T.; Busse, H.; Christensen, H.; Chuvochina, M.; Dedysh, S.N.; Fournier, P.; Konstantinidis, K.T.; Parker, C.T.; Rossello-Mora, R.; et al. Judicial opinions 123–127. Int. J. Syst. Evol. Microbiol. 2022, 72, 005708. [Google Scholar] [CrossRef]
- Brzozowska, M.; Wierzba, A.; Śliwczyński, A.; Myśliwiec, M.; Kozłowski, K.; Wierzba, W. The problem of Lyme borreliosis infections in urban and rural residents in Poland, based on National Health Fund data. Ann. Agric. Environ. Med. 2021, 28, 277–282. [Google Scholar] [CrossRef]
- Thamm, S.; Kalko, E.K.; Wells, K. Ectoparasite infestations of hedgehogs (Erinaceus europaeus) are associated with small-scale landscape structures in an urban-suburban environment. EcoHealth 2009, 6, 404–413. [Google Scholar] [CrossRef]
- Pfäffle, M.; Petney, T.; Skuballa, J.; Taraschewski, H. Comparative population dynamics of a generalist (Ixodes ricinus) and specialist tick (I. hexagonus) species from European hedgehogs. Exp. Appl. Acarol. 2011, 54, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Santos, M.A.; Sgroi, G.; Mendoza-Roldan, J.A.; Khedri, J.; Camarda, A.; Iatta, R.; Sazmand, A.; Otranto, D. Ectoparasites of hedgehogs: From flea mite phoresy to their role as vectors of pathogens. Int. J. Parasitol. Parasites Wildl. 2021, 15, 95–104. [Google Scholar] [CrossRef]
- Probst, J.; Springer, A.; Fingerle, V.; Strube, C. Frequency of Anaplasma phagocytophilum, Borrelia spp., and coinfections in Ixodes ricinus ticks collected from dogs and cats in Germany. Parasites Vectors 2024, 17, 87. [Google Scholar] [CrossRef]
- Zhu, J.J.; Zhang, H.Z.; Hong, R.D.; Yu, D.; Hong, M.; Liu, Z.X.; Li, D.M.; Yin, J.X. Prevalence and genetic diversity of Anaplasma phagocytophilum in wild small mammals from western Yunnan province, China. Front. Vet. Sci. 2024, 11, 1472595. [Google Scholar] [CrossRef]
- Fahrig, L. Habitat fragmentation: A long and tangled tale. Glob. Ecol. Biogeogr. 2019, 28, 33–41. [Google Scholar] [CrossRef]
- Jahfari, S.; Ruyts, S.; Frazer-Mendelewska, E.; Jaarsma, R.; Verheyen, K.; Sprong, H. Melting pot of tick-borne zoonoses: The European hedgehog contributes to the maintenance of various tick-borne diseases in natural cycles in urban and suburban areas. Parasites Vectors 2017, 10, 134. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Guglielmone, A.A.; Nava, S. Worldwide host associations of the tick genus Ixodes suggest relationships based on environmental sharing rather than on co-phylogenetic events. Parasites Vectors 2023, 16, 75. [Google Scholar] [CrossRef]
- Wodecka, B.; Michalik, J.; Grochowalska, R. Red foxes (Vulpes vulpes) are exposed to high diversity of Borrelia burgdorferi sensu lato species infecting fox-derived Ixodes ticks in west-central Poland. Pathogens 2022, 11, 696. [Google Scholar] [CrossRef]
- Wodecka, B.; Kolomiiets, V. Genetic diversity of Borreliaceae species detected in natural populations of Ixodes ricinus ticks in northern Poland. Life 2023, 13, 972. [Google Scholar] [CrossRef]
- Schütte, K.; Springer, A.; Brandes, F.; Reuschel, M.; Fehr, M.; Strube, C. Ectoparasites of European hedgehogs (Erinaceus europaeus) in Germany and their health impact. Parasites Vectors 2024, 17, 2. [Google Scholar] [CrossRef]
- Ruszkowski, J.J.; Hetman, M.; Turlewicz-Podbielska, H.; Pomorska-Mól, M. Hedgehogs as a potential source of zoonotic pathogens—A review and an update of knowledge. Animals 2021, 11, 1754. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, O.; Andersson, M.; Råberg, L. The genetic structure of Borrelia afzelii varies with geographic but not ecological sampling scale. J. Evol. Biol. 2011, 24, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Coipan, C.E.; van Duijvendijk, G.L.A.; Hofmeester, T.R.; Takumi, K.; Sprong, H. The genetic diversity of Borrelia afzelii is not maintained by the diversity of the rodent hosts. Parasites Vectors 2018, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Marianne, J.; Gilbert, L.; Bowman, A.; Forbes, K. The heterogeneity, distribution, and environmental associations of Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis, in Scotland. Front. Public Health 2014, 2, 129. [Google Scholar] [CrossRef]
- Shah, J.S.; Du Cruz, I.; Narciso, W.; Lo, W.; Harris, N.S. Improved sensitivity of Lyme disease western blots prepared with a mixture of Borrelia burgdorferi strains 297 and B31. Chronic Dis. Int. 2014, 1, 7. Available online: https://lymebasics.org/wp-content/uploads/2019/02/t10-Improved-Sensitivity-of-Lyme-disease-Western-Blots.pdf (accessed on 10 November 2024).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Siuda, K. Ticks (Acari: Ixodida) of Poland. Part II: Taxonomy and Distribution; Polskie Towarzystwo Parazytologiczne: Warsaw, Poland, 1993. [Google Scholar]
- Estrada-Peña, A.; Nava, S.; Petney, T. Description of all the stages of Ixodes inopinatus n. sp. (Acari: Ixodidae). Ticks Tick-Borne Dis. 2014, 5, 734–743. [Google Scholar] [CrossRef]


| Tick Species | Total N//n/ | Females N//n/ | Males N//n/ | Nymphs N//n/ | Larvae N//n/ |
|---|---|---|---|---|---|
| I. ricinus | 3/4/0.75 | 3/4/0.75 | 0 | 0 | 0 |
| I. hexagonus | 47/501/10.65 | 47/493/10.5 | 6/8/1.3 | 0 | 0 |
| Ixodes sp. | 24/232/9.6 | 0 | 0 | 22/194/8.8 | 7/38/5.4 |
| Total | 56/737/14.5 | 54/497/9.2 | 6/8/1.3 | 22/194/8.8 | 7/38/5.4 |
| Marker | Primer Sequence 5′-3′ | Amplicon Size (bp) | Reference |
|---|---|---|---|
| flaB | step I FL84F: AGAAGCTTTCTAGTGGGTACAGA FL976R: GATTGGCCTGTGCAATCAT step II FL220F: CAGACAACAGAGGGAAAT FL823R: TCAAGTCTATTTTGGAAAGCACC | 604 | [19] |
| mag/trnI | step I mag-268F: TCTAATTAAAACAGCHTGDGGAYT trnI-20R: TGAACATCCGACCTCAGG step II mag-435F: CCATATAAGCTTCCGTTTCAAC trnI-65R: CTAACCACCTGAGCTATGATCC | 309–1183 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentyna, K.; Beata, W. Molecular Identification of Borreliella Species in Ixodes hexagonus Ticks Infesting Hedgehogs (Erinaceus europaeus and E. roumanicus) in North-Western Poland. Int. J. Mol. Sci. 2025, 26, 58. https://doi.org/10.3390/ijms26010058
Valentyna K, Beata W. Molecular Identification of Borreliella Species in Ixodes hexagonus Ticks Infesting Hedgehogs (Erinaceus europaeus and E. roumanicus) in North-Western Poland. International Journal of Molecular Sciences. 2025; 26(1):58. https://doi.org/10.3390/ijms26010058
Chicago/Turabian StyleValentyna, Kolomiiets, and Wodecka Beata. 2025. "Molecular Identification of Borreliella Species in Ixodes hexagonus Ticks Infesting Hedgehogs (Erinaceus europaeus and E. roumanicus) in North-Western Poland" International Journal of Molecular Sciences 26, no. 1: 58. https://doi.org/10.3390/ijms26010058
APA StyleValentyna, K., & Beata, W. (2025). Molecular Identification of Borreliella Species in Ixodes hexagonus Ticks Infesting Hedgehogs (Erinaceus europaeus and E. roumanicus) in North-Western Poland. International Journal of Molecular Sciences, 26(1), 58. https://doi.org/10.3390/ijms26010058







