Proteomic Profile of Daphnia pulex in Response to Heavy Metal Pollution in Lakes of Northern Patagonia
Abstract
1. Introduction
2. Results
2.1. Protein Extraction and Proteomics
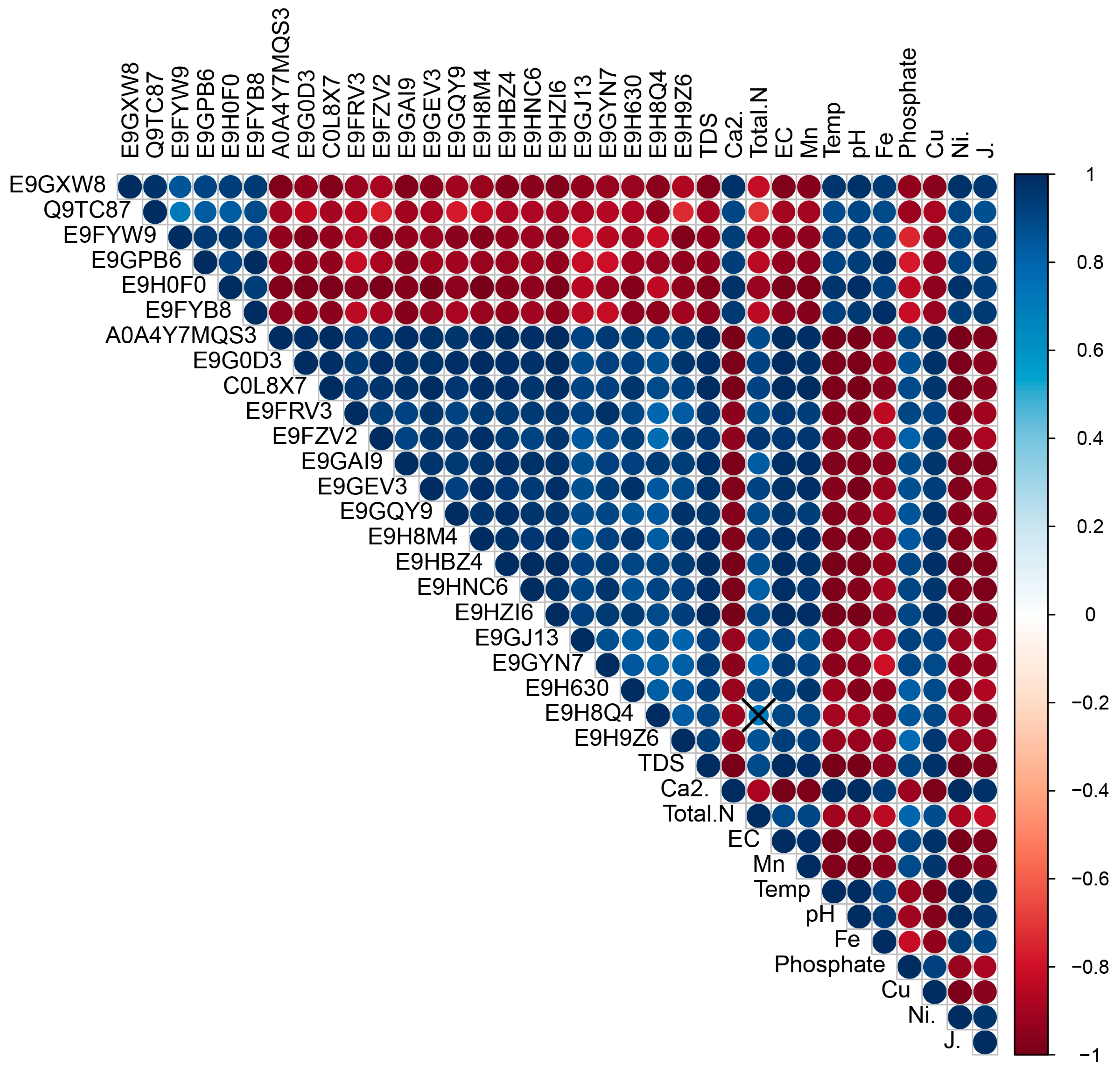
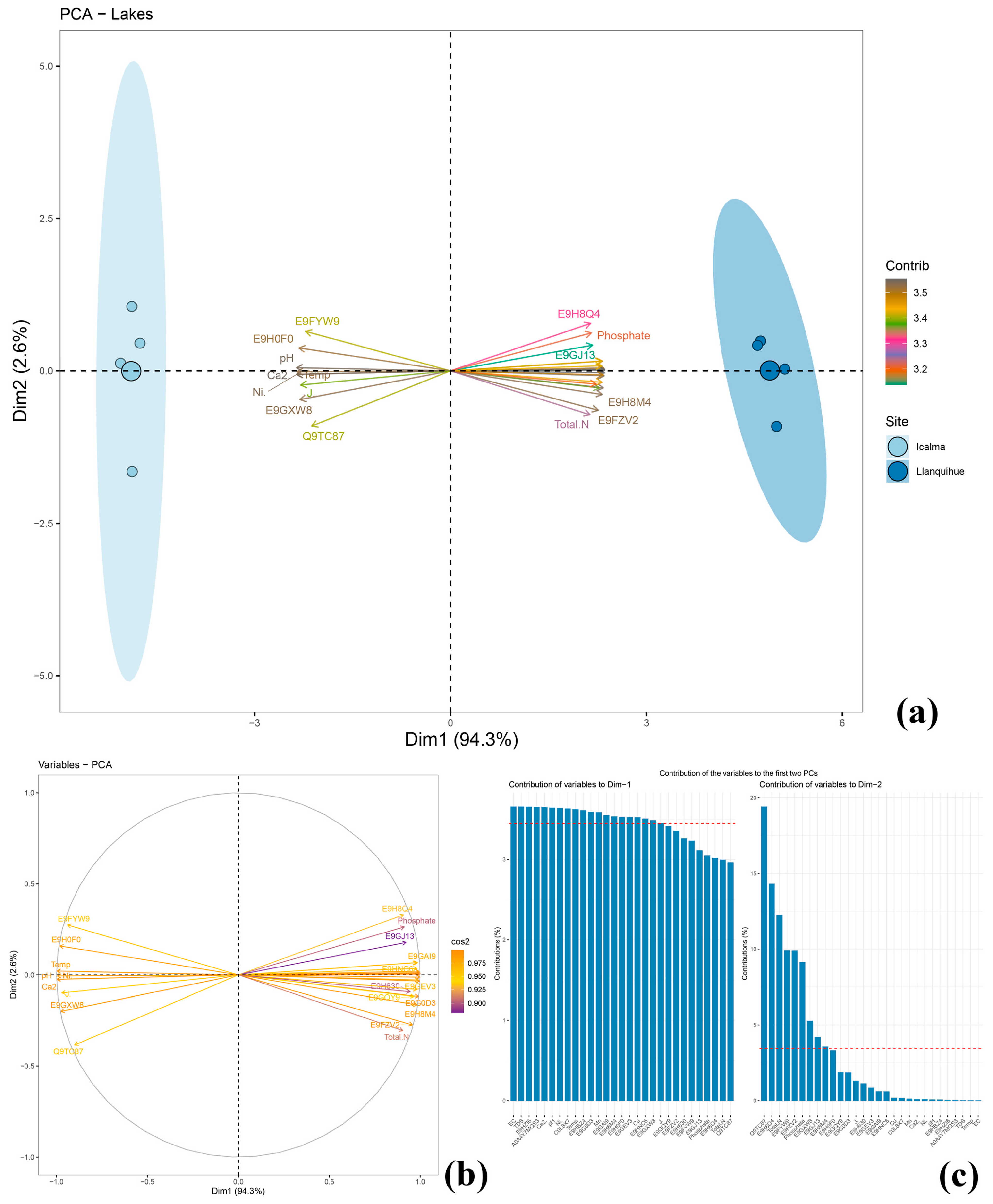
2.2. Relationships of Up- and Downregulated Proteins with Physicochemical and Ecological Factors in Northern Patagonian Lakes
3. Discussion
4. Materials and Methods
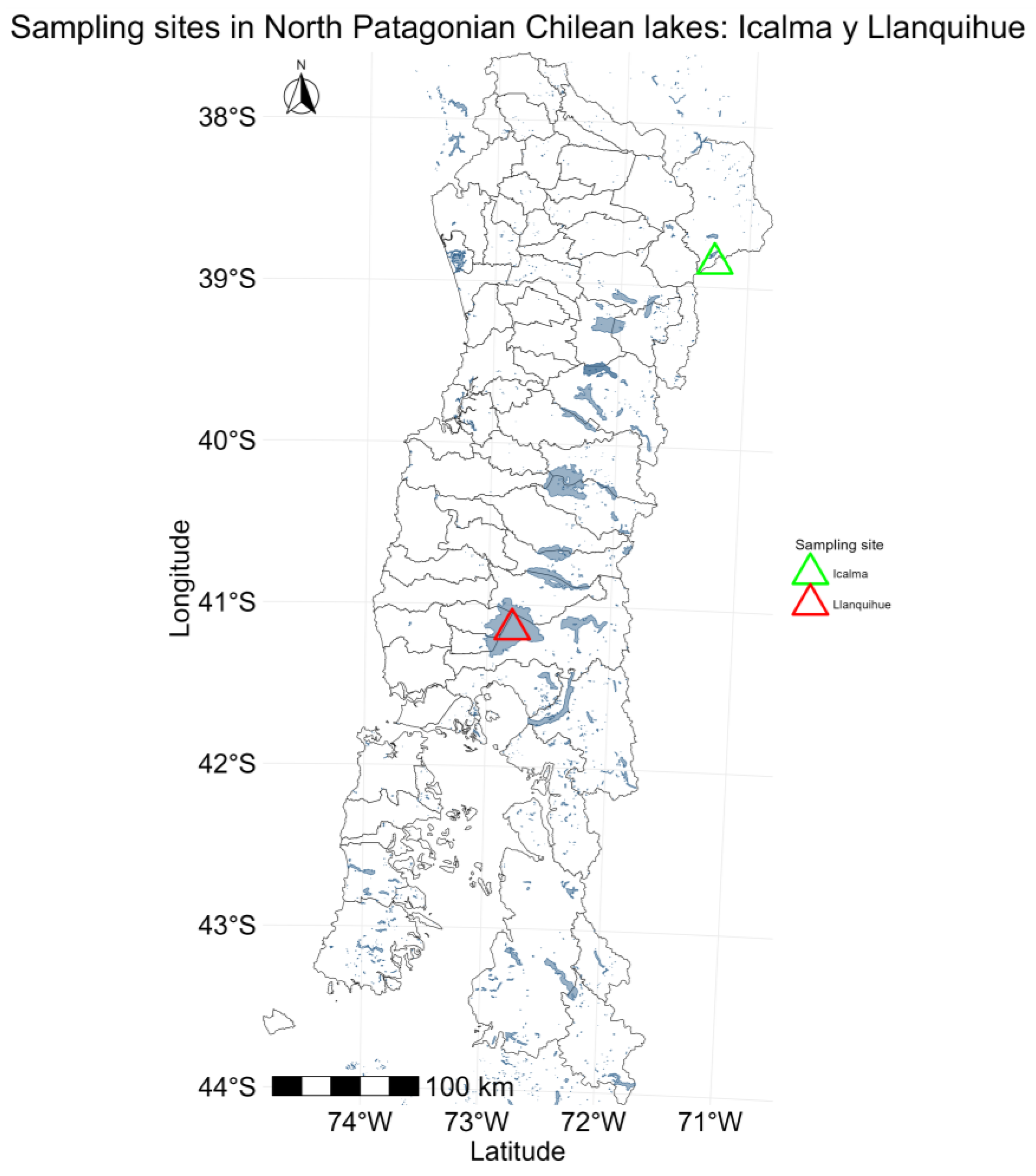
4.1. Study Area and Sampling
4.2. Physicochemical Properties of Study Sites
4.3. Protein Extraction and Quantification
4.4. Proteomic Analysis
4.4.1. Chemicals and Instrumentation
4.4.2. Sample Information
4.4.3. Sample Preparation
4.4.4. NanoLC
4.4.5. Mass Spectrometry
4.4.6. Proteome Data Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater Ecoregions of the World: A New Map of Biogeographic Units for Freshwater Biodiversity Conservation. Bioscience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Tockner, K. Freshwaters: Global Distribution, Biodiversity, Ecosystem Services, and Human Pressures. In Handbook of Water Resources Management: Discourses, Concepts and Examples; Springer International Publishing: Cham, Switzerland, 2021; pp. 489–501. [Google Scholar] [CrossRef]
- Reddy, A.R.; George, S.M.; Rathinasabapathi, G.; Reddy, K.S.; Reddy, P.N.; Reddy, E.J.; Kumar, P.S.; Rajaram, A. Aquatic Plants As Bioindicators for Heavy Metal Content in Surface Water Ecosystems—Lemnaceae Species. Oxid. Commun. 2023, 46, 966–976. [Google Scholar]
- Norambuena, J.-A.; Poblete-Grant, P.; Beltrán, J.F.; De Los Ríos-Escalante, P.; Farías, J.G. Evidence of the Anthropic Impact on a Crustacean Zooplankton Community in Two North Patagonian Lakes. Sustainability 2022, 14, 6052. [Google Scholar] [CrossRef]
- Piwowarska, D.; Kiedrzyńska, E.; Jaszczyszyn, K. A Global Perspective on the Nature and Fate of Heavy Metals Polluting Water Ecosystems, and Their Impact and Remediation. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1436–1458. [Google Scholar] [CrossRef]
- Soetan, O.; Viteritto, M.; Qian, Y.; Feng, H. Evaluation of Toxic Metal Pollution in Freshwater Surficial Sediments Using Environmental Indices and Multivariate Statistical Approaches—A Systematic Review. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100961. [Google Scholar] [CrossRef]
- Gagneten, A.M. Effects of Contamination by Heavy Metals and Eutrophication on Zooplankton, and Their Possible Effects on the Trophic Webs of Freshwater Aquatic Ecosystems. In Eutrophication: Causes, Consequences and Control; Ansari, A., Singh Gill, S., Lanza, G., Rast, W., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 211–223. ISBN 978-90-481-9624-1. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Norambuena, J.-A.; Farías, J.; De los Ríos, P. The Water Flea Daphnia Pulex (Cladocera, Daphniidae), a Possible Model Organism to Evaluate Aspects of Freshwater Ecosystems. Crustaceana 2019, 92, 1415–1426. [Google Scholar] [CrossRef]
- Jamil Emon, F.; Rohani, M.F.; Sumaiya, N.; Tuj Jannat, M.F.; Akter, Y.; Shahjahan, M.; Abdul Kari, Z.; Tahiluddin, A.B.; Goh, K.W. Bioaccumulation and Bioremediation of Heavy Metals in Fishes—A Review. Toxics 2023, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, E.E.; Barnes, R.D. Zoología de Los Invertebrados; McGraw-Hill: New York, NY, USA, 1996; ISBN 968-25-2452-0. [Google Scholar]
- Benzie, J.A.H. The Genus Daphnia (Including Daphniopsis) (Anomopoda: Daphniidae); Dumont, H.J.F., Ed.; Backhuys: Leiden, The Netherlands, 2005; Volume 50, ISBN 90-5782-151-6. [Google Scholar]
- Campos, H. Limnological Study of Araucanian Lakes (Chile). Int. Vereinigung für Theor. und Angew. Limnol. Verhandlungen 1984, 22, 1319–1327. [Google Scholar] [CrossRef]
- De Los Ríos-Escalante, P.R. Crustacean Zooplankton Communities in Chilean Inland Waters. Crustac. Monogr. 2010, 12, 1–117. [Google Scholar]
- Thomasson, K. Araucanian Lakes; Acta Phytogeographica Suecica, Svenska Vaxtgeografiska Sallskapet: Uppsala, Sweden, 1963; Volume 47. [Google Scholar]
- Woelfl, S.; Zagarese, H.; Pedrozo, F. Preface: Limnology of Temperate South America. Limnologica 2007, 37, 1–2. [Google Scholar] [CrossRef][Green Version]
- Aguayo, M.; Pauchard, A.; Azócar, G.; Parra, O. Cambio Del Uso Del Suelo En El Centro Sur de Chile a Fines Del Siglo XX: Entendiendo La Dinámica Espacial y Temporal Del Paisaje. Rev. Chil. Hist. Nat. 2009, 82, 361–374. [Google Scholar] [CrossRef]
- Kamjunke, N.; Nimptsch, J.; Harir, M.; Herzsprung, P.; Schmitt-kopplin, P.; Neu, T.R.; Graeber, D.; Osorio, S.; Valenzuela, J.; Reyes, J.C.; et al. Land-Based Salmon Aquacultures Change the Quality and Bacterial Degradation of Riverine Dissolved Organic Matter. Sci. Rep. 2017, 7, 43739. [Google Scholar] [CrossRef]
- Pizarro, J.; Vergara, P.M.; Cerda, S.; Briones, D. Cooling and Eutrophication of Southern Chilean Lakes. Sci. Total Environ. 2016, 541, 683–691. [Google Scholar] [CrossRef]
- Rozzi, R.; Armesto, J.J.; Figueroa, J. Biodiversidad y Conservación de Los Bosques Nativos de Chile: Una Aproximación Jerárquica. Bosque 1994, 15, 55–64. [Google Scholar] [CrossRef]
- De los Ríos, P.; Soto, D.; Santander-Massa, R.; Acevedo, P. Plankton Crustaceans in Bays with Different Trophic Status in Llanquihue Lake (41° S Chile). Braz. J. Biol. 2017, 77, 469–475. [Google Scholar] [CrossRef]
- Encina, F.; Vega, R.; Lara, G.; De Los Ríos-Escalante, P. Ecological Role of Benthic Crustaceans in Chilean North Patagonian Lakes and Rivers (Araucania Region, 39°S). Crustaceana 2017, 90, 437–447. [Google Scholar] [CrossRef]
- Figueroa, R.; Valdovinos, C.; Araya, E.; Parra, O. Macroinvertebrados Bentónicos Como Indicadores de Calidad de Agua de Ríos Del Sur de Chile. Rev. Chil. Hist. Nat. 2003, 76, 275–285. [Google Scholar] [CrossRef]
- De Los Ríos, P.; Acevedo, P.; Soto, D.; Norambuena, J. Potential Long Term Effects of Climate Changes and Their Effects on Crustacean Inland Water Biodiversity in Southern Patagonia (51–53° S, Chile). In Efecto de Los Cambios Globales Sobre la Biodiversidad; Vanina Volpedo, A., Fernández Reyes, L., Eds.; Red 406RT0285; CYTED—Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo; Madrid, Spain, 2008; pp. 219–231. ISBN 978-987-05-5533-9. [Google Scholar]
- Encina, F.; De Los Ríos Escalante, P.; Salazar, K. Acute Toxicity (LC50) of a Pesticide (Carbendazim) on Two Native Crustacean Zooplankton Species: Daphnia pulex Leydig, 1860 and Tumeodiaptomus Diabolicus (Brehm, 1935) from Northern Patagonian Lakes (Chile). Crustaceana 2017, 90, 199–206. [Google Scholar] [CrossRef]
- Athanasio, C.G.; Chipman, J.K.; Viant, M.R.; Mirbahai, L. Optimisation of DNA Extraction from the Crustacean Daphnia. PeerJ 2016, 4, e2004. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, K.; Deng, D.; Zhang, Y.-N.; Peng, S.; Xu, X. Genetic Diversity of Daphnia pulex in the Middle and Lower Reaches of the Yangtze River. PLoS ONE 2016, 11, e0152436. [Google Scholar] [CrossRef] [PubMed]
- Wilde, M.V.; Brehm, J.; Schwarzer, M.; Stöckl, J.B.; Laforsch, C.; Fröhlich, T. Improving the Proteome Coverage of Daphnia magna—Implications for Future Ecotoxicoproteomics Studies. Proteomics 2022, 22, 2100289. [Google Scholar] [CrossRef]
- Wojewodzic, M.W.; Beaton, M.J. The Future of Environmental Epigenetics: Insights Using the Clonal Water Flea Model. In Advances in Insect Physiology; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 53, pp. 287–312. [Google Scholar]
- Gotelli, N.J.; Ellison, A.M.; Ballif, B.A. Environmental Proteomics, Biodiversity Statistics and Food-Web Structure. Trends Ecol. Evol. 2012, 27, 436–442. [Google Scholar] [CrossRef]
- Gajahin Gamage, N.T.; Miyashita, R.; Takahashi, K.; Asakawa, S.; Senevirathna, J.D.M. Proteomic Applications in Aquatic Environment Studies. Proteomes 2022, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Liorti, A.; Crease, T.; Heyland, A. Interactive Effects of Copper and Calcium in Daphnia pulex. J. Limnol. 2017, 76, 281–291. [Google Scholar] [CrossRef]
- Toei, M.; Saum, R.; Forgac, M. Regulation and Isoform Function of the V-ATPases. Biochemistry 2010, 49, 4715–4723. [Google Scholar] [CrossRef]
- Kumar, S.; Sheokand, N.; Mhadeshwar, M.A.; Raje, C.I.; Raje, M. Characterization of Glyceraldehyde-3-Phosphate Dehydrogenase as a Novel Transferrin Receptor. Int. J. Biochem. Cell Biol. 2012, 44, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Sirover, M.A. Structural Analysis of Glyceraldehyde-3-Phosphate Dehydrogenase Functional Diversity. Int. J. Biochem. Cell Biol. 2014, 57, 20–26. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Z.; Chen, M.; Zhang, M.; Jiao, Y.; Chen, Q.; Zhao, Y. Comparative Proteomic Analysis of Senescence in the Freshwater Cladoceran Daphnia pulex. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 239, 110352. [Google Scholar] [CrossRef]
- Becker, D.; Reydelet, Y.; Lopez, J.A.; Jackson, C.; Colbourne, J.K.; Hawat, S.; Hippler, M.; Zeis, B.; Paul, R.J. The Transcriptomic and Proteomic Responses of Daphnia pulex to Changes in Temperature and Food Supply Comprise Environment-Specific and Clone-Specific Elements. BMC Genom. 2018, 19, 376. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gómez, R.; Weiss, L.C. Distinct Gene Expression Patterns of Two Heat Shock Protein 70 Members During Development, Diapause, and Temperature Stress in the Freshwater Crustacean Daphnia magna. Front. Cell Dev. Biol. 2021, 9, 692517. [Google Scholar] [CrossRef] [PubMed]
- Otte, K.A.; Fröhlich, T.; Arnold, G.J.; Laforsch, C. Proteomic Analysis of Daphnia magna Hints at Molecular Pathways Involved in Defensive Plastic Responses. BMC Genom. 2014, 15, 306. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, I. Gene Expression under Multiple Stressors in Daphnia pulex. Master Thesis, University of Windsor, Windsor, ON, Canada, 2012. Electronic Theses Dissertations. p. 358. Available online: https://scholar.uwindsor.ca/etd/358 (accessed on 3 April 2024).
- Feiner, N.; Radersma, R.; Vasquez, L.; Ringnér, M.; Nystedt, B.; Raine, A.; Tobi, E.W.; Heijmans, B.T.; Uller, T. Environmentally Induced DNA Methylation Is Inherited across Generations in an Aquatic Keystone Species. iScience 2022, 25, 104303. [Google Scholar] [CrossRef]
- Nash, N.; Klymasz-Swartz, A.K.; Nash, M.T.; Sachs, M.; Yoon, G.R.; Weihrauch, D. Impact of Heatwaves and Environmental Ammonia on Energy Metabolism, Nitrogen Excretion, and MRNA Expression of Related Genes in the Indicator Model System Daphnia magna. Aquat. Toxicol. 2022, 249, 106225. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.R.; Pfrender, M.E.; Eads, B.D.; Klaper, R.; Callaghan, A.; Sibly, R.M.; Colson, I.; Jansen, B.; Gilbert, D.; Colbourne, J.K. Daphnia as an Emerging Model for Toxicological Genomics. Adv. Exp. Biol. 2008, 2, 5–7. [Google Scholar] [CrossRef]
- Walsh, M.P. Vascular Smooth Muscle Myosin Light Chain Diphosphorylation: Mechanism, Function, and Pathological Implications. IUBMB Life 2011, 63, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Subramanian, R.R.; Masters, S.C. 14-3-3 Proteins: Structure, Function, and Regulation. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 617–647. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, H.; Coate, J.E.; Ho, E.K.H.; Schaack, S. Thermal Stress and Mutation Accumulation Increase Heat Shock Protein Expression in Daphnia. Evol. Ecol. 2022, 36, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.; Clark, J.; Wilson, P.J.; Little, T.J. Daphnia magna Modifies Its Gene Expression Extensively in Response to Caloric Restriction Revealing a Novel Effect on Haemoglobin Isoform Preference. Mol. Ecol. 2020, 29, 3261–3276. [Google Scholar] [CrossRef] [PubMed]
- Muyssen, B.T.A.; Messiaen, M.; Janssen, C.R. Combined Cadmium and Temperature Acclimation in Daphnia magna: Physiological and Sub-Cellular Effects. Ecotoxicol. Environ. Saf. 2010, 73, 735–742. [Google Scholar] [CrossRef]
- Ukhueduan, B.; Schumpert, C.; Kim, E.; Dudycha, J.L.; Patel, R.C. Relationship between Oxidative Stress and Lifespan in Daphnia pulex. Sci. Rep. 2022, 12, 2354. [Google Scholar] [CrossRef] [PubMed]
- Niemuth, N.J.; Curtis, B.J.; Laudadio, E.D.; Sostare, E.; Bennett, E.A.; Neureuther, N.J.; Mohaimani, A.A.; Schmoldt, A.; Ostovich, E.D.; Viant, M.R.; et al. Energy Starvation in Daphnia magna from Exposure to a Lithium Cobalt Oxide Nanomaterial. Chem. Res. Toxicol. 2021, 34, 2287–2297. [Google Scholar] [CrossRef] [PubMed]
- Vandenbrouck, T.; Soetaert, A.; van der Ven, K.; Blust, R.; De Coen, W. Nickel and Binary Metal Mixture Responses in Daphnia magna: Molecular Fingerprints and (Sub)Organismal Effects. Aquat. Toxicol. 2009, 92, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Sepúlveda, M.S.; Jiang, Q.; Jiao, Y.; Chen, Q.; Huang, Y.; Tian, J.; Zhao, Y. Development of an Adverse Outcome Pathway for Nanoplastic Toxicity in Daphnia pulex Using Proteomics. Sci. Total Environ. 2021, 766, 144249. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Varela, Z.; Franco, D.; Fernández, J.A.; Aboal, J.R. Can Proteomics Contribute to Biomonitoring of Aquatic Pollution? A Critical Review. Environ. Pollut. 2020, 267, 115473. [Google Scholar] [CrossRef]
- Zeis, B.; Lamkemeyer, T.; Paul, R.J.; Nunes, F.; Schwerin, S.; Koch, M.; Schütz, W.; Madlung, J.; Fladerer, C.; Pirow, R. Acclimatory Responses of the Daphnia pulex Proteome to Environmental Changes. I. Chronic Exposure to Hypoxia Affects the Oxygen Transport System and Carbohydrate Metabolism. BMC Physiol. 2009, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Techo, T.; Jindarungrueng, S.; Tatip, S.; Limcharoensuk, T.; Pokethitiyook, P.; Kruatrachue, M.; Auesukaree, C. Vacuolar H+-ATPase Is Involved in Preventing Heavy Metal-Induced Oxidative Stress in Saccharomyces Cerevisiae. Environ. Microbiol. 2020, 22, 2403–2418. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, C.; Wang, Y.; Guo, Y.; Zhao, Y.; Yang, C.; Gao, C. Overexpression of ThVHAc1 and Its Potential Upstream Regulator, ThWRKY7, Improved Plant Tolerance of Cadmium Stress. Sci. Rep. 2016, 6, 18752. [Google Scholar] [CrossRef]
- Dietz, K.J.; Tavakoli, N.; Kluge, C.; Mimura, T.; Sharma, S.S.; Harris, G.C.; Chardonnens, A.N.; Golldack, D. Significance of the V-Type ATPase for the Adaptation to Stressful Growth Conditions and Its Regulation on the Molecular and Biochemical Level. J. Exp. Bot. 2001, 52, 1969–1980. [Google Scholar] [CrossRef]
- Kabala, K.; Janicka-Russak, M.; Reda, M.; Migocka, M. Transcriptional Regulation of the V-ATPase Subunit c and V-PPase Isoforms in Cucumis Sativus under Heavy Metal Stress. Physiol. Plant. 2014, 150, 32–45. [Google Scholar] [CrossRef]
- Reisz, J.A.; Wither, M.J.; Dzieciatkowska, M.; Nemkov, T.; Issaian, A.; Yoshida, T.; Dunham, A.J.; Hill, R.C.; Hansen, K.C.; D’Alessandro, A. Oxidative Modifications of Glyceraldehyde 3-Phosphate Dehydrogenase Regulate Metabolic Reprogramming of Stored Red Blood Cells. Blood 2016, 128, e32–e42. [Google Scholar] [CrossRef] [PubMed]
- Alsafran, M.; Usman, K.; Ahmed, B.; Rizwan, M.; Saleem, M.H.; Al Jabri, H. Understanding the Phytoremediation Mechanisms of Potentially Toxic Elements: A Proteomic Overview of Recent Advances. Front. Plant Sci. 2022, 13, 881242. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, A.; David, E.; Betoulle, S.; Bultelle, F.; Rocher, B.; Barjhoux, I.; Cosio, C. New Insights into Cellular Impacts of Metals in Aquatic Animals. Environments 2020, 7, 46. [Google Scholar] [CrossRef]
- Mattice, A.M.S.; Varma, A.; Storey, K.B. Role of NADP+-Dependent Isocitrate Dehydrogenase from Muscle Tissue of Rana Sylvatica in ROS Defense during Freeze-Tolerance. Biochimie 2023, 210, 14–21. [Google Scholar] [CrossRef]
- Binarová, P.; Tuszynski, J. Tubulin: Structure, Functions and Roles in Disease. Cells 2019, 8, 1294. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Beckerman, A.P. Copper Mediates Life History Responses of Daphnia pulex to Predation Threat. Front. Ecol. Evol. 2022, 10, 983923. [Google Scholar] [CrossRef]
- Suresh, S.; Crease, T.J.; Cristescu, M.E.; Chain, F.J.J. Alternative Splicing Is Highly Variable among Daphnia pulex Lineages in Response to Acute Copper Exposure. BMC Genom. 2020, 21, 433. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat Shock Proteins in Toxicology: How Close and How Far? Life Sci. 2010, 86, 377–384. [Google Scholar] [CrossRef]
- Köhler, H.R.; Rahman, B.; Gräff, S.; Berkus, M.; Triebskorn, R. Expression of the Stress-70 Protein Family (Hsp70) Due to Heavy Metal Contamination in the Slug, Deroceras Reticulatum: An Approach to Monitor Sublethal Stress Conditions. Chemosphere 1996, 33, 1327–1340. [Google Scholar] [CrossRef]
- Shaheen, S.; Majeed, Z.; Mahmood, Q. The Assessment of Metal Resistance through the Expression of Hsp-70 and HO-1 Proteins in Giant Reed. Int. J. Plant Biol. 2023, 14, 687–700. [Google Scholar] [CrossRef]
- Bafana, A.; Dutt, S.; Kumar, A.; Kumar, S.; Ahuja, P.S. The Basic and Applied Aspects of Superoxide Dismutase. J. Mol. Catal. B Enzym. 2011, 68, 129–138. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of Solid Biochar Fuel from Waste Biomass by Hydrothermal Carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Timón-Gómez, A.; Bartley-Dier, E.L.; Fontanesi, F.; Barrientos, A. HIGD-Driven Regulation of Cytochrome C Oxidase Biogenesis and Function. Cells 2020, 9, 2620. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; GS Garland Science Taylor & Francis Group: New York, NY, USA; Abingdon, UK, 2008; ISBN 9780815344643. [Google Scholar]
- Chicherin, I.V.; Dashinimaev, E.; Baleva, M.; Krasheninnikov, I.; Levitskii, S.; Kamenski, P. Cytochrome c Oxidase on the Crossroads of Transcriptional Regulation and Bioenergetics. Front. Physiol. 2019, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xie, L.; Lee, Y.K.; Brede, D.A.; Lyne, F.; Kassaye, Y.; Thaulow, J.; Caldwell, G.; Salbu, B.; Tollefsen, K.E. Integrative Assessment of Low-Dose Gamma Radiation Effects on Daphnia magna Reproduction: Toxicity Pathway Assembly and AOP Development. Sci. Total Environ. 2020, 705, 135912. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Z.; Yu, B.; Sun, Y.; Gu, L.; Zhang, L.; Huang, Y.; Yang, Z. Population Changes of Daphnia Caused by Declined Calcium Concentration: Evidences from Population Dynamics and Sexual Reproduction. Ecotoxicol. Environ. Saf. 2022, 233, 113352. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.M.; Bailey, H.; Canaria, E. Toxicity of Total Dissolved Solids Associated with Two Mine Effluents to Chironomid Larvae and Early Life Stages of Rainbow Trout. Environ. Toxicol. Chem. 2000, 19, 210–214. [Google Scholar] [CrossRef]
- Weber, A.K.; Pirow, R. Physiological Responses of Daphnia pulex to Acid Stress. BMC Physiol. 2009, 9, 9. [Google Scholar] [CrossRef]
- Campos, H.; Stefen, W.; Aguero, G.; Parra, O.; Zúñiga, L. Limnological Study of Lake Llanquihue (Chile) Morphometry, Physics, Chemistry, Plankton and Primary Productivity. Arch. Fur Hydrobiol. Suppl. 1988, 81, 37–67. [Google Scholar]
- Muñoz-Pedreros, A.; Larraín, A. Impacto de La Actividad Silvoagropecuaria Sobre La Calidad Del Paisaje En Un Transecto Del Sur de Chile. Rev. Chil. Hist. Nat. 2002, 75, 673–689. [Google Scholar] [CrossRef]
- Soto, D.; Campos, H. Los Lagos Oligotroficos Del Bosque Templado Humedo Del Sur de Chile. In Ecología de Los Bosques Templados de Chile; Editorial Universita: Santiago, Chile, 1995; pp. 134–148. [Google Scholar]
- De los Ríos-Escalante, P. Efecto de Las Disponibilidades de Recursos Energéticos, Estructurales y de Protección Sobre La Distribución y Abundancia de Copépodos y Cladóceros Zooplanctónicos Lacustres Chilenos; Universidad Austral de Chile: Valdivia, Chile, 2003. [Google Scholar]
- Woelfl, S.; Caputo, L.; Garcia-Chicote, J.; De los Ríos, P. Manuales Para El Muestreo y Bioindicación de Zooplancton; Ambiente, M.d.M., Manuales, S., Eds.; Santiago: Santiago de Chile, Chile, 2018. [Google Scholar]
- Bair, R.; Eaton, A.; Rice, E. Standard Methods: For the Examination of Water and Waste Water, 23rd ed.; Bridgewater, L.L., Ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017; ISBN 78-0-87553-287-5. [Google Scholar]
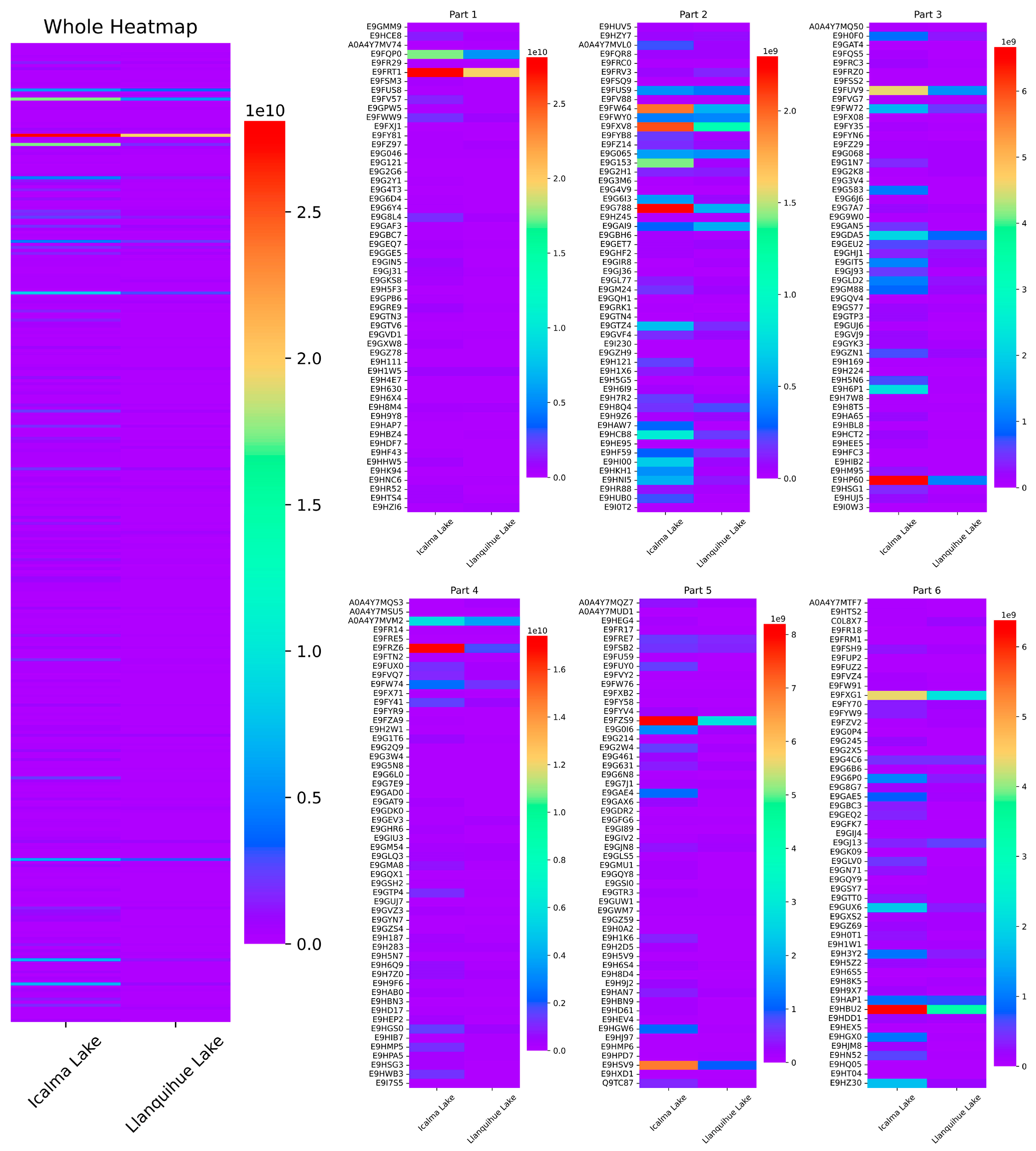

| FASTA Code and Protein Name–Daphnia pulex | Mean LFQ Intensity Icalma Lake | SD | Mean LFQ Intensity Llanquihue Lake | SD | t Student | Effects of High Expression in Daphnia pulex Cells | Reference |
|---|---|---|---|---|---|---|---|
| tr|A0A4Y7MQS3|Calcium-transporting ATPase. | 2,3049,333.33 | 19,962,075.30 | 296,400,000.00 | 7,180,619.75 | 0.0007 | Stress to protection against high dissolved copper concentration. | [32] |
| tr|E9G0D3|EV-type proton ATPase subunit E. | 102,897,333.33 | 10,175,529.93 | 181,240,000.00 | 2,155,643.755 | 0.00202 | Stress due to pH differential in intra- and extra-cellular compartments. | [33] |
| tr|C0L8X7|Glyceraldehyde-3-phosphate dehydrogenase. | 56,312,666.67 | 2,641,977.353 | 188,210,000.00 | 12,966,402.74 | 0.00195 | Its expression depends on extracellular iron concentrations. | [34,35] |
| tr|E9FRV3|Isocitrate dehydrogenase [NADP]. | 70,594,333.33 | 13,921,006.23 | 129,763,333.33 | 5,658,801.40 | 0.01038 | Reducing glutathione disulfide (GSSG) to GSH for antioxidant purposes. | [36] |
| tr|E9FZV2|Aldedh domain-containing protein. | 45,473,666.67 | 7,792,250.92 | 77,216,333.33 | 6,030,186.924 | 0.00074 | No information. | - |
| tr|E9GAI9|Tubulin alpha chain. | 291,610,000.00 | 27,511,075.95 | 544,726,666.67 | 34,310,777.22 | 0.00728 | Stress to changes in temperature and food supply. | [37] |
| tr|E9GEV3|Heat shock 70 kDa protein cognate 4. | 140,560,000.00 | 6,211,094.91 | 289,060,000.00 | 29,163,051.97 | 0.00526 | Stress to changes in temperature. | [38] |
| tr|E9GJ13|Fructose-bisphosphate aldolase. | 373,083,333.33 | 31,550,152.67 | 572,193,333.33 | 67,951,014.95 | 0.02975 | Stress related to metabolic processes. | [39] |
| tr|E9GQY9|40S ribosomal protein SA. | 13,416,333.33 | 3,566,336.82 | 29,646,333.33 | 2,150,024.73 | 0.01879 | Effects on Ribosomal RNA production. | [40,41] |
| tr|E9GYN7|Arginase. | 30,657,666.67 | 3,614,230.95 | 45,341,333.33 | 2,336,978.03 | 0.00278 | Effects on nitrogen metabolism, urea cycle. | [42] |
| tr|E9H630|Myosin regulatory light chain. | 57,037,333.33 | 2,622,803.14 | 78,688,666.67 | 7,572,951.49 | 0.03065 | Effect on muscle fibers. | [43,44] |
| tr|E9H8M4|14_3_3 domain-containing protein. | 348,406,666.67 | 12,185,697.90 | 441,686,666.70 | 13,412,659.44 | 0.00161 | Effects on diverse signaling proteins, including kinases, phosphatases, and transmembrane receptors. | [45] |
| tr|E9H8Q4|Vitellogenin. | 169,703,333.33 | 24,323,168.65 | 230,780,000.00 | 13,865,067.62 | 0.0142 | Effect on egg yolk precursor. | [37] |
| tr|E9H9Z6|40S ribosomal protein S13. | 41,323,000 | 10,951,575.64 | 73,726,333.33 | 3,100,061.343 | 0.02748 | Effects on Ribosomal RNA production. | [40,41] |
| tr|E9HBZ4|Heat shock 70 kDa protein cognate 4. | 36,423,333.33 | 5,107,539.949 | 181,830,000.00 | 13,299,966.17 | 0.00265 | Stress to changes in temperature. | [38] |
| tr|E9HNC6|Heat shock protein 83, 90. | 177,840,000.00 | 6,062,309.79 | 279,873,333.33 | 18,034,783.98 | 0.00591 | Intracellular stress in a climate change of the aquatic environment. | [46] |
| tr|E9HZI6|Superoxide dismutase. | 226,633,333.33 | 4,314,583.80 | 514,356,666.67 | 8,345,719.46 | 0.00012 | Stress response to copper, ammonia, and hypoxia levels. | [47] |
| FASTA Code and Protein Name–Daphnia pulex | Subcellular Location | Mean LFQ Intensity Icalma Lake | SD | Mean LFQ Intensity Llanquihue Lake | SD | t Student | Effects of Down-Regulated in Daphnia pulex | Reference |
|---|---|---|---|---|---|---|---|---|
| tr|E9GXW8|Cytochrome C Oxidase subunit 5A. | Mitochondrion. | 395,303,333.33 | 77,583,748.51 | 31,921,000.00 | 27,890,177.68 | 0.01 | Oxidative Stress, ROS. | [48,49] |
| tr|Q9TC87|Cytochrome C Oxidase subunit 2. | Mitochondrion. | 510,963,333.33 | 158,239,801.04 | 61,080,000.00 | 105,793,663.33 | 0.04 | Oxidative Stress, ROS. | [48,49] |
| tr|E9FYW9|NADH Ubiquinone Oxidoreductase 75 kDa subunit. | Mitochondrion. | 317,076,666.67 | 69,335,851.00 | 121,610,000.00 | 18,139,666.48 | 0.01 | Multi-system disorders. Metal oxide nanomaterials, ROS. | [49,50] |
| tr|E9GPB6|NADH Dehydrogenase [Ubiquinone] flavoprotein 1. | Mitochondrion. | 239,323,333.33 | 51,992,494.01 | 101,170,000.00 | 3,340,119.76 | 0.02 | Multi-system disorders. Metal oxide nanomaterials, ROS. | [49,50] |
| tr|E9H0F0|ATP Synthase subunit gamma. | Mitochondrial proton-transporting ATP synthase complex. | 939,796,666.67 | 66,471,600.95 | 293,256,666.67 | 113,510,292.63 | 0.01 | Decreased ATP production. | [51] |
| tr|E9FYB8|Chitin-binding type-2. | Extracellular region. | 179,673,333.33 | 23,052,571.08 | 34,680,000.00 | 60,067,522.01 | 0.047 | Exoskeleton stability, stress to changes in temperature and food supply. | [37,39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norambuena, J.-A.; Poblete-Grant, P.; Beltrán, J.F.; De los Ríos-Escalante, P.; Aranzaez-Ríos, C.; Farías, J.G. Proteomic Profile of Daphnia pulex in Response to Heavy Metal Pollution in Lakes of Northern Patagonia. Int. J. Mol. Sci. 2025, 26, 417. https://doi.org/10.3390/ijms26010417
Norambuena J-A, Poblete-Grant P, Beltrán JF, De los Ríos-Escalante P, Aranzaez-Ríos C, Farías JG. Proteomic Profile of Daphnia pulex in Response to Heavy Metal Pollution in Lakes of Northern Patagonia. International Journal of Molecular Sciences. 2025; 26(1):417. https://doi.org/10.3390/ijms26010417
Chicago/Turabian StyleNorambuena, Juan-Alejandro, Patricia Poblete-Grant, Jorge F. Beltrán, Patricio De los Ríos-Escalante, Cristian Aranzaez-Ríos, and Jorge G. Farías. 2025. "Proteomic Profile of Daphnia pulex in Response to Heavy Metal Pollution in Lakes of Northern Patagonia" International Journal of Molecular Sciences 26, no. 1: 417. https://doi.org/10.3390/ijms26010417
APA StyleNorambuena, J.-A., Poblete-Grant, P., Beltrán, J. F., De los Ríos-Escalante, P., Aranzaez-Ríos, C., & Farías, J. G. (2025). Proteomic Profile of Daphnia pulex in Response to Heavy Metal Pollution in Lakes of Northern Patagonia. International Journal of Molecular Sciences, 26(1), 417. https://doi.org/10.3390/ijms26010417










