The Unexplored Role of Connexin Hemichannels in Promoting Facioscapulohumeral Muscular Dystrophy Progression
Abstract
1. Introduction
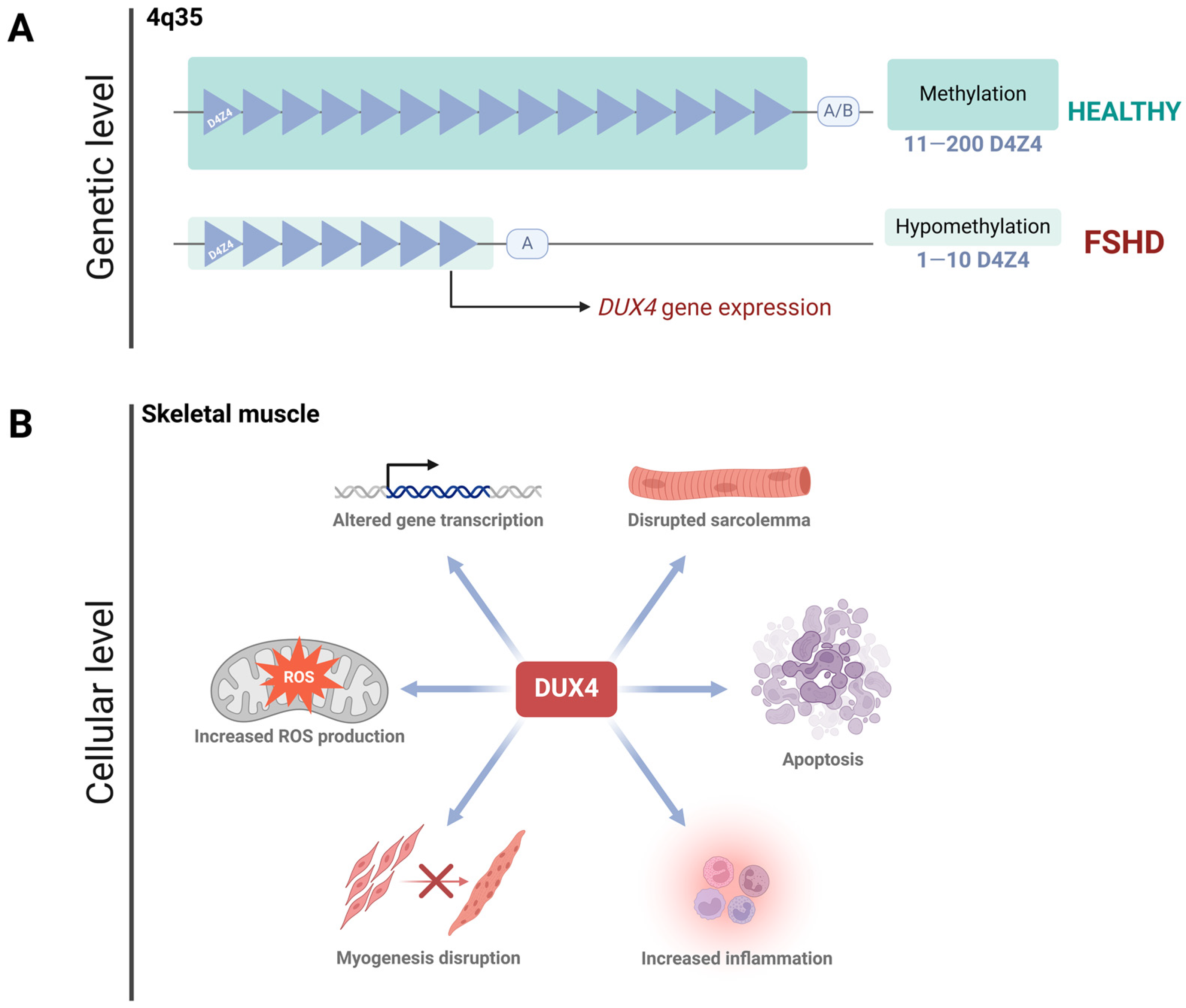
2. Facioscapulohumeral Muscular Dystrophy
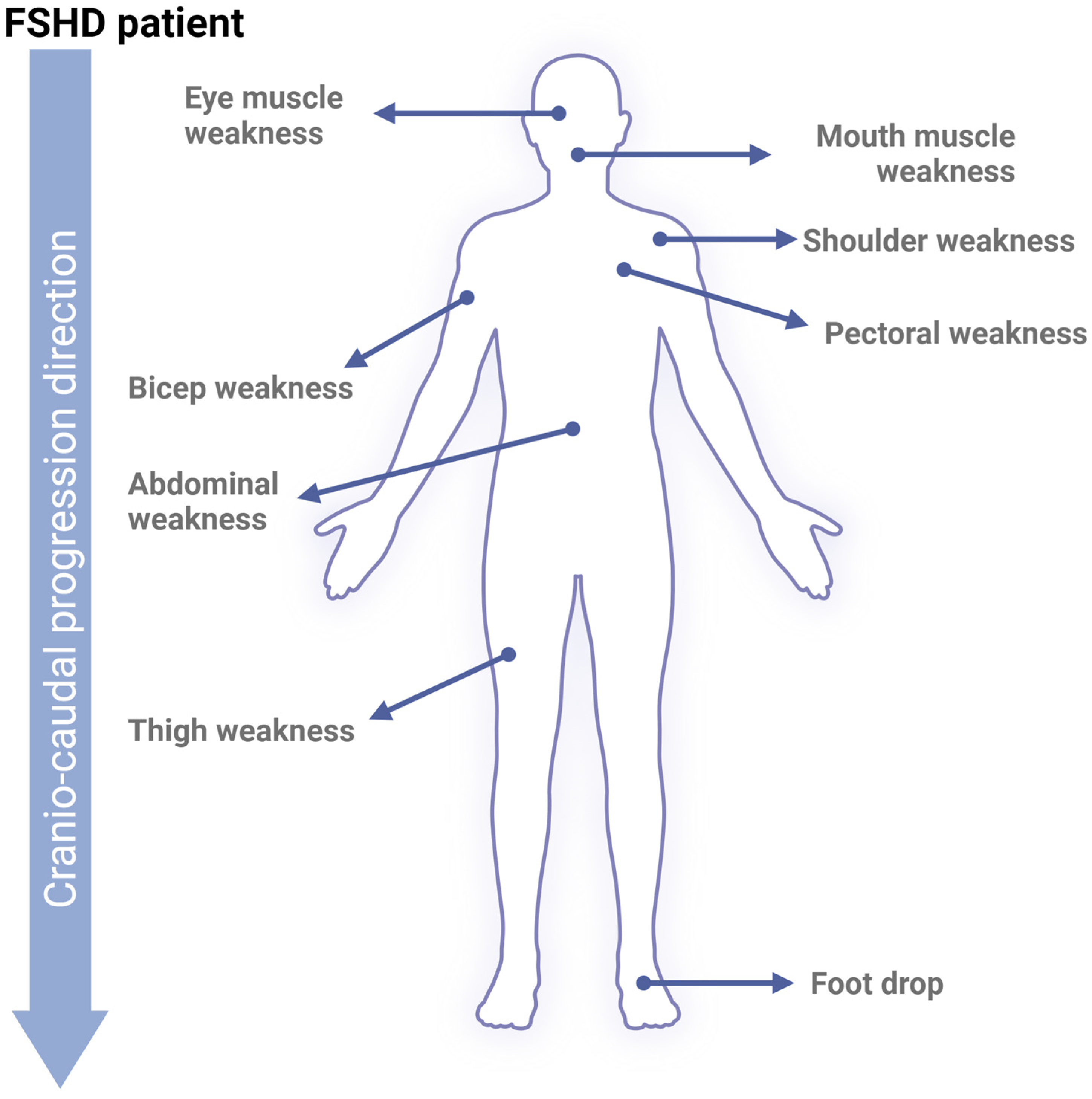
FSHD Progression
3. Connexin Hemichannels
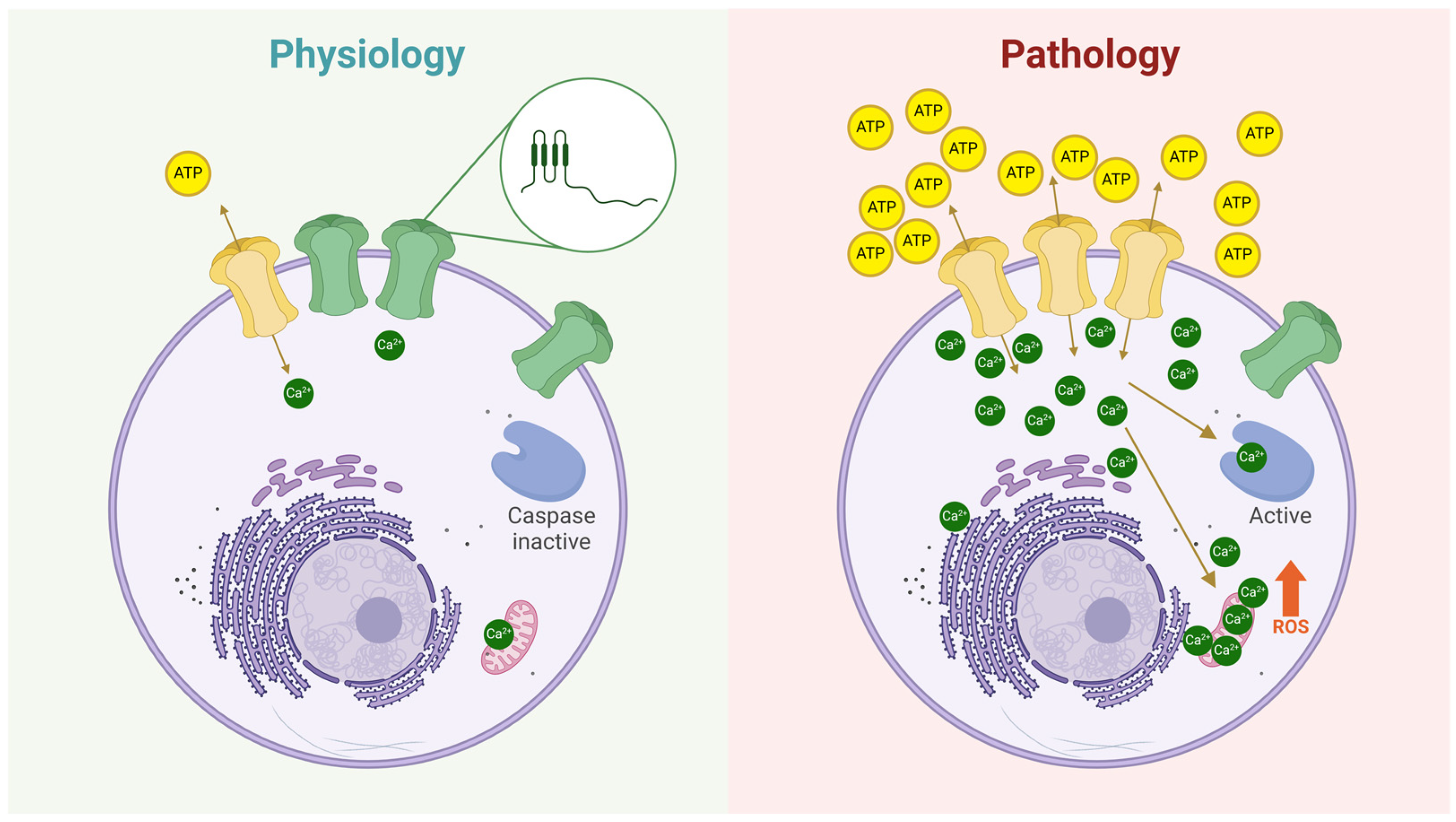
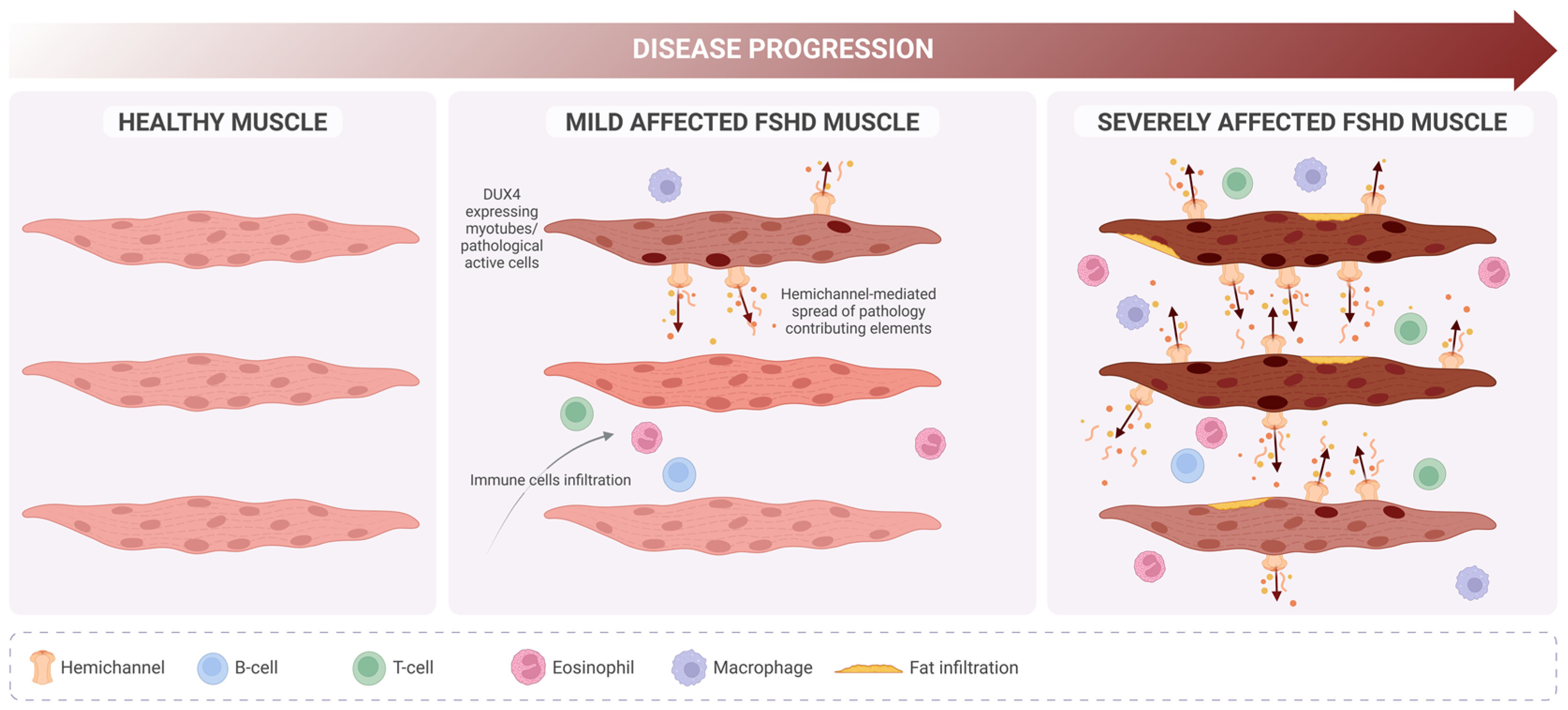
Hemichannels in Skeletal Muscle Atrophy
4. Targeting Hemichannels: A Novel Therapeutic Avenue for FSHD
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tawil, R.; McDermott, M.P.; Mendell, J.R.; Kissel, J.; Griggs, R.C.; FSH-DY Group. Facioscapulohumeral muscular dystrophy (FSHD): Design of natural history study and results of baseline testing. Neurology 1994, 44, 442. [Google Scholar] [CrossRef]
- Zampatti, S.; Colantoni, L.; Strafella, C.; Galota, R.M.; Caputo, V.; Campoli, G.; Pagliaroli, G.; Carboni, S.; Mela, J.; Peconi, C.; et al. Facioscapulohumeral muscular dystrophy (FSHD) molecular diagnosis: From traditional technology to the NGS era. Neurogenetics 2019, 20, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, S.; Salviati, L.; Desnuelle, C. Facioscapulohumeral muscular dystrophy. Biochim. Biophys. Acta 2015, 1852, 607–614. [Google Scholar] [CrossRef]
- Tawil, R.; Mah, J.K.; Baker, S.; Wagner, K.R.; Ryan, M.M.; Corbett, A.; McNamara, S.; van Engelen, B.; Rasko, J.; Raykar, V.; et al. Clinical practice considerations in facioscapulohumeral muscular dystrophy Sydney, Australia, 21 September 2015. Neuromuscul. Disord. 2016, 26, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Griggs, R.C.; Tawil, R.; McDermott, M.; Forrester, J.; Figlewicz, D.; Weiffenbach, B. Monozygotic twins with facioscapulohumeral dystrophy (FSHD): Implications for genotype/phenotype correlation. Muscle Nerve 1995, 18, S50–S55. [Google Scholar] [CrossRef]
- Tupler, R.; Barbierato, L.; Memmi, M.; Sewry, C.A.; De Grandis, D.; Maraschio, P.; Tiepolo, L.; Ferlini, A. Identical de novo mutation at the D4F104S1 locus in monozygotic male twins affected by facioscapulohumeral muscular dystrophy (FSHD) with different clinical expression. J. Med. Genet. 1998, 35, 778–783. [Google Scholar] [CrossRef]
- Banerji, C.R.S. PAX7 target gene repression associates with FSHD progression and pathology over 1 year. Hum. Mol. Genet. 2020, 29, 2124–2133. [Google Scholar] [CrossRef]
- Magdinier, F. Joining mainstream research on Facioscapulohumeral Dystophy: Disease prevalence in China. Lancet Reg. Health West. Pac. 2021, 18, 100328. [Google Scholar] [CrossRef]
- Tassin, A.; Laoudj-Chenivesse, D.; Vanderplanck, C.; Barro, M.; Charron, S.; Ansseau, E.; Chen, Y.W.; Mercier, J.; Coppée, F.; Belayew, A. DUX4 expression in FSHD muscle cells: How could such a rare protein cause a myopathy? J. Cell. Mol. Med. 2013, 17, 76–89. [Google Scholar] [CrossRef]
- Snider, L.; Geng, L.N.; Lemmers, R.J.L.F.; Kyba, M.; Ware, C.B.; Nelson, A.M.; Tawil, R.; Filippova, G.N.; van der Maarel, S.M.; Tapscott, S.J.; et al. Facioscapulohumeral dystrophy: Incomplete suppression of a retrotransposed gene. PLoS Genet. 2010, 6, e1001181. [Google Scholar] [CrossRef]
- Retamal, M.A.; Sáez, J.C. (Eds.) Hemichannels; From the Molecule to the Function; Frontiers Media SA: Lausanne, Switzerland, 2014; Volume 5. [Google Scholar] [CrossRef]
- Delvaeye, T.; Vandenabeele, P.; Bultynck, G.; Leybaert, L.; Krysko, D.V. Therapeutic Targeting of Connexin Channels: New Views and Challenges. Trends Mol. Med. 2018, 24, 1036–1053. [Google Scholar] [CrossRef]
- Sáez, J.C.; Retamal, M.A.; Basilio, D.; Bukauskas, F.F.; Bennett, M.V.L. Connexin-based gap junction hemichannels: Gating mechanisms. Biochim. Biophys. Acta-Biomembr. 2005, 1711, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Cea, L.A.; Puebla, C.; Cisterna, B.A.; Escamilla, R.; Vargas, A.A.; Frank, M.; Martínez-Montero, P.; Prior, C.; Molano, J.; Esteban-Rodríguez, I.; et al. Fast skeletal myofibers of mdx mouse, model of Duchenne muscular dystrophy, express connexin hemichannels that lead to apoptosis. Cell. Mol. Life Sci. 2016, 73, 2583–2599. [Google Scholar] [CrossRef]
- Cea, L.A.; Bevilacqua, J.A.; Arriagada, C.; Cárdenas, A.M.; Bigot, A.; Mouly, V.; Sáez, J.C.; Caviedes, P. The absence of dysferlin induces the expression of functional connexin-based hemichannels in human myotubes. BMC Cell Biol. 2016, 17 (Suppl. 1), 127–136. [Google Scholar] [CrossRef] [PubMed]
- Duranti, E.; Villa, C. Influence of DUX4 Expression in Facioscapulohumeral Muscular Dystrophy and Possible Treatments. Int. J. Mol. Sci. 2023, 24, 9503. [Google Scholar] [CrossRef]
- Teveroni, E.; Pellegrino, M.; Sacconi, S.; Calandra, P.; Cascino, I.; Farioli-Vecchioli, S.; Puma, A.; Garibaldi, M.; Morosetti, R.; Tasca, G.; et al. Estrogens enhance myoblast differentiation in facioscapulohumeral muscular dystrophy by antagonizing DUX4 activity. J. Clin. Investig. 2017, 127, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- Tonini, M.M.O.; Passos-Bueno, M.R.; Cerqueira, A.; Matioli, S.R.; Pavanello, R.; Zatz, M. Asymptomatic carriers and gender differences in facioscapulohumeral muscular dystrophy (FSHD). Neuromuscul. Disord. 2004, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Schätzl, T.; Kaiser, L.; Deigner, H.P. Facioscapulohumeral muscular dystrophy: Genetics, gene activation and downstream signalling with regard to recent therapeutic approaches: An update. Orphanet J. Rare Dis. 2021, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Van Deutekom, J.C.T.; Wljmenga, C.; Van Tlenhoven, E.A.E.; Gruter, A.M.; Hewitt, J.E.; Padberg, G.W.; van Ommen, G.J.B.; Hofker, M.H.; Fronts, R.R. FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit. Hum. Mol. Genet. 1993, 2, 2037–2042. [Google Scholar] [CrossRef]
- Lemmers, R.J.L.F.; Wohlgemuth, M.; Van Der Gaag, K.J.; Van Der Vliet, P.J.; Van Teijlingen, C.M.M.; De Knijff, P.; Padberg, G.W.; Frants, R.R.; Van Der Maarel, S.M. Specific sequence variations within the 4q35 region are associated with facioscapulohumeral muscular dystrophy. Am. J. Hum. Genet. 2007, 81, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Gatica, L.V.; Rosa, A.L. A complex interplay of genetic and epigenetic events leads to abnormal expression of the DUX4 gene in facioscapulohumeral muscular dystrophy. Neuromuscul. Disord. 2016, 26, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Van Overveld, P.G.M.; Lemmers, R.J.F.L.; Sandkuijl, L.A.; Enthoven, L.; Winokur, S.T.; Bakels, F.; Padberg, G.W.; Van Ommen, G.J.B.; Frants, R.R.; Van Der Maarel, S.M. Hypomethylation of D4Z4 in 4q-linked and non-4q-linked facioscapulohumeral muscular dystrophy. Nat. Genet. 2003, 35, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.M.; Garwick, S.E.; Mei, W.; Belayew, A.; Coppee, F.; Ladner, K.J.; Guttridge, D.; Yang, J.; Harper, S.Q. DUX4, a candidate gene for facioscapulohumeral muscular dystrophy, causes p53-dependent myopathy in vivo. Ann. Neurol. 2011, 69, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Corona, E.D.; Jacquelin, D.; Gatica, L.; Rosa, A.L. Multiple protein domains contribute to nuclear import and cell toxicity of DUX4, a candidate pathogenic protein for facioscapulohumeral muscular dystrophy. PLoS ONE 2013, 8, e75614. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.Q.; Nguyen, Q.; Yokota, T. DUX4 Signalling in the Pathogenesis of Facioscapulohumeral Muscular Dystrophy. Int. J. Mol. Sci. 2020, 21, 729. [Google Scholar] [CrossRef]
- Zeng, W.; De Greef, J.C.; Chen, Y.Y.; Chien, R.; Kong, X.; Gregson, H.C.; Winokur, S.T.; Pyle, A.; Robertson, K.D.; Schmiesing, J.A.; et al. Specific Loss of Histone H3 Lysine 9 Trimethylation and HP1γ/Cohesin Binding at D4Z4 Repeats Is Associated with Facioscapulohumeral Dystrophy (FSHD). PLoS Genet. 2009, 5, e1000559. [Google Scholar] [CrossRef] [PubMed]
- Cabianca, D.S.; Casa, V.; Bodega, B.; Xynos, A.; Ginelli, E.; Tanaka, Y.; Gabellini, D. A Long ncRNA Links Copy Number Variation to a Polycomb/Trithorax Epigenetic Switch in FSHD Muscular Dystrophy. Cell 2012, 149, 819. [Google Scholar] [CrossRef]
- Vizoso, M.; Esteller, M. The activatory long non-coding RNA DBE-T reveals the epigenetic etiology of facioscapulohumeral muscular dystrophy. Cell Res. 2012, 22, 1413–1415. [Google Scholar] [CrossRef]
- Himeda, C.L.; Jones, T.I.; Virbasius, C.M.; Zhu, L.J.; Green, M.R.; Jones, P.L. Identification of Epigenetic Regulators of DUX4-fl for Targeted Therapy of Facioscapulohumeral Muscular Dystrophy. Mol. Ther. 2018, 26, 1797. [Google Scholar] [CrossRef] [PubMed]
- Resnick, R.; Wong, C.J.; Hamm, D.C.; Bennett, S.R.; Skene, P.J.; Hake, S.B.; Henikoff, S.; van der Maarel, S.M.; Tapscott, S.J. DUX4-Induced Histone Variants H3.X and H3.Y Mark DUX4 Target Genes for Expression. Cell Rep. 2019, 29, 1812. [Google Scholar] [CrossRef] [PubMed]
- Kujirai, T.; Horikoshi, N.; Sato, K.; Maehara, K.; Machida, S.; Osakabe, A.; Kimura, H.; Ohkawa, Y.; Kurumizaka, H. Structure and function of human histone H3.Y nucleosome. Nucleic Acids Res. 2016, 44, 6127. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, S. The evolution of DUX4 gene regulation and its implication for facioscapulohumeral muscular dystrophy. Biochim. Biophys. Acta-Mol. Basis Dis. 2022, 1868, 166367. [Google Scholar] [CrossRef] [PubMed]
- De Iaco, A.; Planet, E.; Coluccio, A.; Verp, S.; Duc, J.; Trono, D. DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat. Genet. 2017, 49, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Vuoristo, S.; Bhagat, S.; Hydén-Granskog, C.; Yoshihara, M.; Gawriyski, L.; Jouhilahti, E.M.; Ranga, V.; Tamirat, M.; Huhtala, M.; Kirjanov, I.; et al. DUX4 is a multifunctional factor priming human embryonic genome activation. iScience 2022, 25, 104137. [Google Scholar] [CrossRef]
- Banerji, C.R.S.; Zammit, P.S. PAX7 target gene repression is a superior FSHD biomarker than DUX4 target gene activation, associating with pathological severity and identifying FSHD at the single-cell level. Hum. Mol. Genet. 2019, 28, 2224–2236. [Google Scholar] [CrossRef] [PubMed]
- Sasaki-Honda, M.; Jonouchi, T.; Arai, M.; Hotta, A.; Mitsuhashi, S.; Nishino, I.; Matsuda, R.; Sakurai, H. A patient-derived iPSC model revealed oxidative stress increases facioscapulohumeral muscular dystrophy-causative DUX4. Hum. Mol. Genet. 2018, 27, 4024–4035. [Google Scholar] [CrossRef] [PubMed]
- Kowaljow, V.; Marcowycz, A.; Ansseau, E.; Conde, C.B.; Sauvage, S.; Mattéotti, C.; Arias, C.; Corona, E.D.; Nuñez, N.G.; Leo, O.; et al. The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein. Neuromuscul. Disord. 2007, 17, 611–623. [Google Scholar] [CrossRef]
- Banerji, C.R.S.; Zammit, P.S. Pathomechanisms and biomarkers in facioscapulohumeral muscular dystrophy: Roles of DUX4 and PAX7. EMBO Mol. Med. 2021, 13, e13695. [Google Scholar] [CrossRef] [PubMed]
- Lassche, S.; Ottenheijm, C.A.C.; Voermans, N.C.; Westeneng, H.J.; Janssen, B.H.; van der Maarel, S.M.; Hopman, M.T.; Padberg, G.W.; Stienen, G.J.M.; van Engelen, B.G.M. Determining the role of sarcomeric proteins in facioscapulohumeral muscular dystrophy: A study protocol. BMC Neurol. 2013, 13, 144. [Google Scholar] [CrossRef]
- Zernov, N.; Skoblov, M. Genotype-phenotype correlations in FSHD. BMC Med. Genom. 2019, 12, 43. [Google Scholar] [CrossRef]
- Banerji, C.R.S.; Panamarova, M.; Pruller, J.; Figeac, N.; Hebaishi, H.; Fidanis, E.; Saxena, A.; Contet, J.; Sacconi, S.; Severini, S.; et al. Dynamic transcriptomic analysis reveals suppression of PGC1α/ERRα drives perturbed myogenesis in facioscapulohumeral muscular dystrophy. Hum. Mol. Genet. 2019, 28, 1244–1259. [Google Scholar] [CrossRef] [PubMed]
- Hiramuki, Y.; Kure, Y.; Saito, Y.; Ogawa, M.; Ishikawa, K.; Mori-Yoshimura, M.; Oya, Y.; Takahashi, Y.; Kim, D.S.; Arai, N.; et al. Simultaneous measurement of the size and methylation of chromosome 4qA-D4Z4 repeats in facioscapulohumeral muscular dystrophy by long-read sequencing. J. Transl. Med. 2022, 20, 517. [Google Scholar] [CrossRef]
- Gould, T.; Jones, T.I.; Jones, P.L. Precise Epigenetic Analysis Using Targeted Bisulfite Genomic Sequencing Distinguishes FSHD1, FSHD2, and Healthy Subjects. Diagnostics 2021, 11, 1469. [Google Scholar] [CrossRef] [PubMed]
- Balog, J.; Thijssen, P.E.; Shadle, S.; Straasheijm, K.R.; van der Vliet, P.J.; Krom, Y.D.; van den Boogaard, M.L.; de Jong, A.; F Lemmers, R.J.L.; Tawil, R.; et al. Increased DUX4 expression during muscle differentiation correlates with decreased SMCHD1 protein levels at D4Z4. Epigenetics 2015, 10, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Nykänen, S.; Vuoristo, S. DUX4, the rockstar of embryonic genome activation? Int. J. Dev. Biol. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Ferreboeuf, M.; Mariot, V.; Furling, D.; Butler-Browne, G.; Mouly, V.; Dumonceaux, J. Nuclear protein spreading: Implication for pathophysiology of neuromuscular diseases. Hum. Mol. Genet. 2014, 23, 4125–4133. [Google Scholar] [CrossRef]
- Rickard, A.M.; Petek, L.M.; Miller, D.G. Endogenous DUX4 expression in FSHD myotubes is sufficient to cause cell death and disrupts RNA splicing and cell migration pathways. Hum. Mol. Genet. 2015, 24, 5901–5914. [Google Scholar] [CrossRef]
- Hangul, C.; Celik, E.G.; Kaya, H.; Eroglu, O.; Uysal, H.; Karauzum, S.B. Estradiol differentially regulates DUX4, β-catenin and PAX3/PAX7 in primary myoblasts of facioscapulohumeral muscular dystrophy patients. Turk. J. Biochem. 2021, 46, 435–444. [Google Scholar] [CrossRef]
- Hangül, C.; Bozkurt, S.; Bilge, U.; Özdem, S.; Altunbaş, H.; Uysal, H.; Koç, F.; Karaüzüm, S.B. The ratios of estradiol and progesterone to testosterone influence the severity of facioscapulohumeral muscular dystrophy. Neurol. Sci. Neurophysiol. 2020, 37, 190–196. [Google Scholar] [CrossRef]
- Banerji, C.R.S.; Panamarova, M.; Zammit, P.S. DUX4 expressing immortalized FSHD lymphoblastoid cells express genes elevated in FSHD muscle biopsies, correlating with the early stages of inflammation. Hum. Mol. Genet. 2020, 29, 2285–2299. [Google Scholar] [CrossRef]
- Hauerslev, S.; Ørngreen, M.C.; Hertz, J.M.; Vissing, J.; Krag, T.O. Muscle regeneration and inflammation in patients with facioscapulohumeral muscular dystrophy. Acta Neurol. Scand. 2013, 128, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, J.R.; Andersen, G.; Khawajazada, T.; Vissing, C.; Thomsen, C.; Vissing, J. Relationship between muscle inflammation and fat replacement assessed by MRI in facioscapulohumeral muscular dystrophy. J. Neurol. 2019, 266, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Arahata, K.; Ishihara, T.; Fukunaga, H.; Orimo, S.; Lee, J.H.; Goto, K.; Nonaka, I. Inflammatory response in facioscapulohumeral muscular dystrophy (FSHD): Immunocytochemical and genetic analyses. Muscle Nerve 1995, 18, S56–S66. [Google Scholar] [CrossRef]
- van den Heuvel, A.; Lassche, S.; Mul, K.; Greco, A.; San León Granado, D.; Heerschap, A.; Küsters, B.; Tapscott, S.J.; Voermans, N.C.; van Engelen, B.G.M.; et al. Facioscapulohumeral dystrophy transcriptome signatures correlate with different stages of disease and are marked by different MRI biomarkers. Sci. Rep. 2022, 12, 1426. [Google Scholar] [CrossRef]
- Friedman, S.D.; Poliachik, S.L.; Carter, G.T.; Budech, C.B.; Bird, T.D.; Shaw, D.W.W. The magnetic resonance imaging spectrum of facioscapulohumeral muscular dystrophy. Muscle Nerve 2012, 45, 500–506. [Google Scholar] [CrossRef]
- Dahlqvist, J.R.; Poulsen, N.S.; Østergaard, S.T.; Fornander, F.; De Stricker Borch, J.; Danielsen, E.R.; Thomsen, C.; Vissing, J. Evaluation of inflammatory lesions over 2 years in facioscapulohumeral muscular dystrophy. Neurology 2020, 95, E1211–E1221. [Google Scholar] [CrossRef] [PubMed]
- Frisullo, G.; Frusciante, R.; Nociti, V.; Tasca, G.; Renna, R.; Iorio, R.; Patanella, A.K.; Iannaccone, E.; Marti, A.; Rossi, M.; et al. CD8+ T cells in facioscapulohumeral muscular dystrophy patients with inflammatory features at muscle MRI. J. Clin. Immunol. 2011, 31, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Nunes, A.M.; Daoudlarian, D.; Pini, J.; Martinuzzi, E.; Barbosa, S.; Ramirez, M.; Puma, A.; Villa, L.; Cavalli, M.; et al. Identification of Serum Interleukin 6 Levels as a Disease Severity Biomarker in Facioscapulohumeral Muscular Dystrophy. J. Neuromuscul. Dis. 2022, 9, 83–93. [Google Scholar] [CrossRef]
- Wosiski-Kuhn, M.; Caress, J.B.; Cartwright, M.S.; Hawkins, G.A.; Milligan, C. Interleukin 6 (IL6) level is a biomarker for functional disease progression within IL6R358Ala variant groups in amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, P.; Bou Saada, Y.; Dib, C.; Ansseau, E.; Barat, A.; Hamade, A.; Dessen, P.; Robert, T.; Lazar, V.; Louzada, R.A.N.; et al. DUX4-induced constitutive DNA damage and oxidative stress contribute to aberrant differentiation of myoblasts from FSHD patients. Free Radic. Biol. Med. 2016, 99, 244–258. [Google Scholar] [CrossRef]
- Passerieux, E.; Hayot, M.; Jaussent, A.; Carnac, G.; Gouzi, F.; Pillard, F.; Picot, M.C.; Böcker, K.; Hugon, G.; Pincemail, J.; et al. Effects of vitamin C, vitamin E, zinc gluconate, and selenomethionine supplementation on muscle function and oxidative stress biomarkers in patients with facioscapulohumeral dystrophy: A double-blind randomized controlled clinical trial. Free Radic. Biol. Med. 2015, 81, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Söhl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef]
- Maeda, S.; Nakagawa, S.; Suga, M.; Yamashita, E.; Oshima, A.; Fujiyoshi, Y.; Tsukihara, T. Structure of the connexin 26 gap junction channel at 3.5 Å resolution. Nature 2009, 458, 597–602. [Google Scholar] [CrossRef]
- Koval, M. Pathways and control of connexin oligomerization. Trends Cell Biol. 2006, 16, 159–166. [Google Scholar] [CrossRef]
- Thomas, T.; Jordan, K.; Simek, J.; Shao, Q.; Jedeszko, C.; Walton, P.; Laird, D.W. Mechanisms of Cx43 and Cx26 transport to the plasma membrane and gap junction regeneration. J. Cell Sci. 2005, 118, 4451–4462. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.-J.; Liu, X.-Z.; Tu, L.; Sun, Y. Cytomembrane Trafficking Pathways of Connexin 26, 30, and 43. Int. J. Mol. Sci. 2023, 24, 10349. [Google Scholar] [CrossRef]
- Li, F.; Sugishita, K.; Su, Z.; Ueda, I.; Barry, W.H. Activation of connexin-43 hemichannels can elevate [Ca2+]i and [Na+]i in rabbit ventricular myocytes during metabolic inhibition. J. Mol. Cell. Cardiol. 2001, 33, 2145–2155. [Google Scholar] [CrossRef]
- Schalper, K.A.; Sánchez, H.A.; Lee, S.C.; Altenberg, G.A.; Nathanson, M.H.; Sáez, J.C. Connexin 43 hemichannels mediate the Ca2+ influx induced by extracellular alkalinization. Am. J. Physiol. Cell Physiol. 2010, 299. [Google Scholar] [CrossRef] [PubMed]
- Fiori, M.C.; Figueroa, V.; Zoghbi, M.E.; Saéz, J.C.; Reuss, L.; Altenberg, G.A. Permeation of calcium through purified connexin 26 hemichannels. J. Biol. Chem. 2012, 287, 40826–40834. [Google Scholar] [CrossRef] [PubMed]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.G.; Charles, A.C. Intercellular Calcium Signaling in Astrocytes via ATP Release through Connexin Hemichannels. J. Biol. Chem. 2002, 277, 10482–10488. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Riquelme, M.A.; Gu, S.; Jiang, J.X. Connexin hemichannels mediate glutathione transport and protect lens fiber cells from oxidative stress. J. Cell Sci. 2018, 131, jcs212506. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.-C.; Wyeth, M.S.; Baltan-Tekkok, S.; Ransom, B.R. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J. Neurosci. 2003, 23, 3588–3596. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A.; Froger, N.; Palacios-Prado, N.; Ezan, P.; Saez, P.J.; Saez, J.C.; Giaume, C. Cx43 Hemichannels and Gap Junction Channels in Astrocytes Are Regulated Oppositely by Proinflammatory Cytokines Released from Activated Microglia. J. Neurosci. 2007, 27, 13781–13792. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.E.; Saez, J.C.; Bukauskas, F.F.; Bennett, M.V.L. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA 2003, 100, 11388–11393. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.T.; Poudyal, N.; Verdugo, D.A.; Peña, F.; Stehberg, J.; Retamal, M.A. KI04 an Aminoglycosides-Derived Molecule Acts as an Inhibitor of Human Connexin46 Hemichannels Expressed in HeLa Cells. Biomolecules 2023, 13, 411. [Google Scholar] [CrossRef]
- Gaete, P.S.; Kumar, D.; Fernandez, C.I.; Valdez Capuccino, J.M.; Bhatt, A.; Jiang, W.; Lin, Y.-C.; Liu, Y.; Harris, A.L.; Luo, Y.L.; et al. Large-pore connexin hemichannels function like molecule transporters independent of ion conduction. Proc. Natl. Acad. Sci. USA 2024, 121, e2403903121. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A.; Reyes, E.P.; García, I.E.; Pinto, B.; Martínez, A.D.; González, C. Diseases associated with leaky hemichannels. Front. Cell. Neurosci. 2015, 9, 267. [Google Scholar] [CrossRef]
- Liang, G.S.L.L.; De Miguel, M.; Gómez-Hernández, J.M.; Glass, J.D.; Scherer, S.S.; Mintz, M.; Barrio, L.C.; Fischbeck, K.H. Severe neuropathy with leaky connexin32 hemichannels. Ann. Neurol. 2005, 57, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, H.A.; Meşe, G.; Srinivas, M.; White, T.W.; Verselis, V.K. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J. Gen. Physiol. 2010, 136, 47–62. [Google Scholar] [CrossRef]
- Saez, J.; Berthoud, V.; Branes, M.; Martinez, A.; Beyer, E. Plasma membrane channels formed by connexins: Their regulation and functions. Physiol. Rev. 2003, 83, 1359–1400. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sinovas, A.; Sánchez, J.A.; Valls-Lacalle, L.; Consegal, M.; Ferreira-González, I. Connexins in the Heart: Regulation, Function and Involvement in Cardiac Disease. Int. J. Mol. Sci. 2021, 22, 4413. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Rohrbach, S.; Weissmann, N.; Schulz, R. Importance of Cx43 for Right Ventricular Function. Int. J. Mol. Sci. 2021, 22, 987. [Google Scholar] [CrossRef] [PubMed]
- Lucero, C.M.; Andrade, D.C.; Toledo, C.; Díaz, H.S.; Pereyra, K.V.; Diaz-Jara, E.; Schwarz, K.G.; Marcus, N.J.; Retamal, M.A.; Quintanilla, R.A.; et al. Cardiac remodeling and arrhythmogenesis are ameliorated by administration of Cx43 mimetic peptide Gap27 in heart failure rats. Sci. Rep. 2020, 10, 6878. [Google Scholar] [CrossRef]
- Andrade, D.C.; Iturriaga, R.; Toledo, C.; Lucero, C.M.; Díaz, H.S.; Arce-Álvarez, A.; Retamal, M.A.; Marcus, N.J.; Alcayaga, J.; Del Rio, R. Topical Application of Connexin43 Hemichannel Blocker Reduces Carotid Body-Mediated Chemoreflex Drive in Rats. Adv. Exp. Med. Biol. 2018, 1071, 61–68. [Google Scholar] [CrossRef]
- Leybaert, L.; De Smet, M.A.J.; Lissoni, A.; Allewaert, R.; Roderick, H.L.; Bultynck, G.; Delmar, M.; Sipido, K.R.; Witschas, K. Connexin hemichannels as candidate targets for cardioprotective and anti-arrhythmic treatments. J. Clin. Investig. 2023, 133, 6. [Google Scholar] [CrossRef] [PubMed]
- Araya, R.; Eckardt, D.; Riquelme, M.A.; Willecke, K.; Sáez, J.C. Presence and importance of connexin43 during myogenesis. Cell Commun. Adhes. 2003, 10, 451–456. [Google Scholar] [CrossRef]
- Merrifield, P.A.; Laird, D.W. Connexins in skeletal muscle development and disease. Semin. Cell Dev. Biol. 2016, 50, 67–73. [Google Scholar] [CrossRef]
- Cea, L.A.; Riquelme, M.A.; Cisterna, B.A.; Puebla, C.; Vega, J.L.; Rovegno, M.; Sáez, J.C. Connexin- and pannexin-based channels in normal skeletal muscles and their possible role in muscle atrophy. J. Membr. Biol. 2012, 245, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.C.; Cisterna, B.A.; Vargas, A.; Cardozo, C.P. Regulation of pannexin and connexin channels and their functional role in skeletal muscles. Cell. Mol. Life Sci. 2015, 72, 2929–2935. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W. Life cycle of connexins in health and disease. Biochem. J. 2006, 394, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Cea, L.A.; Balboa, E.; Puebla, C.; Vargas, A.A.; Cisterna, B.A.; Escamilla, R.; Regueira, T.; Sáez, J.C. Dexamethasone-induced muscular atrophy is mediated by functional expression of connexin-based hemichannels. Biochim. Biophys. Acta 2016, 1862, 1891–1899. [Google Scholar] [CrossRef]
- Cisterna, B.A.; Vargas, A.A.; Puebla, C.; Sáez, J.C. Connexin hemichannels explain the ionic imbalance and lead to atrophy in denervated skeletal muscles. Biochim. Biophys. Acta 2016, 1862, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Cea, L.A.; Cisterna, B.A.; Puebla, C.; Frank, M.; Figueroa, X.F.; Cardozo, C.; Willecke, K.; Latorre, R.; Sáez, J.C. De novo expression of connexin hemichannels in denervated fast skeletal muscles leads to atrophy. Proc. Natl. Acad. Sci. USA 2013, 110, 16229–16234. [Google Scholar] [CrossRef] [PubMed]
- Cea, L.A.; Balboa, E.; Vargas, A.A.; Puebla, C.; Brañes, M.C.; Escamilla, R.; Regueira, T.; Sáez, J.C. De novo expression of functional connexins 43 and 45 hemichannels increases sarcolemmal permeability of skeletal myofibers during endotoxemia. Biochim. Biophys. Acta-Mol. Basis Dis. 2019, 1865, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.; Saavedra, F.; Cea, L.A.; Ramírez, V.; Escamilla, R.; Vargas, A.A.; Regueira, T.; Sáez, J.C. Vitamin E Blocks Connexin Hemichannels and Prevents Deleterious Effects of Glucocorticoid Treatment on Skeletal Muscles. Int. J. Mol. Sci. 2020, 21, 4094. [Google Scholar] [CrossRef] [PubMed]
- Willebrords, J.; Crespo Yanguas, S.; Maes, M.; Decrock, E.; Wang, N.; Leybaert, L.; Kwak, B.R.; Green, C.R.; Cogliati, B.; Vinken, M. Connexins and their channels in inflammation. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 413–439. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; Wang, H.Q.; Zhang, N.N.; Li, X.M.; Yang, X.Y.; Chen, A.P.; Yan, X.; Zhang, Z.; Chu, S.F.; et al. Inflammation and Connexin 43 profiles in the prefrontal cortex are relevant to stress susceptibility and resilience in mice. Pharmacol. Biochem. Behav. 2024, 239, 173757. [Google Scholar] [CrossRef]
- Sáez, J.C.; Contreras-Duarte, S.; Gómez, G.I.; Labra, V.C.; Santibañez, C.A.; Gajardo-Gómez, R.; Avendaño, B.C.; Díaz, E.F.; Montero, T.D.; Velarde, V.; et al. Connexin 43 Hemichannel Activity Promoted by Pro-Inflammatory Cytokines and High Glucose Alters Endothelial Cell Function. Front. Immunol. 2018, 9, 1899. [Google Scholar] [CrossRef]
- Chanson, M.; Berclaz, P.-Y.; Scerri, I.; Dudez, T.; Wernke-Dollries, K.; Pizurki, L.; Pavirani, A.; Fiedler, M.A.; Suter, S. Regulation of Gap Junctional Communication by a Pro-Inflammatory Cytokine in Cystic Fibrosis Transmembrane Conductance Regulator-Expressing but Not Cystic Fibrosis Airway Cells. Am. J. Pathol. 2001, 158, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A.; Cortés, C.J.; Reuss, L.; Bennett, M.V.L.; Sáez, J.C. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: Induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. USA 2006, 103, 4475–4480. [Google Scholar] [CrossRef] [PubMed]
- Lucero, C.M.; Prieto-Villalobos, J.; Marambio-Ruiz, L.; Balmazabal, J.; Alvear, T.F.; Vega, M.; Barra, P.; Retamal, M.A.; Orellana, J.A.; Gómez, G.I. Hypertensive Nephropathy: Unveiling the Possible Involvement of Hemichannels and Pannexons. Int. J. Mol. Sci. 2022, 23, 15936. [Google Scholar] [CrossRef] [PubMed]
- León-Paravic, C.G.; Figueroa, V.A.; Guzmán, D.J.; Valderrama, C.F.; Vallejos, A.A.; Fiori, M.C.; Altenberg, G.A.; Reuss, L.; Retamal, M.A. Carbon monoxide (CO) is a novel inhibitor of connexin hemichannels. J. Biol. Chem. 2014, 289, 36150–36157. [Google Scholar] [CrossRef] [PubMed]
- Sirago, G.; Candia, J.; Franchi, M.V.; Sarto, F.; Monti, E.; Toniolo, L.; Reggiani, C.; Giacomello, E.; Zampieri, S.; Hartnell, L.M.; et al. Upregulation of Sarcolemmal Hemichannels and Inflammatory Transcripts with Neuromuscular Junction Instability during Lower Limb Unloading in Humans. Biology 2023, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Himeda, C.L.; Jones, P.L. FSHD Therapeutic Strategies: What Will It Take to Get to Clinic? J. Pers. Med. 2022, 12, 865. [Google Scholar] [CrossRef]
- Wang, L.H.; Tawil, R. Current Therapeutic Approaches in FSHD. J. Neuromuscul. Dis. 2021, 8, 441–451. [Google Scholar] [CrossRef]
- Chen, T.H.; Wu, Y.Z.; Tseng, Y.H. Early-Onset Infantile Facioscapulohumeral Muscular Dystrophy: A Timely Review. Int. J. Mol. Sci. 2020, 21, 7783. [Google Scholar] [CrossRef]
- DeSimone, A.M.; Cohen, J.; Lek, M.; Lek, A. Cellular and animal models for facioscapulohumeral muscular dystrophy. Dis. Model. Mech. 2020, 13, dmm046904. [Google Scholar] [CrossRef]
- Statland, J.; Donlin-Smith, C.M.; Tapscott, S.J.; Van Der Maarel, S.M.; Tawil, R. Multiplex Screen of Serum Biomarkers in Facioscapulohumeral Muscular Dystrophy. J. Neuromuscul. Dis. 2014, 1, 181–190. [Google Scholar] [CrossRef]
- Fernández, G.; Arias-Bravo, G.; Bevilacqua, J.A.; Castillo-Ruiz, M.; Caviedes, P.; Sáez, J.C.; Cea, L.A. Myofibers deficient in connexins 43 and 45 expression protect mice from skeletal muscle and systemic dysfunction promoted by a dysferlin mutation. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165800. [Google Scholar] [CrossRef]
- Hernández-Salinas, R.; Vielma, A.Z.; Arismendi, M.N.; Boric, M.P.; Sáez, J.C.; Velarde, V. Boldine prevents renal alterations in diabetic rats. J. Diabetes Res. 2013, 2013, 165800. [Google Scholar] [CrossRef]
- Yi, C.; Ezan, P.; Fernández, P.; Schmitt, J.; Sáez, J.C.; Giaume, C.; Koulakoff, A. Inhibition of glial hemichannels by boldine treatment reduces neuronal suffering in a murine model of Alzheimer’s disease. Glia 2017, 65, 1607–1625. [Google Scholar] [CrossRef]
- Sáez, J.C.; Burrell, J.C.; Cahill, C.M.; Cullen, D.K.; Devi, L.A.; Gilbert, R.J.; Graham, Z.A.; Gurvich, V.J.; Havton, L.A.; Iyengar, R.; et al. Pharmacology of boldine: Summary of the field and update on recent advances. Front. Pharmacol. 2024, 15, 1427147. [Google Scholar] [CrossRef] [PubMed]
- Cea, L.A.; Fernández, G.; Arias-Bravo, G.; Castillo-Ruiz, M.; Escamilla, R.; Brañes, M.C.; Sáez, J.C. Blockade of Hemichannels Normalizes the Differentiation Fate of Myoblasts and Features of Skeletal Muscles from Dysferlin-Deficient Mice. Int. J. Mol. Sci. 2020, 21, 6025. [Google Scholar] [CrossRef] [PubMed]
- Cea, L.A.; Vásquez, W.; Hernández-Salinas, R.; Vielma, A.Z.; Castillo-Ruiz, M.; Velarde, V.; Salgado, M.; Sáez, J.C. Skeletal Muscle Atrophy Induced by Diabetes Is Mediated by Non-Selective Channels and Prevented by Boldine. Biomolecules 2023, 13, 708. [Google Scholar] [CrossRef]
- Burrell, J.C.; Vu, P.T.; Alcott, O.J.B.; Toro, C.A.; Cardozo, C.; Cullen, D.K. Orally administered boldine reduces muscle atrophy and promotes neuromuscular recovery in a rodent model of delayed nerve repair. Front. Cell. Neurosci. 2023, 17, 1240916. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.A.; Toro, C.A.; Harlow, L.; Lavin, K.M.; Cardozo, C.P.; Wende, A.R.; Graham, Z.A. Assessing the impact of boldine on the gastrocnemius using multiomics profiling at 7 and 28 days post-complete spinal cord injury in young male mice. Physiol. Genom. 2023, 55, 297–313. [Google Scholar] [CrossRef]
- Riquelme, M.A.; Kar, R.; Gu, S.; Jiang, J.X. Antibodies targeting extracellular domain of connexins for studies of hemichannels. Neuropharmacology 2013, 75, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Walrave, L.; Pierre, A.; Albertini, G.; Aourz, N.; De Bundel, D.; Van Eeckhaut, A.; Vinken, M.; Giaume, C.; Leybaert, L.; Smolders, I. Inhibition of astroglial connexin43 hemichannels with TAT-Gap19 exerts anticonvulsant effects in rodents. Glia 2018, 66, 1788–1804. [Google Scholar] [CrossRef] [PubMed]
- Linsambarth, S.; Carvajal, F.J.; Moraga-Amaro, R.; Mendez, L.; Tamburini, G.; Jimenez, I.; Verdugo, D.A.; Gómez, G.I.; Jury, N.; Martínez, P.; et al. Astroglial gliotransmitters released via Cx43 hemichannels regulate NMDAR-dependent transmission and short-term fear memory in the basolateral amygdala. FASEB J. 2022, 36, e22134. [Google Scholar] [CrossRef]
- Chen, C.-H.; Mayo, J.N.; Gourdie, R.G.; Johnstone, S.R.; Isakson, B.E.; Bearden, S.E. The connexin 43/ZO-1 complex regulates cerebral endothelial F-actin architecture and migration. Am. J. Physiol. Physiol. 2015, 309, C600–C607. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.; Ghatnekar, G.S.; Grek, C.L.; Moyer, K.E.; Gourdie, R.G. Connexin 43-based therapeutics for dermal wound healing. Int. J. Mol. Sci. 2018, 19, 1778. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Ghatnekar, G.; Gourdie, R.G.; Potts, J.D. Impact of the controlled release of a connexin 43 peptide on corneal wound closure in an STZ model of type I diabetes. PLoS ONE 2014, 9, e86570. [Google Scholar] [CrossRef]
- Che, J.; DePalma, T.J.; Sivakumar, H.; Mezache, L.S.; Tallman, M.M.; Venere, M.; Swindle-Reilly, K.; Veeraraghavan, R.; Skardal, A. αCT1 peptide sensitizes glioma cells to temozolomide in a glioblastoma organoid platform. Biotechnol. Bioeng. 2023, 120, 1108–1119. [Google Scholar] [CrossRef]
- Tardif, J.C. Antioxidants: The good, the bad and the ugly. Can. J. Cardiol. 2006, 22 (Suppl. B), 61B–65B. [Google Scholar] [CrossRef] [PubMed]
- Tyuryaeva, I.; Lyublinskaya, O. Expected and Unexpected Effects of Pharmacological Antioxidants. Int. J. Mol. Sci. 2023, 24, 9303. [Google Scholar] [CrossRef]
- Lamers, C. Overcoming the shortcomings of peptide-based therapeutics. Future Drug Discov. 2022, 4, FDD75. [Google Scholar] [CrossRef]
- Gemel, J.; Kilkus, J.; Dawson, G.; Beyer, E.C. Connecting Exosomes and Connexins. Cancers 2019, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Catoe, H.; Werner, R. MIR-206 regulates connexin43 expression during skeletal muscle development. Nucleic Acids Res. 2006, 34, 5863–5871. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.M.; Ramirez, M.; Jones, T.I.; Jones, P.L. Identification of candidate miRNA biomarkers for facioscapulohumeral muscular dystrophy using DUX4-based mouse models. Dis. Model. Mech. 2021, 14, dmm049016. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Ubilla, M.; Retamal, M.A. The Unexplored Role of Connexin Hemichannels in Promoting Facioscapulohumeral Muscular Dystrophy Progression. Int. J. Mol. Sci. 2025, 26, 373. https://doi.org/10.3390/ijms26010373
Díaz-Ubilla M, Retamal MA. The Unexplored Role of Connexin Hemichannels in Promoting Facioscapulohumeral Muscular Dystrophy Progression. International Journal of Molecular Sciences. 2025; 26(1):373. https://doi.org/10.3390/ijms26010373
Chicago/Turabian StyleDíaz-Ubilla, Macarena, and Mauricio A. Retamal. 2025. "The Unexplored Role of Connexin Hemichannels in Promoting Facioscapulohumeral Muscular Dystrophy Progression" International Journal of Molecular Sciences 26, no. 1: 373. https://doi.org/10.3390/ijms26010373
APA StyleDíaz-Ubilla, M., & Retamal, M. A. (2025). The Unexplored Role of Connexin Hemichannels in Promoting Facioscapulohumeral Muscular Dystrophy Progression. International Journal of Molecular Sciences, 26(1), 373. https://doi.org/10.3390/ijms26010373






