The Potential of Naturally Derived Compounds for Treating Chronic Kidney Disease: A Review of Autophagy and Cellular Senescence
Abstract
1. Traditional Chinese Medicine in CKD: Aging and Senescence Focus
2. Aging and Cellular Senescence
3. The Role of SASP in Aging, Autophagy, and Podocyte
4. The Role of the Gut Microbiome in Longevity
5. Natural Products Used in TCM for CKD
6. Advancing CKD Treatment: Insights into Natural Products, TCM, and Personalized Therapies
| Name (with Active Component) | Common Name | The Common Usage in CKD | Description/ Usefulness | Drawback/ Limitation | Targeting Molecule/ Signaling Pathway |
|---|---|---|---|---|---|
| Turmeric (Curcumin) | - | -diabetic nephropathy | -demonstrated promising renoprotective [49,122,133] ability against nephrotoxicity [73,134] and renal injury [135] -reduces proteinuria in lupus nephritis [136,137] -enhances autophagy of podocytes [122] -significant antioxidant protective effect on renal ischemia-reperfusion injury [138] | -poor adsorption, which affected its efficacy [122] but is not a significant concern [139] | -Transforming growth factor B and interleukin-8 [140] -NF-KB and activation of the JAK2/STAT3 signaling pathway [141] -Nrf2 [49] |
| Resveratrol (polyphenolic) | -diabetic nephropathy | -renoprotection from Adriamycin induced-FSGS [142] -ameliorates podocyte damage [143], the potential treatment approach for diabetic nephropathy patients [144] -Resveratrol’s renoprotective effects work by an activated mechanism to inhibit oxidative stress [145] and apoptosis of mitochondria [143] and podocyte [144,146] | -poor solubility and limited bioavailability [147] -molecular mechanism [144], pharmacokinetic, and pharmacodynamics need further studies [148] | -C3aR and C5aR [142] -Nrf2 activation [145] -sirtuin 1 (sirt 1) activation [145,149] -angiotensin type 1 receptor and NF-κB [149] -downregulates malondialdehyde and inhibit reactive oxygen species (ROS) [143] -5′ adenosine monophosphate-activated protein kinase (AMPK) [144] | |
| Astragalus membranaceus or Astragalus mongholicus (Astragaloside IV) | Huáng Qí | -effective adjuvant therapy used in membranous nephropathy [61] -alternative therapy for the frequent-relapse nephrotic syndrome [66] | -attenuation of podocyte injury [61] -most essential herbs to treat proteinuria [66] -ability to restore actin cytoskeleton in podocytes [61] -reduce chemotherapy toxicity [150], such as cyclophosphamide-induce toxicity [66,151,152] | -side effects are not well understood because they are generally used in combination with other herbs [5] -lack of molecular studies | -reduction in phosphorylation of JNK and ERK and their signaling pathway [61] |
| Coptis chinensis (Berberine) | Huang Lian/ Coptis rhizome | -commonly used to treat diabetes | -berberine exhibits renoprotective effects against various podocyte injury agents [153,154,155,156,157,158] | -lack of studies | -inhibition of RhoA/ROCK signaling was reported -EP4-Gαs-cAMP signaling pathway as significant renoprotective effects of berberine [82] -NF-κB signaling pathway [156] -β-arrestins, intercellular cell adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) [158] -reduces PKC- β [155] |
| Rheum palmatum L. (emodin and rhein) | Rhubarb/Dahuang | -treating diabetic kidney disease [82] | -improvement of renal function and reduction of proteinuria was reported [5] -Rhein could prevent kidney damage [82] -emodin was reported to have a protective action on podocytes [159] | -nausea, vomiting, diarrhea, and abdominal pain were reported [5] | -downregulate tumor necrosis factor-α and interleukin-6 [160] -Rhein was reported as downregulating the wnt/ ß-catenin signaling pathway and upregulating SIRTI [82] -Emodin was reported to reduce glycation of proteins, inhibit cFLIP, TGF-ß1, and p38MAPK pathways [82] -Emodin inhibits the PERK-eIF2α signaling pathway [159] |
| Tripterygium wilfordii Hook F (TWHF) Triptolide (PG-90) (diterpene triepoxide) | Lei Gong Teng | -used to treat glomerulonephritis for more than 30 years in China [5] | -outstanding antiproteinuric effects are proven in animal models [5] -reduces podocyte injury via podocyte apoptosis inhibition [161,162] -improved kidney function compared with cyclophosphamide was reported [163] | -lack of clinical data in English -nephrotoxicity as a side effect was reported due to oxidative stress [164] -insufficient evidence to prove as effective as prednisone and cyclophosphamide [163] | -nuclear factor-kappa B (NF-κB) [165,166] -inhibit C5b-9-induced MAPK activation [78] -Triptolide was reported to decrease chemokine expression and inhibit macrophage infiltration [82] -antioxidative to inhibit reactive oxygen species [82] |
| Ganoderma fungus (lucidum/cochlear) (Triterpenes) | Lingzhi | -various acute kidney diseases and chronic kidney diseases [107] | -Ganoderma lucidum has low toxicity with significant antioxidant activity [102,167] -a successful clinical outcome of suppressing proteinuria in FSGS patients [168] -enhance antioxidant ability with vitamin C and E that successfully suppress proteinuria [169] | -suggested using with caution as potential toxicity was reported [170] | -CD4+, CD25+, Foxp3+, Treg, Interleukin-10, Breg [171] -interleukin-8 [102] |
| Cordyceps sinensis and Cordyceps militaris (cordycepin) | Dong Chong Cao | -commonly used in diabetes nephropathy | -attenuates glomerular damage when used together with TWHF [82,123] -antinephritic function [172,173] and significant nephroprotective effect [87] -cordycepin has excellent effect on anti-hyperuricemic [174] | -molecular mechanism unclear [123] | -podocin, nephrin [82] -Toll-like receptor 4/ nuclear factor kappa B [123,175] |
| Plantago asiatica and Plantago major (Hispidulin) | -diabetes nephropathy | -prevents podocyte apoptosis via autophagy [115] -renoprotective effect and potential therapeutic effects in nephrotic syndrome [112] -Plantago major could improve kidney function and reduce apoptosis [112] -improved proteinuria [112,176] | -unclear exact mechanism -renoprotective effect in nephrotic syndrome was reported in animal models only | -Mitogen-activated protein (MAP) [112] | |
| Schisandra sphenanthera (deoxyschizandrin) | Wuzhi | -commonly reported to be used after renal transplant | -enhanced tacrolimus effect when used in combination [177,178,179,180], while it did not increase the adverse effect [66] -nephrotoxicity protective effect induced by cisplatin [181] | -limited evidence and research on human clinical studies [180]. | -transcription factor NF-E2-related factor 2 [181] |
| Stephania tetrandra S Moore (Tetrandrine) | Fangji | -nephrotic syndrome | -tetrandrine is a practical component in the treatment of nephrotic syndrome [182] -effective combined treatment with glucocorticoids (prednisolone) could be another beneficial approach for the nephrotic syndrome [99,183] | -identification of therapeutic molecule was narrowed down but is still unclear and pending further validation [184] -potential nephrotoxicity [97] | -inhibits RhoA activation [185] |
| Polyphenol (Epigallocatechin-3-gallate (EGCG)) | Green tea | -diabetic nephropathy | -powerful antioxidant against oxidative stress [58,186,187] -promising therapeutic effects on various kidney diseases [8] | -unstable bioavailability is the concern [8] -lack of clinical studies on humans to provide conclusive evidence | -nuclear factor erythroid related-factor 2 (Nrf2) [8,60,188] -nuclear factor κB [8,60] |
| Silybum marianum (silymarin) | milk thistle | -protects against diabetic nephropathy [109] | -promising nephroprotective [108,109,110,189] ability against a wide range of drugs such as cyclosporin [90] -reduces urinary excretion of albumin [190] | -first reported case on the usage of Silybum that was not effective [189,191] -challenging bioavailability to show natural effect [192] | -blocking TNF-induced activation of NF-κB [109] |
| Herba Leonuri Leonurine | Motherwort | -renal fibrosis | -kidney protective effects on animal models [57] -a potential therapeutic drug to prevent podocyte injury from oxidative stress with its antioxidant ability [57] | -lack of clinical studies [193] -minimal scientific studies on kidney | -TGF-β/Smad3 and NF-κB signaling pathways [193] |
| Rutin | -type 1 diabetic mice [194] | -anti-inflammatory effect and anti-oxidative-stress effect on the kidney [195,196] -improved kidney structure [197] | -lack of studies on kidney-related disease and clinical studies | -TGF-β1/Smad3 signaling [198] -ceramide, MAPK, p53, and calpain [195] | |
| Polyporaceae Poria cocos Wolf | Fu-Ling | -commonly used to treat renal fibrosis | -strong ability to inhibit renal fibrosis and podocyte injury [199] | -suppressed TGF-β/Smad pathway [200] |
| Natural Ingredient | References | Disease/ Target | Conclusion | |||
|---|---|---|---|---|---|---|
| Curcumin | Animal studies in kidney autophagy: | Animal Model | Dosage and route of administration | Combination | ||
| [201] | Thirty male Sprague Dawley rats | 300 mg/kg/d, (Intragastric)., for 8 weeks | Treatment with curcumin only | DN | Curcumin treatment protects DN by inducing autophagy and alleviating podocyte EMT, through the PI3k/Akt/mTOR pathway. | |
| [202] | Male Wistar rats, Sprague Dawley (SD) rats, and New Zealand white rabbits | 300 mg/kg/d, (Intragastric)., for 30 days | Treatment with curcumin only | Membranous Nephropathy (MN) | Curcumin enhances kidney health by promoting autophagy and reducing oxidative stress in the kidneys through specific pathways like PI3K/AKT/mTOR and Nrf2/HO-1. | |
| [122] | male balb/c mice | 200 mg/kg/d, (intragastric) for 8 weeks | Treatment with curcumin only | Diabetic Nephropathy (DN) | Curcumin inhibited podocyte apoptosis and accelerated cell autophagy via regulating Beclin1/UVRAG/Bcl2, demonstrating that curcumin exerted significantly protective effects in DN. | |
| [203] | Twenty-four Sprague Dawley male rats, unilateral ureteral obstruction (UUO) rats | 200 mg/kg (gastrogavage) for 14 days | Treatment with curcumin only | Renal interstitial fibrosis (RIF) | Promising treatment for RIF and its antifibrotic effect may be regulated by autophagy and protection of mitochondria function. | |
| [204] | Nude Mice | 100, 200, and 400 mg/kg via oral gavage for 30 days | Treatment with various concentration of curcumin | Renal cell carcinoma | Curcumin is capable of inhibiting the proliferation of renal cell carcinoma by regulating the miR-148/ ADAMTS18 axis via the suppression of autophagy in vitro and in vivo. | |
| Clinical studies with kidney autophagy: | ||||||
| No record found | ||||||
| Clinical studies with kidney patients: | Type of clinical trial | Dosage and route of administration | Combination | |||
| (2011–2017): [48,49,140,205,206] | Chronic kidney disease (CKD)/Diabetic kidney disease (DKD)/ Hemodialysis (HD) | Short-term turmeric supplementation could attenuate proteinuria and has potential to reduce oxidative stress. Lack of clinical studies to verify appropriate dose for long-term safety usage in all stages of CKD. Short-term use of curcumin halts the progression of diabetic kidney disease. Curcumin considered as an effective anti-inflammatory supplement in HD patient group. | ||||
| [137] | randomized, double-blind clinical trial n = 46 | Dosage: 500 mg of curcumin per capsule, taken three times daily after meals (total 1500 mg/day) for 16 weeks. | Adjuvant therapy | DN | Curcumin could be an effective adjuvant therapy for ameliorating proteinuria in type 2 diabetes patients. | |
| [207] | double-blind randomized pilot study n = 31 | Taken orally in100 mL of orange juice containing 12 g of carrot and 2.5 g of turmeric, given after each hemodialysis session (three times per week) for 3 months | nutritional supplement (curcumin-enriched juice) | HD | Short-term treatment with curcumin suggesting that oral supplementation of curcumin may have anti-inflammatory effect in HD patient group. | |
| [208] | single-center, prospective, double-blinded, randomized controlled trial n = 60 | Curcuminoids were administered orally at a dosage of 1500 mg per day, starting 3 days before and continuing 2 days after the coronary procedure for 5 days (3 days prior to and 2 days post procedure). | Curcuminoids were used as an anti-inflammatory and antioxidant supplement in addition to the standard prophylaxis protocol for CI-AKI. | Contrast-induced acute kidney injury (CI-AKI) | Reduces the overall CI-AKI and AKI incidents in CKD patient undergoing elective coronary procedure. | |
| [209] | randomized, double-blind, placebo-controlled trial. n = 43 | Curcumin was administered orally at a dosage of 1 g/day for 12 weeks. | Curcumin was used as a nutritional supplement; the placebo group received corn starch. | HD | Potential effects on antioxidant response, but insufficient to reduce oxidative stress markers and inflammation in hemodialysis patients | |
| In vitro studies: | ||||||
| [210] | Renal fibrosis | Curcumin-induced autophagy extracellular vesicles improved fibrosis condition. | ||||
| Curcumin’s role in autophagy in kidney disease was investigated in animal studies, and there was no record of clinical studies in term of autophagy in kidney disease found so far, to the best of the authors’ knowledge, from 2019 to 2023. There are clinical studies of curcumin in the cancer patient group, and in cardiovascular disease and other diseases such as Alzheimer, but not in kidney autophagy [211]. | ||||||
| Astragaloside IV (AS-IV) | Animal studies in kidney disease | Animal model | Dosage and route of administration | Combination | ||
| [212] | 50 Wistar rats: 33 rats were confirmed with adriamycin nephropathy | Astragaloside: intragastric 150 mg/kg/day for 3 months. Methylprednisolone (MP): oral gavage at a dosage of 20 mg/kg/day for 3 months. | Adjuvant therapy, Methylprednisolone (MP): used as a reference standard therapy for nephrotic syndrome | Adriamycin nephropathy | AS-IV could prevent the progression of kidney injury. | |
| [213] | 48 diabetic male Sprague Dawley rats. | Astragaloside IV: administered via intragastric at doses of 2.5 mg/kg/day, 5 mg/kg/day, and 10 mg/kg/day for 12 weeks. Tempol: administered via drinking water at a concentration of 1 mmol/L for 12 weeks. Insulin: Administered via subcutaneous injection at 6 U/day for 12 weeks. | Adjuvant therapy with tempol: used as a reference antioxidant therapy. Insulin: served as baseline treatment for diabetic rats. | DN | AS-IV could prevent kidney injury in DN rat model. | |
| [214] | 18 male and female Sprague Dawley rats | Astragaloside IV (ASI): administered via intragastric (i.g.) administration at a dosage of 40 mg/kg/day for 8 weeks. | Adjuvant therapy | DN | AS-IV exerts therapeutic effect on DN, potentially through the inhibition of excessive mesangial proliferation and renal fibrosis via the TGF-β1/Smad/miR-192 signaling pathway. | |
| [215] | 32 male Sprague Dawley rats | AS-IV was administered via oral gavage at doses of 20, 40, and 80 mg/kg once daily. Positive control rats received Metformin 200 mg/kg via oral gavage for 8 weeks. | Treatment with astragaloside IV only. Metformin as positive control. | DN | AS-IV could exert protective effect from ER stress-induced apoptosis via the downregulation of p-PERK, ATF4 and CHOP. | |
| [216] | 32 male Wistar rats | AS-IV was administered via intragastric (i.g.) gavage at a dose of 40 mg/kg/day for rats for 11 days. | Treatment with astragaloside IV only. | podocytes | AS-IV could alleviate PAN-induced podocytes injury via partial activation of Wnt/planar cell polarity (PCP) pathway. | |
| [217] | 8-week-old male db/db mice | AS-IV: 5 g/kg in the diet. Enalapril: 0.8 g/kg in the diet.Both compounds were administered as dietary supplements. Combination therapy included both AS-IV and Enalapril at the same doses for 12 weeks. | AS-IV was used alone and in combination with Enalapril (an ACE inhibitor). | DN | AS-IV is more effective when used in combination with angiotensin, converting enzyme inhibitor to exert renal protective effect. | |
| [218] | 16 male Sprague Dawley (SD) rats | Astragaloside IV (AS-IV) dissolved in 0.5% CMC-Na, 80 mg/kg/day administered via oral gavage for 12 weeks. | Treatment with astragaloside IV only. | DN | AS-IV treatment could inhibit inflammation in rats’ kidney; hence, it ameliorated the severity of DN. | |
| [219] | 40 male Sprague Dawley (SD) rats | 40 mg/kg and 80 mg/kg of AS-IV administered daily via intragastric for 10 weeks. | Treatment with astragaloside IV only. | DN | AS-IV can ameliorate renal injury caused by high glucose; it has anti-oxidative-stress, anti-inflammatory, and anti-epithelial-mesenchymal transition (EMT) effects, and can inhibit the Wnt/β-catenin signaling pathway. | |
| [69] | Male diabetic C57BLKS/J-LepR (db/db) mice | 2 mg/kg/day, 6 mg/kg/day, and 18 mg/kg/day of AS-IV administered via oral gavage for 8 weeks. | Treatment with astragaloside IV only. | DN | AS-IV protects podocytes from apoptosis by inhibiting oxidative stress via activating PPARγ-Klotho-FoxO1 signaling pathway. | |
| [220] | 24 Male Sprague Dawley (SD) rats | ADR (adriamycin) was administered intraperitoneally at 4 mg/kg in four equal injections over 5 weeks. AS-IV was administered intragastrically at 10 mg/kg daily for 5 weeks. | AS-IV was combined with ADR treatment | Adriamycin-induced renal damage | ASIV might protect nephrocytes against ADR-induced ferroptosis, potentially via the activations of the Pi3K/Akt and Nrf2 signaling pathways. | |
| [65] | 40 male Sprague Dawley (SD) rats | 40 mg/(kg·d) of AS-IV administered via intragastric infusion for 8 weeks. | No combination. Astragaloside IV and SRT1720 were tested separately. | Kidney aging | AS-IV can delay kidney aging by regulating the SIRT1/p53 signaling pathway. | |
| [221] | 50 db/db mice | AS-IV at 10 mg/kg/day (low dose), 20 mg/kg/day (medium dose), and 40 mg/kg/day (high dose) via oral gavage for 12 weeks. | Treatment with astragaloside IV only. | EMT/DKD | C-X3-C motif ligand 1 (CX3CL1) plays a significant role in the progression of EMT; it is a potential target for AS-IV to alleviate renal tubular EMT. | |
| [68] | 32 8-week-old male Sprague Dawley rats | 40 mg/kg/day and 80 mg/kg/day of AS-IV administered via oral gavage for 12 weeks. | Treatment with astragaloside IV only. | DN | AS-IV upregulate the klotho expression, hence exert a protective effect. | |
| [222] | 40 Male Sprague Dawley rats | Astragaloside IV (AS) dose: 40 mg/kg/day via oral gavage for 8 weeks. | No combination therapy mentioned; AS and UBCS039 were administered as monotherapies in separate groups. | DKD | AS-IV inhibited podocytes pyroptosis in DKD by regulating SIRT6/HIF-1α pathway, thus, ameliorating injury of DKD. | |
| Animal studies in kidney autophagy | ||||||
| [223] | 6-week-old male C57BL/6J mice | Astragaloside IV (AS-IV) doses: 3 mg/kg, 6 mg/kg, and 12 mg/kg via oral gavage for 8 weeks, once daily | Treatment with astragaloside IV only. | DN | AS-IV was suggested to prevent the progression of DN by SERCA2-dependent ER stress attenuation and AMPKα-promoted autophagy induction. | |
| [224] | KK-Ay mice: used as a model for DKD with renal lesions resembling those in human type 2 diabetes mellitus. C57BL/6J mice: used as normal controls. | 40 mg/kg/day of AS-IV via oral gavage for 12 weeks | Treatment with astragaloside IV only. | EMT | AS-IV could exert protective effect on podocyte from EMT via the modulation of SIRT1–NF-κB pathway and autophagy activation. | |
| [225] | 40 Male Sprague Dawley (SD) rats, | Cisplatin: 15 mg/kg, intraperitoneally. AS-IV (oral gavage) Low dose: 40 mg/kg. High dose: 80 mg/kg. AS-IV: administered daily for 7 days. Cisplatin: administered on the 4th day of the 7-day AS-IV treatment. | Cisplatin was combined with AS-IV in the treatment groups | Cisplatin-induced liver and kidney injury | AS-IV induced autophagy and limit the expression of NLRP3 to effectively protect against cisplatin-induced injuries. | |
| [121] | 30 Male Sprague Dawley (SD) rats, | Astragaloside IV (AS-IV): Low dose: 10 mg/kg/day. High dose: 20 mg/kg/day via oral gavage for weeks. Benazepril: 1 mg/kg/day via oral gavage for 7 weeks. | Separate groups treated with the following: low dose of AS-IV (10 mg/kg/day). high dose of AS-IV (20 mg/kg/day). Benazepril (1 mg/kg/day) for comparison. | chronic glomerular nephritis (CGN) | AS-IV improved kidney function, reduced kidney lesion, and was remarkable in inhibiting the activation of PI3K/AKT/AS160 pathway and improving autophagy activation. | |
| [226] | 36 Male Sprague Dawley (SD) rats, | Astragaloside IV (AS-IV): 80 mg/kg/day via oral gavage for 8 weeks. Metformin (Met): 200 mg/kg/day via oral gavage for 8 weeks. | AS-IV and Metformin were administered as separate treatment groups for comparison | Type 2 diabetes liver injury | AS-IV alleviated diabetic liver injury in T2DM rats, and it could promote AMPK/mTOR-mediated autophagy. | |
| [227] | 40 Male Sprague Dawley (SD) rats, | ASIV doses: 40 mg/kg for the ASIV-40 group. 80 mg/kg for the ASIV-80 group. Administration route: oral gavage with ASIV dissolved in 0.5% CMC-Na solution for 4 weeks. | Treatment with astragaloside IV only. | CKD | Astragaloside IV exerts an anti-fibrosis effect and could enhance ALDH2 transcriptional activity. ALDH2-mediated autophagy could be a novel target for treating renal fibrosis. | |
| Clinical studies of autophagy in kidney patients | ||||||
| No records | ||||||
| Clinical trial studies in kidney patients | Type of studies | Dosage and route of administration | Combination | |||
| [228] | Pragmatic, assessor-blind, parallel, randomized controlled clinical trial n = 118 | 30 g/day astragalus daily (oral administration) for 48 weeks. | Adjuvant therapy with antidiabetic agents (e.g., insulin) and renin-angiotensin system (RAS) blockers like ACE inhibitors | DKD | This trial evaluates astragalus’s effectiveness in slowing DKD progression and identifies predictors for personalized use. Preliminary results are promising, with objective outcomes minimizing bias and supporting integration of conventional and Chinese medicine. | |
| In vitro studies | ||||||
| [229] | EMT | AS-IV blocked the mTORC1/p70S6K signaling pathway in renal tubular epithelial cells, hence, ameliorating high glucose-mediated renal tubular EMT. | ||||
| [230] | Glomerular Endothelial Cells | AS-IV can maintain the integrity of the filtration barrier in glomerular endothelial cells under diabetic conditions. | ||||
| [231] | DKD | AS-IV improved mitochondria function and protected podocytes from apoptosis and resistance to oxidative stress-induced diabetic kidney injury. The process was believed to be closely associated with the activation of Nrf2-ARE/TFAM signaling. | ||||
| AS-IV clinical studies are lacking but animal studies and in vitro studies suggested that AS-IV could exert renoprotective effect and activate autophagy. | ||||||
| Epigallocatechin-3-gallate (EGCG) | Animal studies in kidney disease | Animal model | Dosage and route of administration | Combination | ||
| [232] | Male Sprague Dawley (SD) rats | Indomethacin: 10 mg/kg. L-NAME: 10 mg/kg. Iopromide: 1.8 g(I)/kg. EGCG (Epigallocatechin gallate): 5, 10, or 20 mg/kg. ZnPP (HO-1 inhibitor): 30 mg/kg. Administration route: Indomethacin, L-NAME, and iopromide: intravenous (via left external jugular vein). EGCG: intravenous. ZnPP: intraperitoneal injection. | The CIN model included sequential injections of indomethacin, L-NAME, and iopromide. EGCG was tested alone for its protective effects. ZnPP was used as an inhibitor to assess the role of HO-1 in the protective mechanisms. | Contrast-induced nephropathy (CIN) | EGCG is a potent inducer of the antioxidant (heme oxygenase-1) that can protect CIN by ameliorating oxidative stress and inflammation. | |
| [233] | 20 male Wistar rats | EGCG: 50 mg/kg/day was administered via intraperitoneal injection. Duration: EGCG treatment lasted for 9 days. Started 2 days before UUO surgery. Continued during the 72 h obstruction period. Maintained for 5 days after UUO reversal. | Treatment with EGCG only. | Ureteral obstruction (UO) | EGCG was reported to have no significant protective effect on glomerular function when measured post reversal of unilateral ureteral obstruction but attenuated some of the kidney injury markers and pro-inflammatory mediators. | |
| [234] | 24 Dahl salt-sensitive (Dahl/SS) rats, | Epigallocatechin-3-gallate (EGCG): 50 mg/kg body weight via oral gavage twice daily for 6 weeks. | EGCG was the sole treatment in the EGCG group; no other medications were combined. | Renal damage in Dahl rats with salt hypertension | EGCG may exert antioxidant, anti-inflammatory and apoptosis-inducing effect on fibroblasts to attenuate renal damage and salt-sensitive hypertension. | |
| [235] | 30 male Wistar rats | EGCG at 50 mg/kg and 100 mg/kg, administered daily (oral gavage) for 90 days. | EGCG was the sole treatment tested. | Cigarette smoke induced renal and hepatic fibrosis | EGCG ameliorates renal and hepatic oxidative stress and inflammation, and could attenuate renal and hepatic fibrosis. | |
| [59] | 32 Male Sprague Dawley rats | 40 mg/kg/day EGCG for the low-dose group. 80 mg/kg/day EGCG for the high-dose group. Duration: 8 weeks, both high and low dose administered via oral gavage. | EGCG was the sole treatment tested. | Type 2 diabetic rats | EGCGwas reported to have renoprotective effects on type 2 diabetic rats mainly via repression of endoplasmic reticulum stress-mediated NLRP3 inflammasome. | |
| In vitro studies | ||||||
| [236] | Epithelial mesenchymal transition (EMT) | EGCG could prevent the epithelial mesenchymal transition of the renal tubular cells via nrf2 pathway. | ||||
| [237] | Reactive oxygen species | EGCG can improve the antioxidant capacity of the cell to promote repair of the oxidative stress injury. | ||||
| [238] | EMT | EGCG attenuates EMT in renal tubular cells through GSK-3β/β-catenin/Snail1 and Nrf2 pathways. | ||||
| [239] | EMT | EGCG can reverse EMT to mesenchymal-epithelial transition (MET) process in renal cells, to become a potential anti-fibrotic agent to reverse the fibrotic kidney. | ||||
| The clinical studies of ECGC are lacking, and most evidence was established through animal studies and in vitro studies. The disease focus of the in vitro studies of ECGC mainly focus on EMT and no other type of kidney diseases. To the best of the authors’ knowledge, there was no record regarding to the ECGC studies on autophagy in kidney disease found. | ||||||
| Resveratrol | Studies of resveratrol autophagy in kidney disease | Animal model | Dosage and route of administration | Combination | ||
| [240] | 35 Male C57BL/6 mice | Cisplatin: 20 mg/kg, single injection. Resveratrol (RES): 30 mg/kg/day intraperitoneally (i.p.). Ginsenoside Rg1 (Rg1): 20 mg/kg/day i.p. Duration: cisplatin: single injection. RES and Rg1 treatments: administered for 3 days before cisplatin injection and continued for another 3 days post cisplatin treatment (6 days in total). | Combined-treatment group received both RES (30 mg/kg/day) and Rg1 (20 mg/kg/day), simultaneously. | Cisplatin induced-AKI | Resveratrol used together with Rg1 alleviated the kidney damage caused by cisplatin, and reduced autophagy was involved in cisplatin-induced AKI | |
| Clinical trial (Review) | ||||||
| [241] | Over the last 20 years, clinical data suggested that resveratrol benefits human health, but high-quality trials needed. | |||||
| Berberine | Animal studies and in vitro of autophagy in kidney disease | Animal model | Dosage and route of administration | Combination | ||
| [242] | Male Wistar rats: initial population: 90 rats. Used for high-fat diet and streptozotocin (STZ)-induced diabetic nephropathy model. BKS.Cg-Dock7m+/+Leprdb/JNju (db/db) mice: Total: 40 mice (30 db/db mice and 10 control mice). | Rats: HGSD: 25 mg/kg/day and 100 mg/kg/day orally. Berberine: 100 mg/kg/day orally. Mice: HGSD: 40 mg/kg/day and 160 mg/kg/day orally. Duration: Rats: treatment lasted 16 weeks. Mice: treatment lasted 4 weeks | No combinations were reported in this study. Each treatment group received either HGSD or berberine. | Diabetic nephropathy | High bioavailability of berberine might be connected to the activation of AMPK phosphorylation and protect against diabetic kidney dysfunction. | |
| Abstract access only | [243] | Unspecified rats, in 5 groups. Five groups of rats, number per group not explicitly mentioned: 1. Control (Ctrl). 2. BBR-treated group (no CI-AKI). 3. CI-AKI group. 4. CI-AKI + BBR group. 5. CI-AKI + Tasq (HDAC4 inhibitor) group. | Berberine (BBR): Specific dose not mentioned in the abstract, but is referenced as a treatment group. Ioversol: 10 mL/kg to induce CI-AKI. | CI-AKI + BBR: Evaluating the renal protective effects of berberine alone. CI-AKI + Tasq: testing the effects of HDAC4 inhibition for comparison | Contrast-induced kidney injury | The activation of autophagy-related genes may be associated with berberine and play a protective effect and enhance autophagy. |
| [244]-in vitro | High level of glucose induced apoptosis | Berberine alleviating podocyte apoptosis by activating podocyte autophagy. | ||||
| Rutin | Animal studies of autophagy in kidney disease | Animal model | Dosage and route of administration | Combination | ||
| Abstract access only | [245] | Rats (unspecified in abstract) but groups as follows: 1. Control group. 2. VPA-only group. 3. VPA + RUT (50 mg/kg) group. 4. VPA + RUT (100 mg/kg) group. | Valproic Acid (VPA): 500 mg/kg. Rutin (RUT): 50 mg/kg or 100 mg/kg. Route of administration: not specified in abstract, but likely oral gavage. Duration: 14 days | VPA + RUT (50 mg/kg): to evaluate the protective effects of low-dose RUT. VPA + RUT (100 mg/kg): to evaluate the protective effects of high-dose RUT. | Sodium valproate-induced damage | Rutin treatment protected against kidney damage by attenuating VPA-induced oxidative stress, ER stress, inflammation, apoptosis and autophagy. |
| Abstract access only | [246] | db/db mice | Rutin: administered at a dose of 200 mg/kg/day, likely oral (not specified in abstract) gavage for 8 weeks. | No other medications were combined with Rutin in the study. | DKD | Rutin restores autophagy through inhibiting HDAC1 via the PI3K/AKT/mTOR pathway in DKD. |
| The active ingredients or the natural products that are not mentioned in this table but in Table 1 (Rheum palmatum L., Tripterygium wilfordii Hook F, Cordyceps sinensis and Cordyceps militaris, Schisandra sphenanthera, Plantago asiatica and Plantago major, Stephania tetrandra S Moore, Polyporaceae Poria cocos Wolf, Ganoderma, Silybum marianum, and Herba Leonuri) were lacking in direct evidence and studies conducted to investigate autophagy characteristics in kidney-related diseases. | ||||||
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Levin, A.; Okpechi, I.G.; Caskey, F.J.; Yang, C.-W.; Tonelli, M.; Jha, V. Perspectives on early detection of chronic kidney disease: The facts, the questions, and a proposed framework for 2023 and beyond. Kidney Int. 2023, 103, 1004–1008. [Google Scholar] [CrossRef]
- Politano, S.A.; Colbert, G.B.; Hamiduzzaman, N. Nephrotic Syndrome. Prim. Care Clin. Off. Pract. 2020, 47, 597–613. [Google Scholar] [CrossRef]
- Kawachi, H.; Fukusumi, Y. New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin. Exp. Nephrol. 2020, 24, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Deng, Y.; Chen, Y.; Chuang, P.Y.; He, J.C. Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney Int. 2013, 84, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Risso-Gill, I.; Balabanova, D.; Majid, F.; Ng, K.K.; Yusoff, K.; Mustapha, F.; Kuhlbrandt, C.; Nieuwlaat, R.; Schwalm, J.D.; McCready, T.; et al. Understanding the modifiable health systems barriers to hypertension management in Malaysia: A multi-method health systems appraisal approach. BMC Health Serv. Res. 2015, 15, 254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rajiah, K.; Veettil, S.K.; Wei, N.S. A cross-sectional study on knowledge and attitude toward Traditional Chinese Medicine (TCM) among adults in selected regions of Malaysia. J. Complement. Integr. Med. 2015, 12, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Thongboonkerd, V. Protective Effects of Epigallocatechin-3-Gallate from Green Tea in Various Kidney Diseases. Adv. Nutr. 2019, 10, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-C.; Su, Y.-C.; Sun, M.-F.; Huang, S.-T. Chinese Herbal Medicine Improves the Long-Term Survival Rate of Patients With Chronic Kidney Disease in Taiwan: A Nationwide Retrospective Population-Based Cohort Study. Front. Pharmacol. 2018, 9, 1117. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Q.; Hu, H.-H.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Zhao, Y.-Y. Natural products for the prevention and treatment of kidney disease. Phytomedicine 2018, 50, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Zou, X.-R.; Zhang, Y.C. From “Kidneys Govern Bones” to Chronic Kidney Disease, Diabetes Mellitus, and Metabolic Bone Disorder: A Crosstalk between Traditional Chinese Medicine and Modern Science. Evid.-Based Complement. Altern. Med. 2016, 2016, 4370263. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, R.; Melk, A. Molecular mechanisms of renal aging. Kidney Int. 2017, 92, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lerman, L.O. Cellular Senescence. Hypertension 2020, 76, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Teh, Y.M.; Mualif, S.A.; Lim, S.K. A comprehensive insight into autophagy and its potential signaling pathways as a therapeutic target in podocyte injury. Int. J. Biochem. Cell Biol. 2022, 143, 106153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wan, Y.; Chen, R.; Zhang, C.; Li, X.; Meng, F.; Glaser, S.; Wu, N.; Zhou, T.; Li, S.; et al. The emerging role of cellular senescence in renal diseases. J. Cell. Mol. Med. 2020, 24, 2087–2097. [Google Scholar] [CrossRef]
- Sharma, R. Bioactive food components for managing cellular senescence in aging and disease: A critical appraisal and perspectives. PharmaNutrition 2021, 18, 100281. [Google Scholar] [CrossRef]
- Kang, C.; Elledge, S.J. How autophagy both activates and inhibits cellular senescence. Autophagy 2016, 12, 898–899. [Google Scholar] [CrossRef]
- Yin, S.; Zhou, Z.; Fu, P.; Jin, C.; Wu, P.; Ji, C.; Shan, Y.; Shi, L.; Xu, M.; Qian, H. Roles of extracellular vesicles in ageing-related chronic kidney disease: Demon or angel. Pharmacol. Res. 2023, 193, 106795. [Google Scholar] [CrossRef] [PubMed]
- Marechal, A.; Zou, L. DNA Damage Sensing by the ATM and ATR Kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.; Wang, J.; Xiao, M.; Guo, Y.; Tang, Y.; Zhang, J.; Gu, J. Epigenetic Regulation Associated With Sirtuin 1 in Complications of Diabetes Mellitus. Front. Endocrinol. 2021, 11, 598012. [Google Scholar] [CrossRef]
- Wang, W.-J.; Chen, X.-M.; Cai, G.-Y. Cellular senescence and the senescence-associated secretory phenotype: Potential therapeutic targets for renal fibrosis. Exp. Gerontol. 2021, 151, 111403. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Gong, A.Y.; Haller, S.T.; Dworkin, L.D.; Liu, Z.; Gong, R. The ageing kidney: Molecular mechanisms and clinical implications. Ageing Res. Rev. 2020, 63, 101151. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. Current role of mammalian sirtuins in DNA repair. DNA Repair 2019, 80, 85–92. [Google Scholar] [CrossRef]
- Minami, S.; Yamamoto, T.; Yamamoto-Imoto, H.; Isaka, Y.; Hamasaki, M. Autophagy and kidney aging. Prog. Biophys. Mol. Biol. 2023, 179, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Lee, J.-H.; Lee, H.-Y.; Min, K.-J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, B.N.; Thackray, J.K.; Serrano, L. Sirtuins and DNA damage repair: SIRT7 comes to play. Nucleus 2017, 8, 107–115. [Google Scholar] [CrossRef]
- Tharaux, P.L.; Huber, T.B. How many ways can a podocyte die? Semin. Nephrol. 2012, 32, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Ogawa-Akiyama, A.; Sugiyama, H.; Kitagawa, M.; Tanaka, K.; Kano, Y.; Mise, K.; Otaka, N.; Tanabe, K.; Morinaga, H.; Kinomura, M.; et al. Podocyte autophagy is associated with foot process effacement and proteinuria in patients with minimal change nephrotic syndrome. PLoS ONE 2020, 15, e0228337. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Kang, Q.; Wang, Q.; Wang, Q.; Chen, F.; Cheng, K.-W. Resveratrol: Evidence for Its Nephroprotective Effect in Diabetic Nephropathy. Adv. Nutr. 2020, 11, 1555–1568. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, B.; Liu, Z.; Gong, A.Y.; Gunning, W.T.; Ge, Y.; Malhotra, D.; Gohara, A.F.; Dworkin, L.D.; Gong, R. Age-related GSK3β overexpression drives podocyte senescence and glomerular aging. J. Clin. Investig. 2022, 132, e141848. [Google Scholar] [CrossRef]
- Medina Rangel, P.X.; Cross, E.; Liu, C.; Pedigo, C.E.; Tian, X.; Gutiérrez-Calabrés, E.; Nagata, S.; Priyadarshini, A.; Lerner, G.; Bunda, P.; et al. Cell Cycle and Senescence Regulation by Podocyte Histone Deacetylase 1 and 2. J. Am. Soc. Nephrol. 2023, 34, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Sturmlechner, I.; Durik, M.; Sieben, C.J.; Baker, D.J.; van Deursen, J.M. Cellular senescence in renal ageing and disease. Nature Rev. Nephrol. 2017, 13, 77–89. [Google Scholar] [CrossRef]
- Lin, X.; Jin, H.; Chai, Y.; Shou, S. Cellular senescence and acute kidney injury. Pediatr. Nephrol. 2022, 37, 3009–3018. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Liu, Y. Cellular Senescence in Kidney Fibrosis: Pathologic Significance and Therapeutic Strategies. Front. Pharmacol. 2020, 11, 601325. [Google Scholar] [CrossRef]
- Kreidberg, J.A.; Schumacher, V.A. GSK3β and the aging kidney. J. Clin. Investig. 2022, 132, e155885. [Google Scholar] [CrossRef]
- Castillo-Quan, J.I.; Li, L.; Kinghorn, K.J.; Ivanov, D.K.; Tain, L.S.; Slack, C.; Kerr, F.; Nespital, T.; Thornton, J.; Hardy, J.; et al. Lithium Promotes Longevity through GSK3/NRF2-Dependent Hormesis. Cell Rep. 2016, 15, 638–650. [Google Scholar] [CrossRef]
- Zarse, K.; Terao, T.; Tian, J.; Iwata, N.; Ishii, N.; Ristow, M. Low-dose lithium uptake promotes longevity in humans and metazoans. Eur. J. Nutr. 2011, 50, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Zhang, Q.; Liu, X.; Song, Y.; Li, X.; Wang, Z.; Li, C.; Peng, A.; Gong, R. Lithium targeting of AMPK protects against cisplatin-induced acute kidney injury by enhancing autophagy in renal proximal tubular epithelial cells. FASEB J. 2019, 33, 14370–14381. [Google Scholar] [CrossRef] [PubMed]
- Koshida, T.; Gohda, T.; Sugimoto, T.; Asahara, T.; Asao, R.; Ohsawa, I.; Gotoh, H.; Murakoshi, M.; Suzuki, Y.; Yamashiro, Y. Gut Microbiome and Microbiome-Derived Metabolites in Patients with End-Stage Kidney Disease. Int. J. Mol. Sci. 2023, 24, 11456. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, G.; Nigam, S.K.; Vanholder, R.; Verbeke, F. Role of the Microbiome in Gut-Heart-Kidney Cross Talk. Circ. Res. 2023, 132, 1064–1083. [Google Scholar] [CrossRef]
- Siddiqui, R.; Maciver, S.; Elmoselhi, A.; Soares, N.C.; Khan, N.A. Longevity, cellular senescence and the gut microbiome: Lessons to be learned from crocodiles. Heliyon 2021, 7, e08594. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Fan, Y.; Li, A.; Shen, Q.; Wu, J.; Ren, L.; Lu, H.; Ding, S.; Ren, H.; Liu, C.; et al. Alterations of the Human Gut Microbiome in Chronic Kidney Disease. Adv. Sci. 2020, 7, 2001936. [Google Scholar] [CrossRef] [PubMed]
- Nallu, A.; Sharma, S.; Ramezani, A.; Muralidharan, J.; Raj, D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl. Res. 2017, 179, 24–37. [Google Scholar] [CrossRef]
- Mafra, D.; Borges, N.; Alvarenga, L.; Esgalhado, M.; Cardozo, L.; Lindholm, B.; Stenvinkel, P. Dietary Components That May Influence the Disturbed Gut Microbiota in Chronic Kidney Disease. Nutrients 2019, 11, 496. [Google Scholar] [CrossRef]
- Maung, S.C.; Sara, A.E.; Chapman, C.; Cohen, D.; Cukor, D. Sleep disorders and chronic kidney disease. World J. Nephrol. 2016, 5, 224. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Ahern, A.; Carbone, C.; Temko, A.; Claesson, M.J.; Gasbarrini, A.; Tortora, G. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Y.; Wang, X.K.; Li, X.; Ji, K.; Du, S.H.; Liu, Y.; Kong, L.L.; Xu, J.C.; Yang, G.Q.; Chen, D.Q.; et al. Curcumin, as a pleiotropic agent, improves doxorubicin-induced nephrotic syndrome in rats. J. Ethnopharmacol. 2020, 250, 10. [Google Scholar] [CrossRef]
- Jiménez-Osorio, A.S.; García-Niño, W.R.; González-Reyes, S.; Álvarez-Mejía, A.E.; Guerra-León, S.; Salazar-Segovia, J.; Falcón, I.; Montes de Oca-Solano, H.; Madero, M.; Pedraza-Chaverri, J. The Effect of Dietary Supplementation with Curcumin on Redox Status and Nrf2 Activation in Patients With Nondiabetic or Diabetic Proteinuric Chronic Kidney Disease: A Pilot Study. J. Ren. Nutr. 2016, 26, 237–244. [Google Scholar] [CrossRef]
- de Almeida Alvarenga, L.; Leal, V.d.O.; Borges, N.A.; Silva de Aguiar, A.; Faxén-Irving, G.; Stenvinkel, P.; Lindholm, B.; Mafra, D. Curcumin—A promising nutritional strategy for chronic kidney disease patients. J. Funct. Foods 2018, 40, 715–721. [Google Scholar] [CrossRef]
- Avila-Rojas, S.H.; Lira-León, A.; Aparicio-Trejo, O.E.; Reyes-Fermín, L.M.; Pedraza-Chaverri, J. Role of Autophagy on Heavy Metal-Induced Renal Damage and the Protective Effects of Curcumin in Autophagy and Kidney Preservation. Medicina 2019, 55, 360. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kim, W.; Ha, A.W. The effect of curcumin on blood pressure and cognitive impairment in spontaneously hypertensive rats. Nutr. Res. Pract. 2023, 17, 192–205. [Google Scholar] [CrossRef]
- Jacob, A.; Chaves, L.; Eadon, M.T.; Chang, A.; Quigg, R.J.; Alexander, J.J. Curcumin alleviates immune-complex-mediated glomerulonephritis in factor-H-deficient mice. Immunology 2013, 139, 328–337. [Google Scholar] [CrossRef]
- Hellmann, P.H.; Bagger, J.I.; Carlander, K.R.; Hansen, K.B.; Forman, J.L.; Størling, J.; Chabanova, E.; Holst, J.; Vilsbøll, T.; Knop, F.K. No effect of the turmeric root phenol curcumin on prednisolone-induced glucometabolic perturbations in men with overweight or obesity. Endocr. Connect. 2023, 12, e220334. [Google Scholar] [CrossRef]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Mosieniak, G.; Bijoch, Ł.; Sikora, E. The Role of Curcumin in the Modulation of Ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef]
- Hao, M.; Chu, Y.; Lei, J.; Yao, Z.; Wang, P.; Chen, Z.; Wang, K.; Sang, X.; Han, X.; Wang, L.; et al. Pharmacological Mechanisms and Clinical Applications of Curcumin: Update. Aging Dis. 2023, 14, 716–749. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, W.; Qi, J.; Li, Q.; Zhao, M.; Chen, Z.; Zhu, J.; Huang, Z.; Wu, L.; Zhang, B.; et al. Leonurine ameliorates adriamycin-induced podocyte injury via suppression of oxidative stress. Free. Radic. Res. 2018, 52, 952–960. [Google Scholar] [CrossRef]
- Bao, H.; Peng, A. The Green Tea Polyphenol (—)-epigallocatechin-3-gallate and its beneficial roles in chronic kidney disease. J. Transl. Intern. Med. 2016, 4, 99–103. [Google Scholar] [CrossRef]
- Yang, R.; Chen, J.; Jia, Q.; Yang, X.; Mehmood, S. Epigallocatechin-3-gallate ameliorates renal endoplasmic reticulum stress-mediated inflammation in type 2 diabetic rats. Exp. Biol. Med. 2022, 247, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Thongboonkerd, V. Molecular Mechanisms of Epigallocatechin-3-Gallate for Prevention of Chronic Kidney Disease and Renal Fibrosis: Preclinical Evidence. Curr. Dev. Nutr. 2019, 3, nzz101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Deng, Y.; Chen, Y.; Fan, J.; Zhang, M.; Zhong, Y.; Zhu, R.; Wang, L. Astragaloside IV Attenuates Complement Membranous Attack Complex Induced Podocyte Injury Through the MAPK Pathway. Phytother. Res. 2012, 26, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, T.; Yang, W.; Zhang, L.; Wu, S.; Yan, C.; Li, Q. Astragalus membranaceus Injection Suppresses Production of Interleukin-6 by Activating Autophagy through the AMPK-mTOR Pathway in Lipopolysaccharide-Stimulated Macrophages. Oxidative Med. Cell. Longev. 2020, 2020, 1364147. [Google Scholar] [CrossRef]
- Afkhami-Ardakani, M.; Hassanzadeh, S.; Shahrooz, R.; Asadi-Samani, M.; Latifi, E.; Luther, T. Phytotherapy and phytopharmacology for reduction of cyclophosphamide-induced toxicity in the male urinary system. J. Ren. Inj. Prev. 2017, 6, 164–170. [Google Scholar] [CrossRef]
- Xia, M.-L.; Xie, X.-H.; Ding, J.-H.; Du, R.-H.; Hu, G. Astragaloside IV inhibits astrocyte senescence: Implication in Parkinson’s disease. J. Neuroinflammation 2020, 17, 105. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Fang, J.-A.; Li, S.-F.; Hu, Y.-L.; Liu, W.-Y.; Liu, X.-J. Effects of astragaloside IV on delaying kidney aging and its mechanisms. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2022, 38, 448–452. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Wang, L.; Tu, Y.-C.; Zhang, Y.C. Traditional Chinese medicine for refractory nephrotic syndrome: Strategies and promising treatments. Evid.-Based Complement. Altern. Med. 2018, 2018, 8746349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Li, X.; Li, N.; Wang, Q.; Liu, Y.; Liang, Q.; Shao, Z.; Zhang, N.; Zhao, T.; et al. Tangshen Formula Alleviates Hepatic Steatosis by Inducing Autophagy Through the AMPK/SIRT1 Pathway. Front. Physiol. 2019, 10, 494. [Google Scholar] [CrossRef]
- He, J.; Cui, J.; Shi, Y.; Wang, T.; Xin, J.; Li, Y.; Shan, X.; Zhu, Z.; Gao, Y. Astragaloside IV Attenuates High-Glucose-Induced Impairment in Diabetic Nephropathy by Increasing Klotho Expression via the NF-κB/NLRP3 Axis. J. Diabetes Res. 2023, 2023, 7423661. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Fang, J.; Zhu, B.; Wang, L.; Chen, J.; Wang, Y.; Huang, J.; Wang, H.; Yao, X. Astragaloside IV protects against podocyte apoptosis by inhibiting oxidative stress via activating PPARγ-Klotho-FoxO1 axis in diabetic nephropathy. Life Sci. 2021, 269, 119068. [Google Scholar] [CrossRef]
- Bi, F.; Chen, F.; Li, Y.; Wei, A.; Cao, W. Klotho preservation by Rhein promotes toll-like receptor 4 proteolysis and attenuates lipopolysaccharide-induced acute kidney injury. J. Mol. Med. 2018, 96, 915–927. [Google Scholar] [CrossRef]
- Dos Santos, M.; Poletti, P.T.; Milhoransa, P.; Monticielo, O.A.; Veronese, F.V. Unraveling the podocyte injury in lupus nephritis: Clinical and experimental approaches. Semin. Arthritis Rheum. 2017, 46, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Rafiei, H.; Mohammadinejad, R.; Afshar, E.G.; Farkhondeh, T.; Samarghandian, S. Potential therapeutic effects of curcumin mediated by JAK/STAT signaling pathway: A review. Phytother. Res. 2020, 34, 1745–1760. [Google Scholar] [CrossRef] [PubMed]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Tarnowski, M. A Role for Advanced Glycation End Products in Molecular Ageing. Int. J. Mol. Sci. 2023, 24, 9881. [Google Scholar] [CrossRef]
- Liang, X.; Chen, B.; Wang, P.; Ge, Y.; Malhotra, D.K.; Dworkin, L.D.; Liu, Z.; Gong, R. Triptolide potentiates the cytoskeleton-stabilizing activity of cyclosporine A in glomerular podocytes via a GSK3β dependent mechanism. Am. J. Transl. Res. 2020, 12, 800–812. [Google Scholar]
- Li, H.D.; Meng, X.M.; Huang, C.; Zhang, L.; Lv, X.W.; Li, J. Application of Herbal Traditional Chinese Medicine in the Treatment of Acute Kidney Injury. Front. Pharmacol. 2019, 10, 12. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Q.-L.; Feng, Y.-H.; Yang, Y.-F.; Li, X.-Y.; Zuo, J.-P. Triptolide suppresses CD80 and CD86 expressions and IL-12 production in THP-1 cells1. Acta Pharmacol. Sin. 2005, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Li, X.; Lu, Q.; Zhu, Q.; Jiang, H.; Wang, T.; Huang, G.; Xu, A. Application and Mechanisms of Triptolide in the Treatment of Inflammatory Diseases—A Review. Front. Pharmacol. 2019, 10, 1469. [Google Scholar] [CrossRef]
- Teh, Y.M.; Lim, S.K.; Jusoh, N.; Osman, K.; Mualif, S.A. CD80 Insights as Therapeutic Target in the Current and Future Treatment Options of Frequent-Relapse Minimal Change Disease. BioMed Res. Int. 2021, 2021, 6671552. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Wang, K.; Lu, J.; Bao, X.; Wang, R.; Qiu, Y.; Wang, T.; Yu, H. Cellular senescence and cancer: Focusing on traditional Chinese medicine and natural products. Cell Prolif. 2020, 53, e12894. [Google Scholar] [CrossRef]
- Mamun, A.A.; Sufian, M.A.; Uddin, M.S.; Sumsuzzman, D.M.; Jeandet, P.; Islam, M.S.; Zhang, H.-J.; Kong, A.-N.; Sarwar, M.S. Exploring the role of senescence inducers and senotherapeutics as targets for anticancer natural products. Eur. J. Pharmacol. 2022, 928, 174991. [Google Scholar] [CrossRef]
- Wen, Y.; Yan, M.; Zhang, B.; Li, P. Chinese medicine for diabetic kidney disease in China. Nephrology 2017, 22, 50–55. [Google Scholar] [CrossRef]
- Nxumalo, W.; Elateeq, A.A.; Sun, Y. Can Cordyceps cicadae be used as an alternative to Cordyceps militaris and Cordyceps sinensis?—A review. J. Ethnopharmacol. 2020, 257, 112879. [Google Scholar] [CrossRef] [PubMed]
- Combest, W.; Newton, M.; Combest, A.; Kosier, J.H. Effects of herbal supplements on the kidney. Urol. Nurs. 2005, 25, 381–386. [Google Scholar] [PubMed]
- Wei-Yan, W.; Xue-Jie, G.A.O.; Bing-Hua, L.I.U.; Li, Y.; Meng-Li, Y.A.N.; Ke, L.I.U. Cordycep cicadae extracts has a protect effect against H2O2-induced cellular senescence by promoting oxidative stress response in HeLa cells. J. Sichuan Univ. (Nat. Sci. Ed.) 2018, 55, 632–636. [Google Scholar]
- Wang, H.-P.; Liu, C.-W.; Chang, H.-W.; Tsai, J.-W.; Sung, Y.-Z.; Chang, L.-C. Cordyceps sinensis protects against renal ischemia/reperfusion injury in rats. Mol. Biol. Rep. 2013, 40, 2347–2355. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Lu, Y.; Zhang, J.; Yang, M.; Tian, Y.; Dong, J.; Liao, L. Protective effect of Cordyceps sinensis against diabetic kidney disease through promoting proliferation and inhibiting apoptosis of renal proximal tubular cells. BMC Complement. Med. Ther. 2023, 23, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Jiang, Z.; Luo, P.; Liu, L.; Huang, Y.; Wang, H.; Wang, Y.; Long, L.; Tan, X.; et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat. Commun. 2019, 10, 2538. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H. Chinese Herbal Medicine in the Treatment of Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2005, 12, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Yarnell, E. Botanical medicines used for kidney disease in the United States. Iran. J. Kidney Dis. 2012, 6, 407–418. [Google Scholar]
- Zu, C.; Qin, G.; Yang, C.; Liu, N.; He, A.; Zhang, M.; Zheng, X. Low dose Emodin induces tumor senescence for boosting breast cancer chemotherapy via silencing NRARP. Biochem. Biophys. Res. Commun. 2018, 505, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-L.; Li, J.-L.; Wang, S.-H.; Chen, X.; Huang, M.; Bi, H.-C. Co-administration of Wuzhi tablet (Schisandra sphenanthera extract) alters tacrolimus pharmacokinetics in a dose- and time-dependent manner in rats. J. Ethnopharmacol. 2020, 263, 113233. [Google Scholar] [CrossRef]
- Xue, X.-P.; Qin, X.-L.; Xu, C.; Zhong, G.-P.; Wang, Y.; Huang, M.; Bi, H.-C. Effect of Wuzhi Tablet (Schisandra sphenanthera extract) on the Pharmacokinetics of Cyclosporin A in Rats. Phytother. Res. 2013, 27, 1255–1259. [Google Scholar] [CrossRef]
- Zhao, J.; Ding, K.; Hou, M.; Li, Y.; Hou, X.; Dai, W.; Li, Z.; Zhao, J.; Liu, W.; Bai, Z. Schisandra chinensis essential oil attenuates acetaminophen-induced liver injury through alleviating oxidative stress and activating autophagy. Pharm. Biol. 2022, 60, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Zakłos-Szyda, M.; Błasiak, J.; Nowak, A.; Zhang, Z.; Zhang, B. Potential of Schisandra chinensis (Turcz.) Baill. in Human Health and Nutrition: A Review of Current Knowledge and Therapeutic Perspectives. Nutrients 2019, 11, 333. [Google Scholar] [CrossRef]
- Li, X.; Gao, J.; Yu, Z.; Jiang, W.; Sun, W.; Yu, C.; Sun, J.; Wang, C.; Chen, J.; Jing, S.; et al. Regulatory Effect of Anwulignan on the Immune Function Through Its Antioxidation and Anti-Apoptosis in D-Galactose-Induced Aging Mice. Clin. Interv. Aging 2020, 15, 97–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, D.; Gao, Y.; Liang, C.; Zhang, Y.; Ma, Z.; Liu, Y.; Peng, H.; Zhang, Y.; Qin, H.; et al. History of uses, phytochemistry, pharmacological activities, quality control and toxicity of the root of Stephania tetrandra S. Moore: A review. J. Ethnopharmacol. 2020, 260, 112995. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yu, P.; Sheng, L.; Ye, J.; Qin, Z. Fangjifuling Ameliorates Lipopolysaccharide-Induced Renal Injury via Inhibition of Inflammatory and Apoptotic Response in Mice. Cell. Physiol. Biochem. 2018, 49, 2124–2137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-N.; Yang, L.; He, S.-S.; Li, A.-P.; Qin, X.-M. Mechanisms of Chinese medical formula Fangji Huangqi Decoction as an effective treatment of nephrotic syndrome based on systems pharmacology. Chin. Herb. Med. 2019, 11, 281–291. [Google Scholar] [CrossRef]
- Wang, H.; Liu, T.; Li, L.; Wang, Q.; Yu, C.; Liu, X.; Li, W. Tetrandrine is a potent cell autophagy agonist via activated intracellular reactive oxygen species. Cell Biosci. 2015, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Wang, D.; Cui, D.; Yang, Q.; Wang, P.; Yang, W.; Chen, F. Anti-aging function and molecular mechanism of Radix astragali and Radix astragali preparata via network pharmacology and PI3K/Akt signaling pathway. Phytomedicine 2021, 84, 153509. [Google Scholar] [CrossRef]
- Lai, K.N.; Chan, L.Y.Y.; Tang, S.C.W.; Leung, J.C.K. Ganoderma extract prevents albumin-induced oxidative damage and chemokines synthesis in cultured human proximal tubular epithelial cells. Nephrol. Dial. Transplant. 2006, 21, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, B.; Zhao, H.; Feng, J. Emerging roles of Ganoderma lucidum in anti-aging. Aging Dis. 2017, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Naruse, K.; Katsuta, R.; Yajima, A.; Nukada, T.; Watanabe, H.; Ishigami, K. Formal synthesis of cochlearol A, a meroterpenoid with renoprotective activity. Tetrahedron Lett. 2020, 61, 151845. [Google Scholar] [CrossRef]
- Diwan, B.; Sharma, R. Nutritional components as mitigators of cellular senescence in organismal aging: A comprehensive review. Food Sci. Biotechnol. 2022, 31, 1089–1109. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Shin, S.; Cho, E.; Ryu, D.; Garandeau, D.; Chajra, H.; Fréchet, M.; Park, D.; Jung, E. Senotherapeutic-like effect of Silybum marianum flower extract revealed on human skin cells. PLoS ONE 2021, 16, e0260545. [Google Scholar] [CrossRef]
- Geng, X.; Zhong, D.; Su, L.; Lin, Z.; Yang, B. Chapter Nine—Preventive and therapeutic effect of Ganoderma lucidum on kidney injuries and diseases. In Advances in Pharmacology; Du, G., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 87, pp. 257–276. [Google Scholar]
- Cengiz, M. Renoprotective effects of Silybum marianum (L.) Gaertn (Silymarin) on thioacetamide-induced renal injury: Biochemical and histopathological approach. Pak. J. Pharm. Sci. 2018, 31, 2137–2141. [Google Scholar] [PubMed]
- Guzel, S.; Sahinogullari, Z.U.; Canacankatan, N.; Antmen, S.E.; Kibar, D.; Coskun Yilmaz, B. Potential renoprotective effects of silymarin against vancomycin-induced nephrotoxicity in rats. Drug Chem. Toxicol. 2020, 43, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Malkani, N.; Naeem, A.; Ijaz, F.; Mumtaz, S.; Ashraf, S.; Sohail, M.I. Silybum marianum (milk thistle) improves vancomycin induced nephrotoxicity by downregulating apoptosis. Mol. Biol. Rep. 2020, 47, 5451–5459. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Shangguan, J.; Jiang, A.; Ren, A. Research Progress on the Biological Activity of Ganoderic Acids in Ganoderma lucidum over the Last Five Years. Life 2024, 14, 1339. [Google Scholar] [CrossRef]
- Kho, M.; Park, J.; Han, B.; Tan, R.; Yoon, J.; Kim, H.; Ahn, Y.; Lee, Y.; Kang, D.; Lee, H. Plantago asiatica L. Ameliorates Puromycin Aminonucleoside-Induced Nephrotic Syndrome by Suppressing Inflammation and Apoptosis. Nutrients 2017, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Wang, Q.; Zhou, X.; Lu, X.; Liu, T.; Zhan, Y.; Li, P. Chinese Herbal Medicine in Ameliorating Diabetic Kidney Disease via Activating Autophagy. J. Diabetes Res. 2019, 2019, 9030893. [Google Scholar] [CrossRef]
- Lu, Z.; Zhong, Y.; Liu, W.; Xiang, L.; Deng, Y. The Efficacy and Mechanism of Chinese Herbal Medicine on Diabetic Kidney Disease. J. Diabetes Res. 2019, 2019, 2697672. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, S.; Zhang, N.; Huang, W.; Li, X.; Wang, M.; Bai, D.; Han, B. Hispidulin alleviates high-glucose-induced podocyte injury by regulating protective autophagy. Biomed. Pharmacother. 2018, 104, 307–314. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A. Molecular Interactions Between Reactive Oxygen Species and Autophagy in Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3791. [Google Scholar] [CrossRef]
- Xu, W.; Cui, J.; Zhou, F.; Bai, M.; Deng, R.; Wang, W. Leonurine protects against dexamethasone-induced cytotoxicity in pancreatic β-cells via PI3K/Akt signaling pathway. Biochem. Biophys. Res. Commun. 2020, 529, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.; Zhou, B.-H. Leonurine ameliorates D-galactose-induced aging in mice through activation of the Nrf2 signalling pathway. Aging 2019, 11, 7339–7356. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Li, S.; Shen, B.; Ma, L.; Zuo, L.; Gao, Y.; Yang, G. Leonurine Alleviates Cognitive Dysfunction and Reduces Oxidative Stress by Activating Nrf-2 Pathway in Alzheimer’s Disease Mouse Model. Neuropsychiatr. Dis. Treat. 2023, 19, 1347–1357. [Google Scholar] [CrossRef]
- Ma, L.; Fu, R.; Duan, Z.; Lu, J.; Gao, J.; Tian, L.; Lv, Z.; Chen, Z.; Han, J.; Jia, L.; et al. Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol. Res. Pract. 2016, 212, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Chen, J.; Liu, B.; Lin, H.; Bai, L.; Zhang, P.; Chen, D.; Li, H.; Li, J.; Pang, Y.; et al. Protective role of Astragaloside IV in chronic glomerulonephritis by activating autophagy through PI3K/AKT/AS160 pathway. Phytother. Res. 2020, 34, 3236–3248. [Google Scholar] [CrossRef]
- Zhang, P.; Fang, J.; Zhang, J.; Ding, S.; Gan, D. Curcumin Inhibited Podocyte Cell Apoptosis and Accelerated Cell Autophagy in Diabetic Nephropathy via Regulating Beclin1/UVRAG/Bcl2. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 641. [Google Scholar] [CrossRef]
- Sun, T.; Dong, W.; Jiang, G.; Yang, J.; Liu, J.; Zhao, L.; Ma, P. Cordyceps militaris Improves Chronic Kidney Disease by Affecting TLR4/NF-B Redox Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 7850863. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Sim, H.A.; Jung, D.Y.; Lim, E.Y.; Kim, Y.T.; Kim, B.J.; Jung, M.H. Poria cocus Wolf Extract Ameliorates Hepatic Steatosis Through Regulation of Lipid Metabolism, Inhibition of ER Stress, and Activation of Autophagy via AMPK Activation. Int. J. Mol. Sci. 2019, 20, 4801. [Google Scholar] [CrossRef]
- Gong, J.; Jin, J.; Zhao, L.; Li, Y.; Li, Y.; He, Q. Tripterygium glycoside protects against puromycin amino nucleoside-induced podocyte injury by upregulating autophagy. Int. J. Mol. Med. 2018, 42, 115–122. [Google Scholar] [CrossRef]
- Ashaq, A.; Maqbool, M.F.; Maryam, A.; Khan, M.; Shakir, H.A.; Irfan, M.; Qazi, J.I.; Li, Y.; Ma, T. Hispidulin: A novel natural compound with therapeutic potential against human cancers. Phytother. Res. 2021, 35, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, X.; Zhu, W.; Chen, H.; Hu, X.; Jiang, Z.; Xu, Y.; Wang, L.; Zhou, Y.; Chen, P.; et al. SIRT1 ameliorates age-related senescence of mesenchymal stem cells via modulating telomere shelterin. Front. Aging Neurosci. 2014, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Z. Aging Kidney and Aging-Related Disease. In Aging and Aging-Related Diseases; Springer: Singapore, 2018; pp. 169–187. [Google Scholar]
- Gui, Y.; Dai, C. mTOR Signaling in Kidney Diseases. Kidney360 2020, 1, 1319–1327. [Google Scholar] [CrossRef]
- Zhou, S.; Ai, Z.; Li, W.; You, P.; Wu, C.; Li, L.; Hu, Y.; Ba, Y. Deciphering the Pharmacological Mechanisms of Taohe-Chengqi Decoction Extract Against Renal Fibrosis Through Integrating Network Pharmacology and Experimental Validation In Vitro and In Vivo. Front. Pharmacol. 2020, 11, 425. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Xiong, H.; Hu, Q.; Chen, Y.; Zhang, W.; Ma, X.; Zhao, Y. Deciphering the toxicity-effect relationship and action patterns of traditional Chinese medicines from a smart data perspective: A comprehensive review. Front. Pharmacol. 2023, 14, 1278014. [Google Scholar] [CrossRef]
- Ghelani, H.; Razmovski-Naumovski, V.; Chang, D.; Nammi, S. Chronic treatment of curcumin improves hepatic lipid metabolism and alleviates the renal damage in adenine-induced chronic kidney disease in Sprague-Dawley rats. BMC Nephrol. 2019, 20, 431. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.M.; Salem, A. Renoprotective effect of curcumin on acetaminophen-induced nephrotoxicity in rats. J. Chem. Pharm. Res. 2016, 8, 773–779. [Google Scholar]
- Trujillo, J.; Chirino, Y.I.; Molina-Jijón, E.; Andérica-Romero, A.C.; Tapia, E.; Pedraza-Chaverrí, J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol. 2013, 1, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Zanjaninejad, B.; Aflaki, E.; Nazarinia, M.; Azad, F.; Malekmakan, L.; Dehghanzadeh, G.-R. Oral Supplementation of Turmeric Decreases Proteinuria, Hematuria, and Systolic Blood Pressure in Patients Suffering From Relapsing or Refractory Lupus Nephritis: A Randomized and Placebo-controlled Study. J. Ren. Nutr. 2012, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Vanaie, A.; Shahidi, S.; Iraj, B.; Siadat, Z.D.; Kabirzade, M.; Shakiba, F.; Mohammadi, M.; Parvizian, H. Curcumin as a major active component of turmeric attenuates proteinuria in patients with overt diabetic nephropathy. J. Res. Med. Sci. 2019, 24, 77. [Google Scholar] [PubMed]
- Asgharpour, M.; Tolouian, A.; Bhaskar, L.V.; Tolouian, R.; Massoudi, N. Herbal antioxidants and renal ischemic-reperfusion injury; an updated review. Apoptosis 2020, 6, 7. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, L.; Li, G.S.; Wang, J. The anti-inflammatory effects of curcumin on renal ischemia-reperfusion injury in rats. Ren. Fail. 2018, 40, 680–686. [Google Scholar] [CrossRef]
- Liu, G.Y.; Wang, Q.; Shi, Y.; Peng, X.F.; Liu, H.; Peng, Y.M.; He, L.Y. Resveratrol Attenuates Adriamycin-Induced Focal Segmental Glomerulosclerosis through C3aR/C5aR-Sphingosine Kinase 1 Pathway. Pharmacology 2017, 100, 253–260. [Google Scholar] [CrossRef]
- Zhang, T.; Chi, Y.; Kang, Y.; Lu, H.; Niu, H.; Liu, W.; Li, Y. Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC-1α mediated attenuation of mitochondrial oxidative stress. J. Cell. Physiol. 2019, 234, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, R.; Zhao, L.; Ma, S.; Qin, G. Resveratrol ameliorates renal damage by inhibiting oxidative stress-mediated apoptosis of podocytes in diabetic nephropathy. Eur. J. Pharmacol. 2020, 885, 173387. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.-A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-S.; Ding, D.-F.; Chen, S.; Dong, C.-L.; Ye, X.-L.; Yuan, Y.-G.; Feng, Y.-M.; You, N.; Xu, J.-R.; Miao, H.; et al. Resveratrol protects podocytes against apoptosis via stimulation of autophagy in a mouse model of diabetic nephropathy. Sci. Rep. 2017, 7, 45692. [Google Scholar] [CrossRef]
- Llarena, M.; Andrade, F.; Hasnaoui, M.; Portillo, M.P.; Perez-Matute, P.; Arbones-Mainar, J.M.; Hijona, E.; Villanueva-Millan, M.J.; Aguirre, L.; Carpene, C.; et al. Potential renoprotective effects of piceatannol in ameliorating the early-stage nephropathy associated with obesity in obese Zucker rats. J. Physiol. Biochem. 2016, 72, 555–566. [Google Scholar] [CrossRef]
- Huang, D.-D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Lin, S.-L.; Chen, Y.-M.; Wu, V.-C.; Yang, W.-S.; Wu, K.-D. Downregulation of angiotensin type 1 receptor and nuclear factor-κB by sirtuin 1 contributes to renoprotection in unilateral ureteral obstruction. Sci. Rep. 2016, 6, 33705. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; An, X.; Guo, Y.; Gu, J.; Xie, T.; Wu, Q.; Sui, X. Meta-Analysis of Astragalus-Containing Traditional Chinese Medicine Combined With Chemotherapy for Colorectal Cancer: Efficacy and Safety to Tumor Response. Front. Oncol. 2019, 9, 749. [Google Scholar] [CrossRef]
- Guo, L.; Bai, S.-P.; Zhao, L.; Wang, X.-H. Astragalus polysaccharide injection integrated with vinorelbine and cisplatin for patients with advanced non-small cell lung cancer: Effects on quality of life and survival. Med. Oncol. 2012, 29, 1656–1662. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.-H.; Park, S.K.; Chang, M.S. Astragalus membranaceus Ameliorates Reproductive Toxicity Induced by Cyclophosphamide in Male Mice. Phytother. Res. 2012, 26, 1418–1421. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.-J.; Ding, H.-H.; Tang, L.-Q. Berberine as a promising anti-diabetic nephropathy drug: An analysis of its effects and mechanisms. Eur. J. Pharmacol. 2015, 760, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-F.; Zhao, T.-T.; Zhang, H.-J.; Huang, X.-R.; Zhang, W.-K.; Zhang, L.; Yan, M.-H.; Dong, X.; Wang, H.; Wen, Y.-M.; et al. Renoprotective effect of berberine on type 2 diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2015, 42, 662–670. [Google Scholar] [CrossRef]
- Qiu, Y.-Y.; Tang, L.-Q.; Wei, W. Berberine exerts renoprotective effects by regulating the AGEs-RAGE signaling pathway in mesangial cells during diabetic nephropathy. Mol. Cell. Endocrinol. 2017, 443, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zong, G.-n.; Wu, H.; Zhang, K.-q. Podoplanin mediates the renoprotective effect of berberine on diabetic kidney disease in mice. Acta Pharmacol. Sin. 2019, 40, 1544–1554. [Google Scholar] [CrossRef]
- Hasanein, P.; Riahi, H. Preventive use of berberine in inhibition of lead-induced renal injury in rats. Environ. Sci. Pollut. Res. 2018, 25, 4896–4903. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.-Q.; Ni, W.-J.; Cai, M.; Ding, H.-H.; Liu, S.; Zhang, S.-T. Renoprotective effects of berberine and its potential effect on the expression of β-arrestins and intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in streptozocin-diabetic nephropathy rats. J. Diabetes 2016, 8, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Gao, Y.; Wang, X.; Wu, X.; Zou, D.; Zhu, Z.; Han, Z.; Wang, T.; Shi, Y. Emodin mitigates podocytes apoptosis induced by endoplasmic reticulum stress through the inhibition of the PERK pathway in diabetic nephropathy. Drug Des. Dev. Ther. 2018, 12, 2195. [Google Scholar] [CrossRef]
- Zhu, X.-L.; Wang, Y.-J.; Yang, Y.-Ζ.; Yang, R.-C.; Zhu, B.; Zhang, Y.H.; Lin, Y.; Lu, Y.; Li, X.-F.; O’Byrne, K.T. Suppression of lipopolysaccharide-induced upregulation of toll-like receptor 4 by emodin in mouse proximal tubular epithelial cells. Mol. Med. Rep. 2012, 6, 493–500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.; Song, R.; Zhang, Y. Triptolide reduces podocytes injury through blocking ERK and JNK pathways in passive Heymann nephritis (PHN) model. Int. J. Clin. Exp. Med. 2017, 10, 692–699. [Google Scholar]
- Wang, X.W.; Tian, R.M.; Yang, Y.Q.; Wang, K.; Li, E.N.; Han, X.D.; Bao, K.; Mao, W.; Xu, H.T.; Liu, B.; et al. Tripterygium glycoside fraction n2 ameliorates adriamycin-induced nephrotic syndrome in rats by suppressing apoptosis. J. Ethnopharmacol. 2020, 257, 10. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, Z.; Chen, X.; Tang, L.; Zhao, X.; Yuan, Q.; Cai, G. Tripterygium wilfordii Hook F (a traditional Chinese medicine) for primary nephrotic syndrome. Cochrane Database Syst. Rev. 2013, 8, CD008568. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, J.; Li, M.; Guan, C.; Wang, W.; Zhu, S.; Qiu, Y.; Huang, M.; Huang, Z. Role of Nrf2 in protection against triptolide-induced toxicity in rat kidney cells. Toxicol. Lett. 2012, 213, 194–202. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Y. Renal fibrosis in 2015: Understanding the mechanisms of kidney fibrosis. Nat. Rev. Nephrol. 2016, 12, 68. [Google Scholar] [CrossRef]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide Attenuates Inflammatory Response in Membranous Glomerulo-Nephritis Rat via Downregulation of NF-κB Signaling Pathway. Kidney Blood Press. Res. 2016, 41, 901–910. [Google Scholar] [CrossRef]
- Peng, X.; Liu, J.; Wang, C.; Han, Z.; Shu, Y.; Li, X.; Zhou, L.; Qiu, M. Unusual prenylated phenols with antioxidant activities from Ganoderma cochlear. Food Chem. 2015, 171, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Futrakul, N.; Panichakul, T.; Butthep, P.; Futrakul, P.; Jetanalin, P.; Patumraj, S.; Siriviriyakul, P. Ganoderma lucidum suppresses endothelial cell cytotoxicity and proteinuria in persistent proteinuric focal segmental glomerulosclerosis (FSGS) nephrosis. Clin. Hemorheol. Microcirc. 2004, 31, 267–272. [Google Scholar] [PubMed]
- Futrakul, N.; Boonyen, M.; Patumraj, S.; Siriviriyakul, P.; Tosukhowong, P.; Futrakul, P. Treatment of glomerular endothelial dysfunction in steroid-resistant nephrosis with Ganoderma lucidum, vitamins C, E and vasodilators. Clin. Hemorheol. Microcirc. 2003, 29, 205–210. [Google Scholar]
- Gill, S.K.; Rieder, M.J. Toxicity of a traditional Chinese medicine, Ganoderma lucidum, in children with cancer. J. Popul. Ther. Clin. Pharmacol. 2008, 15, 275–285. [Google Scholar]
- Cai, Z.; Wong, C.K.; Dong, J.; Jiao, D.; Chu, M.; Leung, P.C.; Lau, C.B.S.; Lau, C.P.; Tam, L.S.; Lam, C.W.K. Anti-inflammatory activities of Ganoderma lucidum (Lingzhi) and San-Miao-San supplements in MRL/lpr mice for the treatment of systemic lupus erythematosus. Chin. Med. 2016, 11, 23. [Google Scholar] [CrossRef]
- Liu, C.; Song, J.; Teng, M.; Zheng, X.; Li, X.; Tian, Y.; Pan, M.; Li, Y.; Lee, R.J.; Wang, D. Antidiabetic and Antinephritic Activities of Aqueous Extract of Cordyceps militaris Fruit Body in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats. Oxidative Med. Cell. Longev. 2016, 2016, 9685257. [Google Scholar] [CrossRef]
- He, L.-Y.; Niu, S.-Q.; Yang, C.-X.; Tang, P.; Fu, J.-J.; Tan, L.; Li, Y.; Hua, Y.-N.; Liu, S.-J.; Guo, J.-L. Cordyceps proteins alleviate lupus nephritis through modulation of the STAT3/mTOR/NF-kB signaling pathway. J. Ethnopharmacol. 2023, 309, 116284. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Chen, S.; Xie, Y.; Chen, D.; Su, J.; Shuai, O.; Jiao, C.; Zuo, D. Cordycepin, a Characteristic Bioactive Constituent in Cordyceps militaris, Ameliorates Hyperuricemia through URAT1 in Hyperuricemic Mice. Front. Microbiol. 2018, 9, 58. [Google Scholar] [CrossRef]
- Park, S.J.; Jang, H.-J.; Hwang, I.-H.; Kim, J.M.; Jo, E.; Lee, M.-G.; Jang, I.-S.; Joo, J.C. Cordyceps militaris Extract Inhibits the NF-κB pathway and Induces Apoptosis through MKK7-JNK Signaling Activation in TK-10 Human Renal Cell Carcinoma. Nat. Prod. Commun. 2018, 13, 1934578X1801300422. [Google Scholar] [CrossRef]
- Yazd, Z.N.E.; Noshahr, Z.S.; Hosseinian, S.; Shafei, M.N.; Bideskan, A.E.; Mohebbati, R.; Heravi, N.E.; Shahraki, S.; Mahzari, S.; Rad, A.K. Renoprotective effect of Plantago major against proteinuria and apoptosis induced by adriamycin in rat. J. Pharmacopunct. 2019, 22, 35. [Google Scholar] [CrossRef]
- Wei, H.; Tao, X.; Di, P.; Yang, Y.; Li, J.; Qian, X.; Feng, J.; Chen, W. Effects of Traditional Chinese Medicine Wuzhi Capsule on Pharmacokinetics of Tacrolimus in Rats. Drug Metab. Dispos. 2013, 41, 1398. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.-p.; Yan, Q.; Chen, H.-z. Study on the clinical application of wuzhi capsule after renal transplantation. Zhongguo Zhong Xi Yi Jie He Za Zhi 2011, 31, 1213–1215. [Google Scholar] [PubMed]
- Wang, K.; Qu, Q.S.; Zhang, Y.X.; Miao, S.Z.; Jiang, X. Effects of Wuzhi capsule on blood concentration of tacrolimus after renal transplantation. J. Biol. Regul. Homeost. Agents 2016, 30, 155–159. [Google Scholar]
- Yan, L.; Yang, Z.Q.; Shi, Y.Y.; Ren, J.; Yang, C.L.; Wan, Z.L.; Bai, Y.J.; Luo, L.M.; Wang, L.L.; Li, Y. Effects of Wuzhi Capsules on Blood Concentration of Tacrolimus in Renal Transplant Recipients. Ann. Transplant. 2019, 24, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, M.; Zhao, Z.; Sun, X.; Li, J.; Wang, W.; Huang, M.; Huang, Z. Protective effect of Wuzhi tablet (Schisandra sphenanthera extract) against cisplatin-induced nephrotoxicity via Nrf2-mediated defense response. Phytomedicine 2015, 22, 528–535. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, X.; Xie, L.; Cai, B. Pharmacokinetic/pharmacodynamic modelling of effective components of Fangji Huangqi Tang for its treatment of nephrotic syndrome. New J. Chem. 2019, 43, 338–347. [Google Scholar] [CrossRef]
- Xu, W.; Meng, K.; Tu, Y.; Tanaka, S.; Onda, K.; Sugiyama, K.; Hirano, T.; Yamada, H. Tetrandrine potentiates the glucocorticoid pharmacodynamics via inhibiting P-glycoprotein and mitogen-activated protein kinase in mitogen-activated human peripheral blood mononuclear cells. Eur. J. Pharmacol. 2017, 807, 102–108. [Google Scholar] [CrossRef]
- Yang, L.; Li, A.; Chen, M.; Yan, Y.; Liu, Y.; Li, K.; Jia, J.; Qin, X. Comprehensive investigation of mechanism and effective ingredients of Fangji Huangqi Tang by serum pharmacochemistry and network pharmacology. Biomed. Chromatogr. 2020, 34, e4785. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, C.; Yin, J.; Yu, D.; Wan, F.; Tang, X.; Jiang, X. Tetrandrine Suppresses Transient Receptor Potential Cation Channel Protein 6 Overexpression-Induced Podocyte Damage via Blockage of RhoA/ROCK1 Signaling. Drug Des. Dev. Ther. 2020, 14, 361. [Google Scholar] [CrossRef]
- Chen, J.; Du, L.; Li, J.; Song, H. Epigallocatechin-3-gallate attenuates cadmium-induced chronic renal injury and fibrosis. Food Chem. Toxicol. 2016, 96, 70–78. [Google Scholar] [CrossRef]
- Shen, H.; Na, W.; Zhao, H.; Xiao, H.; Guo, F.; Zhao, M. Effects of Epigallocatechin-3-gallate on Oxidative Stress and Inflammatory Factors in the Kidney Tissues of Rats with Paraquat Poisoning. J. China Med. Univ. 2017, 46, 210–213,218. [Google Scholar]
- Mohan, T.; Narasimhan, K.K.S.; Ravi, D.B.; Velusamy, P.; Chandrasekar, N.; Chakrapani, L.N.; Srinivasan, A.; Karthikeyan, P.; Kannan, P.; Tamilarasan, B.; et al. Role of Nrf2 dysfunction in the pathogenesis of diabetic nephropathy: Therapeutic prospect of epigallocatechin-3-gallate. Free. Radic. Biol. Med. 2020, 160, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Dumludag, B.; Derici, M.K.; Sutcuoglu, O.; Ogut, B.; Pasaoglu, O.T.; Gonul, I.I.; Derici, U. Role of silymarin (Silybum marianum) in the prevention of colistin-induced acute nephrotoxicity in rats. Drug Chem. Toxicol. 2020, 45, 568–575. [Google Scholar] [CrossRef]
- Fallahzadeh, M.K.; Dormanesh, B.; Sagheb, M.M.; Roozbeh, J.; Vessal, G.; Pakfetrat, M.; Daneshbod, Y.; Kamali-Sarvestani, E.; Lankarani, K.B. Effect of Addition of Silymarin to Renin-Angiotensin System Inhibitors on Proteinuria in Type 2 Diabetic Patients with Overt Nephropathy: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Kidney Dis. 2012, 60, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, F.; Sadighi, S.; Dashti-Khavidaki, S.; Shahi, F.; Mirzania, M.; Abdollahi, A.; Ghahremani, M.-H. Effect of Silymarin Administration on Cisplatin Nephrotoxicity: Report from A Pilot, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Phytother. Res. 2015, 29, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.; Ayhanci, A.; Kutlu, H.M.; Musmul, A. Potential therapeutic effects of silymarin and silymarin-loaded solid lipidnanoparticles on experimental kidney damage in BALB/c mice: Biochemical and histopathological evaluation. Turk. J. Biol. 2016, 40, 807–814. [Google Scholar] [CrossRef]
- Yang, D.; Jia, W.; Zhu, Y.Z. Leonurine, a Potential Agent of Traditional Chinese Medicine: Recent Updates and Future Perspectives. Nat. Prod. Commun. 2016, 11, 1934578X1601101130. [Google Scholar] [CrossRef]
- Gong, B.; Gou, X.; Han, T.; Qi, Y.; Ji, X.; Bai, J. Protective effects of rutin on kidney in type 1 diabetic mice. Pak. J. Pharm. Sci. 2020, 33, 597–603. [Google Scholar] [PubMed]
- Ma, J.-Q.; Liu, C.-M.; Yang, W. Protective effect of rutin against carbon tetrachloride-induced oxidative stress, inflammation and apoptosis in mouse kidney associated with the ceramide, MAPKs, p53 and calpain activities. Chem.-Biol. Interact. 2018, 286, 26–33. [Google Scholar] [CrossRef]
- Cheng, H.; Bo, Y.; Shen, W.; Tan, J.; Jia, Z.; Xu, C.; Li, F. Leonurine ameliorates kidney fibrosis via suppressing TGF-β and NF-κB signaling pathway in UUO mice. Int. Immunopharmacol. 2015, 25, 406–415. [Google Scholar] [CrossRef]
- Diwan, V.; Brown, L.; Gobe, G.C. The flavonoid rutin improves kidney and heart structure and function in an adenine-induced rat model of chronic kidney disease. J. Funct. Foods 2017, 33, 85–93. [Google Scholar] [CrossRef]
- Wang, B.; Liu, D.; Zhu, Q.-H.; Li, M.; Chen, H.; Guo, Y.; Fan, L.-P.; Yue, L.-S.; Li, L.-Y.; Zhao, M. Rutin ameliorates kidney interstitial fibrosis in rats with obstructive nephropathy. Int. Immunopharmacol. 2016, 35, 77–84. [Google Scholar] [CrossRef]
- Ma, S.-X.; Shang, Y.-Q.; Zhang, H.-Q.; Su, W. Action mechanisms and therapeutic targets of renal fibrosis. J. Nephrol. Adv. 2018, 1, 4. [Google Scholar] [CrossRef]
- Chen, L.; Yang, T.; Lu, D.-W.; Zhao, H.; Feng, Y.-L.; Chen, H.; Chen, D.-Q.; Vaziri, N.D.; Zhao, Y.-Y. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed. Pharmacother. 2018, 101, 670–681. [Google Scholar] [CrossRef]
- Tu, Q.; Li, Y.; Jin, J.; Jiang, X.; Ren, Y.; He, Q. Curcumin Alleviates Diabetic Nephropathy via Inhibiting Podocyte Mesenchymal Transdifferentiation and Inducing Autophagy in Rats and MPC5 Cells; Taylor & Francis Ltd.: Abingdon, UK, 2019; Volume 57, pp. 778–786. [Google Scholar]
- Di Tu, Q.; Jin, J.; Hu, X.; Ren, Y.; Zhao, L.; He, Q. Curcumin Improves the Renal Autophagy in Rat Experimental Membranous Nephropathy via Regulating the PI3K/AKT/mTOR and Nrf2/HO-1 Signaling Pathways. BioMed Res. Int. 2020, 2020, 7069052. [Google Scholar] [CrossRef]
- Lu, M.; Li, H.; Liu, W.; Zhang, X.; Li, L.; Zhou, H. Curcumin attenuates renal interstitial fibrosis by regulating autophagy and retaining mitochondrial function in unilateral ureteral obstruction rats. Basic Clin. Pharmacol. Toxicol. 2021, 128, 594–604. [Google Scholar] [CrossRef]
- Xu, B.; Yuan, C.-W.; Zhang, J.-E. Curcumin Inhibits Proliferation of Renal Cell Carcinoma in vitro and in vivo by Regulating miR-148/ADAMTS18 through Suppressing Autophagy. Chin. J. Integr. Med. 2023, 29, 699–706. [Google Scholar] [CrossRef]
- Yang, H.; Xu, W.; Zhou, Z.; Liu, J.; Li, X.; Chen, L.; Weng, J.; Yu, Z. Curcumin Attenuates Urinary Excretion of Albumin in Type II Diabetic Patients with Enhancing Nuclear Factor Erythroid-Derived 2-Like 2 (Nrf2) System and Repressing Inflammatory Signaling Efficacies. Exp. Clin. Endocrinol. Diabetes 2015, 123, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Samadian, F.; Dalili, N.; Poor-reza Gholi, F.; Fattah, M.; Malih, N.; Nafar, M.; Firoozan, A.; Ahmadpoor, P.; Samavat, S.; Ziaie, S. Evaluation of Curcumin’s effect on inflammation in hemodialysis patients. Clin. Nutr. ESPEN 2017, 22, 19–23. [Google Scholar] [CrossRef]
- Alvarenga, L.; Salarolli, R.; Cardozo, L.F.M.F.; Santos, R.S.; de Brito, J.S.; Kemp, J.A.; Reis, D.; de Paiva, B.R.; Stenvinkel, P.; Lindholm, B.; et al. Impact of curcumin supplementation on expression of inflammatory transcription factors in hemodialysis patients: A pilot randomized, double-blind, controlled study. Clin. Nutr. 2020, 39, 3594–3600. [Google Scholar] [CrossRef] [PubMed]
- Trakarnvanich, T. SUN-025 Curcuminoids can prevent contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures: A randomized controlled trial. Kidney Int. Rep. 2020, 5, S215. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, H.C.N.; Martins, T.F.P.; Santana, N.C.F.e.S.; Braga, C.C.; Silva, M.A.C.; Cunha, L.C.d.; Sugizaki, C.S.d.A.; Freitas, A.T.V.d.S.; Costa, N.A.; Peixoto, M.d.R.G. Antioxidant and anti-inflammatory response to curcumin supplementation in hemodialysis patients: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. ESPEN 2021, 44, 136–142. [Google Scholar] [CrossRef]
- Ahrabi, B.; Abbaszadeh, H.A.; Piryaei, A.; Shekari, F.; Ahmady Roozbahany, N.; Rouhollahi, M.; Azam Sayahpour, F.; Ahrabi, M.; Azimi, H.; Moghadasali, R. Autophagy-induced mesenchymal stem cell-derived extracellular vesicles ameliorated renal fibrosis in an in vitro model. BioImpacts 2023, 13, 359–372. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef]
- Wang, N.; Wei, R.B.; Li, Q.P.; Yang, X.; Chen, X.M. Protective effects of astragaloside in rats with adriamycin nephropathy and underlying mechanism. Chin. J. Nat. Med. 2016, 14, 270–277. [Google Scholar] [CrossRef] [PubMed]
- He, K.Q.; Li, W.Z.; Chai, X.Q.; Yin, Y.Y.; Jiang, Y.; Li, W.P. Astragaloside IV prevents kidney injury caused by iatrogenic hyperinsulinemia in a streptozotocin-induced diabetic rat model. Int. J. Mol. Med. 2018, 41, 1078–1088. [Google Scholar] [CrossRef]
- Mao, Q.; Chen, C.; Liang, H.; Zhong, S.; Cheng, X.; Li, L. Astragaloside IV inhibits excessive mesangial cell proliferation and renal fibrosis caused by diabetic nephropathy via modulation of the TGF-β1/Smad/miR-192 signaling pathway. Exp. Ther. Med. 2019, 18, 3053–3061. [Google Scholar] [CrossRef]
- Ju, Y.; Su, Y.; Chen, Q.; Ma, K.; Ji, T.; Wang, Z.; Li, W.; Li, W. Protective effects of Astragaloside IV on endoplasmic reticulum stress-induced renal tubular epithelial cells apoptosis in type 2 diabetic nephropathy rats. Biomed. Pharmacother. 2019, 109, 84–92. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, B.; Liu, X.; He, L.; Wang, T.; Yu, X.; Kang, Y.; Li, S. Astragaloside IV alleviates puromycin aminonucleoside-induced podocyte cytoskeleton injury through the Wnt/PCP pathway. Am. J. Transl. Res. 2020, 12, 3512–3521. [Google Scholar]
- Zhan, H.; Han, P.; Wang, M.; Wang, Y.; Weng, W.; Yu, X.; Yuan, C.; Li, Y.; Shao, M.; Sun, H. Combination of astragaloside IV and ACEi ameliorates renal injuries in db/db mice. Int. J. Clin. Exp. Pathol. 2020, 13, 827–836. [Google Scholar] [PubMed]
- Zhang, Y.; Tao, C.; Xuan, C.; Jiang, J.; Cao, W. Transcriptomic Analysis Reveals the Protection of Astragaloside IV against Diabetic Nephropathy by Modulating Inflammation. Oxidative Med. Cell. Longev. 2020, 2020, 9542165. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wang, L.; Ding, R.; Zhai, M.; Ge, R.; Zhou, P.; Wang, T.; Fang, H.; Wang, J.; Huang, J. Astragaloside IV acts through multi-scale mechanisms to effectively reduce diabetic nephropathy. Pharmacol. Res. 2020, 157, 104831. [Google Scholar] [CrossRef]
- Qin, L.-Y.; Guan, P.; Wang, J.-X.; Chen, Y.; Zhao, Y.-S.; Yang, S.-C.; Guo, Y.-J.; Wang, N.; Ji, E.-S. Therapeutic Potential of Astragaloside IV Against Adriamycin-Induced Renal Damage in Rats via Ferroptosis. Front. Pharmacol. 2022, 13, 812594. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tang, W.; Liu, W.; Hu, Z.; Pan, C. Astragaloside IV Alleviates Renal Tubular Epithelial-Mesenchymal Transition via CX3CL1-RAF/MEK/ERK Signaling Pathway in Diabetic Kidney Disease. Drug Des. Dev. Ther. 2022, 16, 1605–1620. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Liu, Y.; Zhang, Z.; Hu, Y.; Sun, D.; Li, S.; Fang, J. Astragaloside IV Inhibited Podocyte Pyroptosis in Diabetic Kidney Disease by Regulating SIRT6/HIF-1α Axis. DNA Cell Biol. 2023, 42, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, Y.; Zhang, X.; Zang, Y.; Zhang, Y.; Wang, L.; Wang, H.; Wang, Y.; Cao, A.; Peng, W. Astragaloside IV Protects Against Podocyte Injury via SERCA2-Dependent ER Stress Reduction AMPK Alpha-Regulated Autophagy Induction in Streptozotocin-Induced Diabetic Nephropathy; Nature Publishing Group: London, UK, 2017; Volume 7. [Google Scholar]
- Wang, X.; Gao, Y.; Tian, N.; Wang, T.; Shi, Y.; Xu, J.; Wu, B. Astragaloside IV inhibits glucose-induced epithelial-mesenchymal transition of podocytes through autophagy enhancement via the SIRT–NF-κB p65 axis. Sci. Rep. 2019, 9, 323. [Google Scholar] [CrossRef]
- Qu, X.; Gao, H.; Tao, L.; Zhang, Y.; Zhai, J.; Sun, J.; Song, Y.; Zhang, S. Astragaloside IV protects against cisplatin-induced liver and kidney injury via autophagy-mediated inhibition of NLRP3 in rats. J. Toxicol. Sci. 2019, 44, 167–175. [Google Scholar] [CrossRef]
- Zhu, Y.; Su, Y.; Zhang, J.; Zhang, Y.; Li, Y.; Han, Y.; Dong, X.; Li, W.; Li, W. Astragaloside IV alleviates liver injury in type 2 diabetes due to promotion of AMPK/mTOR-mediated autophagy. Mol. Med. Rep. 2021, 23, 437. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Zhan, Q.; Zeng, Y.; Peng, Z.; He, Q.; Tan, Q.; Cao, W.; Wang, S.; Wang, J. Astragaloside IV Blunts Epithelial–Mesenchymal Transition and G2/M Arrest to Alleviate Renal Fibrosis via Regulating ALDH2-Mediated Autophagy. Cells 2023, 12, 1777. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.; Kwong, A.S.K.; Tsui, P.N.; Cheung, S.C.Y.; Chan, G.C.W.; Choi, W.F.; Yiu, W.H.; Zhang, Y.; Wong, M.M.-Y.; Zhang, Z.-J.; et al. Efficacy, safety and response predictors of adjuvant astragalus for diabetic kidney disease (READY): Study protocol of an add-on, assessor-blind, parallel, pragmatic randomised controlled trial. BMJ Open 2021, 11, e042686. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Liu, C.; Chen, Z.; Wang, D. Astragaloside IV ameliorates high glucose-induced renal tubular epithelial-mesenchymal transition by blocking mTORC1/p70S6K signaling in HK-2 cells. Int. J. Mol. Med. 2019, 43, 709–716. [Google Scholar] [CrossRef]
- Zhao, T.; Tian, J.; Xu, T.; Zhang, X.; Xiang, Q.; Xiong, J.; Gui, D.; Xu, Y. Astragaloside IV Improves the Barrier Damage in Diabetic Glomerular Endothelial Cells Stimulated by High Glucose and High Insulin. Evid.-Based Complement. Altern. Med. 2022, 2022, 7647380. [Google Scholar] [CrossRef]
- Shen, Q.; Fang, J.; Guo, H.; Su, X.; Zhu, B.; Yao, X.; Wang, Y.; Cao, A.; Wang, H.; Wang, L. Astragaloside IV attenuates podocyte apoptosis through ameliorating mitochondrial dysfunction by up-regulated Nrf2-ARE/TFAM signaling in diabetic kidney disease. Free. Radic. Biol. Med. 2023, 203, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Han, Y.; Hu, Y.; Wu, X.; Wang, Y.; Zhang, X.; Fu, J.; Zou, X.; Zhang, J.; Chen, X.; et al. Targeting HO-1 by Epigallocatechin-3-Gallate Reduces Contrast-Induced Renal Injury via Anti-Oxidative Stress and Anti-Inflammation Pathways. PLoS ONE 2016, 11, e0149032. [Google Scholar] [CrossRef]
- Hammad, F.T.; Lubbad, L. The effect of epigallocatechin-3-gallate on the renal dysfunction in the obstructed kidney in the rat. Int. J. Physiol. Pathophysiol. Pharmacol. 2017, 9, 119–126. [Google Scholar] [PubMed]
- Luo, D.; Xu, J.; Chen, X.; Zhu, X.; Liu, S.; Li, J.; Xu, X.; Ma, X.; Zhao, J.; Ji, X. (−)-Epigallocatechin-3-gallate (EGCG) attenuates salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Sci. Rep. 2020, 10, 4783. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Lei, Y.; Liu, M. Potential ameliorative effects of epigallocatechin-3-gallate against cigarette smoke exposure induced renal and hepatic deficits. Ecotoxicol. Environ. Saf. 2020, 191, 110202. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Khamchun, S.; Kapincharanon, C.; Thongboonkerd, V. Protective effect of epigallocatechin-3-gallate (EGCG) via Nrf2 pathway against oxalate-induced epithelial mesenchymal transition (EMT) of renal tubular cells. Sci. Rep. 2016, 6, 30233. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yu, J.; Sun, X.; Qu, S.; Zhang, H.; Hu, M.; Yang, S.; Zhou, P. Impact of epigallocatechin-3-gallate on expression of nuclear factor erythroid 2-related factor 2 and γ-glutamyl cysteine synthetase genes in oxidative stress-induced mouse renal tubular epithelial cells. Mol. Med. Rep. 2018, 17, 7952–7958. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Peerapen, P.; Nilnumkhum, A.; Plumworasawat, S.; Sueksakit, K.; Thongboonkerd, V. Epigallocatechin-3-gallate prevents TGF-β1-induced epithelial-mesenchymal transition and fibrotic changes of renal cells via GSK-3β/β-catenin/Snail1 and Nrf2 pathways. Nephrol. Dial. Transplant. 2019, 34, gfz106-FP080. [Google Scholar] [CrossRef]
- Kanlaya, R.; Kapincharanon, C.; Fong-ngern, K.; Thongboonkerd, V. Induction of mesenchymal-epithelial transition (MET) by epigallocatechin-3-gallate to reverse epithelial-mesenchymal transition (EMT) in SNAI1-overexpressed renal cells: A potential anti-fibrotic strategy. J. Nutr. Biochem. 2022, 107, 109066. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, J.; Tan, R.; Tian, Q.; Guan, L.; Niu, S.; Huang, S.; Huang, J.; Yan, Y.; Xiang, Y. Ginsenoside Rg1 and Resveratrol Alleviate Acute Kidney Injury Induced by Cisplatin via Downregulation of Autophagy in Mice. Yangtze Med. 2021, 5, 12–22. [Google Scholar] [CrossRef]
- Brown, K.; Theofanous, D.; Britton, R.G.; Aburido, G.; Pepper, C.; Sri Undru, S.; Howells, L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. Int. J. Mol. Sci. 2024, 25, 747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Xiao, D.; Zhang, J.; Wang, X.; Guan, F.; Zhang, M.; Chen, L. Highly bioavailable berberine formulation ameliorates diabetic nephropathy through the inhibition of glomerular mesangial matrix expansion and the activation of autophagy. Eur. J. Pharmacol. 2020, 873, 172955. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Li, Q.; Zhou, S.; Yu, R.; Wu, C.; Chen, J.; Xiao, Y.; Chen, H.; Song, J.; Pan, Y.; et al. Berberine ameliorates contrast-induced acute kidney injury by regulating HDAC4-FoxO3a axis-induced autophagy: In vivo and in vitro. Phytother. Res. 2023, 38, 1761–1780. [Google Scholar] [CrossRef]
- Li, C.; Guan, X.-M.; Wang, R.-Y.; Xie, Y.-S.; Zhou, H.; Ni, W.-J.; Tang, L.-Q. Berberine mitigates high glucose-induced podocyte apoptosis by modulating autophagy via the mTOR/P70S6K/4EBP1 pathway. Life Sci. 2020, 243, 117277. [Google Scholar] [CrossRef]
- Kandemir, F.M.; Ileriturk, M.; Gur, C. Rutin protects rat liver and kidney from sodium valproate-induce damage by attenuating oxidative stress, ER stress, inflammation, apoptosis and autophagy. Mol. Biol. Rep. 2022, 49, 6063–6074. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, X.; Liu, Y.; Zhao, T.; Sun, Z.; Liu, P.; Xiang, Q.; Xiong, J.; Du, X.; Yang, X.; et al. Rutin alleviates EndMT by restoring autophagy through inhibiting HDAC1 via PI3K/AKT/mTOR pathway in diabetic kidney disease. Phytomedicine 2023, 112, 154700. [Google Scholar] [CrossRef]
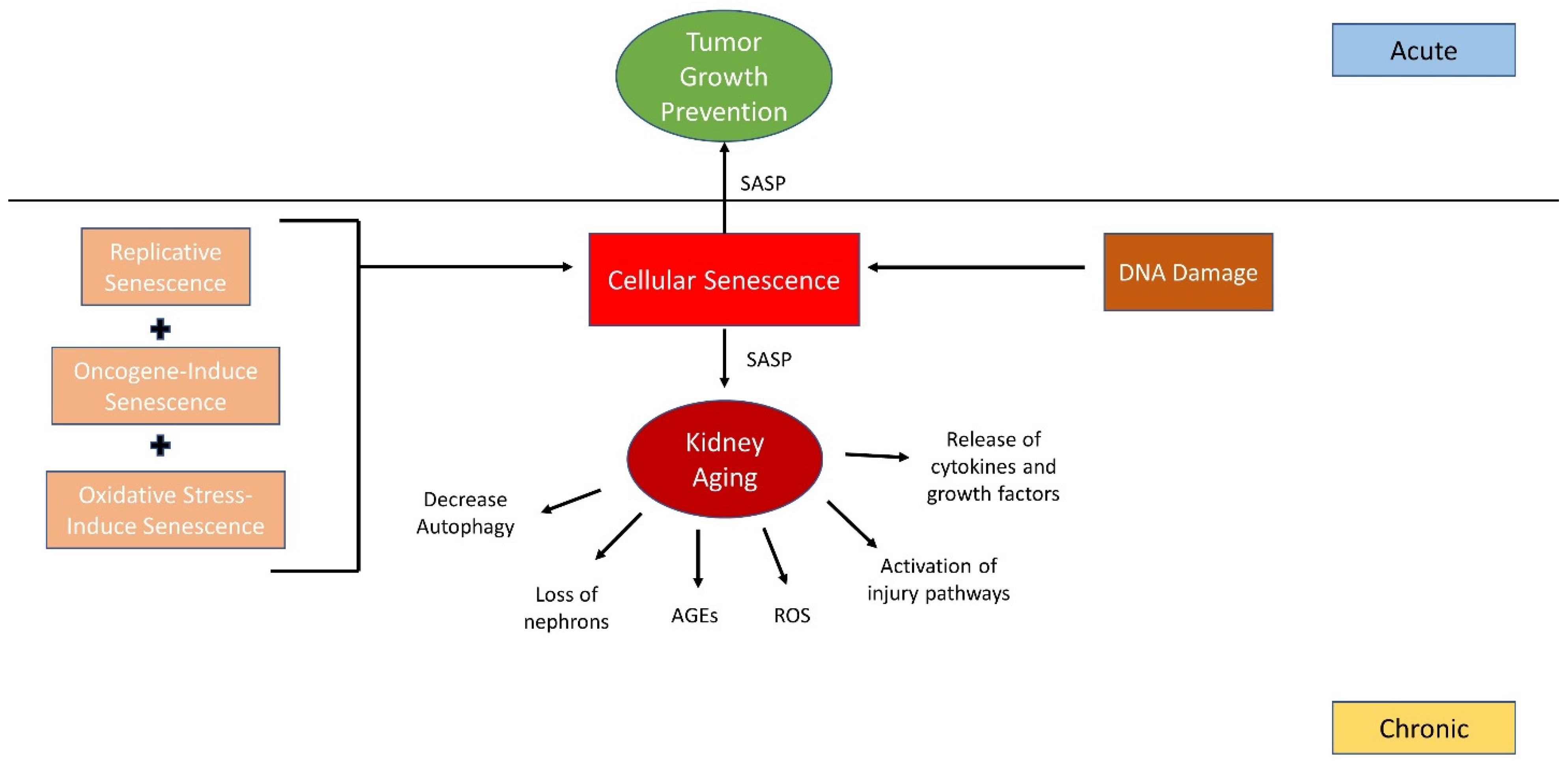
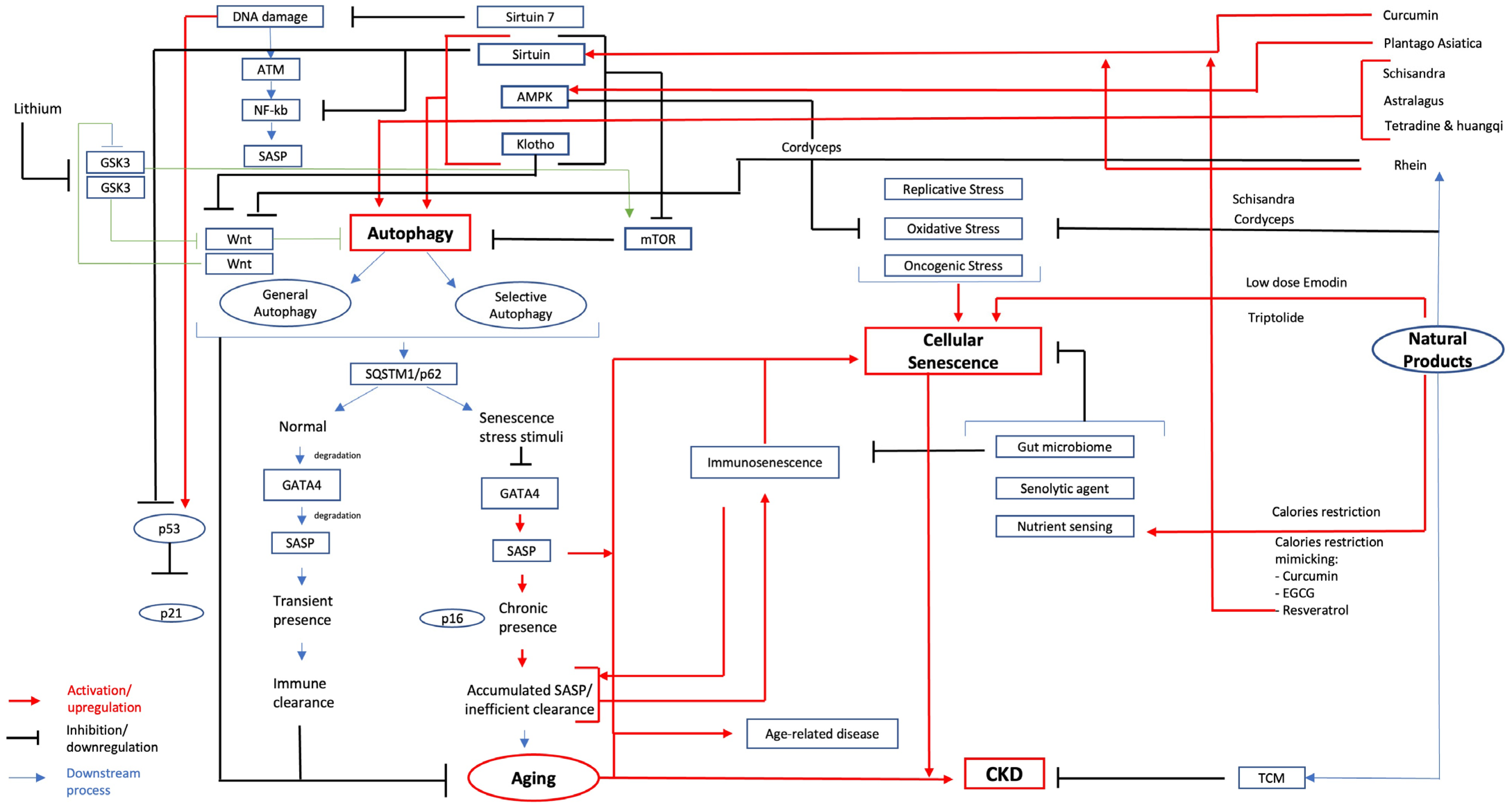
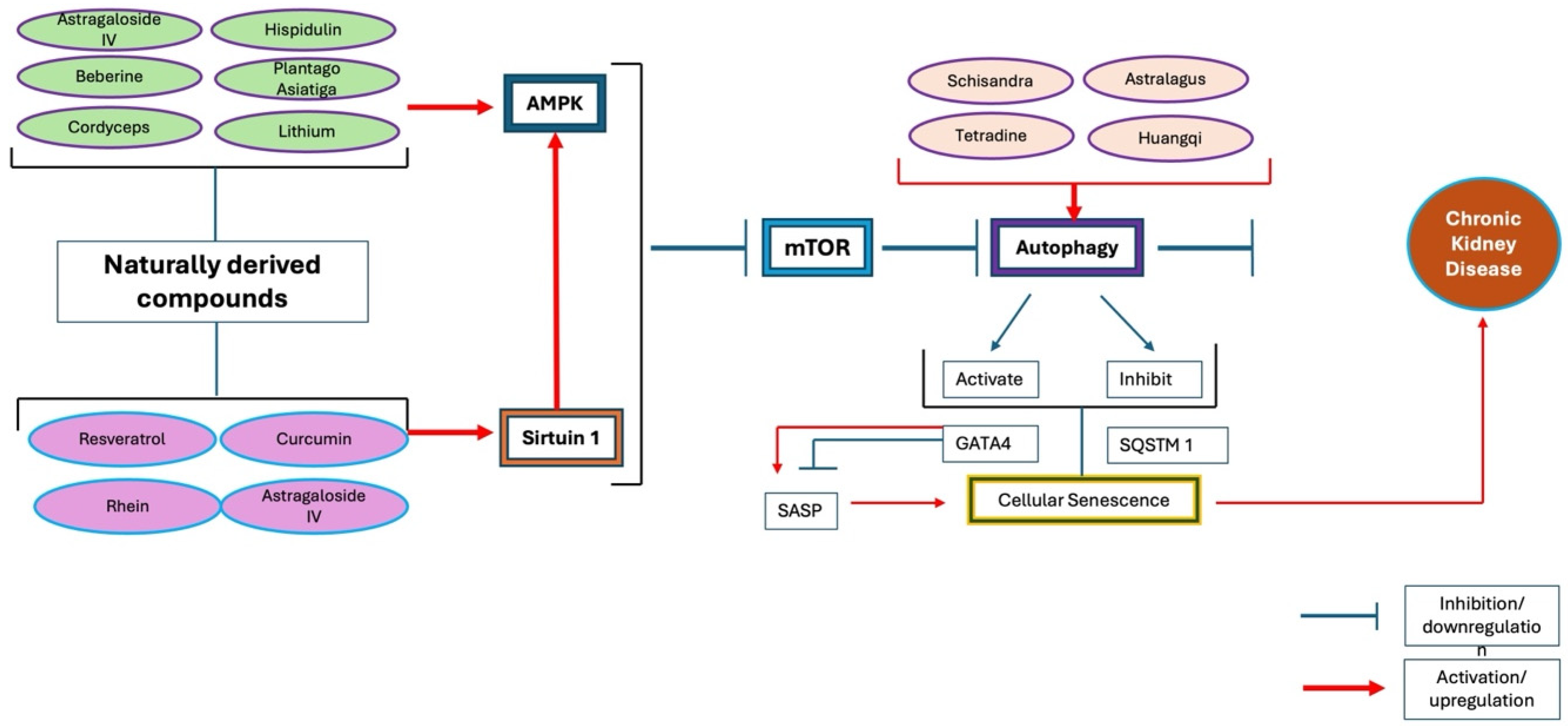
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teh, Y.M.; Mualif, S.A.; Mohd Noh, N.I.; Lim, S.K. The Potential of Naturally Derived Compounds for Treating Chronic Kidney Disease: A Review of Autophagy and Cellular Senescence. Int. J. Mol. Sci. 2025, 26, 3. https://doi.org/10.3390/ijms26010003
Teh YM, Mualif SA, Mohd Noh NI, Lim SK. The Potential of Naturally Derived Compounds for Treating Chronic Kidney Disease: A Review of Autophagy and Cellular Senescence. International Journal of Molecular Sciences. 2025; 26(1):3. https://doi.org/10.3390/ijms26010003
Chicago/Turabian StyleTeh, Yoong Mond, Siti Aisyah Mualif, Nur Izzati Mohd Noh, and Soo Kun Lim. 2025. "The Potential of Naturally Derived Compounds for Treating Chronic Kidney Disease: A Review of Autophagy and Cellular Senescence" International Journal of Molecular Sciences 26, no. 1: 3. https://doi.org/10.3390/ijms26010003
APA StyleTeh, Y. M., Mualif, S. A., Mohd Noh, N. I., & Lim, S. K. (2025). The Potential of Naturally Derived Compounds for Treating Chronic Kidney Disease: A Review of Autophagy and Cellular Senescence. International Journal of Molecular Sciences, 26(1), 3. https://doi.org/10.3390/ijms26010003







