Mechanism and Application of Biomaterials Targeting Reactive Oxygen Species and Macrophages in Inflammation
Abstract
1. Introduction
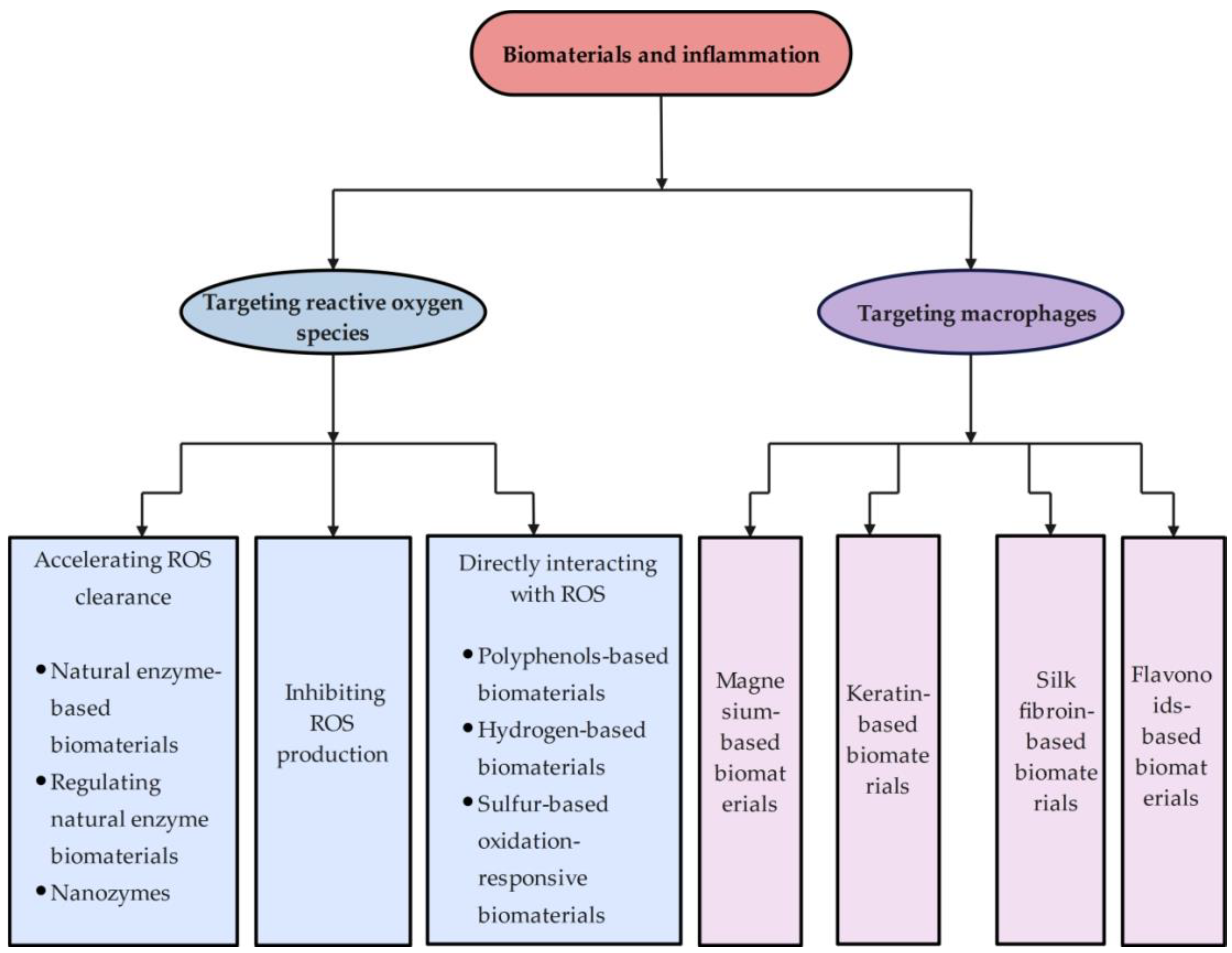
2. Biomaterials Targeting Scavenging Reactive Oxygen Species
2.1. ROS, Inflammation and Biomaterials
2.2. Classification of Biomaterials Targeting Scavenging ROS
2.2.1. Accelerating ROS Clearance
Natural Enzyme-Based Biomaterials
Regulating Natural Enzyme Biomaterials
Nanozymes
| Classification | Actions in Diseases | References |
|---|---|---|
| Natural enzyme-based biomaterials |
| [41,42,43,48] |
| Regulating natural enzyme biomaterials |
| [44,49] |
| Nanozymes |
| [46,47] |
2.2.2. Directly Interacting with ROS
Polyphenol-Based Biomaterials
Hydrogen-Based Biomaterials
Sulfur-Based Oxidation-Responsive Biomaterials
| Classification | Actions in Diseases | References | |
|---|---|---|---|
| Polyphenol-based biomaterials | Resveratrol-based materials |
| [52] |
| Curcumin-based material |
| [53,54] | |
| Epigallocatechin gallate (EGCG) |
| [55,56] | |
| Hydrogen-based biomaterials | - |
| [67,68] |
| Sulfur-based oxidation-responsive materials | Thioether/sulfoxide- contained biomaterials |
| [78,79] |
| Hydrogen sulfide (H₂S) |
| [86,87,88] |
2.2.3. Inhibiting ROS Production
2.3. Manufacture, Application and Action
| Disease | Designed Materials | Actions | References |
|---|---|---|---|
| Periodontitis | CeO2@Ce6 |
| [91] |
| Se-nHA/PC |
| [92] | |
| Lipo-RSV |
| [93] | |
| Diabetic wound healing | HMP hydrogel |
| [94] |
| PtCuTe nanosheets |
| [95] | |
| CoNZs |
| [96] | |
| A thermoreversible hydrogel |
| [97] | |
| GelMA@Mg-POM |
| [98] | |
| Copper enzyme |
| [99] | |
| Osteoarthritis | OHA/HAADH@SeNPs hydrogels |
| [100] |
| PEG-MnO₂ |
| [101] | |
| LDH@TAGel |
| [102] | |
| Fe/ZIF-8/Gel centrase |
| [103] | |
| Cu-EGCG nanosheets |
| [104] | |
| IBD | SOD/CAT nanomedicine |
| [105] |
| CAT mimetic |
| [106] |
3. Biomaterials Targeting Macrophages
3.1. Macrophages, Inflammation and Biomaterials
3.2. Classification, Manufacture, and Application
3.2.1. Magnesium-Based Biomaterials
3.2.2. Keratin-Based Biomaterials
3.2.3. Silk Fibroin-Based Biomaterials
3.2.4. Flavonoid-Based Biomaterials
| Disease | Designed Materials | Actions | Reference | |
|---|---|---|---|---|
| Magnesium-based biomaterials | Wound healing | Electrospun membranes incorporating MgO |
| [138] |
| Diabetic wound healing | PCL/gelatin/MgAC nanofiber membranes |
| [139] | |
| Cardiovascular diseases | SRL/MH/PLGA |
| [140] | |
| Inflamed pulps | Mg-BG |
| [141] | |
| Drug delivery | Mg/PLLA composite microsphere |
| [142] | |
| Keratin-based biomaterials | Cardiac dysfunction after myocardial infarction | Keratin hydrogel |
| [160] |
| Silk fibroin-based biomaterials | Bacterial-infected wound healing | SF/SA hydrogel |
| [172] |
| Bone regeneration | SilMA/nHA hydrogel |
| [173] | |
| Alveolar bone defect | MeSF |
| [174] | |
| Full-thickness burn healing | SF/CMC/AG&GO@PDA hydrogel |
| [175] | |
| Flavonoid-based Biomaterials | Peripheral nerve injuries | FIS/CS/PCL NGCs |
| [190] |
| Wound healing | GSC/PBE@Lut |
| [191] | |
| Acute liver injuries | GC-Que-Lipo |
| [192] | |
| Dental implants | Quercitrin-nanocoating |
| [193] | |
| Blood purification | PSF/SM membranes |
| [194] |
4. Summary and Future
Funding
Conflicts of Interest
References
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Vago, J.P.; Perretti, M.; Teixeira, M.M. Mediators of the Resolution of the Inflammatory Response. Trends Immunol. 2019, 40, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Schönbein, G.W. Analysis of inflammation. Annu. Rev. Biomed. Eng. 2006, 8, 93–151. [Google Scholar] [CrossRef]
- Kobayashi, H.; Higashiura, Y.; Shigetomi, H.; Kajihara, H. Pathogenesis of endometriosis: The role of initial infection and subsequent sterile inflammation (Review). Mol. Med. Rep. 2014, 9, 9–15. [Google Scholar] [CrossRef]
- Vitale, G.; Salvioli, S.; Franceschi, C. Oxidative stress and the ageing endocrine system. Nat. Rev. Endocrinol. 2013, 9, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; ME, L.L. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, H.; Shin, M.R.; Sesti, F. Oxidation of KCNB1 potassium channels in the murine brain during aging is associated with cognitive impairment. Biochem. Biophys. Res. Commun. 2019, 512, 665–669. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxidative Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef]
- Duffield, J.S.; Forbes, S.J.; Constandinou, C.M.; Clay, S.; Partolina, M.; Vuthoori, S.; Wu, S.; Lang, R.; Iredale, J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Investig. 2005, 115, 56–65. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Yao, B.; Yang, S.; Jiang, L.; Wang, S.; Fan, X.; Yin, H.; Wong, K.; Miyazawa, T.; Chen, J.; et al. CSF-1 signaling mediates recovery from acute kidney injury. J. Clin. Investig. 2012, 122, 4519–4532. [Google Scholar] [CrossRef] [PubMed]
- Berse, B.; Brown, L.F.; Van de Water, L.; Dvorak, H.F.; Senger, D.R. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol. Biol. Cell 1992, 3, 211–220. [Google Scholar] [CrossRef]
- Chujo, S.; Shirasaki, F.; Kondo-Miyazaki, M.; Ikawa, Y.; Takehara, K. Role of connective tissue growth factor and its interaction with basic fibroblast growth factor and macrophage chemoattractant protein-1 in skin fibrosis. J. Cell. Physiol. 2009, 220, 189–195. [Google Scholar] [CrossRef]
- Rappolee, D.A.; Mark, D.; Banda, M.J.; Werb, Z. Wound macrophages express TGF-alpha and other growth factors in vivo: Analysis by mRNA phenotyping. Science 1988, 241, 708–712. [Google Scholar] [CrossRef]
- Shimokado, K.; Raines, E.W.; Madtes, D.K.; Barrett, T.B.; Benditt, E.P.; Ross, R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell 1985, 43, 277–286. [Google Scholar] [CrossRef]
- Willenborg, S.; Lucas, T.; van Loo, G.; Knipper, J.A.; Krieg, T.; Haase, I.; Brachvogel, B.; Hammerschmidt, M.; Nagy, A.; Ferrara, N.; et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012, 120, 613–625. [Google Scholar] [CrossRef]
- Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Resolution of liver fibrosis: Basic mechanisms and clinical relevance. Semin. Liver Dis. 2015, 35, 119–131. [Google Scholar] [CrossRef]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef]
- Ren, K.; Torres, R. Role of interleukin-1beta during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef]
- Entman, M.L.; Michael, L.; Rossen, R.D.; Dreyer, W.J.; Anderson, D.C.; Taylor, A.A.; Smith, C.W. Inflammation in the course of early myocardial-ischemia. FASEB J. 1991, 5, 2529–2537. [Google Scholar] [CrossRef]
- Anselmi, A.; Abbate, A.; Girola, F.; Nasso, G.; Biondi-Zoccai, G.G.; Possati, G.; Gaudino, M. Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: A review of evidence. Eur. J. Cardio-Thorac. Surg. 2004, 25, 304–311. [Google Scholar] [CrossRef]
- Schmid-Schönbein, G.W.; Hugli, T.E. A new hypothesis for microvascular inflammation in shock and multiorgan failure: Self-digestion by pancreatic enzymes. Microcirculation 2005, 12, 71–82. [Google Scholar] [CrossRef]
- Suematsu, M.; Suzuki, H.; Delano, F.A.; Schmid-Schönbein, G.W. The inflammatory aspect of the microcirculation in hypertension: Oxidative stress, leukocytes/endothelial interaction, apoptosis. Microcirculation 2002, 9, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Stürmer, T.; Brenner, H.; Koenig, W.; Günther, K.P. Severity and extent of osteoarthritis and low grade systemic inflammation as assessed by high sensitivity C reactive protein. Ann. Rheum. Dis. 2004, 63, 200–205. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A. Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease. Nanotechnol. Rev. 2024, 13, 20240057. [Google Scholar] [CrossRef]
- Tu, Z.; Zhong, Y.; Hu, H.; Shao, D.; Haag, R.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.I.; Lavelle, E.C. Immuno-modulatory biomaterials as anti-inflammatory therapeutics. Biochem. Pharmacol. 2022, 197, 114890. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Mohapatra, A.; Park, I.K. Recent Advances in ROS-Scavenging Metallic Nanozymes for Anti-Inflammatory Diseases: A Review. Chonnam Med. J. 2023, 59, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Panchal, N.K.; Prince Sabina, E. Non-steroidal anti-inflammatory drugs (NSAIDs): A current insight into its molecular mechanism eliciting organ toxicities. Food Chem. Toxicol. 2023, 172, 113598. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhu, B.H.; Deng, Y.T.; Li, T.T.; Tian, Q.Y.; Yuan, Z.G.; Ma, L.; Cheng, C.; Guo, Q.Y.; Qiu, L. Biocatalytic and Antioxidant Nanostructures for ROS Scavenging and Biotherapeutics. Adv. Funct. Mater. 2021, 31, 2101804. [Google Scholar] [CrossRef]
- Wang, H.; Wan, K.; Shi, X. Recent Advances in Nanozyme Research. Adv. Mater. 2019, 31, e1805368. [Google Scholar] [CrossRef] [PubMed]
- Gurung, N.; Ray, S.; Bose, S.; Rai, V. A broader view: Microbial enzymes and their relevance in industries, medicine, and beyond. BioMed Res. Int. 2013, 2013, 329121. [Google Scholar] [CrossRef]
- Choct, M. Enzymes for the feed industry: Past, present and future. World’s Poult. Sci. J. 2006, 62, 5–15. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ridderström, M.; Mannervik, B. Human glutathione transferase A4-4: An alpha class enzyme with high catalytic efficiency in the conjugation of 4-hydroxynonenal and other genotoxic products of lipid peroxidation. Biochem. J. 1998, 330, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, H.M.; Abd El Aziz, A.E.; Abdel Raouf, B.M.; Mohamed, S.A.; Nada, D. Antioxidant-biocompatible and stable catalase-based gelatin-alginate hydrogel scaffold with thermal wound healing capability: Immobilization and delivery approach. 3 Biotech 2022, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Q.; Xu, Q.; Li, B.; Dong, H.; Mou, Y. Polydopamine Modified Ceria Nanorods Alleviate Inflammation in Colitis by Scavenging ROS and Regulating Macrophage M2 Polarization. Int. J. Nanomed. 2023, 18, 4601–4616. [Google Scholar] [CrossRef]
- Wang, M.; He, H.; Liu, D.; Ma, M.; Zhang, Y. Preparation, Characterization and Multiple Biological Properties of Peptide-Modified Cerium Oxide Nanoparticles. Biomolecules 2022, 12, 1277. [Google Scholar] [CrossRef]
- Chen, D.; Liang, Z.; Su, Z.; Huang, J.; Pi, Y.; Ouyang, Y.; Luo, T.; Guo, L. Selenium-Doped Mesoporous Bioactive Glass Regulates Macrophage Metabolism and Polarization by Scavenging ROS and Promotes Bone Regeneration In Vivo. ACS Appl. Mater. Interfaces 2023, 15, 34378–34396. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hou, X.; Li, L.; Guo, J.; Jiang, W.; Shang, W. Application of Metal-Based Nanozymes in Inflammatory Disease: A Review. Front. Bioeng. Biotechnol. 2022, 10, 920213. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, K.; Hu, W.; Chen, J.J.; Lu, X.; Wang, M.; Yang, Y.; Guo, J.; Lu, Y.; Ma, Q. Anti-inflammatory cerium-containing nano-scaled mesoporous bioactive glass for promoting regenerative capability of dental pulp cells. Int. Endod. J. 2024, 57, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, M.; Wang, Z.; Feng, Q.; Gao, H.; Li, Q.; Chen, X.; Cao, X. Bioactive Glasses-Based Nanozymes Composite Macroporous Cryogel with Antioxidative, Antibacterial, and Pro-Healing Properties for Diabetic Infected Wound Repair. Adv. Healthc. Mater. 2023, 12, 2302073. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr. Polym. 2018, 180, 168–174. [Google Scholar] [CrossRef]
- Zhang, D.; Ren, Y.; He, Y.; Chang, R.; Guo, S.; Ma, S.; Guan, F.; Yao, M. In situ forming and biocompatible hyaluronic acid hydrogel with reactive oxygen species-scavenging activity to improve traumatic brain injury repair by suppressing oxidative stress and neuroinflammation. Mater. Today Bio 2022, 15, 100278. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, X.; Tong, Q.; Ran, Y.; Ma, L.; Tan, Y.; Yi, Z.; Li, X. Biocompatible, bacteria-targeting resveratrol nanoparticles fabricated by Mannich molecular condensation for accelerating infected wound healing. J. Mater. Chem. B 2022, 10, 9280–9294. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Fadakar, S.; Aghazadeh, M.; Salehi, R.; Samadi Kafil, H.; Roshangar, L.; Mousavi, E.; Aghazadeh, Z. Synthesis, characterization, and evaluation of curcumin-loaded endodontic reparative material. J. Biochem. Mol. Toxicol. 2021, 35, e22854. [Google Scholar] [CrossRef]
- Han, X.; Luo, R.; Qi, S.; Wang, Y.; Dai, L.; Nie, W.; Lin, M.; He, H.; Ye, N.; Fu, C.; et al. “Dual sensitive supramolecular curcumin nanoparticles” in “advanced yeast particles” mediate macrophage reprogramming, ROS scavenging and inflammation resolution for ulcerative colitis treatment. J. Nanobiotechnol. 2023, 21, 321. [Google Scholar] [CrossRef]
- Ming, P.; Li, B.; Li, Q.; Yuan, L.; Jiang, X.; Liu, Y.; Cai, R.; Zhou, P.; Lan, X.; Tao, G.; et al. Multifunctional sericin-based biomineralized nanoplatforms with immunomodulatory and angio/osteo-genic activity for accelerated bone regeneration in periodontitis. Biomaterials 2025, 314, 122885. [Google Scholar] [CrossRef]
- Tian, P.; Zhao, L.; Kim, J.; Li, X.; Liu, C.; Cui, X.; Liang, T.; Du, Y.; Chen, X.; Pan, H. Dual stimulus responsive borosilicate glass (BSG) scaffolds promote diabetic alveolar bone defectsrepair by modulating macrophage phenotype. Bioact. Mater. 2023, 26, 231–248. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef]
- Bellezza, I.; Grottelli, S.; Gatticchi, L.; Mierla, A.L.; Minelli, A. α-Tocopheryl succinate pre-treatment attenuates quinone toxicity in prostate cancer PC3 cells. Gene 2014, 539, 1–7. [Google Scholar] [CrossRef]
- Hirayama, M.; Ito, M.; Minato, T.; Yoritaka, A.; LeBaron, T.W.; Ohno, K. Inhalation of hydrogen gas elevates urinary 8-hydroxy-2′-deoxyguanine in Parkinson’s disease. Med. Gas Res. 2018, 8, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.D.; Zhang, H.; Wang, L.; Han, Q.; Zhou, S.F.; Liu, P. Molecular hydrogen regulates the expression of miR-9, miR-21 and miR-199 in LPS-activated retinal microglia cells. Int. J. Ophthalmol. 2013, 6, 280–285. [Google Scholar] [CrossRef]
- Mao, Y.F.; Zheng, X.F.; Cai, J.M.; You, X.M.; Deng, X.M.; Zhang, J.H.; Jiang, L.; Sun, X.J. Hydrogen-rich saline reduces lung injury induced by intestinal ischemia/reperfusion in rats. Biochem. Biophys. Res. Commun. 2009, 381, 602–605. [Google Scholar] [CrossRef]
- Chen, X.L.; Zhang, Q.; Zhao, R.; Medford, R.M. Superoxide, H2O2, and iron are required for TNF-alpha-induced MCP-1 gene expression in endothelial cells: Role of Rac1 and NADPH oxidase. Am. J. Physiol.-Heart Circ. Physiol. 2004, 286, H1001–H1007. [Google Scholar] [CrossRef]
- Matei, N.; Camara, R.; Zhang, J.H. Emerging mechanisms and novel applications of hydrogen gas therapy. Med. Gas Res. 2018, 8, 98–102. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Carraway, M.S.; Babiker, A.; Suliman, H.B. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ. Res. 2008, 103, 1232–1240. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A Novel Option in Human Disease Treatment. Oxidative Med. Cell. Longev. 2020, 2020, 8384742. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.L.; Tian, B.; Lin, Y.J.; Korupalli, C.; Lu, M.Y.; Cui, Q.; Wan, D.; Chang, Y.; Sung, H.W. Photosynthesis-inspired H2 generation using a chlorophyll-loaded liposomal nanoplatform to detect and scavenge excess ROS. Nat. Commun. 2020, 11, 534. [Google Scholar] [CrossRef]
- Wan, W.L.; Lin, Y.J.; Chen, H.L.; Huang, C.C.; Shih, P.C.; Bow, Y.R.; Chia, W.T.; Sung, H.W. In Situ Nanoreactor for Photosynthesizing H2 Gas To Mitigate Oxidative Stress in Tissue Inflammation. J. Am. Chem. Soc. 2017, 139, 12923–12926. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Hogg, P.J. Disulfide bonds as switches for protein function. Trends Biochem. Sci. 2003, 28, 210–214. [Google Scholar] [CrossRef]
- Bouillaud, F.; Blachier, F. Mitochondria and sulfide: A very old story of poisoning, feeding, and signaling? Antioxid. Redox Signal. 2011, 15, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, A. Thioredoxin and redox signaling: Roles of the thioredoxin system in control of cell fate. Arch. Biochem. Biophys. 2017, 617, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.A.; Kalinina, E.; Tatarskiy, V.; Shtil, A. The Thioredoxin System of Mammalian Cells and Its Modulators. Biomedicines 2022, 10, 1757. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, W.; Lin, W.; Li, Y.; Liu, X. Reactive oxygen species-responsive nanoplatforms for nucleic acid-based gene therapy of cancer and inflammatory diseases. Biomed. Mater. 2021, 16, 042015. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Mecerreyes, D. Thioether-based ROS responsive polymers for biomedical applications. J. Mater. Chem. B 2022, 10, 7206–7221. [Google Scholar] [CrossRef]
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 5. [Google Scholar] [CrossRef]
- De Abreu Costa, L.; Henrique Fernandes Ottoni, M.; Dos Santos, M.G.; Meireles, A.B.; Gomes de Almeida, V.; de Fátima Pereira, W.; Alves de Avelar-Freitas, B.; Eustáquio Alvim Brito-Melo, G. Dimethyl Sulfoxide (DMSO) Decreases Cell Proliferation and TNF-α, IFN-γ, and IL-2 Cytokines Production in Cultures of Peripheral Blood Lymphocytes. Molecules 2017, 22, 1789. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Yu, J.; Shao, N.; Lu, H.; Guo, J.; Qiu, X.; Zhou, D.; Huang, Y. Absorbable Thioether Grafted Hyaluronic Acid Nanofibrous Hydrogel for Synergistic Modulation of Inflammation Microenvironment to Accelerate Chronic Diabetic Wound Healing. Adv. Healthc. Mater. 2020, 9, 2000198. [Google Scholar] [CrossRef]
- Yoo, D.; Magsam, A.W.; Kelly, A.M.; Stayton, P.S.; Kievit, F.M.; Convertine, A.J. Core-Cross-Linked Nanoparticles Reduce Neuroinflammation and Improve Outcome in a Mouse Model of Traumatic Brain Injury. ACS Nano 2017, 11, 8600–8611. [Google Scholar] [CrossRef]
- Xiao, Q.; Ying, J.; Xiang, L.; Zhang, C. The biologic effect of hydrogen sulfide and its function in various diseases. Medicine 2018, 97, e13065. [Google Scholar] [CrossRef]
- Kimura, Y.; Goto, Y.; Kimura, H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Kimura, Y.; Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004, 18, 1165–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Kimura, Y.; Dargusch, R.; Schubert, D.; Kimura, H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid. Redox Signal. 2006, 8, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, H.; Duan, P.; Liu, K.; Yu, Y.; Wei, W.; Wang, W.; Liu, Y.; Cheng, Q.; Liang, X.; et al. An injectable and adaptable hydrogen sulfide delivery system for modulating neuroregenerative microenvironment. Sci. Adv. 2023, 9, eadi1078. [Google Scholar] [CrossRef]
- Yang, J.; Dong, X.; Wei, W.; Liu, K.; Wu, X.; Dai, H. An injectable hydrogel dressing for controlled release of hydrogen sulfide pleiotropically mediates the wound microenvironment. J. Mater. Chem. B 2024, 12, 5377–5390. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, X.; Shang, Y.; Wang, L.; Shen, J.; Yuan, J. Hydrogen sulfide releasing poly(γ-glutamic acid) biocomposite hydrogel with monitoring, antioxidant, and antibacterial properties for diabetic wound healing. Int. J. Biol. Macromol. 2023, 253, 127053. [Google Scholar] [CrossRef]
- Chocry, M.; Leloup, L. The NADPH Oxidase Family and Its Inhibitors. Antioxid. Redox Signal. 2020, 33, 332–353. [Google Scholar] [CrossRef]
- Zhen, J.; Wan, T.; Sun, G.; Chen, X.; Zhang, S. A ROS-responsive microsphere capsule encapsulated with NADPH oxidase 4 inhibitor ameliorates macrophage inflammation and ferroptosis. Heliyon 2024, 10, e23589. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, X.; Li, X.; Li, W.; Li, C.; Zhou, Y.; Wang, L.; Dong, B. A versatile nanocomposite based on nanoceria for antibacterial enhancement and protection from aPDT-aggravated inflammation via modulation of macrophage polarization. Biomaterials 2021, 268, 120614. [Google Scholar] [CrossRef] [PubMed]
- Ming, P.; Liu, Y.; Yu, P.; Jiang, X.; Yuan, L.; Cai, S.; Rao, P.; Cai, R.; Lan, X.; Tao, G.; et al. A Biomimetic Se-nHA/PC Composite Microsphere with Synergistic Immunomodulatory and Osteogenic Ability to Activate Bone Regeneration in Periodontitis. Small 2024, 20, 2305490. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, Y.; Zhang, X.; Chen, R.; Wei, J.; Hou, J.; Wang, B.; Lai, H.; Huang, Y. Remodeling immune microenvironment in periodontitis using resveratrol liposomes as an antibiotic-free therapeutic strategy. J. Nanobiotechnol. 2021, 19, 429. [Google Scholar] [CrossRef]
- Tu, C.; Lu, H.; Zhou, T.; Zhang, W.; Deng, L.; Cao, W.; Yang, Z.; Wang, Z.; Wu, X.; Ding, J.; et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials 2022, 286, 121597. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ding, S.; Shang, C.; Zhang, C.; Li, M.; Zhang, Q.; Gu, L.; Heng, B.C.; Zhang, S.; Mei, F.; et al. Multifunctional PtCuTe Nanosheets with Strong ROS Scavenging and ROS-Independent Antibacterial Properties Promote Diabetic Wound Healing. Adv. Mater. 2024, 36, 2306292. [Google Scholar] [CrossRef]
- Mandakhbayar, N.; Ji, Y.; El-Fiqi, A.; Patel, K.D.; Yoon, D.S.; Dashnyam, K.; Bayaraa, O.; Jin, G.; Tsogtbaatar, K.; Kim, T.H.; et al. Double hits with bioactive nanozyme based on cobalt-doped nanoglass for acute and diabetic wound therapies through anti-inflammatory and pro-angiogenic functions. Bioact. Mater. 2024, 31, 298–311. [Google Scholar] [CrossRef]
- Qi, Y.; Qian, K.; Chen, J.; E, Y.; Shi, Y.; Li, H.; Zhao, L. A thermoreversible antibacterial zeolite-based nanoparticles loaded hydrogel promotes diabetic wound healing via detrimental factor neutralization and ROS scavenging. J. Nanobiotechnol. 2021, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Wang, Y.; Li, Y.; Wang, Y.; Li, L.; Xiang, H.; Sun, Q.; Yong, Y.; Yang, H.; Jiang, K. Nano-enzyme functionalized hydrogels promote diabetic wound healing through immune microenvironment modulation. Biomater. Sci. 2024, 12, 3851–3865. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, X.; Li, K.; Feng, J.; Liu, X.; Li, Y.; Yang, K.; Li, J.; Ge, S. Phenolic Ligand-Metal Charge Transfer Induced Copper Nanozyme with Reactive Oxygen Species-Scavenging Ability for Chronic Wound Healing. ACS Nano 2024, 18, 7024–7036. [Google Scholar] [CrossRef]
- Hu, W.; Yao, X.; Li, Y.; Li, J.; Zhang, J.; Zou, Z.; Kang, F.; Dong, S. Injectable hydrogel with selenium nanoparticles delivery for sustained glutathione peroxidase activation and enhanced osteoarthritis therapeutics. Mater. Today Bio 2023, 23, 100864. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Adjei, I.M.; Brown, S.B.; Liseth, O.; Sharma, B. Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials 2019, 224, 119467. [Google Scholar] [CrossRef]
- Liu, C.; Sun, Y.; Li, D.; Wang, F.; Wang, H.; An, S.; Sun, S. A multifunctional nanogel encapsulating layered double hydroxide for enhanced osteoarthritis treatment via protection of chondrocytes and ECM. Mater. Today Bio 2024, 26, 101034. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhou, Y.; Wan, Q.; Li, L.; Deng, H.; Yin, Y.; Zhou, Q.; Li, Q.; Cheng, D.; Hu, X.; et al. Nano-enzyme hydrogels for cartilage repair effectiveness based on ternary strategy therapy. J. Mater. Chem. B 2024, 12, 6242–6256. [Google Scholar] [CrossRef]
- Wei, H.; Qin, J.; Huang, Q.; Jin, Z.; Zheng, L.; Zhao, J.; Qin, Z. Epigallocatechin-3-gallate (EGCG) based metal-polyphenol nanoformulations alleviates chondrocytes inflammation by modulating synovial macrophages polarization. Biomed. Pharmacother. 2023, 161, 114366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tao, H.; Lin, Y.; Hu, Y.; An, H.; Zhang, D.; Feng, S.; Hu, H.; Wang, R.; Li, X.; et al. A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease. Biomaterials 2016, 105, 206–221. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Fu, X.; Wang, G.; Wang, Z.; Wu, X.; Ren, J.; Zhao, Y. Microfluidic encapsulated manganese organic frameworks as enzyme mimetics for inflammatory bowel disease treatment. J. Colloid Interface Sci. 2022, 607, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Mijiritsky, E.; Gardin, C.; Ferroni, L.; Lacza, Z.; Zavan, B. Albumin-impregnated bone granules modulate the interactions between mesenchymal stem cells and monocytes under in vitro inflammatory conditions. Mater. Sci. Eng. C 2020, 110, 110678. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jin, H.; Song, Y.; Huang, T.; Cao, J.; Tang, Q.; Zou, Z. Targeting tumor-associated macrophages: A potential treatment for solid tumors. J. Cell. Physiol. 2021, 236, 3445–3465. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liu, Y.; Wen, Q.; Li, Y.; Yu, J.; Xu, Q.; Wan, W.; He, Y.; Ma, C.; Huang, Y.; et al. Experimental study on preparation and anti-tumor efficiency of nanoparticles targeting M2 macrophages. Drug Deliv. 2021, 28, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Sims, N.A.; Walsh, N.C. GP130 cytokines and bone remodelling in health and disease. BMB Rep. 2010, 43, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Z.Y.; Zhang, Y.Y.; Yang, H.L. IL-6 Contributes to the Defective Osteogenesis of Bone Marrow Stromal Cells from the Vertebral Body of the Glucocorticoid-Induced Osteoporotic Mouse. PLoS ONE 2016, 11, e0154677. [Google Scholar] [CrossRef] [PubMed]
- Siegel, P.M.; Massagué, J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer 2003, 3, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Liu, L.; Rung, S.; Wang, Y.; Ma, Y.; Hu, C.; Zhao, X.; Man, Y.; Qu, Y. Modulation of foreign body reaction and macrophage phenotypes concerning microenvironment. J. Biomed. Mater. Res. Part A 2020, 108, 127–135. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Crowe, M.J.; Doetschman, T.; Greenhalgh, D.G. Delayed wound healing in immunodeficient TGF-beta 1 knockout mice. J. Investig. Dermatol. 2000, 115, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Mossman, B.T. Generation of superoxide (O2−.) from alveolar macrophages exposed to asbestiform and nonfibrous particles. Cancer Res. 1987, 47, 1681–1686. [Google Scholar]
- Anderson, J.M. Biological responses to materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Labow, R.S.; Meek, E.; Santerre, J.P. Hydrolytic degradation of poly(carbonate)-urethanes by monocyte-derived macrophages. Biomaterials 2001, 22, 3025–3033. [Google Scholar] [CrossRef]
- Wiggins, M.J.; Wilkoff, B.; Anderson, J.M.; Hiltner, A. Biodegradation of polyether polyurethane inner insulation in bipolar pacemaker leads. J. Biomed. Mater. Res. 2001, 58, 302–307. [Google Scholar] [CrossRef]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Klopfleisch, R. Macrophage reaction against biomaterials in the mouse model—Phenotypes, functions and markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Romani, A.M. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef]
- Wolf, F.I.; Trapani, V. Cell (patho)physiology of magnesium. Clin. Sci. 2008, 114, 27–35. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Chen, Y.; Hong, H.; Shen, Y.; Wang, Y.; Sun, J.; Wang, X. The “Yin and Yang” of Immunomodulatory Magnesium-Enriched Graphene Oxide Nanoscrolls Decorated Biomimetic Scaffolds in Promoting Bone Regeneration. Adv. Healthc. Mater. 2021, 10, 2000631. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, X.; Tan, L.; Friis, T.; Wu, C.; Crawford, R.; Xiao, Y. Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate. Biomaterials 2014, 35, 8553–8565. [Google Scholar] [CrossRef]
- Libako, P.; Nowacki, W.; Castiglioni, S.; Mazur, A.; Maier, J.A. Extracellular magnesium and calcium blockers modulate macrophage activity. Magnes. Res. 2016, 29, 11–21. [Google Scholar] [CrossRef]
- Chakraborty Banerjee, P.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium Implants: Prospects and Challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, W.; Chen, Z.; Ding, Y.; Sun, B.; Wang, H.; Mo, X.; Wu, J. Mechanisms of magnesium oxide-incorporated electrospun membrane modulating inflammation and accelerating wound healing. J. Biomed. Mater. Res. Part A 2023, 111, 132–151. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Sun, B.; Wang, H.; Mo, X.; El-Newehy, M.; Abdulhameed, M.M.; Yao, H.; Liang, C.; Wu, J. Electrospun membranes chelated by metal magnesium ions enhance pro-angiogenic activity and promote diabetic wound healing. Int. J. Biol. Macromol. 2024, 259, 129283. [Google Scholar] [CrossRef]
- Jeong, D.W.; Park, W.; Bedair, T.M.; Kang, E.Y.; Kim, I.H.; Park, D.S.; Sim, D.S.; Hong, Y.J.; Koh, W.G.; Jeong, M.H.; et al. Augmented re-endothelialization and anti-inflammation of coronary drug-eluting stent by abluminal coating with magnesium hydroxide. Biomater. Sci. 2019, 7, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, C.; Yan, X.; Li, X.; Chen, X.; Mai, S. Odontogenic and anti-inflammatory effects of magnesium-doped bioactive glass in vital pulp therapy. Biomed. Mater. 2024, 19, 045026. [Google Scholar] [CrossRef]
- Yang, F.; Niu, X.; Gu, X.; Xu, C.; Wang, W.; Fan, Y. Biodegradable Magnesium-Incorporated Poly(l-lactic acid) Microspheres for Manipulation of Drug Release and Alleviation of Inflammatory Response. ACS Appl. Mater. Interfaces 2019, 11, 23546–23557. [Google Scholar] [CrossRef]
- Pichler, K.; Fischerauer, S.; Ferlic, P.; Martinelli, E.; Brezinsek, H.P.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Immunological Response to Biodegradable Magnesium Implants. Jom 2014, 66, 573–579. [Google Scholar] [CrossRef]
- Peng, Q.; Li, K.; Han, Z.; Wang, E.; Xu, Z.; Liu, R.; Tian, Y. Degradable magnesium-based implant materials with anti-inflammatory activity. J. Biomed. Mater. Res. Part A 2013, 101, 1898–1906. [Google Scholar] [CrossRef]
- Wu, J.; Jin, L.; Tan, J.Y.; Chen, X.F.; Wang, Q.Q.; Yuan, G.Y.; Chen, T.X. The effects of a biodegradable Mg-based alloy on the function of VSMCs via immunoregulation of macrophages through Mg-induced responses. Ann. Transl. Med. 2021, 9, 1292. [Google Scholar] [CrossRef]
- Witte, F.; Ulrich, H.; Rudert, M.; Willbold, E. Biodegradable magnesium scaffolds: Part 1: Appropriate inflammatory response. J. Biomed. Mater. Res. Part A 2007, 81, 748–756. [Google Scholar] [CrossRef]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J., 2nd; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef]
- Costantino, M.D.; Schuster, A.; Helmholz, H.; Meyer-Rachner, A.; Willumeit-Römer, R.; Luthringer-Feyerabend, B.J.C. Inflammatory response to magnesium-based biodegradable implant materials. Acta Biomater. 2020, 101, 598–608. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, H.; Wang, L.; Jiang, D.; Wang, W.; Yuan, G.; Pei, J.; Jia, W. The beneficial potential of magnesium-based scaffolds to promote chondrogenesis through controlled Mg2+ release in eliminating the destructive effect of activated macrophages on chondrocytes. Biomater. Adv. 2022, 134, 112719. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Balaji, S.; Sehgal, P.K. Industrial applications of keratins—A review. J. Sci. Ind. Res. 2007, 66, 710–715. [Google Scholar]
- Chilakamarry, C.R.; Mahmood, S.; Saffe, S.; Arifin, M.A.B.; Gupta, A.; Sikkandar, M.Y.; Begum, S.S.; Narasaiah, B. Extraction and application of keratin from natural resources: A review. 3 Biotech 2021, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A. Biological importance and pharmaceutical significance of keratin: A review. Int. J. Biol. Macromol. 2022, 219, 395–413. [Google Scholar] [CrossRef]
- Fearing, B.V.; Van Dyke, M.E. In vitro response of macrophage polarization to a keratin biomaterial. Acta Biomater. 2014, 10, 3136–3144. [Google Scholar] [CrossRef]
- Sierpinski, P.; Garrett, J.; Ma, J.; Apel, P.; Klorig, D.; Smith, T.; Koman, L.A.; Atala, A.; Van Dyke, M. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 2008, 29, 118–128. [Google Scholar] [CrossRef]
- Poranki, D.; Whitener, W.; Howse, S.; Mesen, T.; Howse, E.; Burnell, J.; Greengauz-Roberts, O.; Molnar, J.; Van Dyke, M. Evaluation of skin regeneration after burns in vivo and rescue of cells after thermal stress in vitro following treatment with a keratin biomaterial. J. Biomater. Appl. 2014, 29, 26–35. [Google Scholar] [CrossRef]
- Pace, L.A.; Plate, J.F.; Mannava, S.; Barnwell, J.C.; Koman, L.A.; Li, Z.; Smith, T.L.; Van Dyke, M. A human hair keratin hydrogel scaffold enhances median nerve regeneration in nonhuman primates: An electrophysiological and histological study. Tissue Eng. Part A 2014, 20, 507–517. [Google Scholar] [CrossRef]
- Vázquez, N.; Chacón, M.; Meana, Á.; Menéndez-Menéndez, Y.; Ferrero-Gutierrez, A.; Cereijo-Martín, D.; Naveiras, M.; Merayo-Lloves, J. Keratin-chitosan membranes as scaffold for tissue engineering of human cornea. Histol. Histopathol. 2015, 30, 813–821. [Google Scholar] [PubMed]
- Qiao, D.F.; Lu, Y.M.; Fu, W.Y.; Piao, Y.J. Degradation of human hair keratin scaffold implanted for repairing injured skeletal muscles. Di 1 jun yi da xue xue bao/Acad. J. First Med. Coll. PLA 2002, 22, 902–904. [Google Scholar]
- Tachibana, A.; Nishikawa, Y.; Nishino, M.; Kaneko, S.; Tanabe, T.; Yamauchi, K. Modified keratin sponge: Binding of bone morphogenetic protein-2 and osteoblast differentiation. J. Biosci. Bioeng. 2006, 102, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Wang, X.; Zhang, L.; Zhao, X.; Li, J.; Cheng, K.; Zhang, J. The amelioration of cardiac dysfunction after myocardial infarction by the injection of keratin biomaterials derived from human hair. Biomaterials 2011, 32, 9290–9299. [Google Scholar] [CrossRef]
- Waters, M.; VandeVord, P.; Van Dyke, M. Keratin biomaterials augment anti-inflammatory macrophage phenotype in vitro. Acta Biomater. 2018, 66, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Silk fibroin protein and chitosan polyelectrolyte complex porous scaffolds for tissue engineering applications. Carbohydr. Polym. 2011, 85, 325–333. [Google Scholar] [CrossRef]
- Kasoju, N.; Bora, U. Silk fibroin in tissue engineering. Adv. Healthc. Mater. 2012, 1, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Panilaitis, B.; Bellas, E.; Kaplan, D.L. Functionalized silk biomaterials for wound healing. Adv. Healthc. Mater. 2013, 2, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Yang, Y.T.; He, J.H.; Li, M.; Guo, B.L. Novel supramolecular self-healing silk fibroin-based hydrogel via host-guest interaction as wound dressing to enhance wound healing. Chem. Eng. J. 2021, 417, 128278. [Google Scholar] [CrossRef]
- Liang, A.; Zhang, M.; Luo, H.; Niu, L.; Feng, Y.; Li, M. Porous Poly(Hexamethylene Biguanide) Hydrochloride Loaded Silk Fibroin Sponges with Antibacterial Function. Materials 2020, 13, 285. [Google Scholar] [CrossRef]
- Ruiz-Alcaraz, A.J.; Núñez-Sánchez, M.; Asensio Ruiz, M.A.; Martínez-Sánchez, M.A.; Oliva-Bolarín, A.; Martínez Martínez, T.; Pérez Cuadrado, J.J.; Ramos-Molina, B.; Lozano-Pérez, A.A. Optimizing the Preparation of Silk Fibroin Nanoparticles and Their Loading with Polyphenols: Towards a More Efficient Anti-Inflammatory Effect on Macrophages. Pharmaceutics 2023, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Yuan, X.F.; Bayat, A. Electrospun silk fibroin fiber diameter influences in vitro dermal fibroblast behavior and promotes healing of ex vivo wound models. J. Tissue Eng. 2014, 5, 2041731414551661. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nogales, A.; Lozano-Pérez, A.A.; Aznar-Cervantes, S.D.; Algieri, F.; Garrido-Mesa, J.; Garrido-Mesa, N.; Vezza, T.; Utrilla, M.P.; Cenis, J.L.; Rodríguez-Cabezas, M.E.; et al. Effect of aqueous and particulate silk fibroin in a rat model of experimental colitis. Int. J. Pharm. 2016, 511, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Song, D.W.; Kim, S.H.; Kim, H.H.; Lee, K.H.; Ki, C.S.; Park, Y.H. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: Implications for wound healing. Acta Biomater. 2016, 39, 146–155. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Guo, C.; Qiu, M.; Cheng, L.; Chen, K.; Qi, J.; Deng, L.; He, C.; Li, X.; et al. Vascularized polypeptide hydrogel modulates macrophage polarization for wound healing. Acta Biomater. 2023, 155, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Ruan, L.; Jiang, G.; Nie, L.; Shavandi, A.; Sun, Y.; Xu, J.; Shao, X.; Zhu, J. Regenerated silk fibroin and alginate composite hydrogel dressings loaded with curcumin nanoparticles for bacterial-infected wound closure. Biomater. Adv. 2023, 149, 213405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, D.; Wu, R.; Li, L.; Shi, T.; Shangguang, Z.; Lin, H.; Chen, G.; Wang, Z.; Liu, W. An injectable and photocurable methacrylate-silk fibroin/nano-hydroxyapatite hydrogel for bone regeneration through osteoimmunomodulation. Int. J. Biol. Macromol. 2024, 263, 129925. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, X.; Ai, Y. Pro-angiogenic photo-crosslinked silk fibroin hydrogel: A potential candidate for repairing alveolar bone defects. J. Appl. Oral Sci. 2023, 31, e20230158. [Google Scholar] [CrossRef]
- Babaluei, M.; Mojarab, Y.; Mottaghitalab, F.; Farokhi, M. Injectable hydrogel based on silk fibroin/carboxymethyl cellulose/agarose containing polydopamine functionalized graphene oxide with conductivity, hemostasis, antibacterial, and anti-oxidant properties for full-thickness burn healing. Int. J. Biol. Macromol. 2023, 249, 126051. [Google Scholar] [CrossRef]
- Park, Y.R.; Sultan, M.T.; Park, H.J.; Lee, J.M.; Ju, H.W.; Lee, O.J.; Lee, D.J.; Kaplan, D.L.; Park, C.H. NF-κB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 2018, 67, 183–195. [Google Scholar] [CrossRef]
- Kim, D.W.; Hwang, H.S.; Kim, D.S.; Sheen, S.H.; Heo, D.H.; Hwang, G.; Kang, S.H.; Kweon, H.; Jo, Y.Y.; Kang, S.W.; et al. Effect of silk fibroin peptide derived from silkworm Bombyx mori on the anti-inflammatory effect of Tat-SOD in a mice edema model. BMB Rep. 2011, 44, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A., Jr. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Singh, A.K.; Kumar, R.; Croley, C.R.; Pandey, A.K.; Coy-Barrera, E.; Kumar Patra, J.; Das, G.; Kerry, R.G.; Annunziata, G.; et al. Targeting Inflammation by Flavonoids: Novel Therapeutic Strategy for Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4957. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Di Benedetto, R.; Filesi, C.; Masella, R. Polyphenols, intracellular signalling and inflammation. Ann.-Ist. Super. Di Sanita 2007, 43, 394–405. [Google Scholar]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Yoon, J.H.; Baek, S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef]
- Lago, J.H.; Toledo-Arruda, A.C.; Mernak, M.; Barrosa, K.H.; Martins, M.A.; Tibério, I.F.; Prado, C.M. Structure-activity association of flavonoids in lung diseases. Molecules 2014, 19, 3570–3595. [Google Scholar] [CrossRef]
- Read, M.A. Flavonoids: Naturally occurring anti-inflammatory agents. Am. J. Pathol. 1995, 147, 235–237. [Google Scholar]
- Fang, J.; Xu, B.; Jin, X.; Chen, F.; Liu, S.; Liu, S.; Wang, S.; Zhang, F.; Song, K.; Wang, J.; et al. Nerve Guide Conduits Integrated with Fisetin-Loaded Chitosan Hydrogels for Reducing Oxidative Stress, Inflammation, and Nerve Regeneration. Macromol. Biosci. 2024, 24, 2300476. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Ding, Y.; Fan, X.; Ye, L.; Pan, Q.; Zhang, B.; Li, P.; Luo, K.; Hu, B.; et al. A Double Network Composite Hydrogel with Self-Regulating Cu2+/Luteolin Release and Mechanical Modulation for Enhanced Wound Healing. ACS Nano 2024, 18, 17251–17266, Erratum in ACS Nano 2024, 18, 34415–34418. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, D.; Xing, Z.; Zhao, C.; Wang, L.; Fan, Y.; Nie, H.; Liu, H. Quercetin loaded liposomes modified with galactosylated chitosan prevent LPS/D-GalN induced acute liver injury. Mater. Sci. Eng. C 2021, 131, 112527. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M.; Pacha-Olivenza, M.A.; Fernández-Calderón, M.C.; Córdoba, A.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Quercitrin-nanocoated titanium surfaces favour gingival cells against oral bacteria. Sci. Rep. 2016, 6, 22444. [Google Scholar] [CrossRef]
- Wang, P.; Yang, N.; Luo, Y.; Wang, G.; Zhou, S.; Huang, S.; Chen, L.; Zhao, Y. Silymarin modified polysulfone hollow fiber membranes with antioxidant, anti-M1 macrophage polarization and hemocompatibility for blood purification. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 1785–1799. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Wang, S.; Lin, D. Mechanism and Application of Biomaterials Targeting Reactive Oxygen Species and Macrophages in Inflammation. Int. J. Mol. Sci. 2025, 26, 245. https://doi.org/10.3390/ijms26010245
Yu M, Wang S, Lin D. Mechanism and Application of Biomaterials Targeting Reactive Oxygen Species and Macrophages in Inflammation. International Journal of Molecular Sciences. 2025; 26(1):245. https://doi.org/10.3390/ijms26010245
Chicago/Turabian StyleYu, Mengxuan, Shouli Wang, and Doudou Lin. 2025. "Mechanism and Application of Biomaterials Targeting Reactive Oxygen Species and Macrophages in Inflammation" International Journal of Molecular Sciences 26, no. 1: 245. https://doi.org/10.3390/ijms26010245
APA StyleYu, M., Wang, S., & Lin, D. (2025). Mechanism and Application of Biomaterials Targeting Reactive Oxygen Species and Macrophages in Inflammation. International Journal of Molecular Sciences, 26(1), 245. https://doi.org/10.3390/ijms26010245





