Roles of Mature Domain Targeting Signals (MTSs) for Protein Translocation and Secretion in Lactococcus lactis
Abstract
1. Introduction
2. Results
2.1. Effects of Fusion Protein Overproduction on Cell Growth
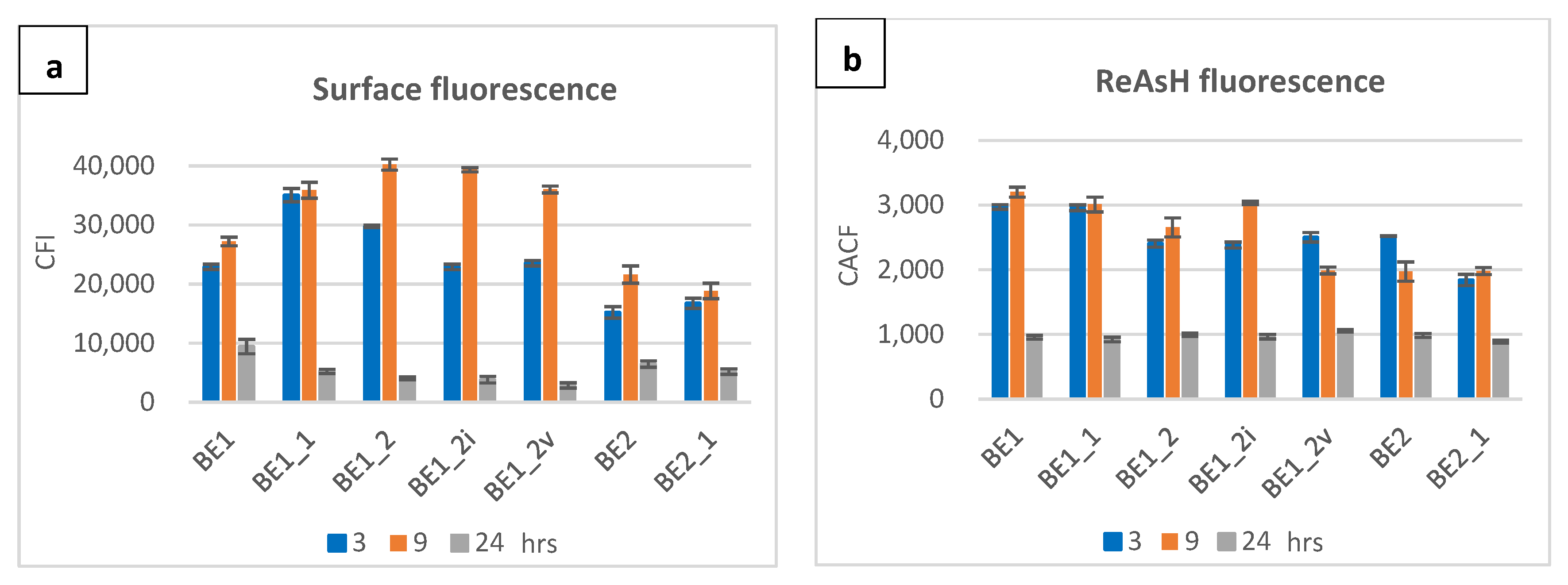
2.2. Localization of Fusion Proteins
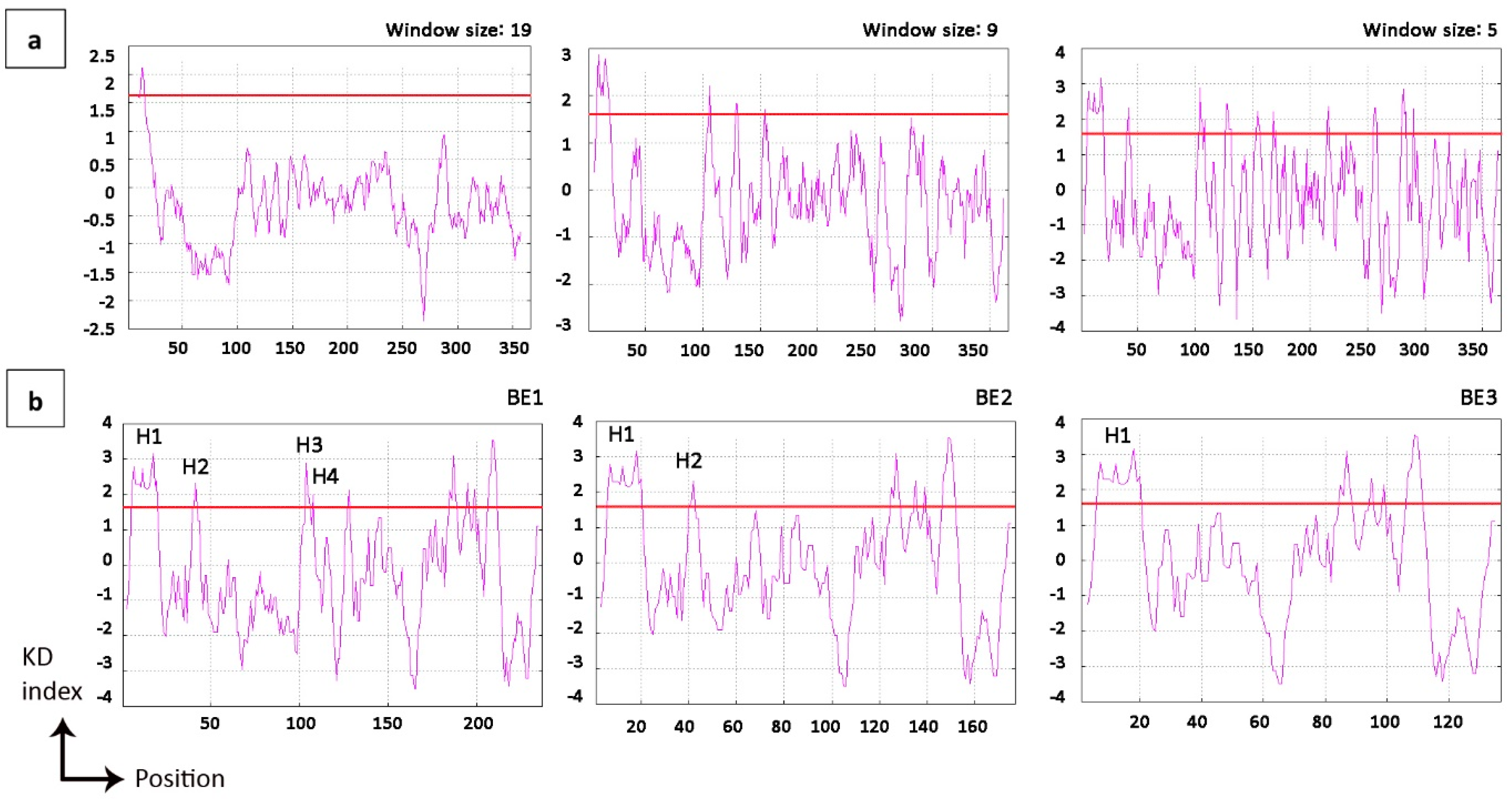
2.3. Modification of Mature Domain Targeting Signals
3. Discussion
4. Materials and Methods
4.1. Chemicals and Media
4.2. Strain Constructions
4.3. Cultivation and Protein Production
4.4. Hydropathy Evaluation
4.5. Staining and Detection of Membrane-Anchored and Intracellular Proteins
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jørgensen, C.M.; Vrang, A.; Madsen, S.M. Recombinant protein expression in Lactococcus lactis using the P170 expression system. FEMS Microbiol. Lett. 2014, 351, 170–178. [Google Scholar] [CrossRef]
- Arnau, J.; Hjerl-Hansen, E.; Israelsen, H. Heterologous gene expression of bovine plasmin in Lactococcus lactis. Appl. Microbiol. Biotechnol. 1997, 48, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chatel, J.-M.; Langella, P.; Adel-Patient, K.; Commissaire, J.; Wal, J.-M.; Corthier, G. Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin. Clin. Diagn. Lab. Immunol. 2001, 8, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Steidler, L.; Robinson, K.; Chamberlain, L.; Schofield, K.M.; Remaut, E.; Le Page, R.W.F.; Wells, J.M. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect. Immun. 1998, 66, 3183–3189. [Google Scholar] [CrossRef] [PubMed]
- Steidler, L.; Wells, J.M.; Raeymaekers, A.; Vandekerckhove, J.; Fiers, W.; Remaut, E. Secretion of biologically active murine interleukin-2 by Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 1995, 61, 1627–1629. [Google Scholar] [CrossRef]
- Drouault, S.; Corthier, G.; Ehrlich, S.D.; Renault, P. Expression of the Staphylococcus hyicus lipase in Lactococcus lactis. Appl. Environ. Microbiol. 2000, 66, 588–598. [Google Scholar] [CrossRef]
- Gaeng, S.; Scherer, S.; Neve, H.; Loessner, M.J. Gene cloning and expression and secretion of Listeria monocytogenes bacteriophage-lytic enzymes in Lactococcus lactis. Appl. Environ. Microbiol. 2000, 66, 2951–2958. [Google Scholar] [CrossRef]
- Ribeiro, L.A.; Azevedo, V.; Le Loir, Y.; Oliveira, S.C.; Dieye, Y.; Piard, J.-C.; Gruss, A.; Langella, P. Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: A first step towards food-grade live vaccines against brucellosis. Appl. Environ. Microbiol. 2002, 68, 910–916. [Google Scholar] [CrossRef]
- Blobel, G.; Dobberstein, B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 1975, 67, 835–851. [Google Scholar] [CrossRef]
- Hegde, R.S.; Bernstein, H.D. The surprising complexity of signal sequences. Trends Biochem. Sci. 2006, 31, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, K.E.; Sardis, M.F.; Tsirigotaki, A.; Koukaki, M.; Šoštarić, N.; Konijnenberg, A.; Sobott, F.; Kalodimos, C.G.; Karamanou, S.; Economou, A. Preprotein mature domains contain translocase targeting signals that are essential for secretion. J. Cell Biol. 2017, 216, 1357–1369. [Google Scholar] [CrossRef]
- Derman, A.I.; Puziss, J.W.; Bassford Jr, P.J.; Beckwith, J. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 1993, 12, 879–888. [Google Scholar] [CrossRef]
- Le Loir, Y.; Nouaille, S.; Commissaire, J.; Brétigny, L.; Gruss, A.; Langella, P. Signal peptide and propeptide optimization for heterologous protein secretion in Lactococcus lactis. Appl. Environ. Microbiol. 2001, 67, 4119–4127. [Google Scholar] [CrossRef] [PubMed]
- Bieker, K.L.; Phillips, G.J.; Silhavy, T.J. The sec and prl genes of Escherichia coli. J. Bioenerg. Biomembr. 1990, 22, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Gouridis, G.; Karamanou, S.; Gelis, I.; Kalodimos, C.G.; Economou, A. Signal peptides are allosteric activators of the protein translocase. Nature 2009, 462, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Lill, R.; Dowhan, W.; Wickner, W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell 1990, 60, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Prinz, W.A.; Spiess, C.; Ehrmann, M.; Schierle, C.; Beckwith, J. Targeting of signal sequenceless proteins for export in Escherichia coli with altered protein translocase. EMBO J. 1996, 15, 5209–5217. [Google Scholar] [CrossRef]
- Berlec, A.; Zadravec, P.; Jevnikar, Z.; Štrukelj, B. Identification of candidate carrier proteins for surface display on Lactococcus lactis by theoretical and experimental analyses of the surface proteome. Appl. Environ. Microbiol. 2011, 77, 1292–1300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoang, M.N.; Peterbauer, C. Double-Labeling Method for Visualization and Quantification of Membrane-Associated Proteins in Lactococcus lactis. Int. J. Mol. Sci. 2023, 24, 586. [Google Scholar] [CrossRef]
- Guruprasad, K.; Reddy, B.B.; Pandit, M.W. Correlation between stability of a protein and its dipeptide composition: A novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng. Des. Sel. 1990, 4, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Chandra, G. Physicochemical characterization and functional analysis of some snake venom toxin proteins and related non-toxin proteins of other chordates. Bioinformation 2012, 8, 891. [Google Scholar] [CrossRef] [PubMed]
- Magdeldin, S.; Yoshida, Y.; Li, H.; Maeda, Y.; Yokoyama, M.; Enany, S.; Zhang, Y.; Xu, B.; Fujinaka, H.; Yaoita, E.; et al. Murine colon proteome and characterization of the protein pathways. BioData Min. 2012, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.Y.; Yang, J.-R. Analysis and prediction of highly effective antiviral peptides based on random forests. PLoS ONE 2013, 8, e70166. [Google Scholar] [CrossRef]
- Georgiou, G. Optimizing the production of recombinant proteins in microorganisms. AIChE J. 1988, 34, 1233–1248. [Google Scholar] [CrossRef]
- Goff, S.A.; Goldberg, A.L. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell 1985, 41, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.Z. Bacterial Stress Response. In The Prokaryotes: Volume 2: Ecophysiology and Biochemistry; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer New York: New York, NY, USA, 2006; pp. 1012–1027. ISBN 978-0-387-30742-8. [Google Scholar]
- Textbook of Biotechnology; Tata McGraw Hill Education: New York, NY, USA, 2012; ISBN 9780071070072.
- Geertsma, E.R.; Poolman, B. High-throughput cloning and expression in recalcitrant bacteria. Nat. Methods 2007, 4, 705–707. [Google Scholar] [CrossRef] [PubMed]
- MoBiTec GmbH. NICE® Expression System for Lactococcus lactis. Available online: https://www.mobitec.com/media/mobitec/category-preview/vector-systems/pdf/3/NICE_Expression_System-Handbook.pdf (accessed on 8 January 2019).
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Deber, C.M.; Wang, C.; Liu, L.; Prior, A.S.; Agrawal, S.; Muskat, B.L.; Cuticchia, A.J. TM Finder: A prediction program for transmembrane protein segments using a combination of hydrophobicity and nonpolar phase helicity scales. Protein Sci. 2001, 10, 212–219. [Google Scholar] [CrossRef] [PubMed]



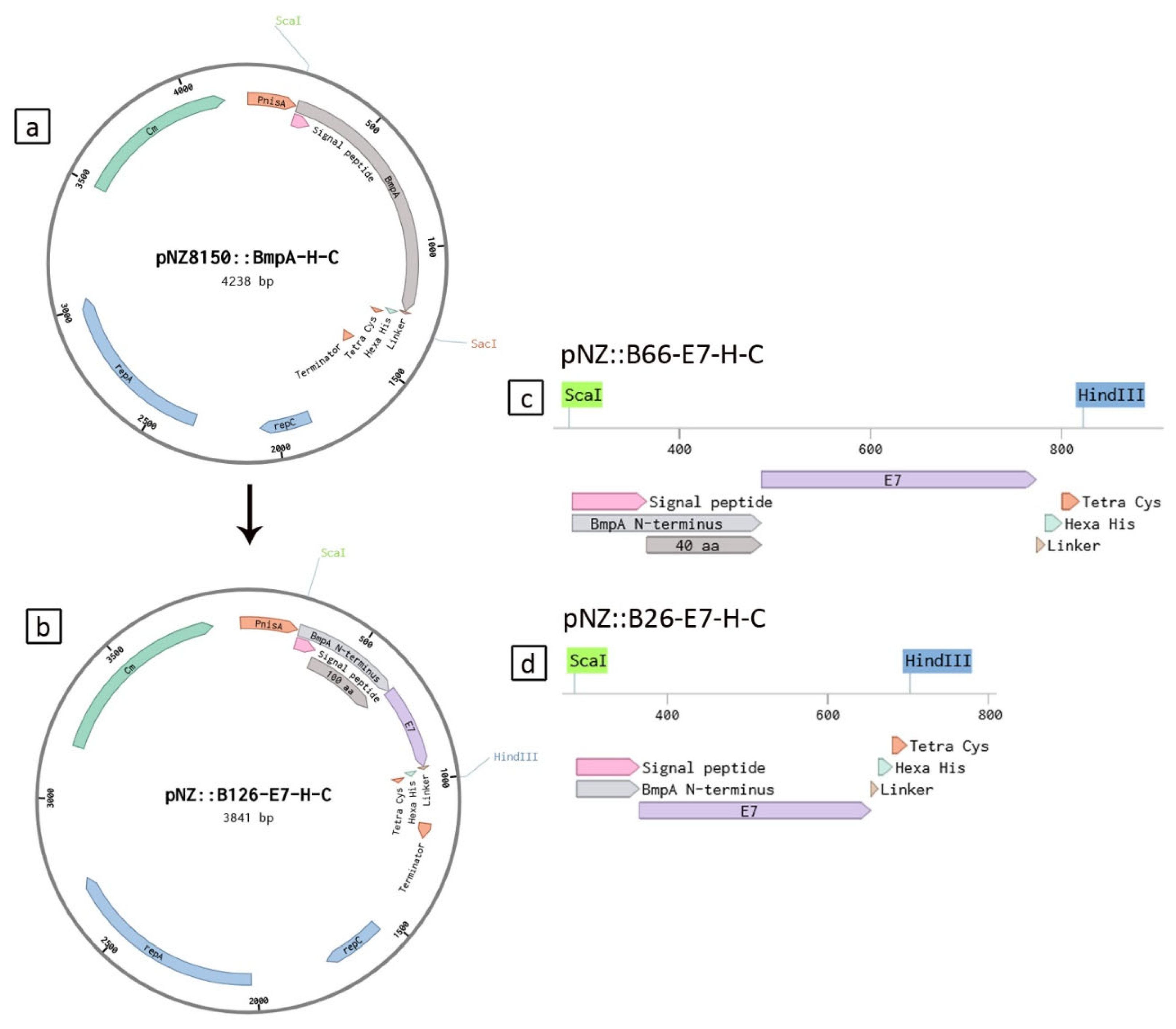
| Strains | Protein of Interest |
|---|---|
| BmpA | Recombinant BmpA with 6-His and 4-Cys tags |
| BE1 | Fusion protein: N-terminal 126 aa of BmpA with full-length E7 with 6-His and 4-Cys tags |
| BE2 | Fusion protein: N-terminal 66 aa of BmpA with full-length E7 with 6-His and 4-Cys tags |
| BE3 | Fusion protein: N-terminal 26 aa of BmpA with full-length E7 with 6-His and 4-Cys tags |
| BE1_1 | BE1 fusion protein with modifications of Q111A and D112A in between H3 and H4 |
| BE1_2 | BE1 fusion protein with combined modifications of K39A and T44A at H2 and Q111A and D112A in between H3 and H4 |
| BE1_2i | BE1_2 protein with additional modifications at H4: T114I and T115I |
| BE1_2v | BE1_2 protein with additional modifications at H4: T114V and T115V |
| BE1_3 | BE1 fusion protein with modifications of L102G and L103G at H3 |
| BE1_4 | BE1 fusion protein with combined modifications of V43G at H2 and L102G and L103G at H3 |
| BE2_1 | BE2 fusion protein with modifications of K39A and T44A at H2 |
| BE2_2 | BE2 fusion protein with modifications of V43G at H2 |
| Protein | AA | Mol. Weight (Dalton) | Theor. pI (*) | Instability Index (**) | Aliphatic Index | GRAVY (***) |
|---|---|---|---|---|---|---|
| B | 366 | 38,453 | 8.75 | 12.93 | 74.13 | −0.312 |
| E7 | 111 | 12,219 | 5.31 | 47.53 | 83.42 | −0.132 |
| BE1 | 237 | 25,595 | 6.43 | 34.10 | 74.56 | −0.297 |
| BE2 | 177 | 19,018 | 6.03 | 41.82 | 85.42 | −0.076 |
| BE3 | 137 | 14,868 | 6.02 | 45.99 | 91.82 | 0.055 |
| BE1_1 | 237 | 25,494 | 6.69 | 31.91 | 75.40 | −0.252 |
| BE1_2 | 237 | 25,407 | 6.43 | 32.27 | 76.24 | −0.218 |
| BE1_2i | 237 | 25,445 | 6.43 | 32.27 | 79.54 | −0.173 |
| BE1_2v | 237 | 25,417 | 6.43 | 32.27 | 78.69 | −0.176 |
| BE1_3 | 237 | 25,482 | 6.43 | 34.62 | 71.27 | −0.332 |
| BE1_4 | 237 | 25,441 | 6.43 | 34.98 | 70.04 | −0.352 |
| BE2_1 | 177 | 18,931 | 5.85 | 42.30 | 86.55 | −0.030 |
| BE2_2 | 177 | 18,976 | 6.03 | 42.30 | 83.79 | −0.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, M.N.; Peterbauer, C. Roles of Mature Domain Targeting Signals (MTSs) for Protein Translocation and Secretion in Lactococcus lactis. Int. J. Mol. Sci. 2025, 26, 219. https://doi.org/10.3390/ijms26010219
Hoang MN, Peterbauer C. Roles of Mature Domain Targeting Signals (MTSs) for Protein Translocation and Secretion in Lactococcus lactis. International Journal of Molecular Sciences. 2025; 26(1):219. https://doi.org/10.3390/ijms26010219
Chicago/Turabian StyleHoang, Mai Ngoc, and Clemens Peterbauer. 2025. "Roles of Mature Domain Targeting Signals (MTSs) for Protein Translocation and Secretion in Lactococcus lactis" International Journal of Molecular Sciences 26, no. 1: 219. https://doi.org/10.3390/ijms26010219
APA StyleHoang, M. N., & Peterbauer, C. (2025). Roles of Mature Domain Targeting Signals (MTSs) for Protein Translocation and Secretion in Lactococcus lactis. International Journal of Molecular Sciences, 26(1), 219. https://doi.org/10.3390/ijms26010219







