Calcium and Magnesium Regulation of Kernel Sugar Content in Maize: Role of Endogenous Hormones and Antioxidant Enzymes
Abstract
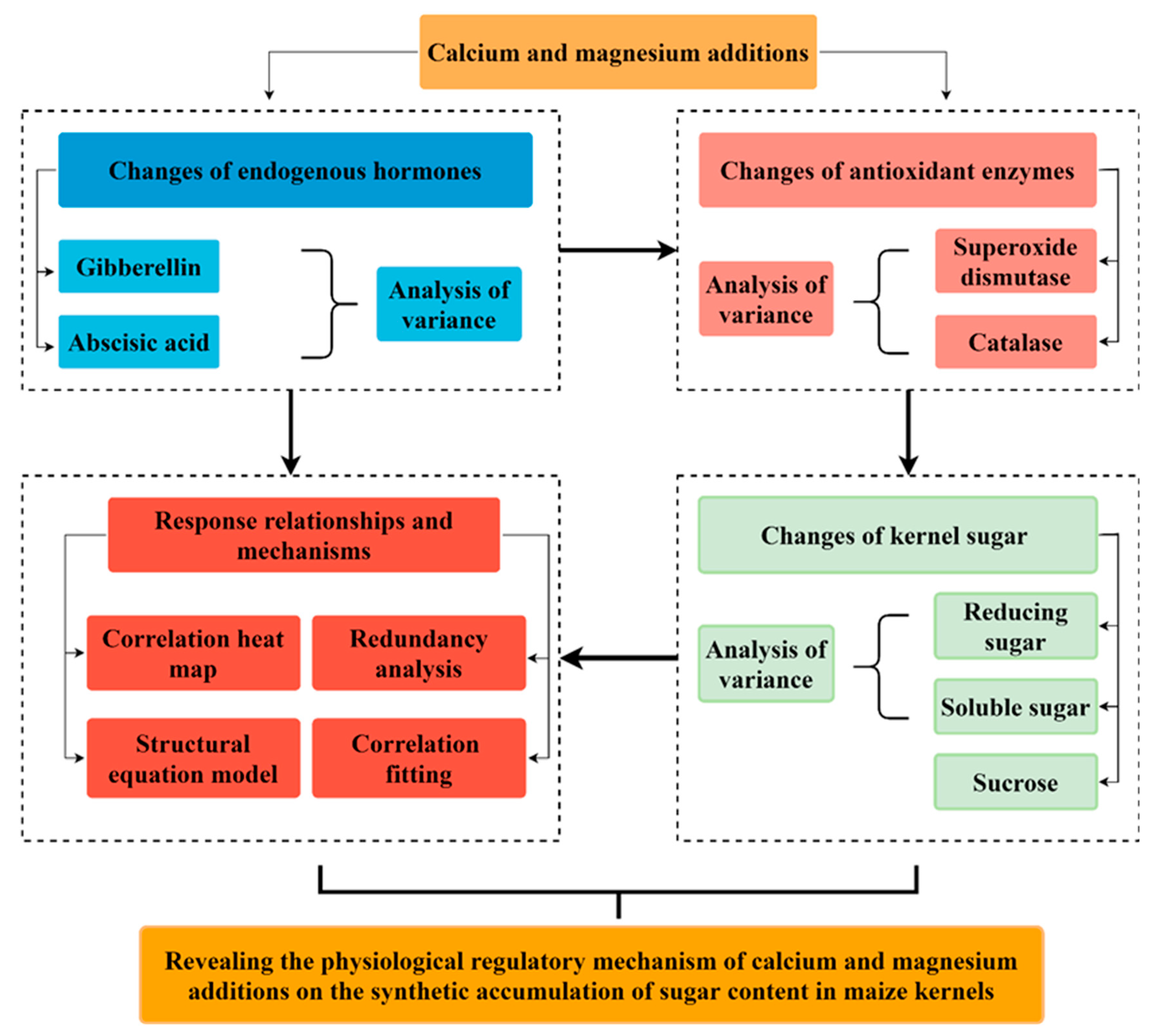
1. Introduction
2. Results
2.1. Changes in Endogenous Hormones and Antioxidant Enzymes in Maize Leaves
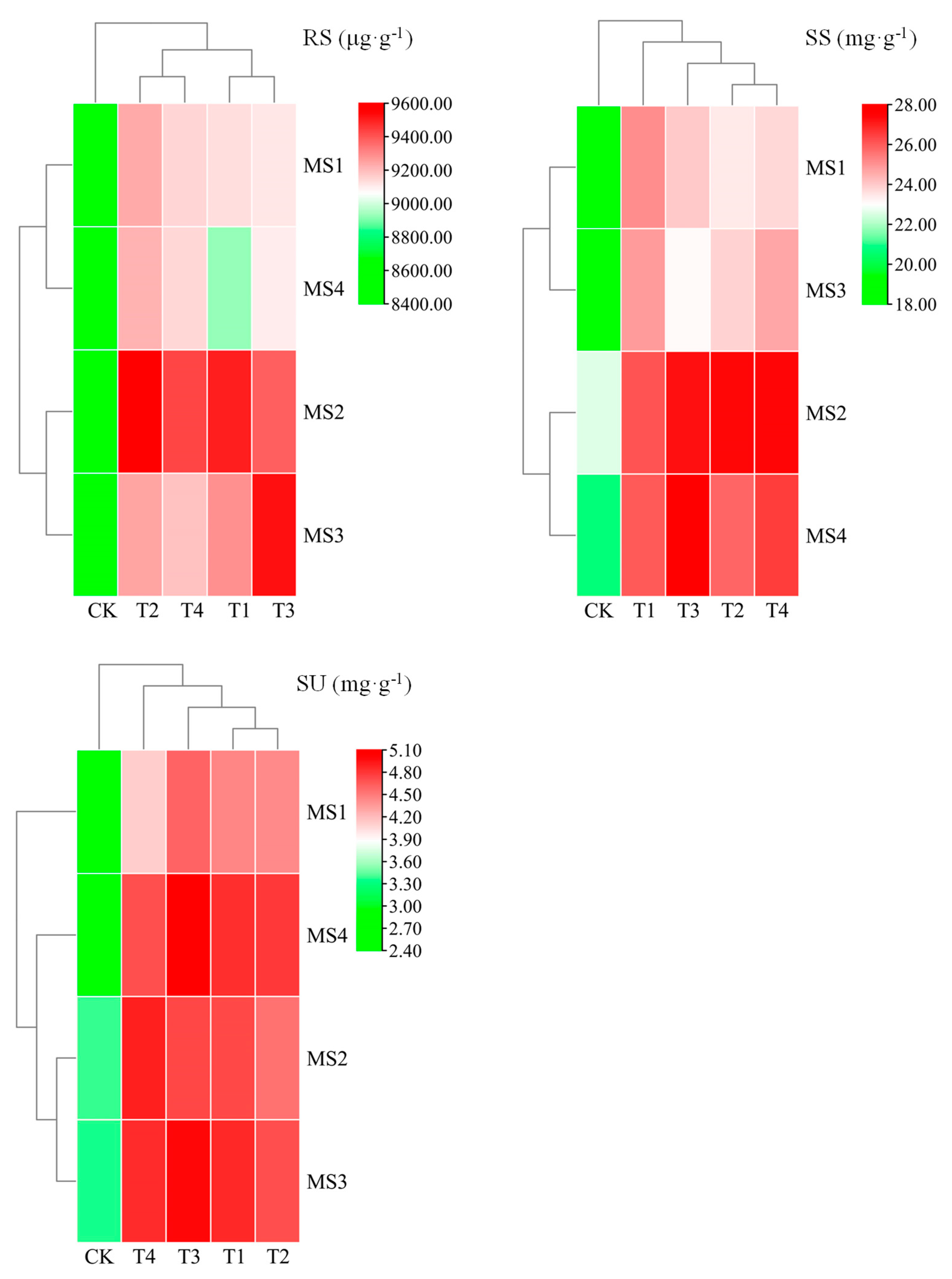
2.2. Variation in and Correlation of Sugar Content in Maize Kernels
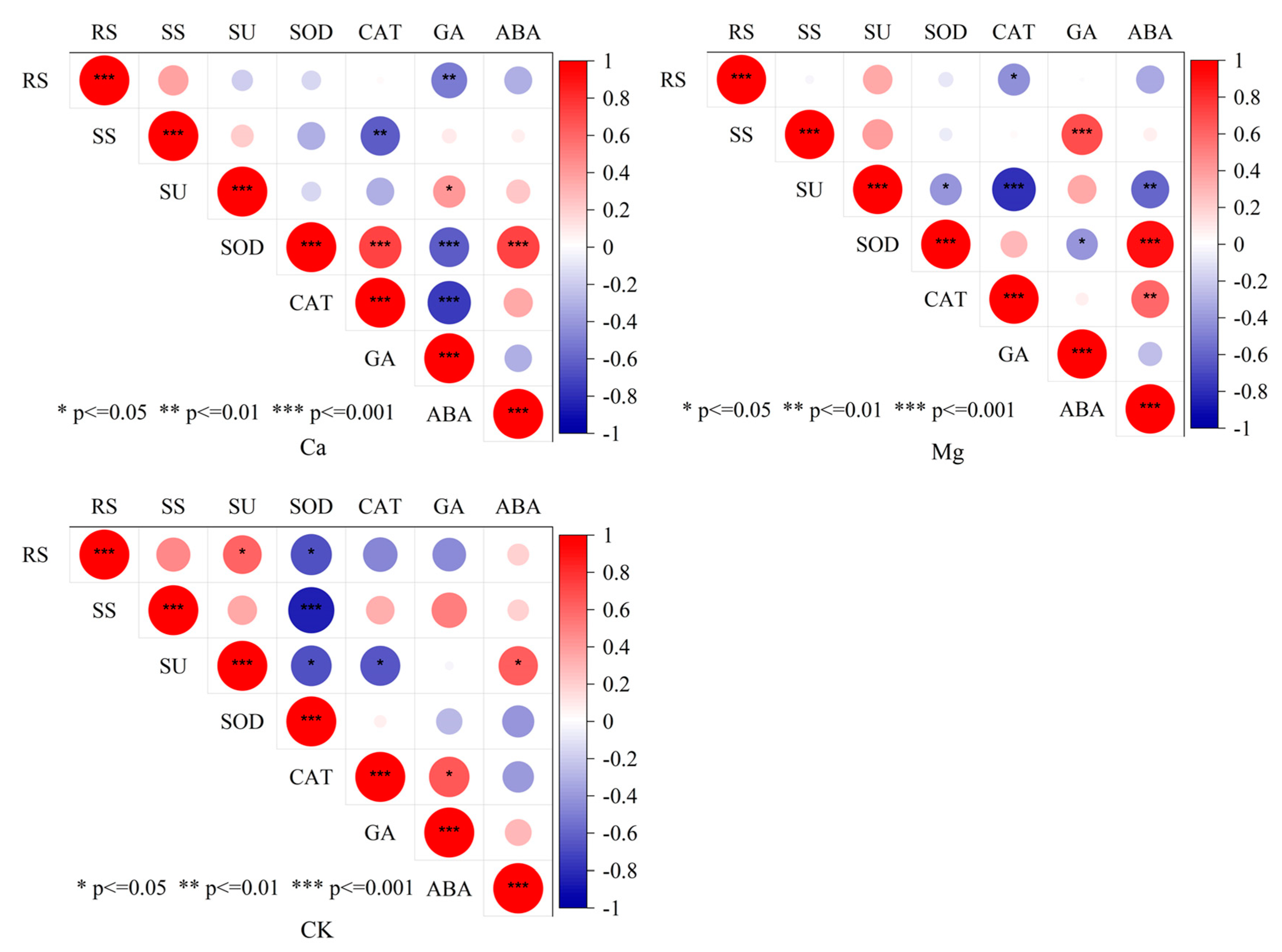
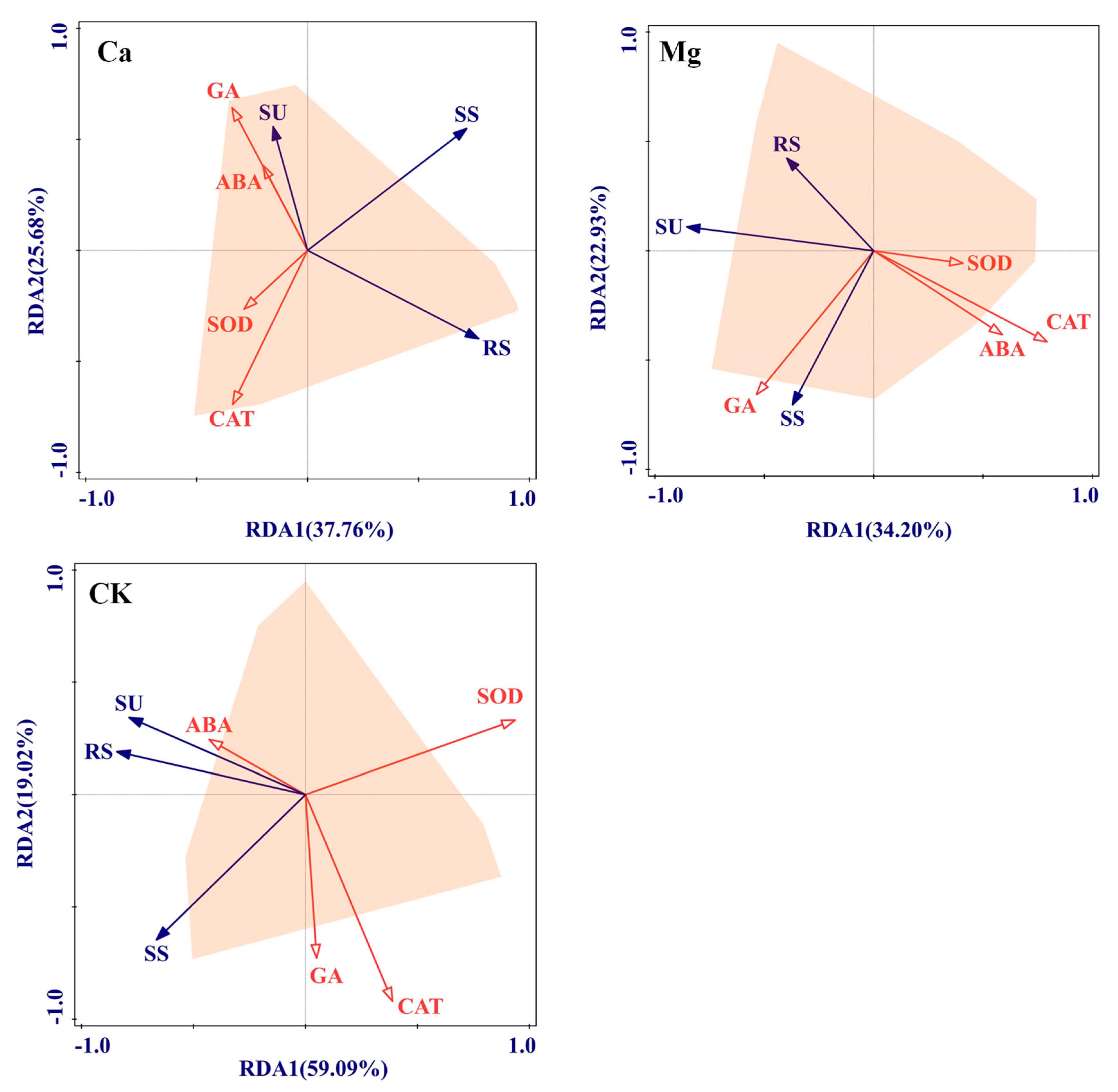
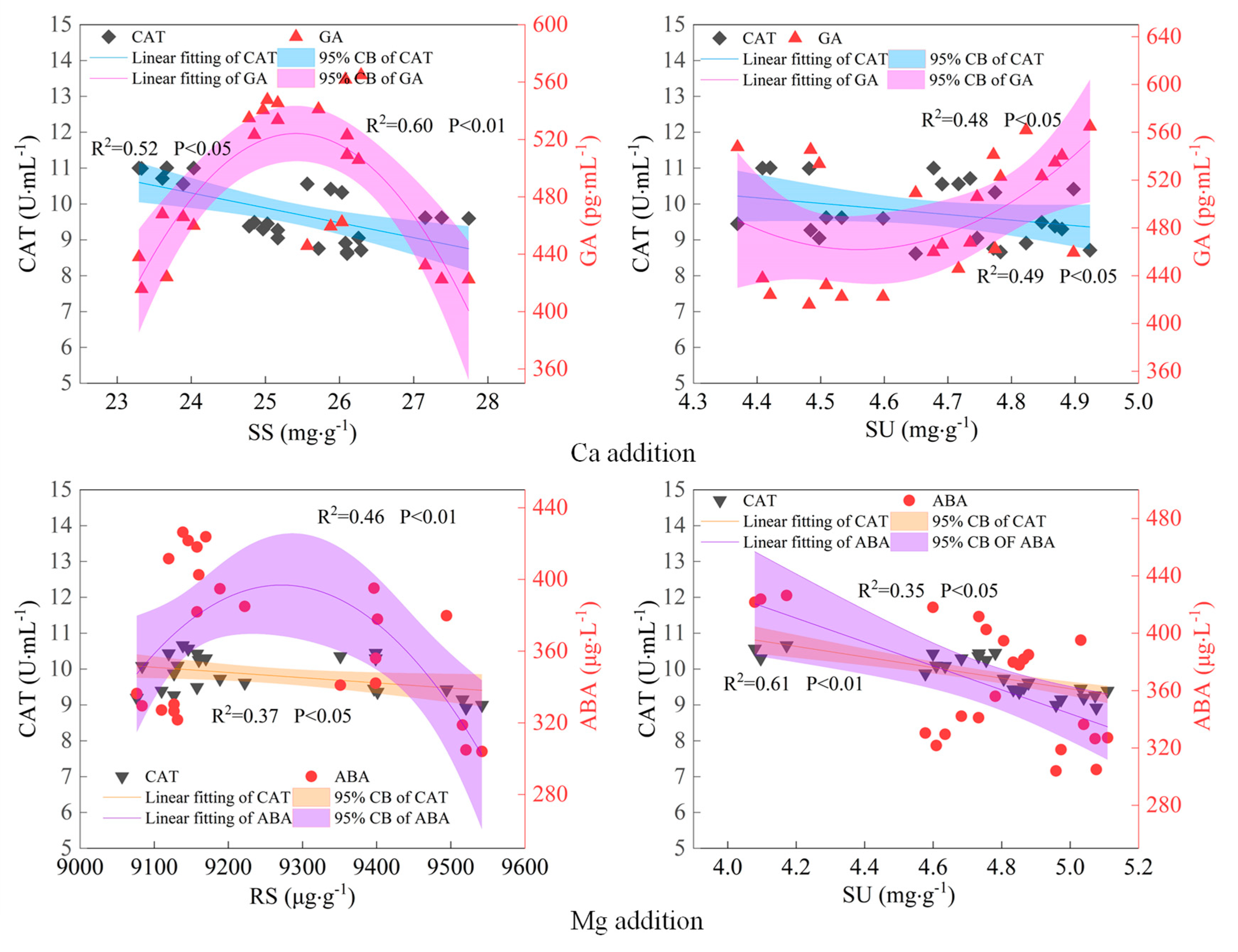
2.3. Physiological Driving Mechanism of Sugar Content Accumulation in Maize Kernels
3. Discussion
3.1. Effects of Ca and Mg Supplementation on Endogenous Hormones and Antioxidant Enzymes in Maize Leaves
3.2. Effect of Ca and Mg Supplementation on the Sugar Content of Maize Kernels
3.3. Physiological Mechanism of Ca and Mg Supplementation in Regulating Sugar Content of Maize Kernels
4. Materials and Methods
4.1. Experimental Planning
- (1)
- Experimental Site Location and Climate
- (2)
- Soil Characteristics
- (3)
- Test Material
- (4)
- Test design and treatment
- (5)
- Cultivation Method and Physical Dimensions
- (6)
- Fertilization and Sample Collection
4.2. Index Measurement and Methods
4.2.1. Test Sample Collection
4.2.2. Determination Methods
- (1)
- Leaf ABA and GA contents determined using the high-performance liquid chromatography (HPLC) method [44]
- (2)
- Leaf SOD activity determined using the nitrogen blue tetrazolium (NBT) photoreduction method; CAT activity determined using UV spectrophotometry [46]
- (3)
- Kernel sugar content determined using the anthrone colorimetric method [48]
4.3. Statistical Methods
5. Conclusions
- (1)
- The high−Mg treatment significantly increased the SOD activity and antioxidant capacity in maize leaves, while the low−Ca and Mg treatments resulted in lower SOD activity with limited effects. The high−Ca treatment was the most effective in enhancing CAT activity during the milk ripening to ripening stage. The low−Ca treatments promoted GA synthesis at the late ripening stage, whereas the high−Ca and Mg treatments inhibited GA at the early ripening stage. The high−Mg treatments also increased the ABA content, potentially enhancing stress tolerance, while the low−Ca treatments had no significant effect on the ABA content.
- (2)
- The high−Ca treatment was the most effective in enhancing the RS content in maize kernels, while the low−Mg treatment was the most effective in increasing the SU content. Both the low- and high−Mg treatments increased the SS content, indicating the importance of Mg for the energy supply.
- (3)
- The core physiological pathway regulating the sugar content in maize kernels after Ca supplementation was “GA−CAT−SU and SS”, with GA negatively regulating CAT activity, which in turn, negatively regulated the SU and SS contents. After Mg supplementation, the pathway was “ABA−CAT−SU and SS”, with ABA positively regulating CAT activity, which then negatively regulated the SU and SS contents. In the control treatments, endogenous hormones affected CAT activity, but the regulation by GA and ABA was opposite to that observed with Ca and Mg supplementation. The relationship between endogenous hormones and the maize kernel sugar content was complex, exhibiting a quadratic nonlinear relationship rather than a simple linear one.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.Q.; Xiang, R.H.; Li, Z.G. The Essential role of H2S-ABA crosstalk in maize thermotolerance through the ROS-scavenging system. Int. J. Mol. Sci. 2023, 24, 12264. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Zhao, W.; Lv, Z.; Liu, L.; Ali, S.; Chen, B.; Hu, W.; Zhou, Z.; Wang, Y. Auxin and abscisic acid play important roles in promoting glucose metabolism of reactivated young kernels of maize (Zea mays L.). Physiol. Plant. 2023, 175, e14019. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Rady, M. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol. Environ. Saf. 2019, 182, 109378. [Google Scholar] [CrossRef]
- Libron, J.; Cardona, D.; Mateo, J.; Beltran, A.; Tuaño, A.; Laude, T. Nutritional properties and phenolic acid profile of selected Philippine pigmented maize with high antioxidant activity. J. Food Compos. Anal. 2021, 101, 103954. [Google Scholar] [CrossRef]
- Tayyab, N.; Naz, R.; Yasmin, H.; Nosheen, A.; Keyani, R.; Sajjad, M.; Hassan, M.; Roberts, T. Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate drought-induced stress in maize. PLoS ONE 2020, 15, e0232269. [Google Scholar]
- Zhao, M.; Meng, Y.; Wang, Y.; Sun, G.; Liu, X.; Li, J.; Wei, S.; Gu, W. Exogenous hemin alleviates cadmium stress in maize by enhancing sucrose and nitrogen metabolism and regulating endogenous hormones. Int. J. Phytoremediat. 2022, 25, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ji, J.; Zhang, S.; Xiao, W.; Guan, C.; Wang, G.; Wang, Y. Changes in the phenolic compound content and antioxidant activity in developmental maize kernels and expression profiles of phenolic biosynthesis-related genes. J. Cereal Sci. 2020, 96, 103113. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, J.; Sun, L.; Huang, B.; Ding, C.; Gu, Y.; Liao, J.; Hu, C.; Zhang, Z.; Yuan, S.; et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Liu, N.; Xue, Y.; Guo, Z.; Li, W.; Tang, J. Genome-wide association study identifies candidate genes for starch content regulation in maize kernels. Front. Plant Sci. 2016, 7, 1046. [Google Scholar] [CrossRef]
- Chen, B.; Feng, S.; Hou, J.; Zhu, Y.; Bao, F.; Han, H.; Tan, H.; Wang, G.; Zhao, F. Genome-wide transcriptome analysis revealing the genes related to sugar metabolism in kernels of sweet corn. Metabolites 2022, 12, 1254. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Y.; Luo, H.; Li, D.; Liu, C.; Song, J.; Zhang, Z.; Liu, C.; Niu, L. Effect of NaCl stress and supplemental CaCl2 on carotenoid accumulation in germinated yellow maize kernels. Food Chem. 2019, 125779. [Google Scholar] [CrossRef]
- Das, A.; Singh, V. Antioxidative free and bound phenolic constituents in botanical fractions of Indian specialty maize (Zea mays L.) genotypes. Food Chem. 2016, 201, 298–306. [Google Scholar] [CrossRef]
- Zabed, H.; Faruq, G.; Sahu, J.; Boyce, A.; Ganesan, P. A comparative study on normal and high sugary corn genotypes for evaluating enzyme consumption during dry-grind ethanol production. Chem. Eng. J. 2016, 287, 691–703. [Google Scholar] [CrossRef]
- Raza, M.; Saeed, F.; Afzaal, M.; Imran, A.; Niaz, B.; Hussain, M.; Rasheed, A.; Mukhtar, M.; Waleed, M.; Jbawi, E. Comparative study of cross- and uncross-linked arabinoxylans extracted from maize bran with special reference to their structural and antioxidant potential. Int. J. Food Prop. 2022, 25, 2495–2504. [Google Scholar] [CrossRef]
- Chen, G.; Chen, B.; Song, D. Co-microbiological regulation of phenolic release through solid-state fermentation of corn kernels (Zea mays L.) to improve their antioxidant activity. LWT Food Sci. Technol. 2021, 142, 111003. [Google Scholar] [CrossRef]
- Ali, R.; Gul, H.; Rauf, M.; Arif, M.; Hamayun, M.H.; Khilji, S.; Ud-Din, A.; Sajid, Z.; Lee, I. Growth-promoting endophytic fungus (Stemphylium lycopersici) ameliorates salt stress tolerance in maize by balancing ionic and metabolic status. Front. Plant Sci. 2022, 13, 890565. [Google Scholar] [CrossRef]
- Zhou, X.; Li, R.; Shen, H.; Yang, L. Effect of exogenous plant growth regulators and rejuvenation measures on the endogenous hormone and enzyme activity responses of Acer mono maxim in cuttage rooting. Int. J. Mol. Sci. 2023, 24, 11883. [Google Scholar] [CrossRef]
- Ahmad, S.; Cui, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Su, W.; Javed, T.; El-Serehy, H.; Jia, Z.; et al. Exogenous application of melatonin induces tolerance to salt stress by improving the photosynthetic efficiency and antioxidant defense system of maize seedling. J. Plant Growth Regul. 2020, 40, 1270–1283. [Google Scholar] [CrossRef]
- Fu, J.; Li, L.; Wang, S.; Yu, N.; Shan, H.; Shi, Z.; Li, F.; Zhong, X. Effect of gibberellic acid on photosynthesis and oxidative stress response in maize under weak light conditions. Front. Plant Sci. 2023, 14, 1128780. [Google Scholar] [CrossRef] [PubMed]
- Doll, N.; Depège-Fargeix, N.; Rogowsky, P.; Widiez, T. Signaling in early maize kernel development. Mol. Plant 2017, 103, 375–388. [Google Scholar] [CrossRef]
- Li, J.; Zhao, M.; Liu, L.; Guo, X.; Pei, Y.; Wang, C.; Song, X. Exogenous sorbitol application confers drought tolerance to maize seedlings through up-regulating antioxidant system and endogenous sorbitol biosynthesis. Plants 2023, 12, 2456. [Google Scholar] [CrossRef]
- Urias-Lugo, D.; Heredia, J.; Serna-Saldívar, S.; Muy-Rangel, M.; Valdez-Torres, J. Total phenolics, total anthocyanins and antioxidant capacity of native and elite blue maize hybrids (Zea mays L.). CyTA J. Food 2015, 13, 336–339. [Google Scholar] [CrossRef]
- Hu, S.; Wang, M.; Zhang, X.; Chen, W.; Song, X.; Fu, X.; Fang, H.; Xu, J.; Xiao, Y.; Li, Y.; et al. Genetic basis of kernel starch content decoded in a maize multi-parent population. Plant Biotechnol. J. 2021, 19, 2192–2205. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Luo, H.; Xu, H.; Zhou, Z.; Li, D.; Bao, Y.; Fu, Q.; Song, J.; Jiao, Y.; Zhang, Z. Effect of exogenous methyl jasmonate on physiological and carotenoid composition of yellow maize sprouts under NaCl stress. Food Chem. 2021, 361, 130177. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Li, W.; Wen, T.; Li, T.; Guo, X.; Liu, R. Anthocyanin accumulation, biosynthesis and antioxidant capacity of black sweet corn (Zea mays L.) during kernel development over two growing seasons. J. Cereal Sci. 2020, 95, 103065. [Google Scholar] [CrossRef]
- Yang, L.; Fountain, J.; Ji, P.; Ni, X.; Chen, S.; Lee, R.; Kemerait, R.; Guo, B. Deciphering drought-induced metabolic responses and regulation in developing maize kernels. Plant Biotechnol. J. 2018, 16, 1616–1628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Wang, Z.; Shen, W.; Xu, X. Protective effects of hydrogen-rich water on the photosynthetic apparatus of maize seedlings (Zea mays L.) as a result of an increase in antioxidant enzyme activities under high light stress. Plant Growth Regul. 2015, 77, 43–56. [Google Scholar] [CrossRef]
- Rohman, M.; Begum, S.; Talukder, M.; Akhi, A.; Amiruzzaman, M.; Ahsan, A.; Hossain, Z. Drought sensitive maize inbred shows more oxidative damage and higher ROS scavenging enzymes, but not glyoxalases than a tolerant one at seedling stage. Plant Omics 2016, 9, 220–232. [Google Scholar] [CrossRef]
- Saikaew, K.; Lertrat, K.; Meenune, M.; Tangwongchai, R. Effect of high-pressure processing on colour, phytochemical contents and antioxidant activities of purple waxy corn (Zea mays L. var. ceratina) kernels. Food Chem. 2018, 243, 328–337. [Google Scholar] [CrossRef]
- Rady, M.; Hemida, K. Sequenced application of ascorbate-proline-glutathione improves salt tolerance in maize seedlings. Ecotoxicol. Environ. Saf. 2016, 133, 252–259. [Google Scholar] [CrossRef]
- Anwar, A.; Bai, L.; Miao, L.; Liu, Y.; Li, S.; Yu, X.; Li, Y. 24-Epibrassinolide ameliorates endogenous hormone levels to enhance low-temperature stress tolerance in cucumber seedlings. Int. J. Mol. Sci. 2018, 19, 2497. [Google Scholar] [CrossRef]
- Anjum, S.; Ashraf, U.; Khan, I.; Tanveer, M.; Shahid, M.; Shakoor, A.; Wang, L. Phyto-toxicity of chromium in maize: Oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 2017, 27, 262–273. [Google Scholar] [CrossRef]
- Ahmad, I.; Kamran, M.; Meng, X.; Ali, S.; Bilegjargal, B.; Cai, T.; Liu, T.; Han, Q. Effects of plant growth regulators on seed filling, endogenous hormone contents and maize production in semiarid regions. J. Plant Growth Regul. 2019, 38, 1467–1480. [Google Scholar] [CrossRef]
- Zabed, H.; Boyce, A.; Faruq, G.; Sahu, J. A comparative evaluation of agronomic performance and kernel composition of normal and high sugary corn genotypes (Zea mays L.) grown for dry-grind ethanol production. Ind. Crop. Prod. 2016, 94, 9–19. [Google Scholar] [CrossRef]
- Yetişsin, F. Exogenous acetone O-(2-naphthylsulfonyl) oxime improves the adverse effects of excess copper by copper detoxification systems in maize. Int. J. Phytoremediat. 2023, 25, 2001–2013. [Google Scholar] [CrossRef] [PubMed]
- Okant, M.; Kaya, C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Res. 2019, 26, 11864–11874. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Zhang, Y.; Cui, J.; Zhang, H.; Wang, H.; Jin, H.; Lu, P.; He, L.; Zhou, Q.; Yu, J.; et al. An enhanced interaction of graft and exogenous SA on photosynthesis, phytohormone, and transcriptome analysis in tomato under salinity stress. Int. J. Mol. Sci. 2024, 25, 10799. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.; Afzal, S.; Dawood, M.; Abbasi, G.; Khan, M.; Kamran, M.; Zhran, M.; Hayat, M.; Aslam, M.; Rafay, M. Exogenous melatonin mitigates chromium toxicity in maize seedlings by modulating antioxidant system and suppresses chromium uptake and oxidative stress. Environ. Geochem. Health 2021, 44, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Gao, Z.; Shen, S.; Liang, X.; Zhao, X.; Lin, S.; Zhou, S. Regulation of maize kernel weight and carbohydrate metabolism by abscisic acid applied at the early and middle post-pollination stages in vitro. J. Plant Physiol. 2017, 216, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Martínez, M.; Otero-Pappatheodorou, J.; Serna-Saldívar, S.; García-Lara, S. Antioxidant activity and characterization of protein fractions and hydrolysates from normal and quality protein maize kernels. J. Cereal Sci. 2017, 76, 85–91. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Liu, B.; Zhang, L.; Zhang, W.; Chen, R.; Wang, L. Overexpression of the maize γ-tocopherol methyltransferase gene (ZmTMT) increases α-tocopherol content in transgenic Arabidopsis and maize seeds. Transgenic Res. 2019, 29, 95–104. [Google Scholar] [CrossRef]
- Pal, S.; Bhattacharjee, P. Supercritical carbon dioxide extraction of lutein from yellow maize (Zea mays) kernels: Process optimization based on lutein content, antioxidant activity, and ω-6/ω-3 fatty acid ratio. Food Sci. Biotechnol. 2017, 26, 1511–1521. [Google Scholar] [CrossRef]
- Blandino, M.; Alfieri, M.; Giordano, D.; Vanara, F.; Redaelli, R. Distribution of bioactive compounds in maize fractions obtained in two different types of large scale milling processes. J. Cereal Sci. 2017, 77, 251–258. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Bai, L.; Zhang, S.; Wang, Y. Melatonin enhances thermotolerance of maize seedlings (Zea mays L.) by modulating antioxidant defense, methylglyoxal detoxification, and osmoregulation systems. Protoplasma 2018, 256, 471–490. [Google Scholar] [CrossRef]
- Wang, H.; Liu, P.; Zhang, J.; Zhao, B.; Ren, B. Endogenous hormones inhibit differentiation of young ears in maize (Zea mays L.) under heat stress. Front. Plant Sci. 2020, 11, 533046. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gong, S.; Fu, L.; Hu, G.; Li, G.; Hu, S.; Yang, J. The involvement of antioxidant enzyme system, nitrogen metabolism and osmoregulatory substances in alleviating salt stress in inbred maize lines and hormone regulation mechanisms. Plants 2022, 11, 1547. [Google Scholar] [CrossRef]
- Karalija, E.; Selović, A. The effect of hydro and proline seed priming on growth, proline and sugar content, and antioxidant activity of maize under cadmium stress. Environ. Sci. Pollut. Res. 2018, 25, 33370–33380. [Google Scholar] [CrossRef]
- Chen, K.; Ali, S.; Chen, Y.M.; Sohail, A.; Jan, A.I.; Fahad, S. Effect of ridge-covering mulching materials on hormonal changes, antioxidative enzyme activities and production of maize in semi-arid regions of China. Agric. Water Manag. 2018, 204, 281–291. [Google Scholar] [CrossRef]
- Liu, B.; Long, S.; Liu, K.; Zhu, T.; Gong, J.; Gao, S.; Wang, R.; Zhang, L.; Liu, T.; Xu, Y. Paclobutrazol ameliorates low-light-induced damage by improving photosynthesis, antioxidant defense system, and regulating hormone levels in tall fescue. Int. J. Mol. Sci. 2022, 23, 9966. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Shang, X.; Jin, X.; Wang, X.; Xing, Y. Calcium and Magnesium Regulation of Kernel Sugar Content in Maize: Role of Endogenous Hormones and Antioxidant Enzymes. Int. J. Mol. Sci. 2025, 26, 200. https://doi.org/10.3390/ijms26010200
He Z, Shang X, Jin X, Wang X, Xing Y. Calcium and Magnesium Regulation of Kernel Sugar Content in Maize: Role of Endogenous Hormones and Antioxidant Enzymes. International Journal of Molecular Sciences. 2025; 26(1):200. https://doi.org/10.3390/ijms26010200
Chicago/Turabian StyleHe, Zhaoquan, Xue Shang, Xiaoze Jin, Xiukang Wang, and Yingying Xing. 2025. "Calcium and Magnesium Regulation of Kernel Sugar Content in Maize: Role of Endogenous Hormones and Antioxidant Enzymes" International Journal of Molecular Sciences 26, no. 1: 200. https://doi.org/10.3390/ijms26010200
APA StyleHe, Z., Shang, X., Jin, X., Wang, X., & Xing, Y. (2025). Calcium and Magnesium Regulation of Kernel Sugar Content in Maize: Role of Endogenous Hormones and Antioxidant Enzymes. International Journal of Molecular Sciences, 26(1), 200. https://doi.org/10.3390/ijms26010200





