Harnessing Mesenchymal Stromal Cells for Advanced Wound Healing: A Comprehensive Review of Mechanisms and Applications
Abstract
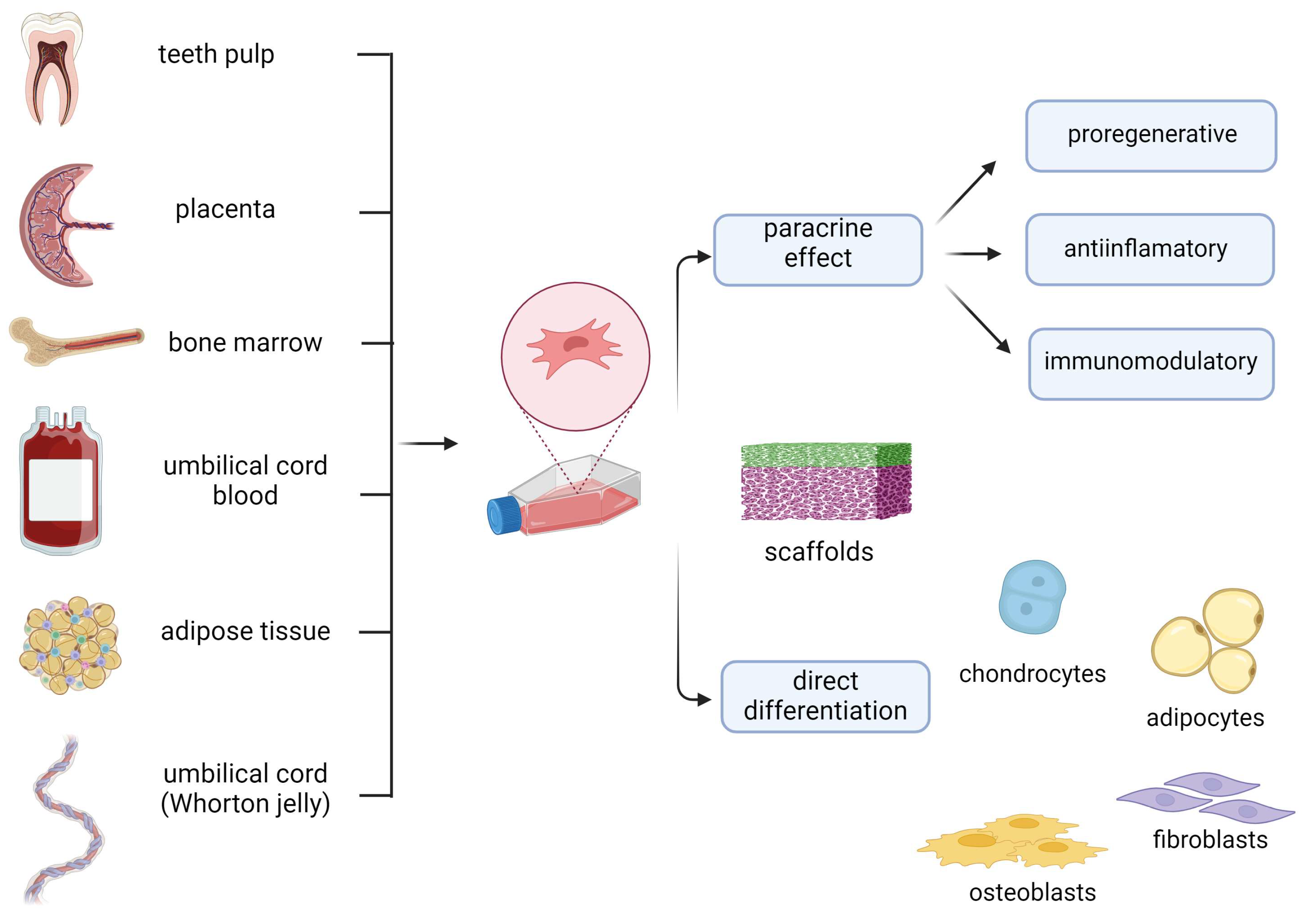
1. Introduction
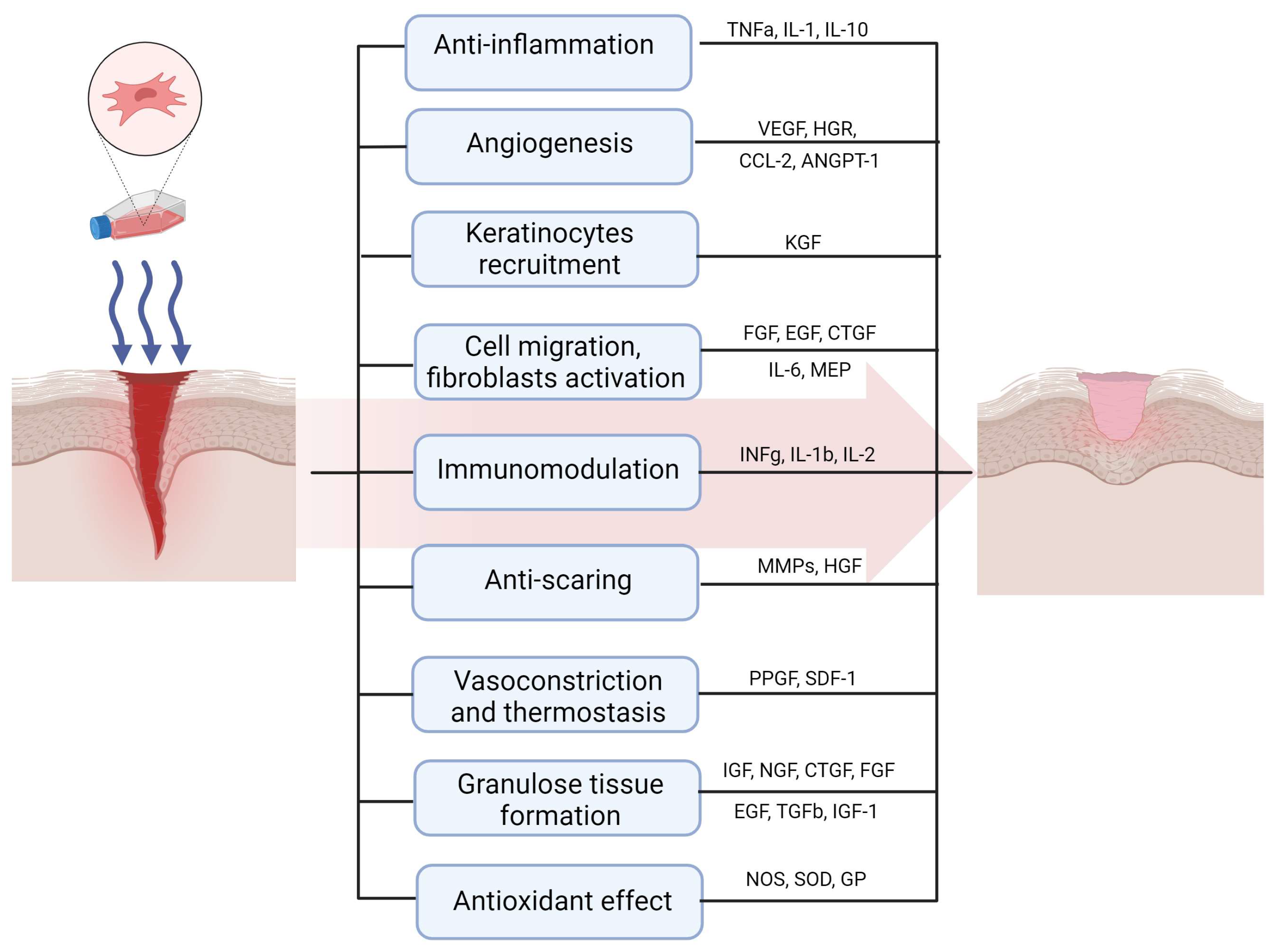
2. Mechanisms of Therapeutic Effect of MSCs in Wound Healing
3. Chronic Wounds
4. Diabetic Wounds
5. Burns
6. Non-Union Fractures
7. Pressure Ulcers
8. Lower Extremity Venous Ulcers
9. Epidermolysis Bullosa
10. Advantages and Limitations of MSC’s Application in Wound Healing
11. Summary and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Y.-Z.; Gou, M.; Da, L.-C.; Zhang, W.-Q.; Xie, H.-Q. Mesenchymal stem cells for chronic wound healing: Current status of preclinical and clinical studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Mirhaj, M.; Labbaf, S.; Tavakoli, M.; Seifalian, A.M. Emerging treatment strategies in wound care. Int. Wound J. 2022, 19, 1934–1954. [Google Scholar] [CrossRef] [PubMed]
- Isakson, M.; De Blacam, C.; Whelan, D.; McArdle, A.; Clover, A.J.P. Mesenchymal stem cells and cutaneous wound healing: Current evidence and future potential. Stem Cells Int. 2015, 2015, 831095. [Google Scholar] [CrossRef]
- Ong, H.T.; Dilley, R.J. Novel non-angiogenic role for mesenchymal stem cell-derived vascular endothelial growth factor on keratinocytes during wound healing. Cytokine Growth Factor Rev. 2018, 44, 69–79. [Google Scholar] [CrossRef]
- Millán-Rivero, J.E.; Martínez, C.M.; Romecín, P.A.; Aznar-Cervantes, S.D.; Carpes-Ruiz, M.; Cenis, J.L.; Moraleda, J.M.; Atucha, N.M.; García-Bernal, D. Silk fibroin scaffolds seeded with Wharton’s jelly mesenchymal stem cells enhance re-epithelialization and reduce formation of scar tissue after cutaneous wound healing. Stem Cell Res. Ther. 2019, 10, 126. [Google Scholar] [CrossRef]
- Wu, S.; Sun, S.; Fu, W.; Yang, Z.; Yao, H.; Zhang, Z. The Role and Prospects of Mesenchymal Stem Cells in Skin Repair and Regeneration. Biomedicines 2024, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Sopata, M.; Jawień, A.; Mrozikiewicz-Rakowska, B.; Augusewicz, Z.; Bakowska, M.; Samson, I.; Gabriel, M.; Grzela, T.; Karpiński, T.; Kuberka, I.; et al. Wytyczne postępowania miejscowego w ranach niezakażonych, zagrożonych infekcją oraz zakażonych—Przegląd dostępnych substancji przeciwdrobnoustrojowych stosowanych w leczeniu ran. Zalecenia Polskiego Towarzystwa Leczenia Ran. Leczenie Ran 2020, 17, 1–21. [Google Scholar] [CrossRef]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M.A. Concise Review: Role of Mesenchymal stem cells in wound Repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Cappuzzello, C.; Doni, A.; Dander, E.; Pasqualini, F.; Nebuloni, M.; Bottazzi, B.; Mantovani, A.; Biondi, A.; Garlanda, C.; D’Amico, G. Mesenchymal stromal Cell-Derived PTX3 promotes wound healing via fibrin remodeling. J. Investig. Dermatol. 2016, 136, 293–300. [Google Scholar] [CrossRef]
- Gofron, M.; Mrozikiewicz-Rakowska, B.; Sieńko, D.; Czupryniak, L. Możliwości zwiększenia efektywności terapii leczenia ran za pomocą mezenchymalnych komórek macierzystych u osób chorych na cukrzycę. Diabetol. Prakt. 2021, 7, 162–170. [Google Scholar] [CrossRef]
- Ramos-Gonzalez, G.; Salazar, L.; Wittig, O.; Diaz-Solano, D.; Cardier, J.E. The effects of mesenchymal stromal cells and platelet-rich plasma treatments on cutaneous wound healing. Arch. Dermatol. Res. 2022, 315, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fang, T.; Qi, Y.; Yin, X.; Di, T.; Feng, G.; Lei, Z.; Zhang, Y.; Huang, Z. Combined use of mesenchymal stromal cell sheet transplantation and local injection of SDF-1 for bone repair in a rat nonunion model. Cell Transplant. 2016, 25, 1801–1817. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Breitbach, M.; Schildberg, F.A.; Hesse, M.; Fleischmann, B.K. Bone marrow CD73+ mesenchymal stem cells display increased stemness in vitro and promote fracture healing in vivo. Bone Rep. 2021, 15, 101133. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, C.; Zhao, L.; Chen, K.; He, H.; Mo, Z. Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. Int. J. Endocrinol. 2013, 2013, 592454. [Google Scholar] [CrossRef]
- Lee, D.E.; Ayoub, N.; Agrawal, D.K. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Guillamat-Prats, R. The role of MSC in wound healing, scarring and regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef] [PubMed]
- Saheli, M.; Bayat, M.; Ganji, R.; Hendudari, F.; Kheirjou, R.; Pakzad, M.; Najar, B.; Piryaei, A. Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Arch. Dermatol. Res. 2019, 312, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Concise Review: Clinical Translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl. Med. 2011, 1, 44–50. [Google Scholar] [CrossRef]
- An, Y.; Liu, W.J.; Xue, P.; Ma, Y.; Zhang, L.Q.; Zhu, B.; Qi, M.; Li, L.Y.; Zhang, Y.J.; Wang, Q.T.; et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death Dis. 2018, 9, 58. [Google Scholar] [CrossRef]
- Palma, M.B.; Luzzani, C.; Andrini, L.B.; Riccillo, F.; Buero, G.; Pelinski, P.; Inda, A.M.; Errecalde, A.L.; Miriuka, S.; Carosella, E.D.; et al. Wound healing by allogeneic transplantation of specific subpopulation from human umbilical cord mesenchymal stem cells. Cell Transplant. 2021, 30, 096368972199377. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.; Johnson, V.; Impastato, R.; Coy, J.; Strumpf, A.; Dow, S. Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells. Stem Cells Transl. Med. 2019, 9, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Menocal, L.; Shareef, S.; Salgado, M.; Shabbir, A.; Van Badiavas, E. Role of whole bone marrow, whole bone marrow cultured cells, and mesenchymal stem cells in chronic wound healing. Stem Cell Res. Ther. 2015, 6, 24. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, J.; Niu, X.; Hu, G.; Guo, S.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 2015, 13, 49. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, G.; Meng, F.; Wang, W.; Hao, P.; Xiang, Y.; Wang, Y.; Han, R.; Li, F.; Wang, L.; et al. Paracrine effects of mesenchymal stem cells on the activation of keratocytes. Br. J. Ophthalmol. 2017, 101, 1583–1590. [Google Scholar] [CrossRef]
- Aryan, A.; Bayat, M.; Bonakdar, S.; Taheri, S.; Haghparast, N.; Bagheri, M.; Piryaei, A.; Abdollahifar, M.-A. Human bone marrow mesenchymal stem cell conditioned medium promotes wound healing in deep Second-Degree burns in male rats. Cells Tissues Organs 2018, 206, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Huang, S.; Gao, D.; Xie, J.; Liu, N.; Fu, X. Age-associated changes in regenerative capabilities of mesenchymal stem cell: Impact on chronic wounds repair. Int. Wound J. 2015, 13, 1252–1259. [Google Scholar] [CrossRef]
- Rangatchew, F.; Vester-Glowinski, P.; Rasmussen, B.S.; Haastrup, E.; Munthe-Fog, L.; Talman, M.-L.; Bonde, C.; Drzewiecki, K.T.; Fischer-Nielsen, A.; Holmgaard, R. Mesenchymal stem cell therapy of acute thermal burns: A systematic review of the effect on inflammation and wound healing. Burns 2021, 47, 270–294. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.S.; Moon, J.-H.; Jun, E.K.; Kim, J.; Maeng, I.; Kim, J.S.; Lee, J.H.; Baik, C.S.; Kim, A.; Cho, K.S.; et al. Secretory profiles and wound healing effects of human amniotic Fluid–Derived mesenchymal stem cells. Stem Cells Dev. 2010, 19, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Zeugolis, D.I.; O’Brien, T. Scaffold-based delivery of mesenchymal stromal cells to diabetic wounds. Stem Cell Res. Ther. 2022, 13, 426. [Google Scholar] [CrossRef]
- Kosol, W.; Kumar, S.; Marrero-BerrÍos, I.; Berthiaume, F. Medium conditioned by human mesenchymal stromal cells reverses low serum and hypoxia-induced inhibition of wound closure. Biochem. Biophys. Res. Commun. 2020, 522, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, P.; Shukla, M.; Meena, P.; Kakkar, A.; Khatri, N.; Nagar, R.K.; Kumar, M.; Saraswat, S.K.; Shrivastava, S.; Datt, R.; et al. Mesenchymal stem cells are prospective novel off-the-shelf wound management tools. Drug Deliv. Transl. Res. 2021, 12, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Di Silvestre, D.; Mastracci, L.; Grillo, F.; Grisoli, P.; Marrubini, G.; Nardini, M.; Mastrogiacomo, M.; Sorlini, M.; Rossi, R.; et al. GMP-compliant sponge-like dressing containing MSC lyo-secretome: Proteomic network of healing in a murine wound model. Eur. J. Pharm. Biopharm. 2020, 155, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Brossard, C.; Pouliet, A.-L.; Lefranc, A.; Benadjaoud, M.; Santos, M.D.; Demarquay, C.; Buard, V.; Benderitter, M.; Simon, J.-M.; Milliat, F.; et al. Mesenchymal stem cells limit vascular and epithelial damage and restore the impermeability of the urothelium in chronic radiation cystitis. Stem Cell Res. Ther. 2023, 14, 5. [Google Scholar] [CrossRef]
- Li, H.; Ziemer, M.; Stojanovic, I.; Saksida, T.; Maksimovic-Ivanic, D.; Mijatovic, S.; Djmura, G.; Gajic, D.; Koprivica, I.; Krajnovic, T.; et al. Mesenchymal stem cells from mouse hair follicles reduce hypertrophic scarring in a murine wound healing model. Stem Cell Rev. Rep. 2022, 18, 2028–2044. [Google Scholar] [CrossRef]
- Jiang, D.; Scharffetter-Kochanek, K. Mesenchymal stem cells adaptively respond to environmental cues thereby improving granulation tissue formation and wound healing. Front. Cell Dev. Biol. 2020, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Huerta, C.T.; Ortiz, Y.Y.; Li, Y.; Ribieras, A.J.; Voza, F.; Le, N.; Dodson, C.; Wang, G.; Vazquez-Padron, R.I.; Liu, Z.-J.; et al. Novel Gene-Modified Mesenchymal stem cell therapy reverses impaired wound healing in ischemic limbs. Ann. Surg. 2023, 278, 383–395. [Google Scholar] [CrossRef]
- Badiavas, E.V. Treatment of chronic wounds with Bone Marrow–Derived cells. Arch. Dermatol. 2003, 139, 510. [Google Scholar] [CrossRef]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous bone Marrow–Derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Mitsuno, H.; Nonaka, I.; Sen, Y.; Kawanishi, K.; Inada, Y.; Takakura, Y.; Okuchi, K.; Nonomura, A. Wound therapy by marrow mesenchymal cell transplantation. Plast. Reconstr. Surg. 2008, 121, 860–877. [Google Scholar] [CrossRef]
- Dash, S.N.; Dash, N.R.; Guru, B.; Mohapatra, P.C. Towards reaching the target: Clinical application of mesenchymal stem cells for diabetic foot ulcers. Rejuvenation Res. 2013, 17, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Masłowski, L.; Paprocka, M.; Czyżewska-Buczyńska, A.; Bielawska-Pohl, A.; Duś, D.; Grendziak, R.; Witkiewicz, W.; Czarnecka, A. Autotransplantation of the adipose Tissue-Derived mesenchymal stromal cells in therapy of venous stasis ulcers. Arch. Immunol. Et Ther. Exp. 2020, 68, 5. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Mossell, J.; Suo, Z. Extracellular vesicles: Emerging frontiers in wound healing. Med. Res. Rev. 2022, 42, 2102–2125. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Wu, Y.; Song, G.; Azizi, R.; Zamani, A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: A comprehensive review. Stem Cell Res. Ther. 2022, 13, 24. [Google Scholar] [CrossRef]
- Eiro, N.; Fraile, M.; González-Jubete, A.; González, L.O.; Vizoso, F.J. Mesenchymal (STEM) stromal cells based as new therapeutic alternative in inflammatory bowel disease: Basic mechanisms, experimental and clinical evidence, and challenges. Int. J. Mol. Sci. 2022, 23, 8905. [Google Scholar] [CrossRef]
- Carvello, M.; Lightner, A.; Yamamoto, T.; Kotze, P.G.; Spinelli, A. Mesenchymal stem cells for perianal Crohn’s disease. Cells 2019, 8, 764. [Google Scholar] [CrossRef]
- Jodheea-Jutton, A.; Hindocha, S.; Bhaw-Luximon, A. Health economics of diabetic foot ulcer and recent trends to accelerate treatment. Foot 2022, 52, 101909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, L.; Wang, X.; Zhou, X.; Zhang, X.; Li, L.; Wu, J.; Kou, M.; Cai, C.; Lian, Q.; et al. Topical and intravenous administration of human umbilical cord mesenchymal stem cells in patients with diabetic foot ulcer and peripheral arterial disease: A phase I pilot study with a 3-year follow-up. Stem Cell Res. Ther. 2022, 13, 451. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Liang, H.; Cai, Y.; Li, X.; Yan, L.; Zhou, L.; Shan, L.; Wang, H. Human umbilical cord-derived mesenchymal stem cells not only ameliorate blood glucose but also protect vascular endothelium from diabetic damage through a paracrine mechanism mediated by MAPK/ERK signaling. Stem Cell Res. Ther. 2022, 13, 258. [Google Scholar] [CrossRef]
- Zhuge, Y.; Regueiro, M.M.; Tian, R.; Li, Y.; Xia, X.; Vazquez-Padron, R.; Elliot, S.; Thaller, S.R.; Liu, Z.-J.; Velazquez, O.C. The effect of estrogen on diabetic wound healing is mediated through increasing the function of various bone marrow-derived progenitor cells. J. Vasc. Surg. 2018, 68, 127S–135S. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Lian, W.; Jin, Y.; Cao, C.; Han, S.; Yang, X.; Zhao, S.; Li, M.; Zhao, H. Role and effect of vein-transplanted human umbilical cord mesenchymal stem cells in the repair of diabetic foot ulcers in rats. Acta Biochim. Et Biophys. Sin. 2020, 52, 620–630. [Google Scholar] [CrossRef]
- Moon, K.-C.; Suh, H.-S.; Kim, K.-B.; Han, S.-K.; Young, K.-W.; Lee, J.-W.; Kim, M.-H. Potential of Allogeneic Adipose-Derived Stem Cell–Hydrogel complex for treating diabetic foot ulcers. Diabetes 2019, 68, 837–846. [Google Scholar] [CrossRef]
- Qin, H.; Zhu, X.; Zhang, B.; Zhou, L.; Wang, W. Clinical evaluation of human umbilical cord mesenchymal stem cell transplantation after angioplasty for diabetic foot. Exp. Clin. Endocrinol. Diabetes 2016, 124, 497–503. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Lei, X.; Tan, J.; Xie, H. Mesenchymal stem cell-based therapy for burn wound healing. Burn. Trauma 2021, 9, tkab002. [Google Scholar] [CrossRef]
- Lykov, A.P.; Bondarenko, N.A.; Poveshchenko, O.V.; Miller, T.V.; Poveshchenko, A.F.; Surovtseva, M.A.; Bgatova, N.P.; Konenkov, V.I. Biomedical cellular product for wound healing. Integr. Obes. Diabetes 2016, 2, 176–179. [Google Scholar] [CrossRef]
- Kudinov, V.A.; Artyushev, R.I.; Zurina, I.M.; Lapshin, R.D.; Snopova, L.B.; Mukhina, I.V.; Grinakovskaya, O.S.; Saburina, I.N. Antimicrobial and regenerative effects of placental multipotent mesenchymal stromal cell Secretome-Based Chitosan gel on infected burns in rats. Pharmaceuticals 2021, 14, 1263. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, M.; Peng, Y.; Chen, X.; Sun, J.; Huang, F.; Fan, Z.; Zhou, H.; Wu, X.; Yu, G.; et al. Improvement in Poor Graft Function after Allogeneic Hematopoietic Stem Cell Transplantation upon Administration of Mesenchymal Stem Cells from Third-Party Donors: A Pilot Prospective Study. Cell Transplant. 2013, 23, 1087–1098. [Google Scholar] [CrossRef]
- Clover, A.J.P.; Kumar, A.H.S.; Isakson, M.; Whelan, D.; Stocca, A.; Gleeson, B.M.; Caplice, N.M. Allogeneic mesenchymal stem cells, but not culture modified monocytes, improve burn wound healing. Burns 2015, 41, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.; Makaram, N.; Simpson, A.; Keating, J. Fracture nonunion in long bones: A literature review of risk factors and surgical management. Injury 2021, 52, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Poundarik, A.A.; Cabral, J.M.S.; Da Silva, C.L.; Vashishth, D. Biomimetic matrices for rapidly forming mineralized bone tissue based on stem cell-mediated osteogenesis. Sci. Rep. 2018, 8, 14388. [Google Scholar] [CrossRef]
- Ko, K.I.; Coimbra, L.S.; Tian, C.; Alblowi, J.; Kayal, R.A.; Einhorn, T.A.; Gerstenfeld, L.C.; Pignolo, R.J.; Graves, D.T. Diabetes reduces mesenchymal stem cells in fracture healing through a TNFα-mediated mechanism. Diabetologia 2015, 58, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.E.; Bindl, R.; Heilmann, A.; Erbacher, A.; Müller, I.; Brenner, R.E.; Ignatius, A. Systemic mesenchymal stem cell administration enhances bone formation in fracture repair but not load-induced bone formation. Eur. Cells Mater. 2015, 29, 22–34. [Google Scholar] [CrossRef]
- Myers, T.J.; Yan, Y.; Granero-Molto, F.; Weis, J.A.; Longobardi, L.; Li, T.; Li, Y.; Contaldo, C.; Ozkhan, H.; Spagnoli, A. Systemically delivered insulin-like growth factor-I enhances mesenchymal stem cell-dependent fracture healing. Growth Factors 2012, 30, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Wang, Y.; Liang, Q.; Mao, X. Preclinical and Clinical Amelioration of Bone Fractures with Mesenchymal Stromal Cells: A Systematic Review and Meta-Analysis. Cell Transplant. 2022, 31, 096368972110517. [Google Scholar] [CrossRef] [PubMed]
- Katahira, Y.; Murakami, F.; Inoue, S.; Miyakawa, S.; Sakamoto, E.; Furusaka, Y.; Watanabe, A.; Sekine, A.; Kuroda, M.; Hasegawa, H.; et al. Protective effects of conditioned media of immortalized stem cells from human exfoliated deciduous teeth on pressure ulcer formation. Front. Immunol. 2023, 13, 010700. [Google Scholar] [CrossRef]
- Bukowska, J.; Alarcon Uquillas, A.; Wu, X.; Frazier, T.; Walendzik, K.; Vanek, M.; Gaupp, D.; Bunnell, B.A.; Kosnik, P.; Mehrara, B.; et al. Safety and Efficacy of Human Adipose-Derived Stromal/Stem Cell Therapy in an Immunocompetent Murine Pressure Ulcer Model. Stem Cells Dev. 2020, 29, 440–451. [Google Scholar] [CrossRef] [PubMed]
- de la Garza-Rodea, A.S.; Knaän-Shanzer, S.; van Bekkum, D.W. Pressure ulcers: Description of a new model and use of mesenchymal stem cells for repair. Dermatology 2011, 223, 266–284. [Google Scholar] [CrossRef]
- Deng, C.L.; Yao, Y.Z.; Liu, Z.Y.; Wang, B.; Wang, D.L.; Wei, Z.R. Effects of adipose-derived mesenchymal stem cells from type 2 diabetes mellitus patients on wound healing of pressure ulcers in mice. Zhonghua Shao Shang Za Zhi 2019, 35, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Alexandrushkina, N.; Nimiritsky, P.; Eremichev, R.; Popov, V.; Arbatskiy, M.; Danilova, N.; Malkov, P.; Akopyan, Z.; Tkachuk, V.; Makarevich, P. Cell Sheets from Adipose Tissue MSC Induce Healing of Pressure Ulcer and Prevent Fibrosis via Trigger Effects on Granulation Tissue Growth and Vascularization. Int. J. Mol. Sci. 2020, 21, 5567. [Google Scholar] [CrossRef] [PubMed]
- Sario, G.D.; Borna, S.; Eldaly, A.S.; Quinones-Hinojosa, A.; Zubair, A.C.; Ho, O.A.; Forte, A.J. Mesenchymal Stromal Cell Healing Outcomes in Clinical and Pre-Clinical Models to Treat Pressure Ulcers: A Systematic Review. J. Clin. Med. 2023, 12, 7545. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Nie, J.; Duan, L.; Qiao, X.; Sui, Y. Umbilical cord mesenchymal stem cells combined with autologous platelet-rich plasma for lower extremity venous ulcers: A case report and literature review. Medicine 2024, 103, e40433. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawi, M.; Ghoneim, B.; Westby, D.; Jones, D.; Tawfick, W.; Walsh, S.R. Adipose-derived stem cells in patients with venous ulcers. Syst. Rev. Vasc. 2023, 31, 989–993. [Google Scholar] [CrossRef]
- Kerstan, A.; Dieter, K.; Niebergall-Roth, E.; Dachtler, A.K.; Kraft, K.; Stücker, M.; Daeschlein, G.; Jünger, M.; Görge, T.; Meyer-Pannwitt, U.; et al. Allogeneic ABCB5+ mesenchymal stem cells for treatment-refractory chronic venous ulcers: A phase I/IIa clinical trial. JID Innov. 2022, 2, 100067. [Google Scholar] [CrossRef]
- Nita, M.; Pliszczyński, J.; Eljaszewicz, A.; Moniuszko, M.; Ołdak, T.; Woźniak, K.; Majewski, S.; Kowalewski, C.; Kamiński, A.; Śladowski, D.; et al. Surgical treatment of wounds using stem cells in epidermolysis Bullosa (EB). In IntechOpen eBooks; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Maseda, R.; Martínez-Santamaría, L.; Sacedón, R.; Butta, N.; Del Carmen De Arriba, M.; García-Barcenilla, S.; García, M.; Illera, N.; Pérez-Conde, I.; Carretero, M.; et al. Beneficial effect of systemic allogeneic adipose derived mesenchymal cells on the clinical, inflammatory and immunologic status of a patient with recessive dystrophic epidermolysis Bullosa: A case report. Front. Med. 2020, 7, 576558. [Google Scholar] [CrossRef]
- Riedl, J.; Popp, C.; Eide, C.; Ebens, C.; Tolar, J. Mesenchymal stromal cells in wound healing applications: Role of the secretome, targeted delivery and impact on recessive dystrophic epidermolysis bullosa treatment. Cytotherapy 2021, 23, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lee, S.-J.; Kim, S.-E.; Kim, K.; Cho, B.; Roh, K.; Kim, S.-C. Intravenous allogeneic umbilical cord blood–derived mesenchymal stem cell therapy in recessive dystrophic epidermolysis bullosa patients. JCI Insight 2021, 6, e143606. [Google Scholar] [CrossRef] [PubMed]
- Rashidghamat, E.; Kadiyirire, T.; Ayis, S.; Petrof, G.; Liu, L.; Pullabhatla, V.; Ainali, C.; Guy, A.; Aristodemou, S.; McMillan, J.R.; et al. Phase I/II open-label trial of intravenous allogeneic mesenchymal stromal cell therapy in adults with recessive dystrophic epidermolysis bullosa. J. Am. Acad. Dermatol. 2019, 83, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Kühl, T.; Mezger, M.; Hausser, I.; Handgretinger, R.; Bruckner-Tuderman, L.; Nyström, A. High local concentrations of intradermal MSCs restore skin integrity and facilitate wound healing in dystrophic epidermolysis Bullosa. Mol. Ther. 2015, 23, 1368–1379. [Google Scholar] [CrossRef]
- Kiritsi, D.; Dieter, K.; Niebergall-Roth, E.; Fluhr, S.; Daniele, C.; Esterlechner, J.; Sadeghi, S.; Ballikaya, S.; Erdinger, L.; Schauer, F.; et al. Clinical trial of ABCB5+ mesenchymal stem cells for recessive dystrophic epidermolysis bullosa. JCI Insight 2021, 6, e151922. [Google Scholar] [CrossRef]
- Różycka-Baczyńska, A.M.; Stepaniec, I.M.; Warzycha, M.; Zdolińska-Malinowska, I.; Oldak, T.; Rozwadowska, N.; Kolanowski, T.J. Development of a novel gene expression panel for the characterization of MSCs for increased biological safety. J. Appl. Genet. 2024. [Google Scholar] [CrossRef]
- Alsultan, A.; Farge, D.; Kili, S.; Forte, M.; Weiss, D.J.; Grignon, F.; Boelens, J.J. International Society for Cell and Gene Therapy Clinical Translation Committee recommendations on mesenchymal stromal cells in graft-versus-host disease: Easy manufacturing is faced with standardizing and commercialization challenges. Cytotherapy 2024, 26, 1132–1140. [Google Scholar] [CrossRef]
- Sukmana, B.I.; Margiana, R.; Almajidi, Y.Q.; Almalki, S.G.; Hjazi, A.; Shahab, S.; Romero-Parra, R.M.; Alazbjee, A.A.A.; Alkhayyat, A.; John, V. Supporting wound healing by mesenchymal stem cells (MSCs) therapy in combination with scaffold, hydrogel, and matrix; State of the art. Pathol. Res. Pract. 2023, 248, 154575. [Google Scholar] [CrossRef]
- Liu, C.; Pei, M.; Li, Q.; Zhang, Y. Decellularized extracellular matrix mediates tissue construction and regeneration. Front. Med. 2022, 16, 56–82. [Google Scholar] [CrossRef] [PubMed]
| Condition | Method of Cell Delivery | Combination Agent | Autologous/ Allogeneic | Cell Source | Phase | NCT Number |
|---|---|---|---|---|---|---|
| Radiation-induced rectal injury | Local application by means of endoscopy | - | Allogeneic | Umbilical cord | 1 | 05939778 |
| Anterior cruciate ligament reconstruction using mesenchymal stem cells and collagen matrix carrier | Local implantation | Porous bovine collagen matrix carrier | Autologous | Anterior cruciate ligament | N/A | 05582226 |
| Venous leg | Local transplantation | Silver ion dressing | Allogeneic | Umbilical cord | N/A | 05319106 |
| Dystrophic epidermolysis bullosa | Local application of MSC dressing | Dressing for dystrophic epidermolysis bullosa wound | Allogeneic | N/A | 2 | 05157958 |
| Skin grafts in donor site wounds | MSC injection into the de-epithelialized area and the surrounding 0.5 cm subcutaneous region. | - | Allogeneic | Umbilical cord | N/A | 05984628 |
| Condition | Method of Cell Delivery | Combination Agent | Autologous/ Allogeneic | Cell Source | Phase | NCT Number |
|---|---|---|---|---|---|---|
| Mandible fractures | Local application on the fracture site within the surgical procedure | - | Autologous | Adipose tissue | 3 | 02755922 |
| Tibial closed diaphyseal fractures | Local application in the fractured site | - | Allogeneic | Adipose tissue | 2 | 02140528 |
| Tendon injury | Ultrasound-guided injection at the injury site | - | Allogeneic | Adipose tissue | 1 | 01856140 |
| Heel injury | Local application | Skin graft | Allogeneic | Umbilical cord | 1 | NCT04219657 |
| Poor healing after uterus injury | Intrauterine injection | - | Allogeneic | Umbilical cord | 1 | 03386708 |
| Second degree burn wounds of less than 20% of the total body surface area | Local application | - | Allogeneic | N/A | 1 | 02104713 |
| Chronic wounds in diabetic foot syndrome | Direct application onto the prepared wound bed | Fibrin gel | Allogeneic | Adipose tissue | 1–2 | 03865394 |
| Non-union of long bone fractures | Local application in fractured zone | - | Autologous | Bone marrow | 1 | 01206179 |
| Non-united tibial and femoral fractures | Injection in non-union site | - | N/A | Bone marrow | 2 | 01788059 |
| Tendon injury | Local injection under ultrasound guidance | Fibrin glue + range of motion exercise | Allogeneic | Adipose tissue | 2 | 02298023 |
| Distal tibial fractures | Local implantation at the fracture site | MSC carrier | Autologous | N/A | 1–2 | 00250302 |
| Ocular corneal burn | Subconjunctival injection | - | N/A | Bone marrow | 2 | 02325843 |
| Fracture non-union healing | Local application of in vitro-expanded MSC | Carrier | Autologous | Bone marrow | N/A | 02177565 |
| Chronic ulcer wounds | Topical application of Wharton jelly MSC culture medium | Gel carrier | Allogeneic | Umbilical cord | 1 | 04134676 |
| Knee articular cartilage injury | Local application | - | Allogeneic | Umbilical cord | 3 | 01041001 |
| Deep second-degree burn wound | Local application | hydrogel sheet | Allogeneic | Adipose tissue | 1 | 02394873 |
| Long bones non-union | Percutaneous application around fracture ends | - | Autologous | Adipose tissue | 1–2 | 04340284 |
| Knee articular cartilage injury or defect | Local application | - | Allogeneic | Umbilical cord | 3 | 01626677 |
| Support of autologous chondron transplantation with instant MSC | Filling of cartilage defect | Glue carrier, autologous chondrons | Allogeneic | N/A | 1–2 | 02037204 |
| Tibial shaft fracture | Local injection | - | Autologous | Bone marrow | N/A | 00512434 |
| Mandibular distraction osteogenesis | Local injection | - | N/A | Bone marrow | N/A | 03861650 |
| Severe epidermolysis bullosa | Serial infusions | Allogeneic hematopoietic stem cell transplant | Allogeneic (related) | N/A | 2 | 02582775 |
| Severe epidermolysis bullosa | Infusions | Allogeneic hematopoietic stem cell transplant | Allogeneic | N/A | 1–2 | 01033552 |
| Recessive dystrophic epidermolysis bullosa | Infusions | - | Allogeneic | Umbilical cord blood | 1–2 | 04520022 |
| Epidermolysis bullosa | Local application | hydrogel sheet | Allogeneic | Adipose tissue | 1–2 | 02579369 |
| Epidermolysis bullosa | Local application | hydrogel sheet | Allogeneic | Adipose tissue | 1–2 | 03183934 |
| Treatment-refractory chronic venous ulcers | Topical application | - | Allogeneic | Skin derived | 1–2 | 03257098 |
| Recessive dystrophic epidermolysis bullosa | Infusions | - | Allogeneic | Skin derived | 1–2A | 03529877 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasadiuk, K.; Kolanowski, T.; Kowalewski, C.; Wozniak, K.; Oldak, T.; Rozwadowska, N. Harnessing Mesenchymal Stromal Cells for Advanced Wound Healing: A Comprehensive Review of Mechanisms and Applications. Int. J. Mol. Sci. 2025, 26, 199. https://doi.org/10.3390/ijms26010199
Nasadiuk K, Kolanowski T, Kowalewski C, Wozniak K, Oldak T, Rozwadowska N. Harnessing Mesenchymal Stromal Cells for Advanced Wound Healing: A Comprehensive Review of Mechanisms and Applications. International Journal of Molecular Sciences. 2025; 26(1):199. https://doi.org/10.3390/ijms26010199
Chicago/Turabian StyleNasadiuk, Khrystyna, Tomasz Kolanowski, Cezary Kowalewski, Katarzyna Wozniak, Tomasz Oldak, and Natalia Rozwadowska. 2025. "Harnessing Mesenchymal Stromal Cells for Advanced Wound Healing: A Comprehensive Review of Mechanisms and Applications" International Journal of Molecular Sciences 26, no. 1: 199. https://doi.org/10.3390/ijms26010199
APA StyleNasadiuk, K., Kolanowski, T., Kowalewski, C., Wozniak, K., Oldak, T., & Rozwadowska, N. (2025). Harnessing Mesenchymal Stromal Cells for Advanced Wound Healing: A Comprehensive Review of Mechanisms and Applications. International Journal of Molecular Sciences, 26(1), 199. https://doi.org/10.3390/ijms26010199






