Dead or Alive: Exploratory Analysis of Selected Apoptosis- and Autophagy-Related Proteins in Human Endometrial Stromal Cells of Fertile Females and Their Potential Role During Embryo Implantation
Abstract
1. Introduction
2. Results
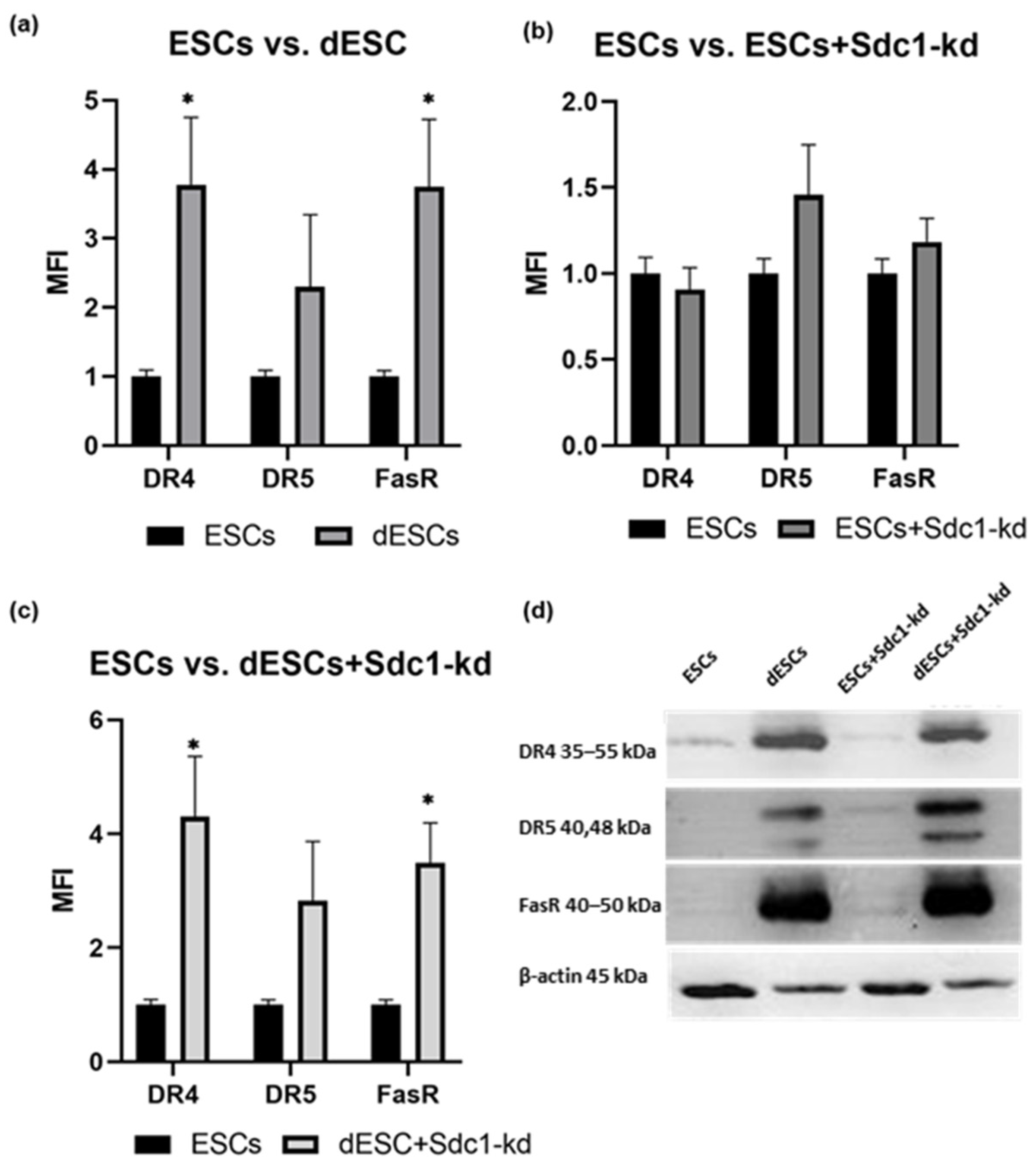
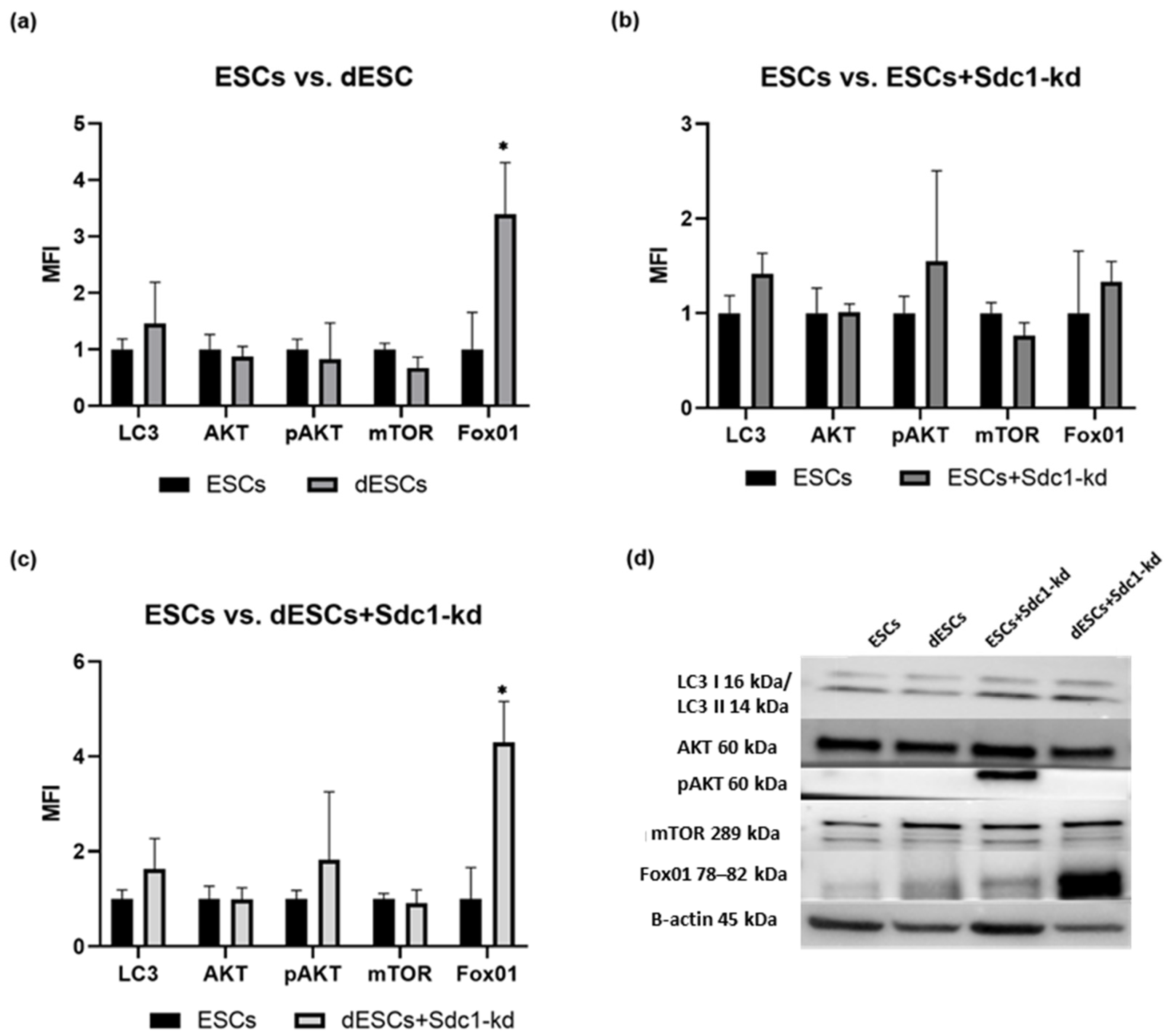
2.1. Analysis of Apoptosis- and Autophagy-Related Proteins in ESCs of Fertile Females Concerning Decidualization and Reduction in Sdc1
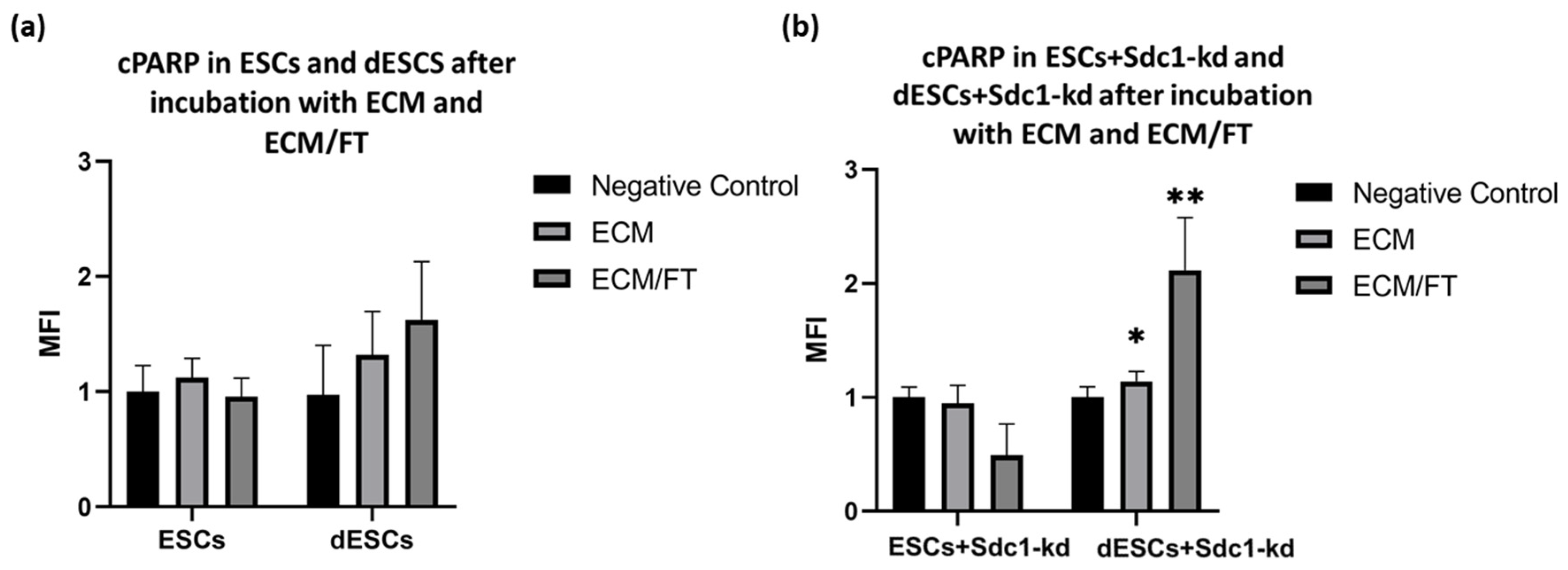
2.2. Analysis of Apoptosis- and Autophagy-Related Proteins in ESCs of Fertile Females After Incubation with FasL, TRAIL, and ECM for 24 h Concerning Decidualization and Sdc1 Reduction
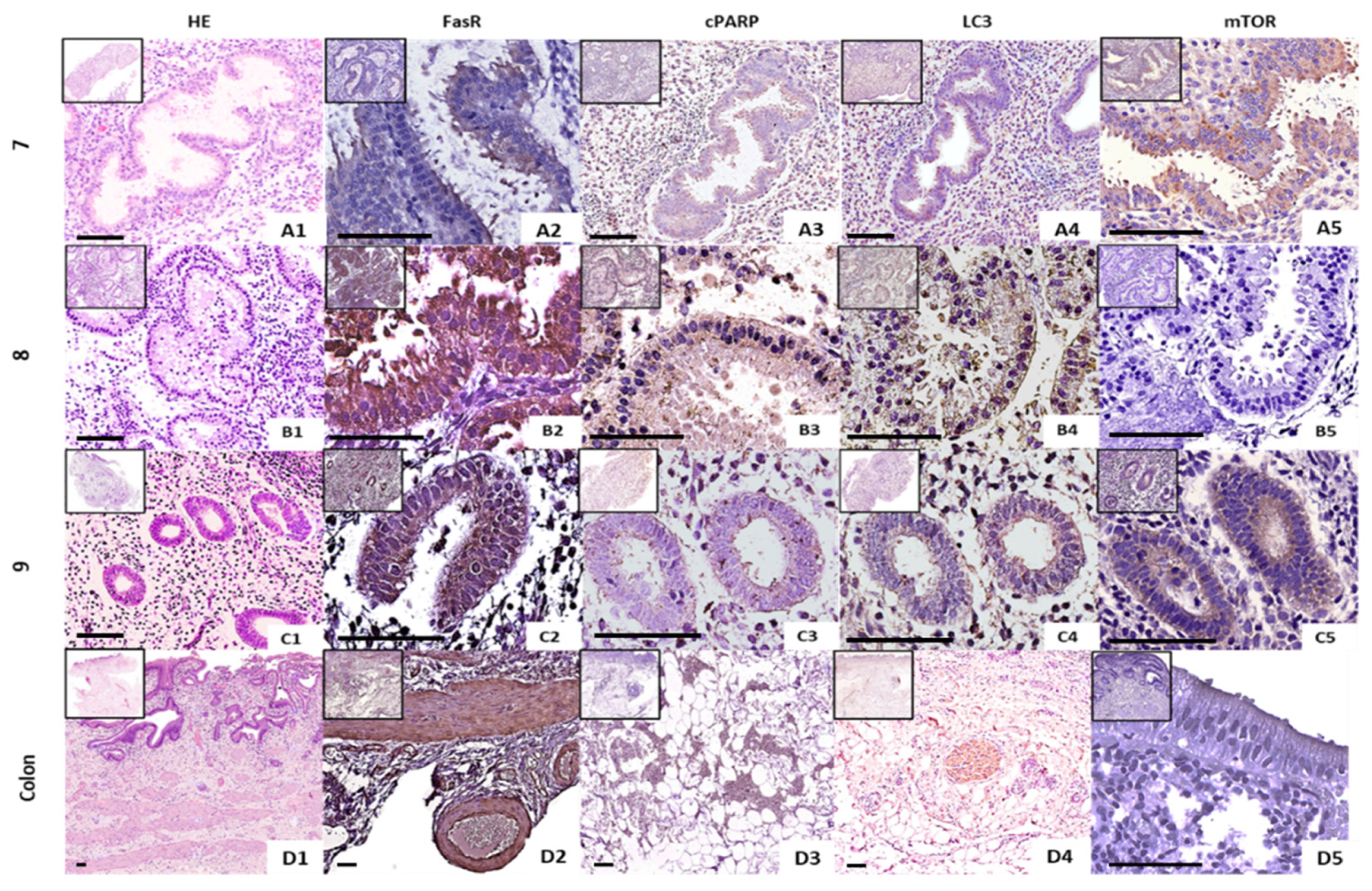
2.3. Spatial Expression of Several Apoptosis- and Autophagy-Related Proteins in Formalin-Fixed, Paraffin-Embedded Endometrial Tissues of Fertile Females
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Primary Cell Isolation, Cell Culture Conditions, Transfection, and Decidualization
4.3. Western Blot Analysis of Apoptosis- and Autophagy-Related Proteins
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muter, J.; Lynch, V.J.; McCoy, R.C.; Brosens, J.J. Human embryo implantation. Development 2023, 150, dev201507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.D.; Ruane, P.T. Embryo-epithelium interactions during implantation at a glance. J. Cell Sci. 2017, 130, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Bogdan, A.; Balassa, T.; Csabai, T.; Szekeres-Bartho, J. The decidua-the maternal bed embracing the embryo- maintains the pregnancy. Semin. Immunopathol. 2016, 38, 635–649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lockshin, R.A.; Zakeri, Z. Apoptosis, autophagy, and more. Int. J. Biochem. Cell Biol. 2004, 36, 2405–2419. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.-L. Doctor Jekyll and Mister Hyde: Autophagy can promote both cell survival and cell death. Cell Death Differ. 2005, 12 (Suppl. 2), 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A. Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis 2008, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, J.; Rutherford, T.; Naftolin, F.; Brown, S.; Mor, G. Hormonal regulation of apoptosis and the Fas and Fas ligand system in human endometrial cells. Mol. Hum. Reprod. 2002, 8, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Boeddeker, S.J.; Baston-Buest, D.M.; Fehm, T.; Kruessel, J.; Hess, A. Decidualization and syndecan-1 knockdown sensitize endometrial stromal cells to apoptosis induced by embryonic stimuli. PLoS ONE 2015, 10, e0121103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kapiteijn, K.; Koolwijk, P.; van der Weiden, R.M.F.; van Nieuw Amerongen, G.; Plaisier, M.; van Hinsbergh, V.W.M.; Helmerhorst, F.M. Human embryo-conditioned medium stimulates in vitro endometrial angiogenesis. Fertil. Steril. 2006, 85 (Suppl. 1), 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Boeddeker, S.J.; Baston-Buest, D.M.; Altergot-Ahmad, O.; Kruessel, J.S.; Hess, A.P. Syndecan-1 knockdown in endometrial epithelial cells alters their apoptotic protein profile and enhances the inducibility of apoptosis. Mol. Hum. Reprod. 2014, 20, 567–578. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Germeyer, A.; Klinkert, M.S.; Huppertz, A.-G.; Clausmeyer, S.; Popovici, R.M.; Strowitzki, T.; von Wolff, M. Expression of syndecans, cell-cell interaction regulating heparan sulfate proteoglycans, within the human endometrium and their regulation throughout the menstrual cycle. Fertil. Steril. 2007, 87, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Kokawa, K.; Shikone, T.; Nakano, R. Apoptosis in the human uterine endometrium during the menstrual cycle. J. Clin. Endocrinol. Metab. 1996, 81, 4144–4147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weimar, C.H.; Macklon, N.S.; Uiterweer, E.D.P.; Brosens, J.J.; Gellersen, B. The motile and invasive capacity of human endometrial stromal cells: Implications for normal and impaired reproductive function. Hum. Reprod. Update 2013, 19, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Gores, G.J. Life and death by death receptors. FASEB J. 2009, 23, 1625–1637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mills, K.R.; Reginato, M.; Debnath, J.; Queenan, B.; Brugge, J.S. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 3438–3443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, J.; Jo, M.; Lee, E.; Oh, Y.K.; Choi, D. The role of autophagy in human endometrium. Biol. Reprod. 2012, 86, 70. [Google Scholar] [CrossRef] [PubMed]
- Datan, E.; Shirazian, A.; Benjamin, S.; Matassov, D.; Tinari, A.; Malorni, W.; Lockshin, R.A.; Garcia-Sastre, A.; Zakeri, Z. mTOR/p70S6K signaling distinguishes routine, maintenance-level autophagy from autophagic cell death during influenza A infection. Virology 2014, 452–453, 175–190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, H.L.; Mei, J.; Chang, K.K.; Zhou, W.J.; Huang, L.Q.; Li, M.Q. Autophagy in endometriosis. Am. J. Transl. Res. 2017, 9, 4707–4725. [Google Scholar] [PubMed] [PubMed Central]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grinius, L.; Kessler, C.; Schroeder, J.; Handwerger, S. Forkhead transcription factor FOXO1A is critical for induction of human decidualization. J. Endocrinol. 2006, 189, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yao, Z.; Klionsky, D.J. How to control self-digestion: Transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 2015, 25, 354–363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, S.; Jeong, H.; Yu, S.W. Autophagy is a decisive process for cell death. Exp. Mol. Med. 2020, 52, 921–930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gubbiotti, M.A.; Iozzo, R.V. Proteoglycans regulate autophagy via outside-in signaling: An emerging new concept. Matrix Biol. 2015, 48, 6–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.M.; Kim, J.S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Citrinovitz, A.C.M.; Strowitzki, T.; Germeyer, A. Decreased Autophagy Impairs Decidualization of Human Endometrial Stromal Cells: A Role for ATG Proteins in Endometrial Physiology. Int. J. Mol. Sci. 2019, 20, 3066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ochoa-Bernal, M.A.; Fazleabas, A.T. Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int. J. Mol. Sci. 2020, 21, 1973. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fluhr, H.; Spratte, J.; Bredow, M.; Heidrich, S.; Zygmunt, M. Constitutive activity of Erk1/2 and NF-κB protects human endometrial stromal cells from death receptor-mediated apoptosis. Reprod. Biol. 2013, 13, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, J.-J.; He, J.-L.; Liu, X.-Q.; Chen, X.-M.; Ding, Y.-B.; Tong, C.; Peng, C.; Geng, Y.-Q.; Wang, Y.-X.; et al. Endometrial autophagy is essential for embryo implantation during early pregnancy. J. Mol. Med. 2020, 98, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Popli, P.; Sun, A.J.; Kommagani, R. The Multifaceted Role of Autophagy in Endometrium Homeostasis and Disease. Reprod. Sci. 2022, 29, 1054–1067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devis-Jauregui, L.; Eritja, N.; Davis, M.L.; Matias-Guiu, X.; Llobet-Navàs, D. Autophagy in the physiological endometrium and cancer. Autophagy 2021, 17, 1077–1095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Fu, L.-J.; Liu, X.-Q.; Hu, Z.-Y.; Jiang, Y.; Gao, R.-F.; Feng, Q.; Lan, X.; Geng, Y.-Q.; Chen, X.-M.; et al. nm23 regulates decidualization through the PI3K-Akt-mTOR signaling pathways in mice and humans. Hum. Reprod. 2016, 31, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Fabi, F.; Grenier, K.; Parent, S.; Adam, P.; Tardif, L.; Leblanc, V.; Asselin, E. Regulation of the PI3K/Akt pathway during decidualization of endometrial stromal cells. PLoS ONE 2017, 12, e0177387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joswig, A.; Gabriel, H.-D.; Kibschull, M.; Winterhager, E. Apoptosis in the uterine epithelium and decidua in response to implantation: Evidence for two different pathways. Reprod. Biol. Endocrinol. 2003, 1, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelleher, A.M.; DeMayo, F.J.; Spencer, T.E. Uterine Glands: Developmental Biology and Functional Roles in Pregnancy. Endocr. Rev. 2019, 40, 1424–1445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bielfeld, A.P.; Pour, S.J.; Poschmann, G.; Stühler, K.; Krüssel, J.-S.; Baston-Büst, D.M. A Proteome Approach Reveals Differences between Fertile Women and Patients with Repeated Implantation Failure on Endometrial Level—Does hCG Render the Endometrium of RIF Patients? Int. J. Mol. Sci. 2019, 20, 425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Degasperi, A.; Birtwistle, M.R.; Volinsky, N.; Rauch, J.; Kolch, W.; Kholodenko, B.N. Evaluating strategies to normalize biological replicates of Western blot data. PLoS ONE 2014, 9, e87293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheliga, I.; Baston-Buest, D.M.; Haramustek, D.; Knebel, A.; Kruessel, J.-S.; Bielfeld, A.P. Dead or Alive: Exploratory Analysis of Selected Apoptosis- and Autophagy-Related Proteins in Human Endometrial Stromal Cells of Fertile Females and Their Potential Role During Embryo Implantation. Int. J. Mol. Sci. 2025, 26, 175. https://doi.org/10.3390/ijms26010175
Scheliga I, Baston-Buest DM, Haramustek D, Knebel A, Kruessel J-S, Bielfeld AP. Dead or Alive: Exploratory Analysis of Selected Apoptosis- and Autophagy-Related Proteins in Human Endometrial Stromal Cells of Fertile Females and Their Potential Role During Embryo Implantation. International Journal of Molecular Sciences. 2025; 26(1):175. https://doi.org/10.3390/ijms26010175
Chicago/Turabian StyleScheliga, Iwona, Dunja M. Baston-Buest, Djamila Haramustek, Alexandra Knebel, Jan-Steffen Kruessel, and Alexandra P. Bielfeld. 2025. "Dead or Alive: Exploratory Analysis of Selected Apoptosis- and Autophagy-Related Proteins in Human Endometrial Stromal Cells of Fertile Females and Their Potential Role During Embryo Implantation" International Journal of Molecular Sciences 26, no. 1: 175. https://doi.org/10.3390/ijms26010175
APA StyleScheliga, I., Baston-Buest, D. M., Haramustek, D., Knebel, A., Kruessel, J.-S., & Bielfeld, A. P. (2025). Dead or Alive: Exploratory Analysis of Selected Apoptosis- and Autophagy-Related Proteins in Human Endometrial Stromal Cells of Fertile Females and Their Potential Role During Embryo Implantation. International Journal of Molecular Sciences, 26(1), 175. https://doi.org/10.3390/ijms26010175




