Dysregulated Signaling Pathways in Canine Mammary Tumor and Human Triple Negative Breast Cancer: Advances and Potential Therapeutic Targets
Abstract
1. Introduction
2. Epidermal Growth Factor Receptor (EGFR) Signaling Pathway
2.1. Structure and Biological Function of EGFR
2.2. Mechanisms of Action of EGFR in HBC
2.3. Mechanisms of EGFR Action in CMT
2.4. Drugs Targeting EGFR
2.4.1. EGFR Tyrosine Kinase Inhibitors (TKIs)
2.4.2. Monoclonal Antibodies
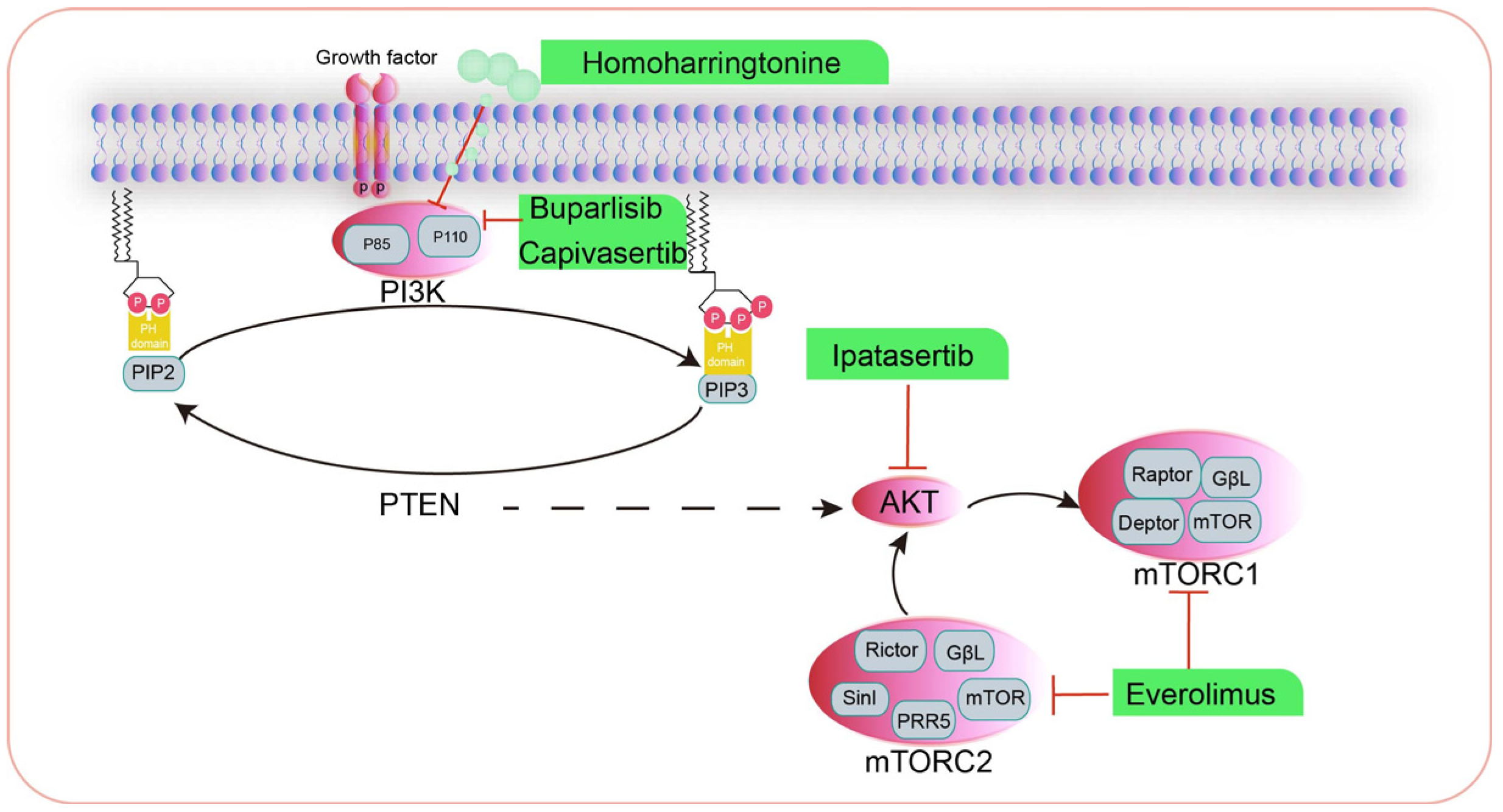
3. The Phosphatidylinositol 3-Kinase/Protein Kinase B/Mammalian Target of Rapamycin (PI3K/AKT/mTOR) Signaling Pathway
3.1. Inhibitors Targeting PI3K for the Treatment of HBC
3.2. Inhibitors Targeting AKT for the Treatment of Human HBC
3.3. Inhibitors Targeting mTOR for the Treatment of Human HBC
3.4. Drugs That Modulate the PI3K/AKT/mTOR Signaling Pathway in CMT
4. Cell Death Signaling Pathway
4.1. Classical Tumor Cell Pyroptosis Pathways
4.2. Non-Classical Pathways That Regulate Inflammatory Cell Death
4.3. Other Pathways
4.4. Drugs That Induce Cellular Pyroptosis in HBC
4.5. Drugs That Induce Cellular Apoptosis in CMT
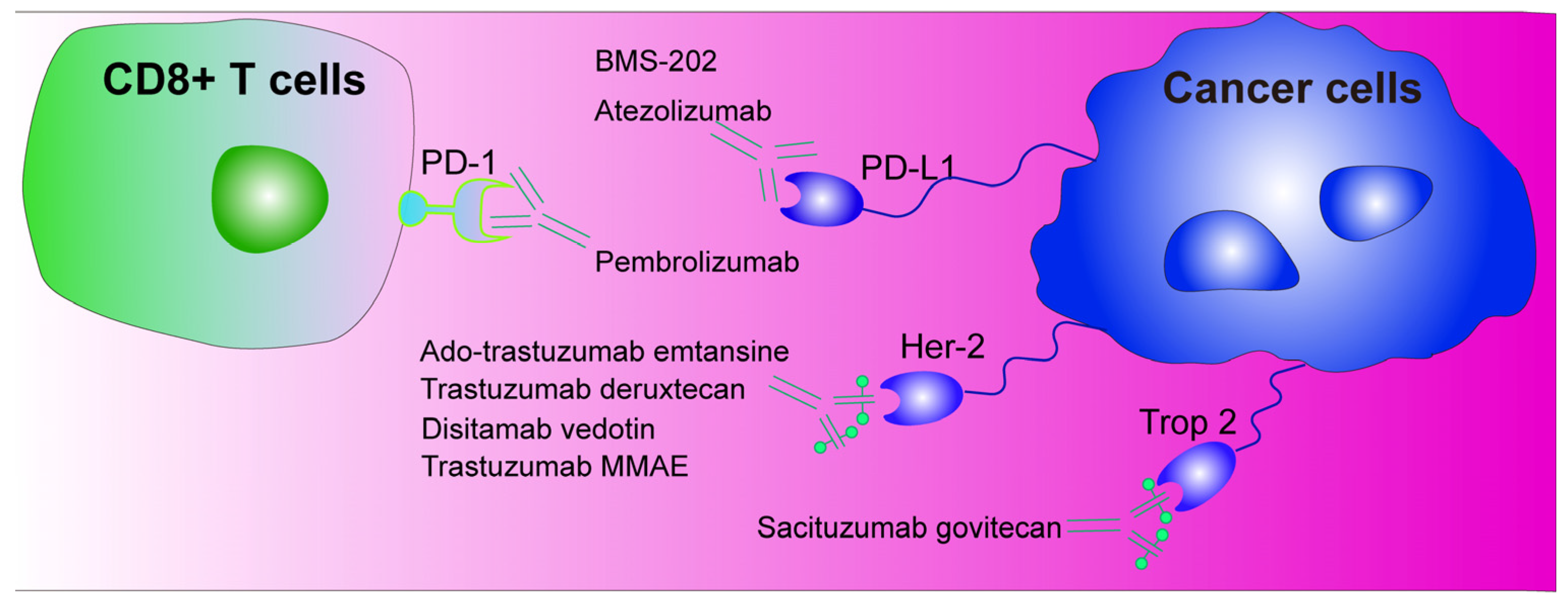
5. Immunotherapy with Programmed Death-1/Programmed Death Ligand-1 (PD-1/PD-L1) as Immune Checkpoints
6. Antibody–Drug Conjugates (ADCs)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez-Rodriguez, E.J.; Taboada-Taboada, R.; Garcia-Martin, A.; Sanchez-Gomez, C.; Saez-Gutierrez, S.; Rihuete-Galve, M.I.; Fonseca-Sánchez, E. Study on the additional financial burden of breast cancer disease on cancer patients and their families. Financial toxicity in cancer. Front. Public Health 2024, 12, 1324334. [Google Scholar] [CrossRef]
- Escala-Garcia, M.; Morra, A.; Canisius, S.; Chang-Claude, J.; Kar, S.; Zheng, W.; Bojesen, S.E.; Easton, D.; Pharoah, P.D.P.; Schmidt, M.K. Breast cancer risk factors and their effects on survival: A Mendelian randomisation study. BMC Med. 2020, 18, 327. [Google Scholar] [CrossRef]
- Cordeiro, Y.G.; Mulder, L.M.; van Zeijl, R.J.M.; Paskoski, L.B.; van Veelen, P.; de Ru, A.; Strefezzi, R.F.; Heijs, B.; Fukumasu, H. Proteomic Analysis Identifies FNDC1, A1BG, and Antigen Processing Proteins Associated with Tumor Heterogeneity and Malignancy in a Canine Model of Breast Cancer. Cancers 2021, 13, 5901. [Google Scholar] [CrossRef]
- Varney, D.; O’Neill, D.; O’Neill, M.; Church, D.; Stell, A.; Beck, S.; Smalley, M.J.; Brodbelt, D. Epidemiology of mammary tumors in bitches under veterinary care in the UK in 2016. Vet. Rec. 2023, 193, e3054. [Google Scholar] [CrossRef]
- Nosalova, N.; Huniadi, M.; Horňáková, Ľ.; Valenčáková, A.; Horňák, S.; Nagoos, K.; Vozar, J.; Cizkova, D. Canine Mammary Tumors: Classification, Biomarkers, Traditional and Personalized Therapies. Int. J. Mol. Sci. 2024, 25, 2891. [Google Scholar] [CrossRef]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Domrazek, K.; Jurka, P. The Novel Diagnostic Techniques and Biomarkers of Canine Mammary Tumors. Vet. Sci. 2022, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, E.A. Both ends of the leash—The human links to good dogs with bad genes. N. Engl. J. Med. 2012, 367, 636–646. [Google Scholar] [CrossRef]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002–2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef]
- Levi, M.; Muscatello, L.V.; Brunetti, B.; Benazzi, C.; Parenti, F.; Gobbo, F.; Avallone, G.; Bacci, B.; Zambon, E.; Valenti, P.; et al. High Intrinsic Expression of P-glycoprotein and Breast Cancer Resistance Protein in Canine Mammary Carcinomas Regardless of Immunophenotype and Outcome. Animals 2021, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, C.L.; Engelman, J.A. ERBB receptors: From oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014, 25, 282–303. [Google Scholar] [CrossRef]
- Drago, J.Z.; Ferraro, E.; Abuhadra, N.; Modi, S. Beyond HER2: Targeting the ErbB receptor family in breast cancer. Cancer Treat. Rev. 2022, 109, 102436. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, H.; Nilsson, M.B.; Robichaux, J.P.; He, J.; Poteete, A.; Jiang, H.; Heeke, S.; Elamin, Y.Y.; Shibata, Y.; Matsumoto, S.; et al. HER4 and EGFR Activate Cell Signaling in NRG1 Fusion-Driven Cancers: Implications for HER2-HER3-specific Versus Pan-HER Targeting Strategies. J. Thorac. Oncol. 2024, 19, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.; Lembach, K.J.; Morrison, M.M.; Cohen, S. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J. Biol. Chem. 1975, 250, 4297–4304. [Google Scholar] [CrossRef]
- Amelia, T.; Kartasasmita, R.E.; Ohwada, T.; Tjahjono, D.H. Structural Insight and Development of EGFR Tyrosine Kinase Inhibitors. Molecules 2022, 27, 819. [Google Scholar] [CrossRef]
- Weinberg, F.; Peckys, D.B.; de Jonge, N. EGFR Expression in HER2-Driven Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9008. [Google Scholar] [CrossRef]
- Wollman, A.J.M.; Fournier, C.; Llorente-Garcia, I.; Harriman, O.; Payne-Dwyer, A.L.; Shashkova, S.; Zhou, P.; Liu, T.C.; Ouaret, D.; Wilding, J.; et al. Critical roles for EGFR and EGFR-HER2 clusters in EGF binding of SW620 human carcinoma cells. J. R. Soc. Interface 2022, 19, 20220088. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.S.; Javed Shaikh, M.A.; Afzal, O.; Alfawaz Altamimi, A.S.; Almalki, W.H.; Alzarea, S.I.; Kazmi, I.; Al-Abbasi, F.A.; Singh, S.K.; Dua, K.; et al. An overview of epithelial growth factor receptor (EGFR) inhibitors in cancer therapy. Chem. Biol. Interact. 2022, 366, 110108. [Google Scholar] [CrossRef] [PubMed]
- Cicek, E.; Circir, A.; Oyken, M.; Akbulut Caliskan, O.; Dioken, D.N.; Guntekin Ergun, S.; Cetin-Atalay, R.; Sapmaz, A.; Ovaa, H.; Sahin, O.; et al. EGF-SNX3-EGFR axis drives tumor progression and metastasis in triple-negative breast cancers. Oncogene 2022, 41, 220–232. [Google Scholar] [CrossRef]
- Berger, A.J.; Renner, C.M.; Hale, I.; Yang, X.; Ponik, S.M.; Weisman, P.S.; Masters, K.S.; Kreeger, P.K. Scaffold stiffness influences breast cancer cell invasion via EGFR-linked Mena upregulation and matrix remodeling. Matrix Biol. 2020, 85–86, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Z.; Binzel, D.W.; Guo, P.; Williams, T.M. Targeting oncogenic KRAS in non-small cell lung cancer with EGFR aptamer-conjugated multifunctional RNA nanoparticles. Mol. Ther. Nucleic Acids 2023, 33, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Jiang, Z.; Gu, P.; Guo, B.; Li, J.; Cheng, S.; Ba, Q.; Wang, H. Cadmium promotes colorectal cancer metastasis through EGFR/Akt/mTOR signaling cascade and dynamics. Sci. Total Environ. 2023, 899, 165699. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, J.M.; Jeong, D.S.; Kim, M.H. Transcriptional activation of EGFR by HOXB5 and its role in breast cancer cell invasion. Biochem. Biophys. Res. Commun. 2018, 503, 2924–2930. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Jiang, Y.Z.; Wei, Y.; Ell, B.; Sheng, X.; Esposito, M.; Kang, J.; Hang, X.; Zheng, H.; Rowicki, M.; et al. Tinagl1 Suppresses Triple-Negative Breast Cancer Progression and Metastasis by Simultaneously Inhibiting Integrin/FAK and EGFR Signaling. Cancer Cell 2019, 35, 64–80.e7. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.P.; Qiao, S.; Jiang, S.; Hu, J.L.; Wang, T.T.; Liu, W.W.; Qin, Y.; Wang, Y.N.; Zheng, L.S.; Zhang, J.C.; et al. Protein Tyrosine Kinase 7 Regulates EGFR/Akt Signaling Pathway and Correlates with Malignant Progression in Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 699889. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Semba, T.; Manyam, G.C.; Wang, J.; Shao, S.; Bertucci, F.; Finetti, P.; Krishnamurthy, S.; Phi, L.T.H.; Pearson, T.; et al. EGFR is a master switch between immunosuppressive and immunoactive tumor microenvironment in inflammatory breast cancer. Sci. Adv. 2022, 8, eabn7983. [Google Scholar] [CrossRef]
- Kim, S.; You, D.; Jeong, Y.; Yu, J.; Kim, S.W.; Nam, S.J.; Lee, J.E. Berberine down-regulates IL-8 expression through inhibition of the EGFR/MEK/ERK pathway in triple-negative breast cancer cells. Phytomedicine 2018, 50, 43–49. [Google Scholar] [CrossRef]
- Mei, C.; Zhang, X.; Zhi, Y.; Liang, Z.; Xu, H.; Liu, Z.; Liu, Y.; Lyu, Y.; Wang, H. Isorhamnetin Regulates Programmed Death Ligand-1 Expression by Suppressing the EGFR-STAT3 Signaling Pathway in Canine Mammary Tumors. Int. J. Mol. Sci. 2024, 25, 670. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Hung, M.C.; Yamaguchi, H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res. 2016, 6, 1609–1623. [Google Scholar] [PubMed]
- Roskoski, R., Jr. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol. Res. 2019, 139, 395–411. [Google Scholar] [CrossRef]
- Girgert, R.; Emons, G.; Gründker, C. 17β-estradiol-induced growth of triple-negative breast cancer cells is prevented by the reduction of GPER expression after treatment with gefitinib. Oncol. Rep. 2017, 37, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, C.M.; Hu, Z.; Kronenberger, T.; Küblbeck, J.; Kinnen, F.J.M.; Hesse, S.S.; Malik, A.; Kudolo, M.; Niess, R.; Gehringer, M.; et al. Gefitinib-Tamoxifen Hybrid Ligands as Potent Agents against Triple-Negative Breast Cancer. J. Med. Chem. 2022, 65, 4616–4632. [Google Scholar] [CrossRef] [PubMed]
- Taurin, S.; Rosengren, R.J. Raloxifene potentiates the effect of gefitinib in triple-negative breast cancer cell lines. Med. Oncol. 2022, 40, 45. [Google Scholar] [CrossRef]
- Fenn, K.; Maurer, M.; Lee, S.M.; Crew, K.D.; Trivedi, M.S.; Accordino, M.K.; Hershman, D.L.; Kalinsky, K. Phase 1 Study of Erlotinib and Metformin in Metastatic Triple-Negative Breast Cancer. Clin. Breast Cancer 2020, 20, 80–86. [Google Scholar] [CrossRef]
- Goel, P.N.; Zhang, H.; Murali, R.; Zheng, C.; Ji, M.Q.; Patterson, A.; Grover, P.; Greene, M. Dual kinase inhibitor for EGFR mutants and ErbB2 limit breast cancer. Biochem. Biophys. Res. Commun. 2023, 651, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Jabbarzadeh Kaboli, P.; Luo, S.; Chen, Y.; Jomhori, M.; Imani, S.; Xiang, S.; Wu, Z.; Li, M.; Shen, J.; Zhao, Y.; et al. Pharmacotranscriptomic profiling of resistant triple-negative breast cancer cells treated with lapatinib and berberine shows upregulation of PI3K/Akt signaling under cytotoxic stress. Gene 2022, 816, 146171. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yang, B.; An, Q.; Assaraf, Y.G.; Cao, X.; Xia, J. Acquired resistance to third-generation EGFR-TKIs and emerging next-generation EGFR inhibitors. Innovation 2021, 2, 100103. [Google Scholar] [CrossRef]
- Zubair, T.; Bandyopadhyay, D. Small Molecule EGFR Inhibitors as Anti-Cancer Agents: Discovery, Mechanisms of Action, and Opportunities. Int. J. Mol. Sci. 2023, 24, 2651. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Mezei, T.; Hajdu, M.; Czigléczki, G.; Lotz, G.; Kocsis, J.; Kulka, J.; Horváth, A. Sterile, abscess-like cerebral lesion during trastuzumab therapy after HER2 status switch in a triple negative breast cancer patient: A case report and literature review. BMC Cancer 2020, 20, 615. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef]
- Pópulo, H.; Lopes, J.M.; Soares, P. The mTOR signalling pathway in human cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Saura, C.; Barroso-Sousa, R.; Guo, H.; Ciruelos, E.; Bermejo, B.; Gavilá, J.; Serra, V.; Prat, A.; Paré, L.; et al. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2020, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Abraham, J.; Chan, S.; Wheatley, D.; Brunt, A.M.; Nemsadze, G.; Baird, R.D.; Park, Y.H.; Hall, P.S.; Perren, T.; et al. Capivasertib Plus Paclitaxel Versus Placebo Plus Paclitaxel as First-Line Therapy for Metastatic Triple-Negative Breast Cancer: The PAKT Trial. J. Clin. Oncol. 2020, 38, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Dent, R.; Im, S.A.; Espié, M.; Blau, S.; Tan, A.R.; Isakoff, S.J.; Oliveira, M.; Saura, C.; Wongchenko, M.J.; et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017, 18, 1360–1372. [Google Scholar] [CrossRef]
- Anand, K.; Patel, T.; Niravath, P.; Rodriguez, A.; Darcourt, J.; Belcheva, A.; Boone, T.; Ensor, J.; Chang, J. Targeting mTOR and DNA repair pathways in residual triple negative breast cancer post neoadjuvant chemotherapy. Sci. Rep. 2021, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.; Hortobagyi, G.N.; Campone, M.; Pritchard, K.I.; Lebrun, F.; Ito, Y.; Noguchi, S.; Perez, A.; Rugo, H.S.; Deleu, I.; et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Overall survival results from BOLERO-2†. Ann. Oncol. 2014, 25, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Farmaki, E.; Nath, A.; Emond, R.; Karimi, K.L.; Grolmusz, V.K.; Cosgrove, P.A.; Bild, A.H. ONC201/TIC10 enhances durability of mTOR inhibitor everolimus in metastatic ER+ breast cancer. Elife 2023, 12, e85898. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, C.; Liang, Z.; Zhi, Y.; Xu, H.; Wang, H.; Dong, H. Homoharringtonine induces apoptosis of mammary carcinoma cells by inhibiting the AKT/mTOR signaling pathway. Vet. Comp. Oncol. 2024, 22, 57–69. [Google Scholar] [CrossRef]
- Kim, T.M.; Yang, I.S.; Seung, B.J.; Lee, S.; Kim, D.; Ha, Y.J.; Seo, M.K.; Kim, K.K.; Kim, H.S.; Cheong, J.H.; et al. Cross-species oncogenic signatures of breast cancer in canine mammary tumors. Nat. Commun. 2020, 11, 3616. [Google Scholar] [CrossRef]
- Zychlinsky, A.; Prevost, M.C.; Sansonetti, P.J. Shigella flexneri induces apoptosis in infected macrophages. Nature 1992, 358, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Monack, D.M.; Raupach, B.; Hromockyj, A.E.; Falkow, S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA 1996, 93, 9833–9838. [Google Scholar] [CrossRef]
- Boise, L.H.; Collins, C.M. Salmonella-induced cell death: Apoptosis, necrosis or programmed cell death? Trends Microbiol. 2001, 9, 64–67. [Google Scholar] [CrossRef]
- Monteleone, M.; Stanley, A.C.; Chen, K.W.; Brown, D.L.; Bezbradica, J.S.; von Pein, J.B.; Holley, C.L.; Boucher, D.; Shakespear, M.R.; Kapetanovic, R.; et al. Interleukin-1β Maturation Triggers Its Relocation to the Plasma Membrane for Gasdermin-D-Dependent and -Independent Secretion. Cell Rep. 2018, 24, 1425–1433. [Google Scholar] [CrossRef]
- Yan, H.; Luo, B.; Wu, X.; Guan, F.; Yu, X.; Zhao, L.; Ke, X.; Wu, J.; Yuan, J. Cisplatin Induces Pyroptosis via Activation of MEG3/NLRP3/caspase-1/GSDMD Pathway in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2021, 17, 2606–2621. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, F.; Ji, P.; Wei, M.; Yin, C.; Yang, A.; Yang, G.; Zhao, J. Efficient Delivery of GSDMD-N mRNA by Engineered Extracellular Vesicles Induces Pyroptosis for Enhanced Immunotherapy. Small 2023, 19, e2204031. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Jin, S.; Tong, D.; Liu, X.; Liu, Y.; Zheng, J. Enhancing the Anti-Tumor Efficacy of NK Cells on Canine Mammary Tumors through Resveratrol Activation. Animals 2024, 14, 1636. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Burgk, J.L.; Gaidt, M.M.; Schmidt, T.; Ebert, T.S.; Bartok, E.; Hornung, V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur. J. Immunol. 2015, 45, 2911–2917. [Google Scholar] [CrossRef]
- Rühl, S.; Broz, P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur. J. Immunol. 2015, 45, 2927–2936. [Google Scholar] [CrossRef]
- Sahoo, P.; Jana, P.; Kundu, S.; Mishra, S.; Chattopadhyay, K.; Mukherjee, A.; Ghosh, C.K. Quercetin@Gd3+ doped Prussian blue nanocubes induce the pyroptotic death of MDA-MB-231 cells: Combinational targeted multimodal therapy, dual modal MRI, intuitive modelling of r1-r2 relaxivities. J. Mater. Chem. B 2023, 11, 6646–6663. [Google Scholar] [CrossRef]
- An, H.; Heo, J.S.; Kim, P.; Lian, Z.; Lee, S.; Park, J.; Hong, E.; Pang, K.; Park, Y.; Ooshima, A.; et al. Tetraarsenic hexoxide enhances generation of mitochondrial ROS to promote pyroptosis by inducing the activation of caspase-3/GSDME in triple-negative breast cancer cells. Cell Death Dis. 2021, 12, 159. [Google Scholar] [CrossRef]
- Yang, X.; Cui, X.; Wang, G.; Zhou, M.; Wu, Y.; Du, Y.; Li, X.; Xu, T. HDAC inhibitor regulates the tumor immune microenvironment via pyroptosis in triple negative breast cancer. Mol. Carcinog. 2024, 63, 1800–1813. [Google Scholar] [CrossRef]
- Tang, J.; Bei, M.; Zhu, J.; Xu, G.; Chen, D.; Jin, X.; Huang, J.; Dong, J.; Shi, L.; Xu, L.; et al. Acute cadmium exposure induces GSDME-mediated pyroptosis in triple-negative breast cancer cells through ROS generation and NLRP3 inflammasome pathway activation. Environ. Toxicol. Pharmacol. 2021, 87, 103686. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Y.; Qi, L.; Li, L.; Song, D.; Gan, J.; Li, Y.; Ling, X.; Song, C. Dihydroartemisinin mediating PKM2-caspase-8/3-GSDME axis for pyroptosis in esophageal squamous cell carcinoma. Chem. Biol. Interact. 2021, 350, 109704. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chang, C.W.; Huang, J.; Zeng, S.; Zhang, X.; Hung, M.C.; Hou, J. Gasdermin C sensitizes tumor cells to PARP inhibitor therapy in cancer models. J. Clin. Invest. 2024, 134, e166841. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Fan, J.; Zhou, N.; Liang, J.; Xiao, C.; Tong, C.; Wang, W.; Liu, B. Biomimetic Prussian blue nanocomplexes for chemo-photothermal treatment of triple-negative breast cancer by enhancing ICD. Biomaterials 2023, 303, 122369. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, Y.; Hou, G.; Lei, H.; Liu, L.; Huang, X.; Sun, S.; Liu, L.; Liu, X.; Na, J.; et al. Zinc-Iron Bimetallic Peroxides Modulate the Tumor Stromal Microenvironment and Enhance Cell Immunogenicity for Enhanced Breast Cancer Immunotherapy Therapy. ACS Nano 2024, 18, 10542–10556. [Google Scholar] [CrossRef]
- Huang, D.; Zou, Y.; Huang, H.; Yin, J.; Long, S.; Sun, W.; Du, J.; Fan, J.; Chen, X.; Peng, X. A PROTAC Augmenter for Photo-Driven Pyroptosis in Breast Cancer. Adv. Mater. 2024, 36, e2313460. [Google Scholar] [CrossRef]
- Brandi, A.; de Faria Lainetti, P.; Elias, F.; Rodrigues, M.M.P.; Fagundes Moraes, L.; Laufer-Amorim, R.; de Camargo, L.S.; Salles Gomes, C.O.M.; Fonseca-Alves, C.E. Firocoxib as a Potential Neoadjuvant Treatment in Canine Patients with Triple-Negative Mammary Gland Tumors. Animals 2022, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Gherman, M.L.; Zanoaga, O.; Budisan, L.; Raduly, L.; Berindan-Neagoe, I. Doxorubicin as a Potential Treatment Option in Canine Mammary Tumors. Vet. Sci. 2023, 10, 654. [Google Scholar] [CrossRef] [PubMed]
- Kuruoglu, F.E.; Ekici, Z.M.; Nak, D.; Ozyigit, M.O.; Kupeli, Z.A.; Koca, D. Investigation of efficacy of two different chemotherapy protocols used in neoadjuvant chemotherapy in clinical stages II-IV canine malignant mammary tumours. Vet. Comp. Oncol. 2024, 22, 284–294. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Chamoto, K.; Hatae, R.; Honjo, T. Current issues and perspectives in PD-1 blockade cancer immunotherapy. Int. J. Clin. Oncol. 2020, 25, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Zhang, Y.; Eoh, K.J.; Sharma, R.; Sanmamed, M.F.; Wu, J.; Choi, J.; Park, H.S.; Iwasaki, A.; Kaftan, E.; et al. The Combination of MEK Inhibitor with Immunomodulatory Antibodies Targeting Programmed Death 1 and Programmed Death Ligand 1 Results in Prolonged Survival in Kras/p53-Driven Lung Cancer. J. Thorac. Oncol. 2019, 14, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhou, T.; Shi, H.; Yao, M.; Zhang, D.; Qian, H.; Zeng, Q.; Wang, Y.; Jin, F.; Chai, C.; et al. Progranulin induces immune escape in breast cancer via up-regulating PD-L1 expression on tumor-associated macrophages (TAMs) and promoting CD8+ T cell exclusion. J. Exp. Clin. Cancer Res. 2021, 40, 4. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef]
- Zak, K.M.; Grudnik, P.; Magiera, K.; Dömling, A.; Dubin, G.; Holak, T.A. Structural Biology of the Immune Checkpoint Receptor PD-1 and Its Ligands PD-L1/PD-L2. Structure 2017, 25, 1163–1174. [Google Scholar] [CrossRef]
- Zeng, B.; Pian, L.; Liu, Y.; Wang, S.; Wang, N.; Liu, C.; Wu, H.; Wan, H.; Chen, L.; Huang, W.; et al. Preparation and effects of functionalized liposomes targeting breast cancer tumors using chemotherapy, phototherapy, and immunotherapy. J. Nanobiotechnology 2024, 22, 558. [Google Scholar] [CrossRef]
- Kwapisz, D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol. Immunother. 2021, 70, 607–617. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Hegg, R.; Chung, W.P.; Im, S.A.; Jacot, W.; Ganju, V.; Chiu, J.W.Y.; Xu, B.; Hamilton, E.; Madhusudan, S.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 2023, 401, 105–117. [Google Scholar] [CrossRef]
- Abdollahpour-Alitappeh, M.; Lotfinia, M.; Bagheri, N.; Sineh Sepehr, K.; Habibi-Anbouhi, M.; Kobarfard, F.; Balalaie, S.; Foroumadi, A.; Abbaszadeh-Goudarzi, G.; Abbaszadeh-Goudarzi, K.; et al. Trastuzumab-monomethyl auristatin E conjugate exhibits potent cytotoxic activity in vitro against HER2-positive human breast cancer. J. Cell. Physiol. 2019, 234, 2693–2704. [Google Scholar] [CrossRef]
- Spring, L.M.; Tolaney, S.M.; Fell, G.; Bossuyt, V.; Abelman, R.O.; Wu, B.; Maheswaran, S.; Trippa, L.; Comander, A.; Mulvey, T.; et al. Response-guided neoadjuvant sacituzumab govitecan for localized triple-negative breast cancer: Results from the NeoSTAR trial. Ann. Oncol. 2024, 35, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, C.; Fleming, C.; O’Leary, D.P.; Hassan, F.; Kelly, L.; O’Sullivan, M.J.; Corrigan, M.A.; Redmond, H.P. Association of Circulating Tumor DNA With Disease-Free Survival in Breast Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2020, 3, e2026921. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Y.; Zhou, X.; Shen, P.; Xue, R.; Zhang, M. Disitamab vedotin: A novel antibody-drug conjugates for cancer therapy. Drug Deliv. 2022, 29, 1335–1344. [Google Scholar] [CrossRef] [PubMed]

| Pyroptosis Inducer | Cell Line | Pyroptosis Pathway | Year |
|---|---|---|---|
| Cadmium [62] | MDAMB231 | Caspase-3/GSDME | 2021 |
| Dihydroartemisinin [63] | MCF7, MDAMB231 | Caspase-3/GSDME | 2021 |
| Cinobufagin [64] | MDA-MB-231, 4T1 | Caspase-3/GSDME | 2023 |
| Fe-ZnO2@HA [65] | 4T1 | Caspase-1/GSDMD | 2024 |
| PARPi [66] | MDAMB436, HCC1937 | Caspase-8/GSDMC | 2024 |
| L@NBMZ [67] | MCF-7 | Caspase-3/GSDME | 2024 |
| Drug | Pathway | Cell Species | Outcome |
|---|---|---|---|
| Gefitinib [29] | EGFR tyrosine kinase inhibitors | TNBC | G1 cell cycle arrest |
| Raloxifene [32] | EGFR tyrosine kinase inhibitors | TNBC | Cell apoptosis |
| Lapatinib [35] | EGFR tyrosine kinase inhibitors | (EGFR+, HER-2+) HBC | Downregulation of CDK6 and DNMT1 |
| Trastuzumab [37] | EGFR monoclonal antibodies | HER2+ HBC | Cell apoptosis |
| Buparlisib [42] | PI3K inhibitor | TNBC | Cell apoptosis |
| Capivasertib [43] | AKT1-3 inhibitor | TNBC | Cell cycle arrest |
| Ipatasertib [44] | AKT inhibitor | TNBC | Cell cycle arrest |
| Homoharringtonine [48] | PI3K/AKT/mTOR inhibitor | CMT | Cell apoptosis |
| BMS-202 [76] | PD-1/PD-L1 inhibitor | TNBC | Cell apoptosis |
| Atezolizumab [79,80] | PD-L1 inhibitor | TNBC | Cell autophagy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, C.; Liu, Y.; Liu, Z.; Zhi, Y.; Jiang, Z.; Lyu, X.; Wang, H. Dysregulated Signaling Pathways in Canine Mammary Tumor and Human Triple Negative Breast Cancer: Advances and Potential Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 145. https://doi.org/10.3390/ijms26010145
Mei C, Liu Y, Liu Z, Zhi Y, Jiang Z, Lyu X, Wang H. Dysregulated Signaling Pathways in Canine Mammary Tumor and Human Triple Negative Breast Cancer: Advances and Potential Therapeutic Targets. International Journal of Molecular Sciences. 2025; 26(1):145. https://doi.org/10.3390/ijms26010145
Chicago/Turabian StyleMei, Chen, Ying Liu, Zhenyi Liu, Yan Zhi, Zhaoling Jiang, Xueze Lyu, and Hongjun Wang. 2025. "Dysregulated Signaling Pathways in Canine Mammary Tumor and Human Triple Negative Breast Cancer: Advances and Potential Therapeutic Targets" International Journal of Molecular Sciences 26, no. 1: 145. https://doi.org/10.3390/ijms26010145
APA StyleMei, C., Liu, Y., Liu, Z., Zhi, Y., Jiang, Z., Lyu, X., & Wang, H. (2025). Dysregulated Signaling Pathways in Canine Mammary Tumor and Human Triple Negative Breast Cancer: Advances and Potential Therapeutic Targets. International Journal of Molecular Sciences, 26(1), 145. https://doi.org/10.3390/ijms26010145






