Pulsed Electric Field (PEF) Treatment Results in Growth Promotion, Main Flavonoids Extraction, and Phytochemical Profile Modulation of Scutellaria baicalensis Georgi Roots
Abstract
1. Introduction
2. Results and Discussion
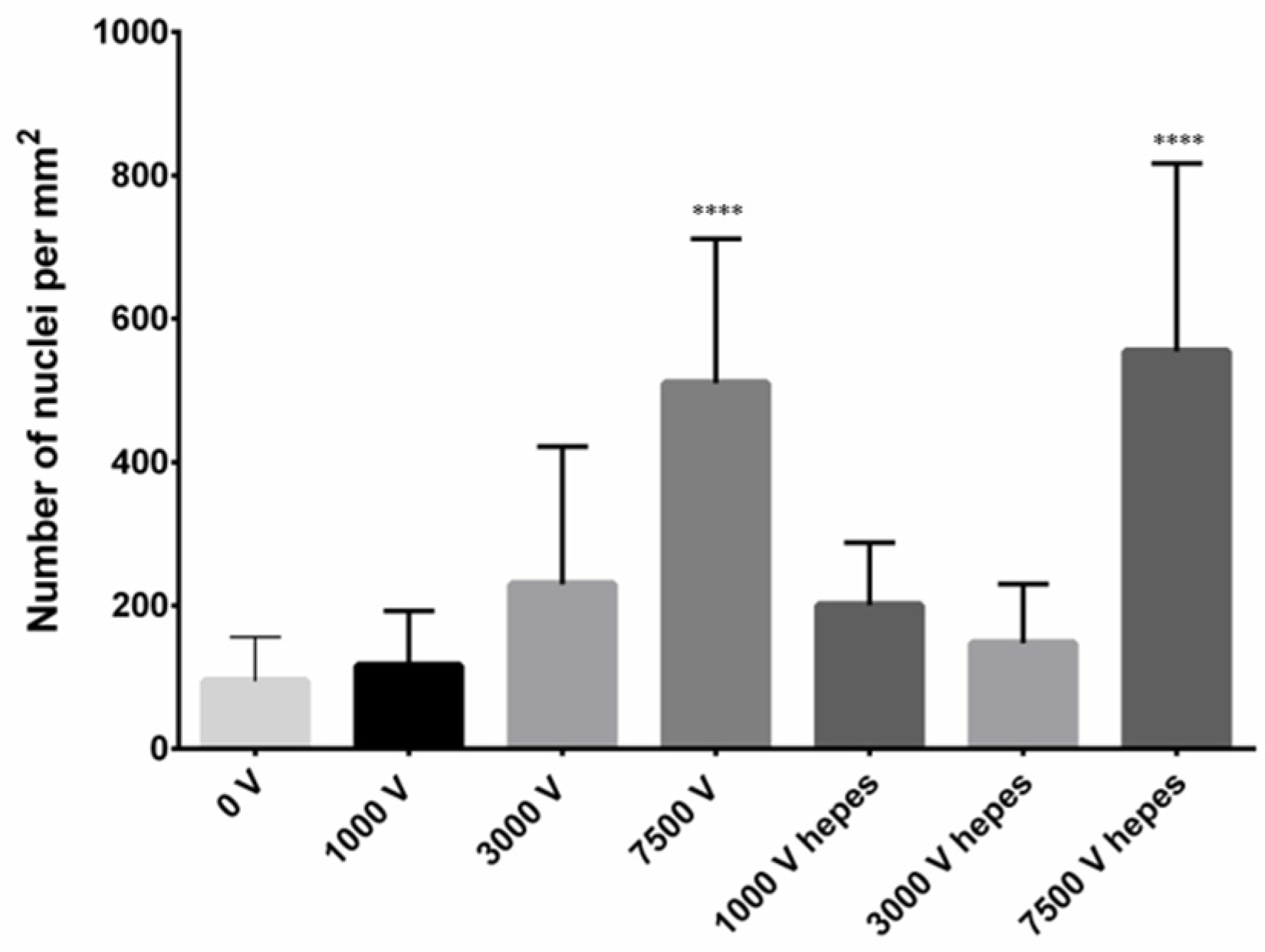
2.1. Confocal Microscopy
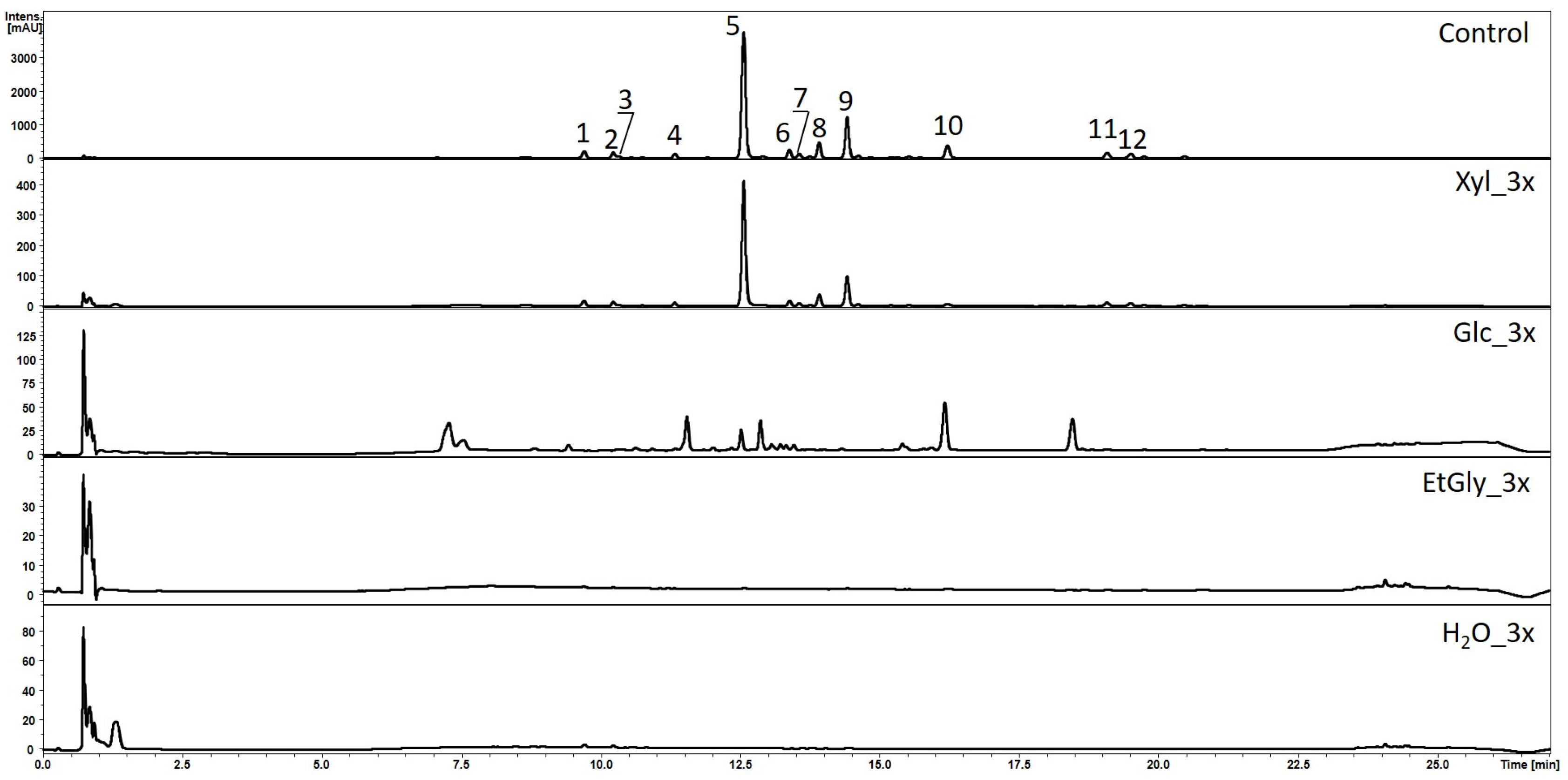
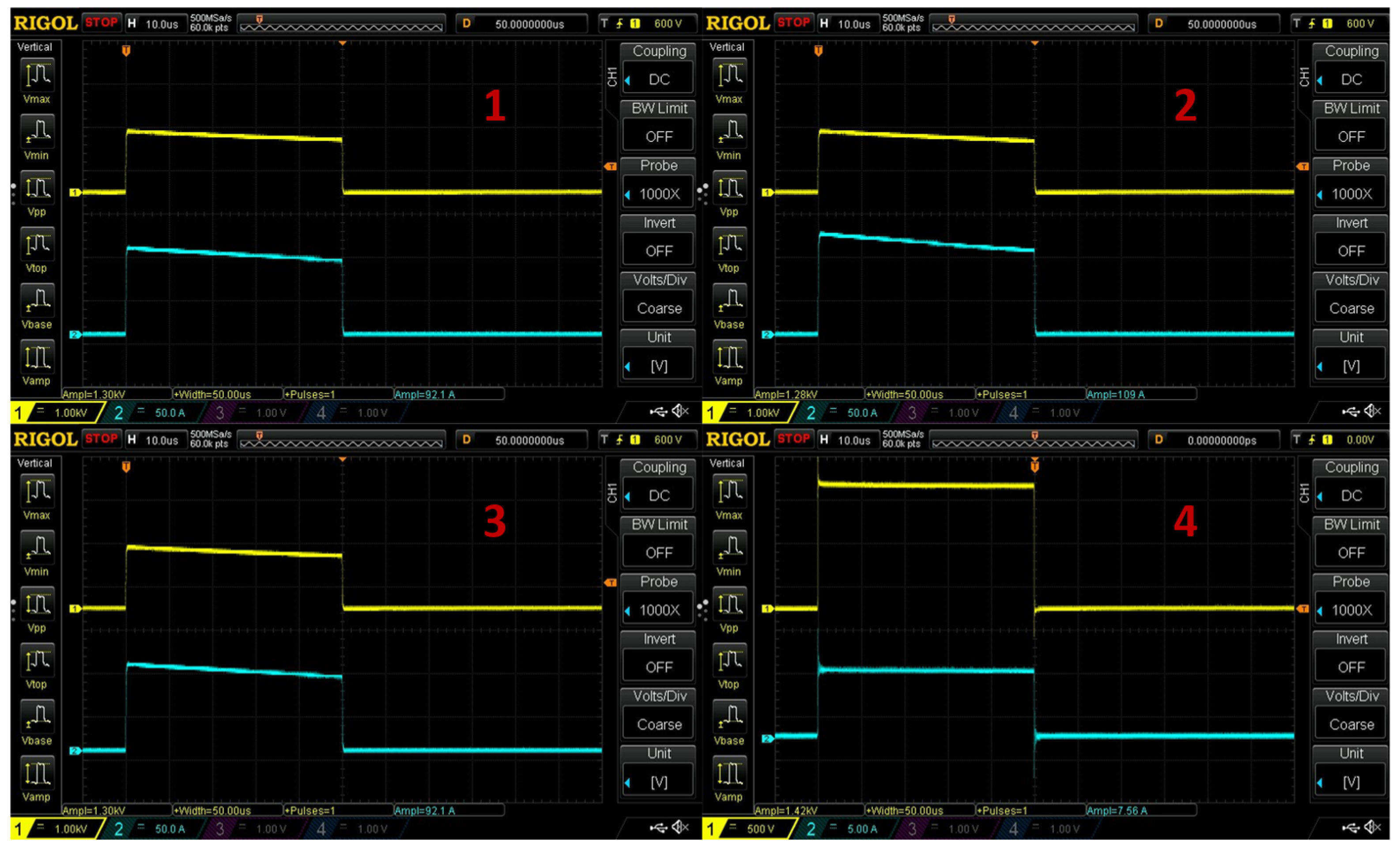
2.2. Compound Extraction from Living S. baicalensis Roots
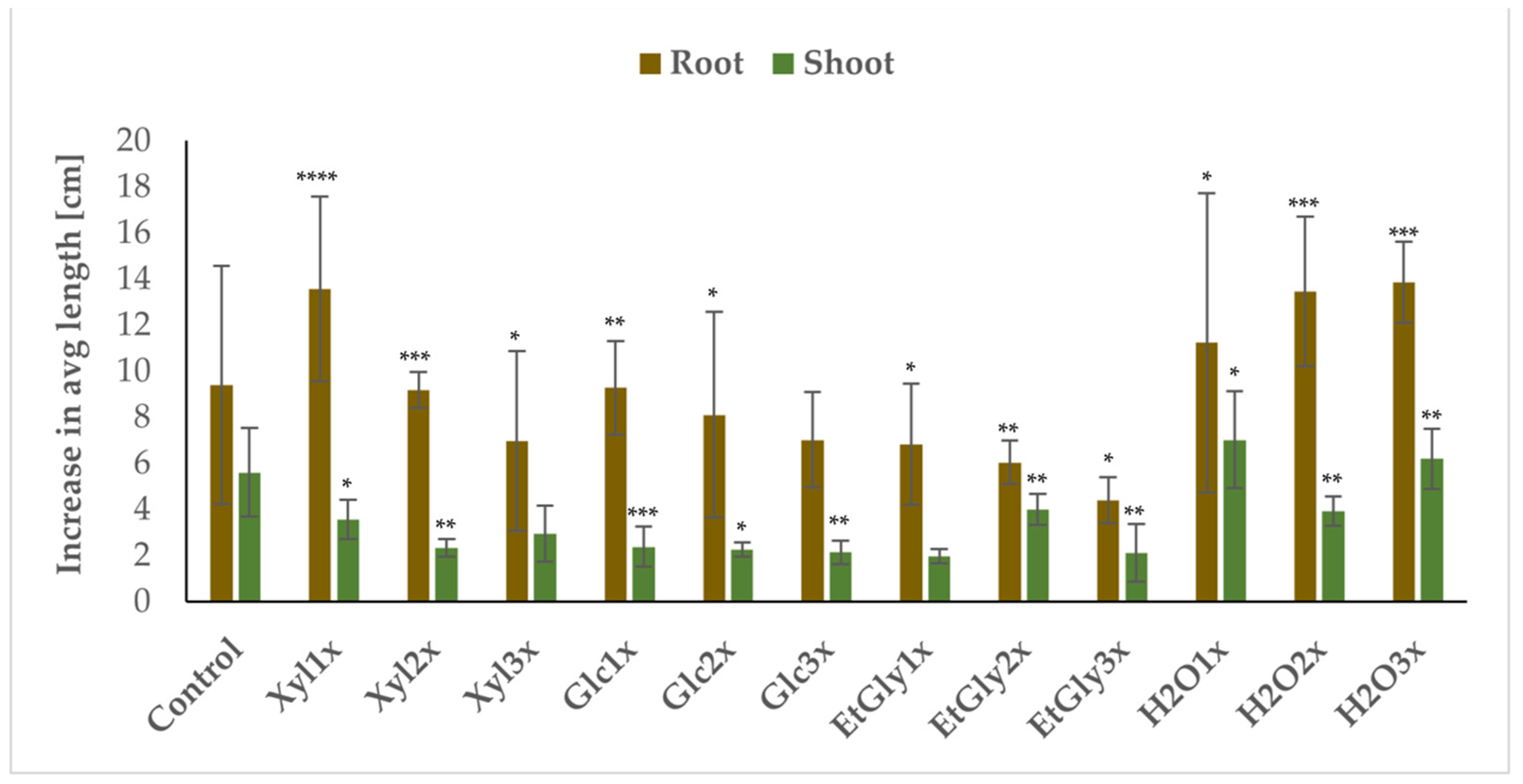
2.3. Impact of PEF Treatment on Plant Growth and Development
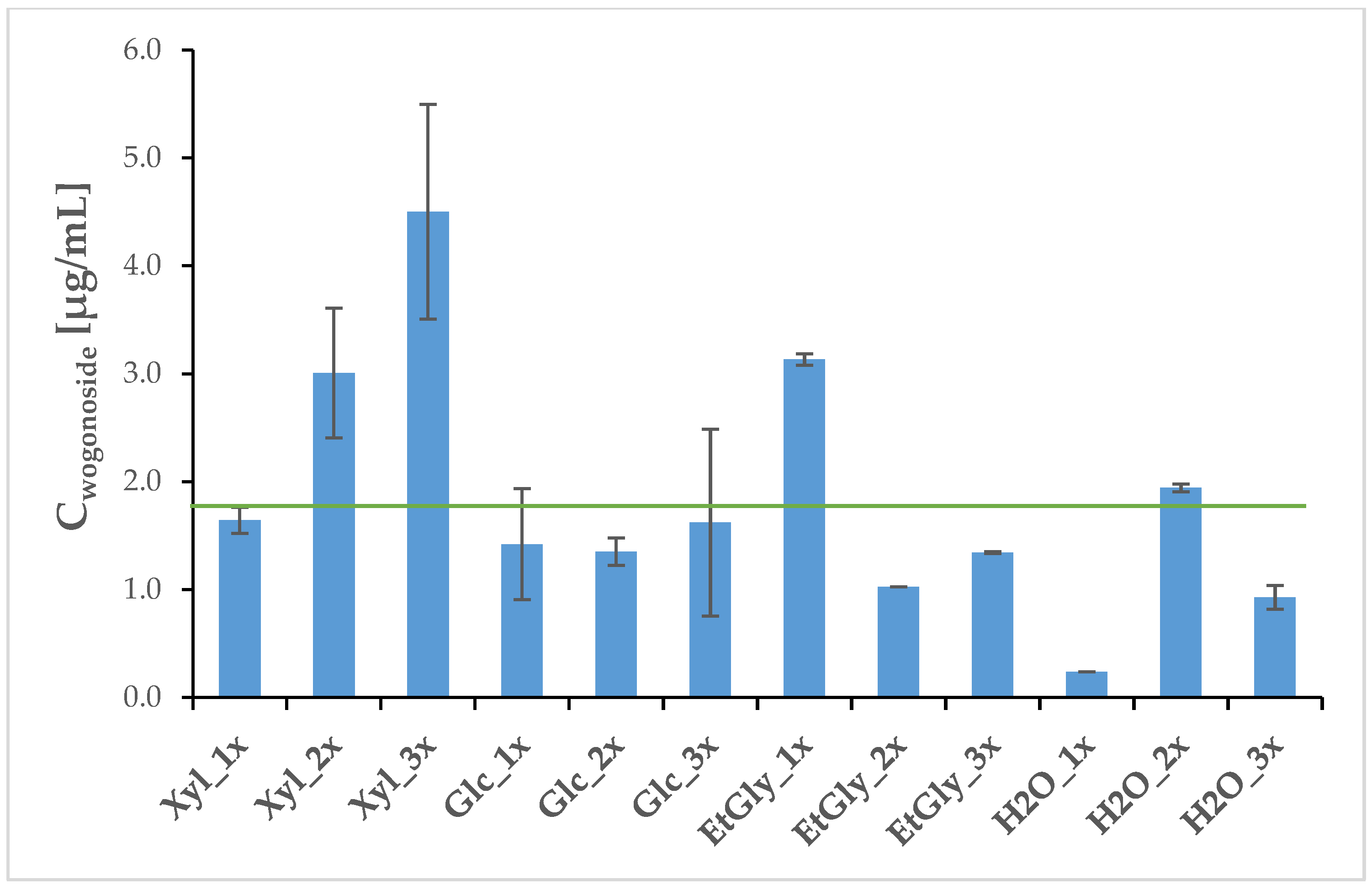
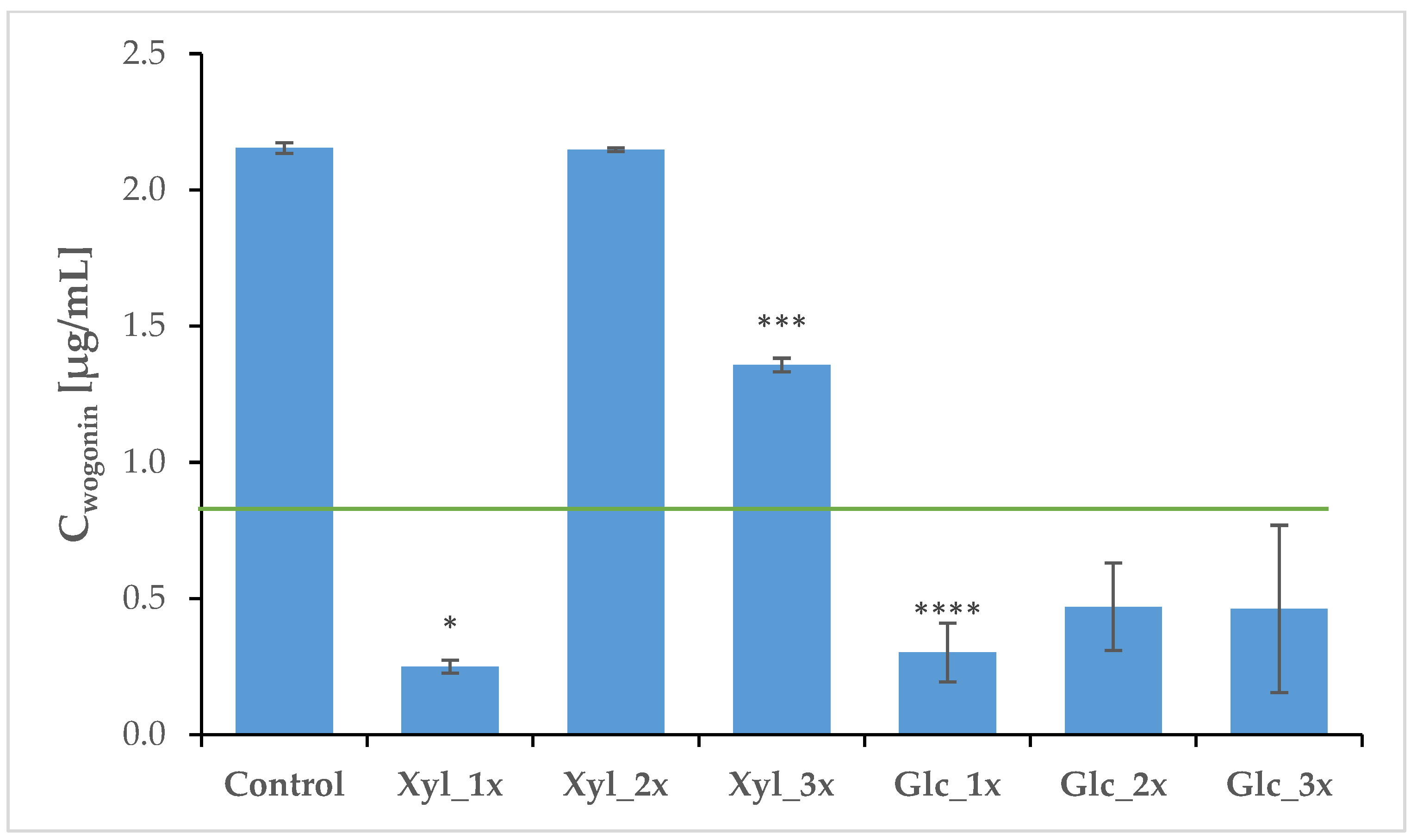
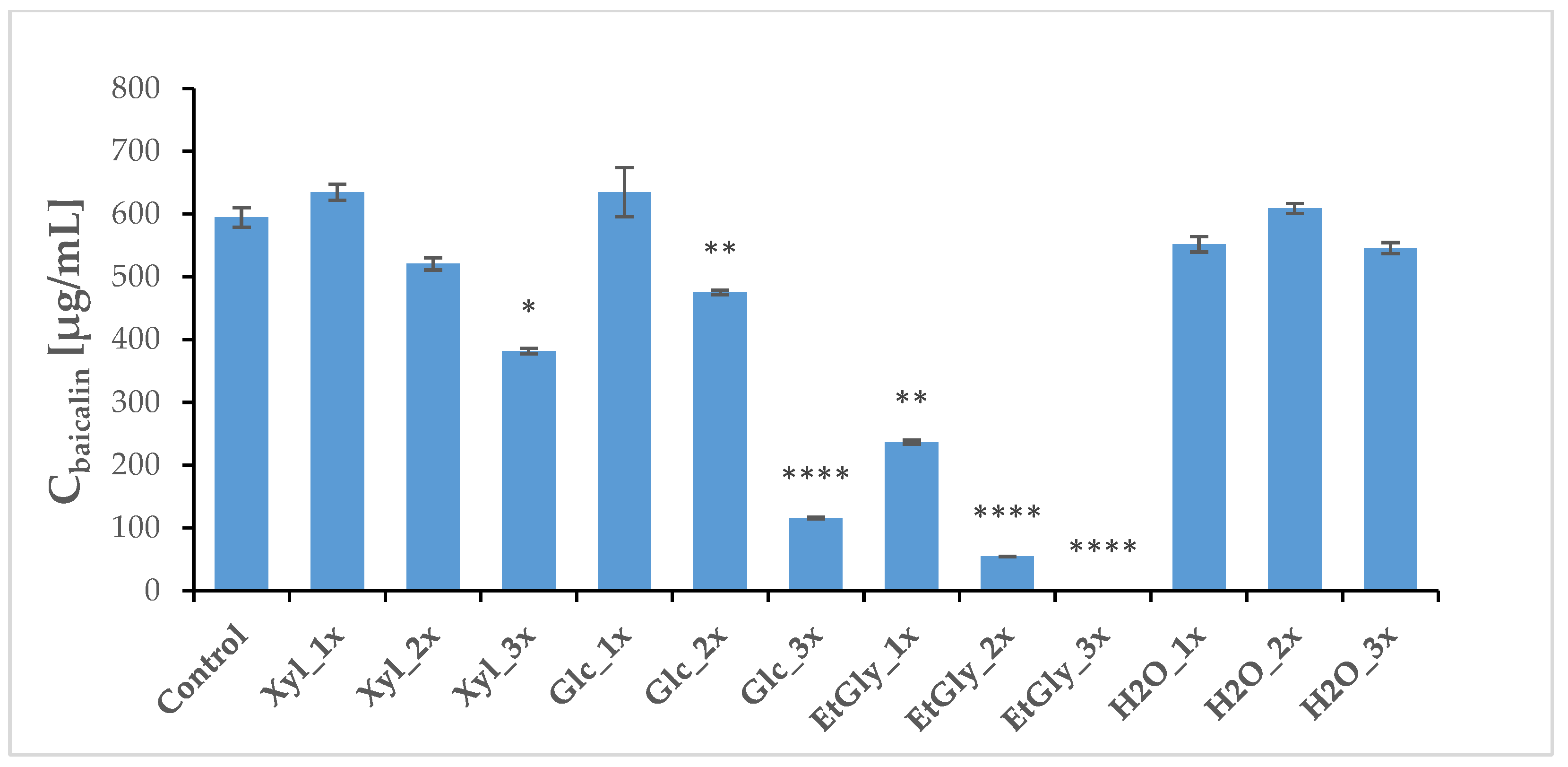
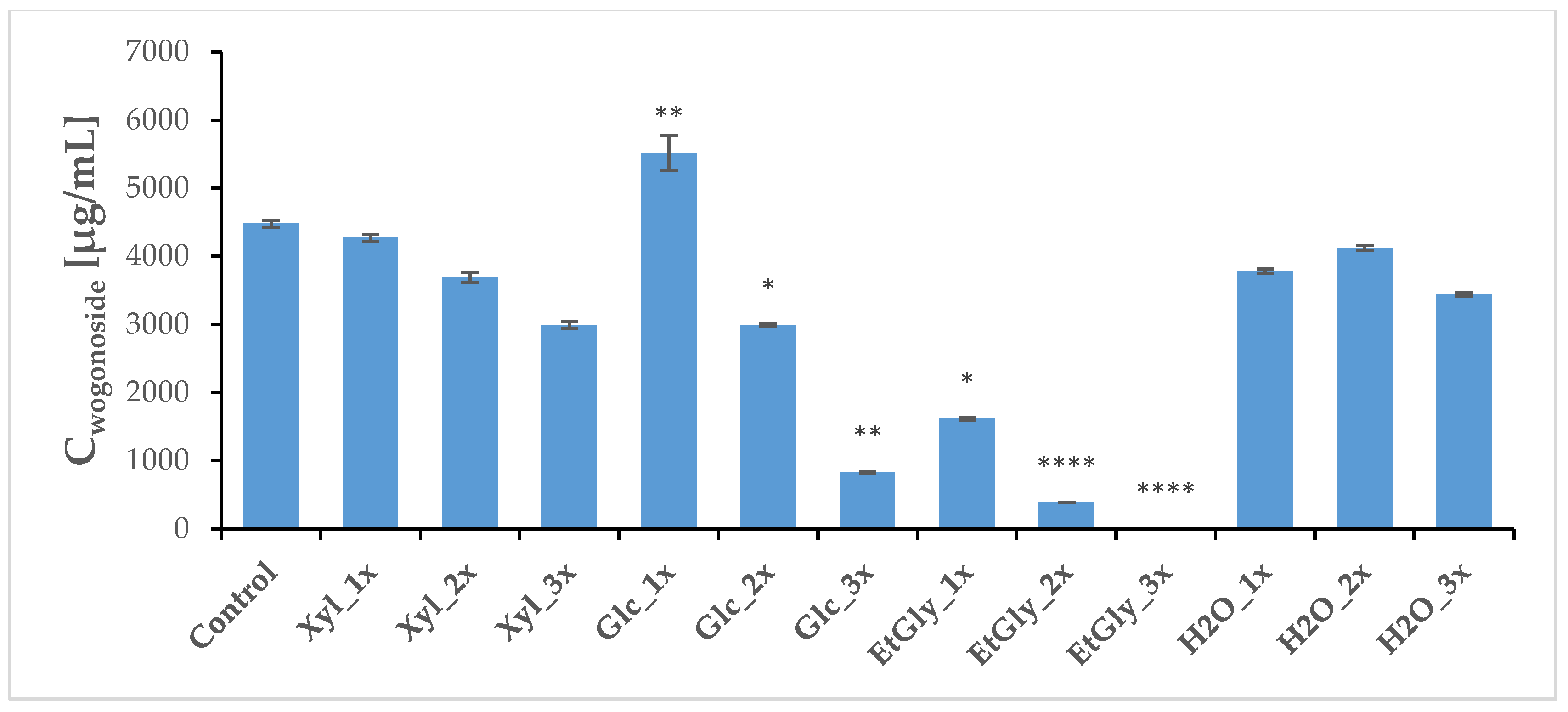
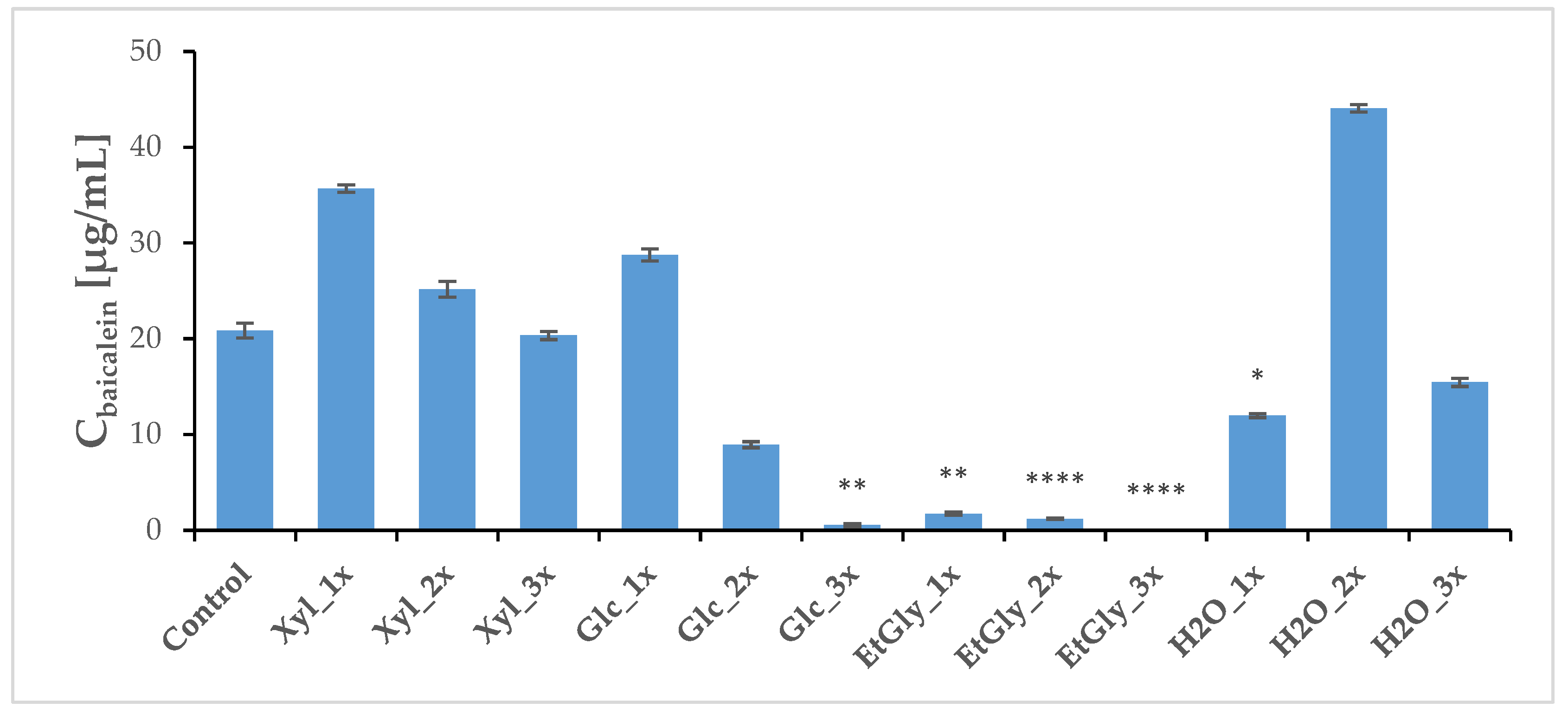
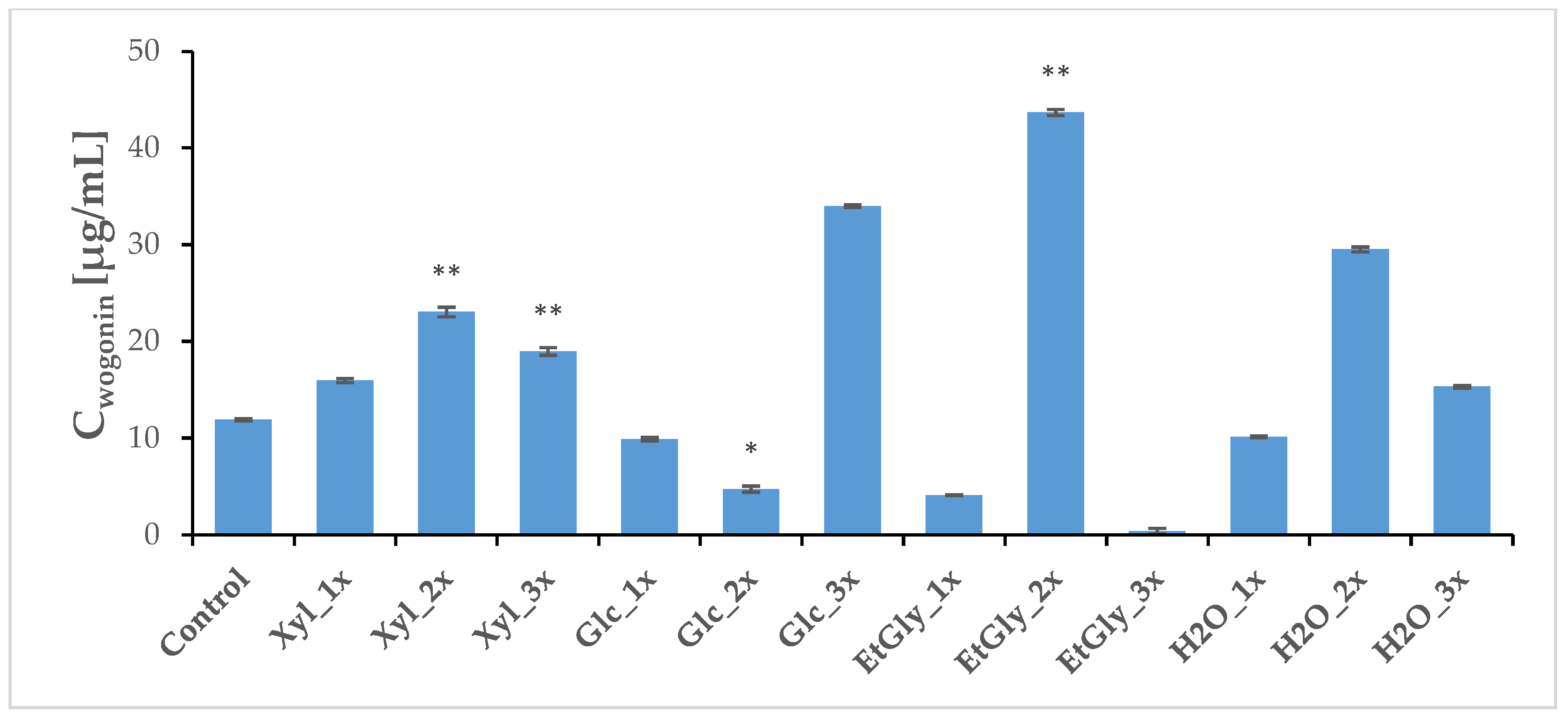
2.4. Phytochemical Profile Modulation as a Result of PEF Treatment
3. Materials and Methods
3.1. Plant Cultivation
3.2. Confocal Microscopy Imaging
3.3. NADES Preparation
3.4. PEF Treatment
3.5. Preparation of Extracts for HPLC-MS Analysis
3.6. HPLC-MS Analysis
3.7. Materials
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Zheng, S.; Wang, X.; Wang, J.; Ali shah, S.B.; Wang, Y.; Gao, R.; Xu, Z. Advances in Pharmacology, Biosynthesis, and Metabolic Engineering of Scutellaria-Specialized Metabolites. Crit. Rev. Biotechnol. 2024, 44, 302–318. [Google Scholar] [CrossRef]
- Xu, X.; Xu, H.; Shang, Y.; Zhu, R.; Hong, X.; Song, Z.; Yang, Z. Development of the General Chapters of the Chinese Pharmacopoeia 2020 Edition: A Review. J. Pharm. Anal. 2021, 11, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, Y. Jp Xvii. In The Japanese Pharmacopoeia; The Ministry of Health, Labour and Welfare: Tokyo, Japan, 2016. [Google Scholar]
- Patient Health Protection. Reflection Paper on the Level of Purification of Extracts to Be Considered as Herbal Preparations Reflection Paper on the Level of Purification of Extracts to Be Considered as Herbal Preparations Table of Contents; European Medicines Agency: Amsterdam, The Netherlands, 2010; Volume 44. [Google Scholar]
- ISO 4564:2023(en); Traditional Chinese Medicine—Scutellaria baicalensis root. International Organization for Standardization: Geneva, Switzerland, 2023.
- Skołucka, N.; Saczko, J.; Kotulska, M.; Kulbacka, J.; Choromańska, A. Elektroporacja i Jej Zastosowanie. Pol. Merkur. Lek. 2010, 28, 501–504. [Google Scholar]
- Nikyar, A.; Bolhassani, A. Electroporation: An Effective Method For In Vivo Gene Delivery. Drug Deliv. Lett. 2022, 12, 35–45. [Google Scholar] [CrossRef]
- Rosazza, C.; Meglic, S.H.; Zumbusch, A.; Rols, M.-P.; Miklavčič, D. Gene Electrotransfer: A Mechanistic Perspective. Curr. Gene Ther. 2016, 16, 98–129. [Google Scholar] [CrossRef]
- Hossain, M.B.; Aguiló-Aguayo, I.; Lyng, J.G.; Brunton, N.P.; Rai, D.K. Effect of Pulsed Electric Field and Pulsed Light Pre-Treatment on the Extraction of Steroidal Alkaloids from Potato Peels. Innov. Food Sci. Emerg. Technol. 2015, 29, 9–14. [Google Scholar] [CrossRef]
- Leong, S.Y.; Oey, I.; Burritt, D.J. Pulsed Electric Field Improves the Bioprotective Capacity of Purées for Different Coloured Carrot Cultivars against H2O2-Induced Oxidative Damage. Food Chem. 2016, 196, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Luengo, E.; Álvarez, I.; Raso, J. Improving Carotenoid Extraction from Tomato Waste by Pulsed Electric Fields. Front. Nutr. 2014, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Ko, M.J.; Park, C.H.; Chung, M.S. Application of Pulsed Electric Field as a Pre-Treatment for Subcritical Water Extraction of Quercetin from Onion Skin. Foods 2022, 11, 1069. [Google Scholar] [CrossRef]
- Leong, S.Y.; Burritt, D.J.; Oey, I. Evaluation of the Anthocyanin Release and Health-Promoting Properties of Pinot Noir Grape Juices after Pulsed Electric Fields. Food Chem. 2016, 196, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Sirithunyalug, J.; Somwongin, S.; Punyoyai, C.; Laothaweerungsawat, N.; Marsup, P.; Neimkhum, W.; Yawootti, A. Enhancement of the Antioxidant, Anti-Tyrosinase, and Anti-Hyaluronidase Activity of Morus alba L. Leaf Extract by Pulsed Electric Field Extraction. Molecules 2020, 25, 2212. [Google Scholar] [CrossRef]
- Hendrawan, Y.; Sabrinauly; Hawa, L.C.; Rachmawati, M.; Argo, B.D. Analysis of the Phenol and Flavonoid Content from Basil Leaves (Ocimum americanum L.) Extract Using Pulsed Electric Field (PEF) Pre-Treatment. Agric. Eng. Int. CIGR J. 2019, 21, 149–158. [Google Scholar]
- Athanasiadis, V.; Lakka, A.; Palaiogiannis, D.; Pappas, V.M.; Bozinou, E.; Ntourtoglou, G.; Makris, D.P.; Dourtoglou, V.G.; Lalas, S.I. Pulsed Electric Field and Salvia officinalis L. Leaves: A Successful Combination for the Extraction of High Value Added Compounds. Foods 2021, 10, 2014. [Google Scholar] [CrossRef] [PubMed]
- Zderic, A.; Zondervan, E.; Meuldijk, J. Breakage of Cellular Tissue by Pulsed Electric Field: Extraction of Polyphenols from Fresh Tea Leaves. In Proceedings of the Chemical Engineering Transactions; Italian Association of Chemical Engineering—AIDIC, Milan, Italy, 2–5 June 2013; Volume 32, pp. 1795–1800. [Google Scholar]
- Eleršek, T.; Flisar, K.; Likozar, B.; Klemenčič, M.; Golob, J.; Kotnik, T.; Miklavčič, D. Electroporation as a Solvent-Free Green Technique for Non-Destructive Extraction of Proteins and Lipids From Chlorella vulgaris. Front. Bioeng. Biotechnol. 2020, 8, 443. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kwon, H.J.; Lee, D.U. Effects of Pulsed Electric Field (PEF) Treatment on Physicochemical Properties of Panax ginseng. Innov. Food Sci. Emerg. Technol. 2019, 58, 102232. [Google Scholar] [CrossRef]
- Grzelka, K.; Matkowski, A.; Ślusarczyk, S. Electrostimulation Improves Plant Growth and Modulates the Flavonoid Profile in Aeroponic Culture of Scutellaria baicalensis Georgi. Front. Plant Sci. 2023, 14, 1142624. [Google Scholar] [CrossRef]
- Stanisz, M.; Stanisz, B.J.; Cielecka-Piontek, J. A Comprehensive Review on Deep Eutectic Solvents: Their Current Status and Potential for Extracting Active Compounds from Adaptogenic Plants. Molecules 2024, 29, 4767. [Google Scholar] [CrossRef] [PubMed]
- Rezaeinezhad, A.; Eslami, P.; Afrasiabpour, G.; Mirmiranpour, H.; Ghomi, H. Effect of Pulsed Electric Field on Diabetes-Induced Glycated Enzyme, Oxidative Stress, and Inflammatory Markers In Vitro and In Vivo. J. Phys. D Appl. Phys. 2022, 55, 015401. [Google Scholar] [CrossRef]
- Su, W.; Wang, Q.; Li, J.; Qiu, Z.; Qiu, Y. Effects of Pulsed Electric Field Technology on the Nutritional Value and Biological Function of Plant Food. Front. Sustain. Food Syst. 2024, 8, 1385533. [Google Scholar] [CrossRef]
- Pei, T.; Yan, M.; Huang, Y.; Wei, Y.; Martin, C.; Zhao, Q. Specific Flavonoids and Their Biosynthetic Pathway in Scutellaria baicalensis. Front. Plant Sci. 2022, 13, 866282. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ren, G.; Wu, F.; Chen, J.; Jiang, D.; Liu, C. Root-Specific Flavones and Critical Enzyme Genes Involved in Their Synthesis Changes Due to Drought Stress on Scutellaria baicalensis. Front. Ecol. Evol. 2023, 11, 1113823. [Google Scholar] [CrossRef]
- Lebovka, N. Electrotechnologies for Extraction from Food Plants and Biomaterials; Food Engineering Series; Springer: New York, NY, USA, 2009; ISBN 978-0-387-79373-3. [Google Scholar]
- Mahnič-Kalamiza, S.; Vorobiev, E.; Miklavčič, D. Electroporation in Food Processing and Biorefinery. J. Membr. Biol. 2014, 247, 1279–1304. [Google Scholar] [CrossRef]
- Asavasanti, S.; Stroeve, P.; Barrett, D.M.; Jernstedt, J.A.; Ristenpart, W.D. Enhanced Electroporation in Plant Tissues via Low Frequency Pulsed Electric Fields: Influence of Cytoplasmic Streaming. Biotechnol. Prog. 2012, 28, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Saczko, J.; Nowak, M.; Skołucka, N.; Kulbacka, J.; Kotulska, M. The Effects of the Electro-Photodynamic In Vitro Treatment on Human Lung Adenocarcinoma Cells. Bioelectrochemistry 2010, 79, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Kiełbik, A.; Szewczyk, A.; Rembiałkowska, N.; Novickij, V.; Tarek, M.; Saczko, J.; Kulbacka, J. Oxidative Effects During Irreversible Electroporation of Melanoma Cells-In Vitro Study. Molecules 2020, 26, 154. [Google Scholar] [CrossRef]
- Sözer, E.B.; Pocetti, C.F.; Vernier, P.T. Asymmetric Patterns of Small Molecule Transport After Nanosecond and Microsecond Electropermeabilization. J. Membr. Biol. 2018, 251, 197–210. [Google Scholar] [CrossRef]
- Rukavina, I.; Rodrigues, M.J.; Pereira, C.G.; Mansinhos, I.; Romano, A.; Ślusarczyk, S.; Matkowski, A.; Custódio, L. Greener Is Better: First Approach for the Use of Natural Deep Eutectic Solvents (Nades) to Extract Antioxidants from the Medicinal Halophyte Polygonum maritimum L. Molecules 2021, 26, 6136. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Ostermeier, R.; Giersemehl, P.; Siemer, C.; Töpfl, S.; Jäger, H. Influence of Pulsed Electric Field (PEF) Pre-Treatment on the Convective Drying Kinetics of Onions. J. Food Eng. 2018, 237, 110–117. [Google Scholar] [CrossRef]
- Giancaterino, M.; Jaeger, H. Impact of Pulsed Electric Fields (PEF) Treatment on the Peeling Ability of Tomatoes and Kiwi Fruits. Front. Food Sci. Technol. 2023, 3, 1152111. [Google Scholar] [CrossRef]
- Eing, C.J.; Bonnet, S.; Pacher, M.; Puchta, H.; Frey, W. Effects of Nanosecond Pulsed Electric Field Exposure on Arabidopsis thaliana. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1322–1328. [Google Scholar] [CrossRef]
- Sonoda, T.; Takamura, N.; Wang, D.; Namihira, T.; Akiyama, H. Growth Control of Leaf Lettuce Using Pulsed Electric Field. IEEE Trans. Plasma Sci. 2014, 42, 3202–3208. [Google Scholar] [CrossRef]
- Bozinou, E.; Karageorgou, I.; Batra, G.; Dourtoglou, V.G.; Lalas, S.I. Pulsed Electric Field Extraction and Antioxidant Activity Determination of Moringa oleifera Dry Leaves: A Comparative Study with Other Extraction Techniques. Beverages 2019, 5, 8. [Google Scholar] [CrossRef]
- Kalyniukova, A.; Holuša, J.; Musiolek, D.; Sedlakova-Kadukova, J.; Płotka-Wasylka, J.; Andruch, V. Application of Deep Eutectic Solvents for Separation and Determination of Bioactive Compounds in Medicinal Plants. Ind. Crops Prod. 2021, 172, 114047. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2023, 12, 56. [Google Scholar] [CrossRef]
- Ye, H.; Huang, L.L.; Chen, S.D.; Zhong, J.J. Pulsed Electric Field Stimulates Plant Secondary Metabolism in Suspension Cultures of Taxus Chinensis. In Proceedings of the Biotechnology and Bioengineering, New York, NY, USA, 20 December 2004; Volume 88, pp. 788–795. [Google Scholar]
- Wu, J.; Lv, S.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.; Ma, F. Advances in the Study of the Function and Mechanism of the Action of Flavonoids in Plants under Environmental Stresses. Planta 2023, 257, 108. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Met. 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]

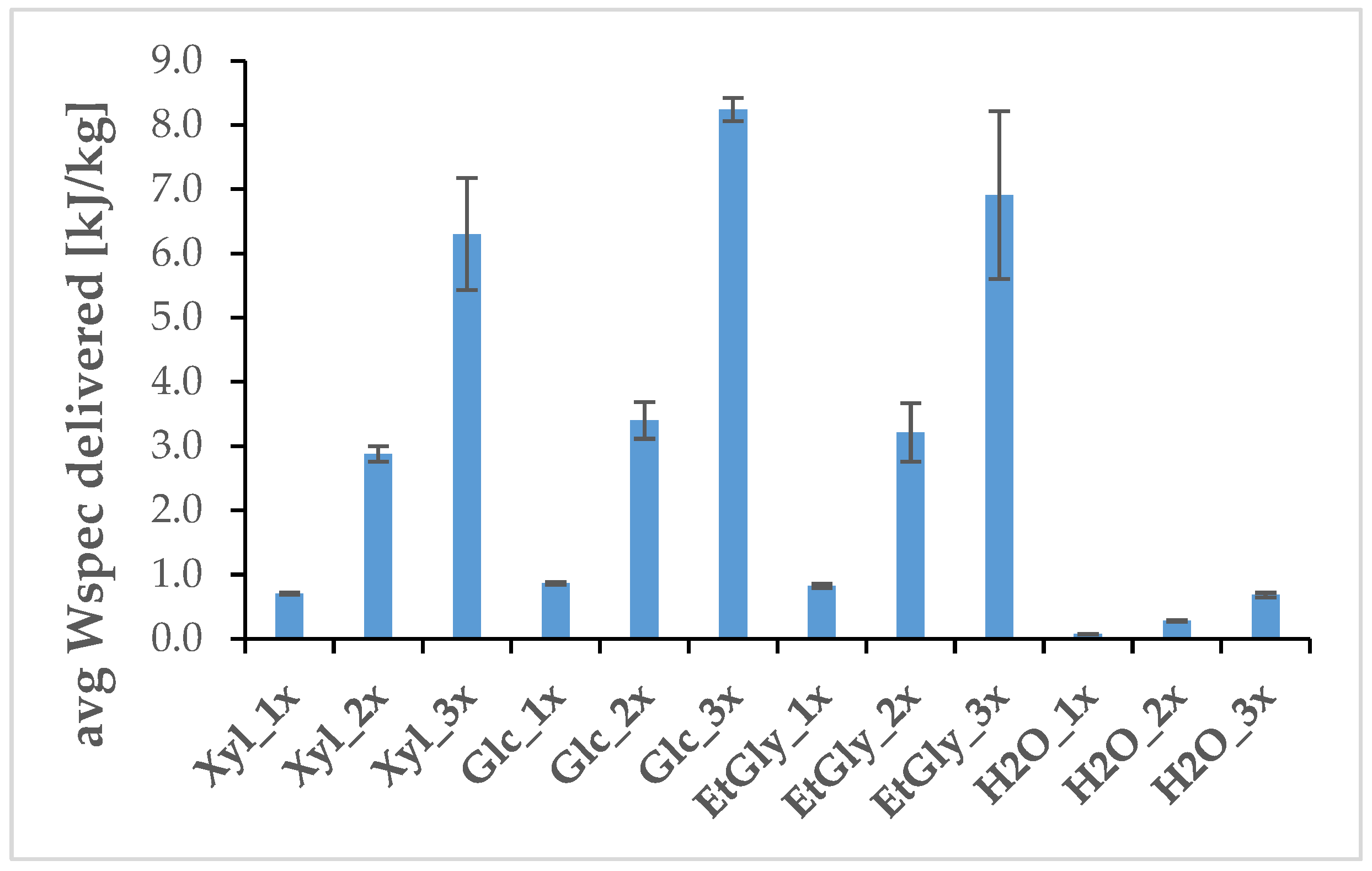

| Subgroup Name | Solvent Used, NADES Media (1–4) |
|---|---|
| Xyl_1x | 1—choline chloride/xylose (1:2) + 30% water EC = 7.97 mS/cm |
| Xyl_2x | |
| Xyl_3x | |
| Glc_1x | 2—choline chloride/glucose (1:2) + 30% water EC = 9.72 mS/cm |
| Glc_2x | |
| Glc_3x | |
| EtGly_1x | 3—choline chloride/ethylene glycol (1:2) EC = 8.61 mS/cm |
| EtGly_2x | |
| EtGly_3x | |
| H2O_1x | 4—tap water EC = 0.7 mS/cm |
| H2O_2x | |
| H2O_3x |
| No. | Compound Name | Rt [min] | UV | m/z [M − H]- | Error [ppm] | Formula | MS2 Ion | m/z [M + H]+ | MS2 Ion | LOQ [µg/mL] | LOD [µg/mL] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6-C-Arabinose-8-C-glucose-chrysin | 9.7 | 215, 275, 315 | 547.1447 | 0.1 | C26H28O13 | 337 (100), 367 (83), 457 (13), 309 (12), 427 (10) | 549.1602 | 309 (100), 375 (94), 321 (90), 279 (72) | ||
| 2 | 6-C-Glucose-8-C-arabinose-chrysin | 10.2 | 215, 275, 315 | 547.1446 | 0.1 | C26H28O13 | 337 (100), 367 (68), 427 (44), 457 (27) | 549.1597 | 351 (100), 271 (35) | ||
| 3 | Scutellarein-7-O-β-D-glucuronide-5-glucoside | 10.4 | 284, 333 | 623.1987 | 0.3 | C29H36O15 | 461 (50), 285 (24) | 625.1997 | 161 (80) | ||
| 4 | Scutellarein glucoside | 11.4 | 210, 270 | 447.0935 | 0.1 | C21H20O11 | 285 (100) | 449.1459 | 287 (100) | ||
| 5 | Baicalin a | 12.6 | 225, 280, 315 | 445.0771 | 0.3 | C21H18O11 | 269 (100), 251 (8) | 447.0922 | 271 (100), 253 (2) | 0.55 | 0.18 |
| 6 | Oroxylin A 7-O-β-D-glucuronide a | 13.8 | 210, 270, 310 | 459.0928 | −1.2 | C22H22O11 | 268 (100), 283 (16), 269 (13) | 461.1084 | 270 (100), 285 (73) | 0.41 | 0.37 |
| 7 | Chrysin-glucuronide | 13.9 | 270, 312 | 429.0821 | 0.3 | C21H18O10 | 253 (100) | 431.0975 | 255 (100) | ||
| 8 | Wogonoside a | 14.4 | 220, 275 | 459.0929 | 0.9 | C22H22O11 | 268 (100), 283 (15), 269 (13), 284 (2) | 461.1085 | 270 (100), 285 (85) | 0.41 | 0.17 |
| 9 | Baicalein a | 14.8 | 215, 275, 320 | 269.0457 | 1.1 | C15H10O5 | 223 (24), 241 (20), 195 (17), 169 (15), 197 (12), 251 (11), 271 (8) | 271.0603 | 123 (79), 253 (21), 169 (20), 103 (8) | 0.45 | 0.16 |
| 10 | Norwogonin | 16.4 | 220, 280 | 269.0453 | 0.4 | C15H10O5 | 197 (100), 171 (40), 213 (31) | 271.0604 | 169 (100), 139 (12), 123 (9), 141 (8) | ||
| 11 | Wogonin a | 19.1 | 210, 275 | 283.0616 | 0.3 | C16H12O5 | 268 (100), 163 (23), 184,239 | 285.0753 | 270 (100), 179 (8), 252 (7) | 0.62 | 0.2 |
| 12 | Oroxylin A a | 19.5 | 215, 270, 320 | 283.0613 | −0.5 | C16H12O5 | 268 (100), 165 (9), 184 (7), 240 | 285.0754 | 270 (100), 168 (46), 140 (9), 242 (8), 224 (3) | 0.72 | 0.22 |
| Group | Wogonoside [μg/mL] | SD | Wogonin [μg/mL] | SD |
|---|---|---|---|---|
| Control | 41.02 | 0.34 | 2.15 | 0.01 |
| Xyl_1x | 1.64 **** | 0.12 | 0.25 * | 0.02 |
| Xyl_2x | 3.01 **** | 0.60 | 2.14 | 0.01 |
| Xyl_3x | 4.50 **** | 0.99 | 1.35 *** | 0.02 |
| Glc_1x | 1.42 **** | 0.51 | 0.30 **** | 0.10 |
| Glc_2x | 1.35 **** | 0.12 | 0.47 | 0.14 |
| Glc_3x | 1.62 **** | 0.86 | 0.47 | 0.15 |
| EtGly_1x | 3.13 **** | 0.05 | ND 1 | |
| EtGly_2x | 1.02 **** | 0.001 | ND | |
| EtGly_3x | 1.34 **** | 0.008 | ND | |
| H2O_1x | 0.23 **** | 0.0009 | ND | |
| H2O_2x | 1.94 **** | 0.03 | ND | |
| H2O_3x | 0.93 **** | 0.10 | ND |
| Group | Avg Root Length Increase [cm] | SD | Avg Root Wet Mass [g] | SD | Avg Root Dry Mass [g] | SD | Avg Shoot Length Increase [cm] | SD | PSR | PGD |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 9.41 | 5.16 | 0.41 | 0.19 | 0.11 | 0.03 | 5.61 | 1.92 | 100% | 80% |
| Xyl_1x | 13.59 | 4.00 | 0.70 | 0.33 | 0.13 | 0.04 | 3.56 * | 0.85 | 80% | 60% |
| Xyl_2x | 9.20 | 0.78 | 0.71 | 0.45 | 0.17 | 0.06 | 2.33 ** | 0.39 | 60% | 60% |
| Xyl_3x | 6.97 | 3.89 | 0.59 | 0.41 | 0.13 | 0.05 | 2.95 * | 1.21 | 60% | 40% |
| Glc_1x | 9.28 | 2.03 | 0.68 | 0.39 | 0.15 | 0.03 | 2.38 * | 0.87 | 80% | 60% |
| Glc_2x | 8.12 | 4.47 | 0.32 | 0.19 | 0.11 | 0.03 | 2.25 ** | 0.31 | 80% | 40% |
| Glc_3x | 7.03 | 2.06 | 0.30 | 0.16 | 0.08 * | 0.04 | 2.14 ** | 0.51 | 60% | 20% |
| EtGly_1x | 6.83 | 2.63 | 0.40 | 0.21 | 0.11 | 0.02 | 1.97 ** | 0.32 | 60% | 60% |
| EtGly_2x | 6.04 | 0.93 | 0.24 | 0.13 | 0.09 * | 0.03 | 4.01 * | 0.68 | 60% | 20% |
| EtGly_3x | 4.41 * | 0.98 | 0.39 | 0.28 | 0.09 * | 0.03 | 2.11 | 1.24 | 60% | 20% |
| H2O_1x | 11.24 | 6.49 | 0.74 | 0.57 | 0.15 | 0.09 | 7.03 | 2.11 | 60% | 60% |
| H2O_2x | 13.47 | 3.24 | 0.56 | 0.39 | 0.12 | 0.05 | 3.93 | 0.63 | 60% | 60% |
| H2O_3x | 13.87 * | 1.76 | 0.88 * | 0.41 | 0.17* | 0.04 | 6.20 | 1.31 | 80% | 80% |
| Group | Wogonoside [μg/mL] | SD | Baicalin [μg/mL] | SD | Wogonin [μg/mL] | SD | Baicalein [μg/mL] | SD |
|---|---|---|---|---|---|---|---|---|
| Control | 4480.56 | 50.03 | 594.57 | 15.52 | 11.91 | 0.10 | 20.86 | 0.77 |
| Xyl_1x | 4269.87 | 51.88 | 635.04 | 12.95 | 15.96 | 0.22 | 35.69 | 0.38 |
| Xyl_2x | 3695.17 | 75.42 | 520.95 | 9.87 | 23.05 ** | 0.50 | 25.17 | 0.83 |
| Xyl_3x | 2991.40 | 51.77 | 381.86 * | 4.56 | 18.97 ** | 0.40 | 20.33 | 0.43 |
| Glc_1x | 5520.29 ** | 258.41 | 635.14 | 39.40 | 9.91 | 0.18 | 28.75 | 0.63 |
| Glc_2x | 2992.32 * | 14.61 | 475.34 ** | 3.65 | 4.73 * | 0.31 | 8.94 | 0.32 |
| Glc_3x | 832.81 ** | 11.98 | 115.83 **** | 1.51 | 33.98 | 0.15 | 0.54 ** | 0.16 |
| EtGly_1x | 1617.56 * | 20.53 | 236.94 ** | 2.96 | 4.11 | 0.04 | 1.73 ** | 0.15 |
| EtGly_2x | 387.01 **** | 3.20 | 54.84 **** | 0.39 | 43.68 ** | 0.33 | 1.21 **** | 0.05 |
| EtGly_3x | 4.18 **** | 0.09 | 0.29 **** | 0.04 | 0.37 | 0.29 | 0.02 **** | 0.01 |
| H2O_1x | 3780.64 | 33.06 | 552.01 | 12.19 | 10.14 | 0.07 | 11.98 * | 0.20 |
| H2O_2x | 4124.55 | 32.43 | 609.29 | 7.90 | 29.53 | 0.26 | 44.07 | 0.39 |
| H2O_3x | 3444.95 | 28.09 | 546.07 | 8.69 | 15.32 | 0.13 | 15.45 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzelka, K.; Matkowski, A.; Chodaczek, G.; Jaśpińska, J.; Pawlikowska-Bartosz, A.; Słupski, W.; Lechniak, D.; Szumacher-Strabel, M.; Olorunlowu, S.; Szulc, K.; et al. Pulsed Electric Field (PEF) Treatment Results in Growth Promotion, Main Flavonoids Extraction, and Phytochemical Profile Modulation of Scutellaria baicalensis Georgi Roots. Int. J. Mol. Sci. 2025, 26, 100. https://doi.org/10.3390/ijms26010100
Grzelka K, Matkowski A, Chodaczek G, Jaśpińska J, Pawlikowska-Bartosz A, Słupski W, Lechniak D, Szumacher-Strabel M, Olorunlowu S, Szulc K, et al. Pulsed Electric Field (PEF) Treatment Results in Growth Promotion, Main Flavonoids Extraction, and Phytochemical Profile Modulation of Scutellaria baicalensis Georgi Roots. International Journal of Molecular Sciences. 2025; 26(1):100. https://doi.org/10.3390/ijms26010100
Chicago/Turabian StyleGrzelka, Kajetan, Adam Matkowski, Grzegorz Chodaczek, Joanna Jaśpińska, Anna Pawlikowska-Bartosz, Wojciech Słupski, Dorota Lechniak, Małgorzata Szumacher-Strabel, Segun Olorunlowu, Karolina Szulc, and et al. 2025. "Pulsed Electric Field (PEF) Treatment Results in Growth Promotion, Main Flavonoids Extraction, and Phytochemical Profile Modulation of Scutellaria baicalensis Georgi Roots" International Journal of Molecular Sciences 26, no. 1: 100. https://doi.org/10.3390/ijms26010100
APA StyleGrzelka, K., Matkowski, A., Chodaczek, G., Jaśpińska, J., Pawlikowska-Bartosz, A., Słupski, W., Lechniak, D., Szumacher-Strabel, M., Olorunlowu, S., Szulc, K., Cieślak, A., & Ślusarczyk, S. (2025). Pulsed Electric Field (PEF) Treatment Results in Growth Promotion, Main Flavonoids Extraction, and Phytochemical Profile Modulation of Scutellaria baicalensis Georgi Roots. International Journal of Molecular Sciences, 26(1), 100. https://doi.org/10.3390/ijms26010100







