Aspartate β-Hydroxylase Is Upregulated in Head and Neck Squamous Cell Carcinoma and Regulates Invasiveness in Cancer Cell Models
Abstract
1. Introduction
2. Results
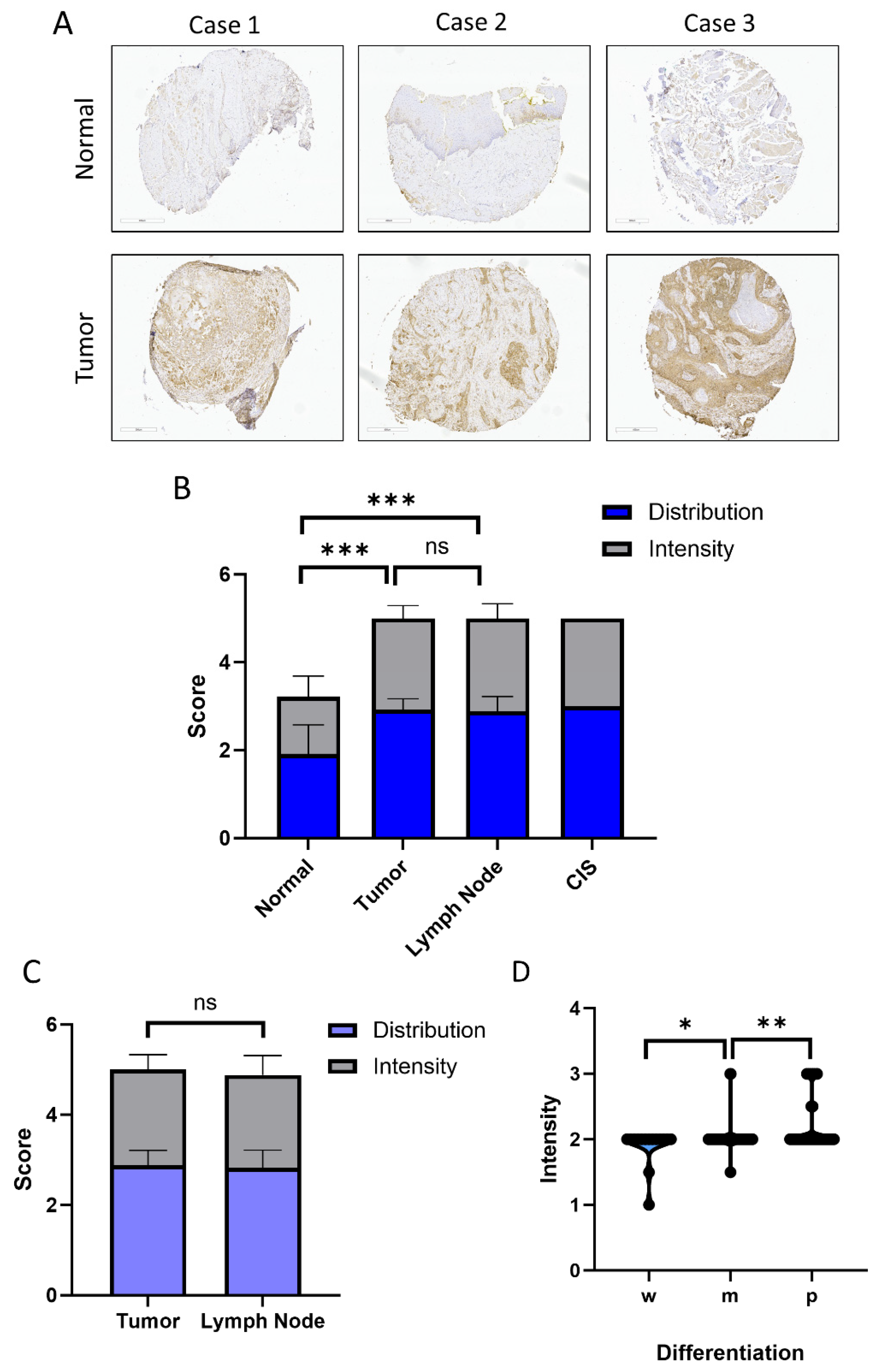
2.1. Expression of ASPH in HNSCC Tissues
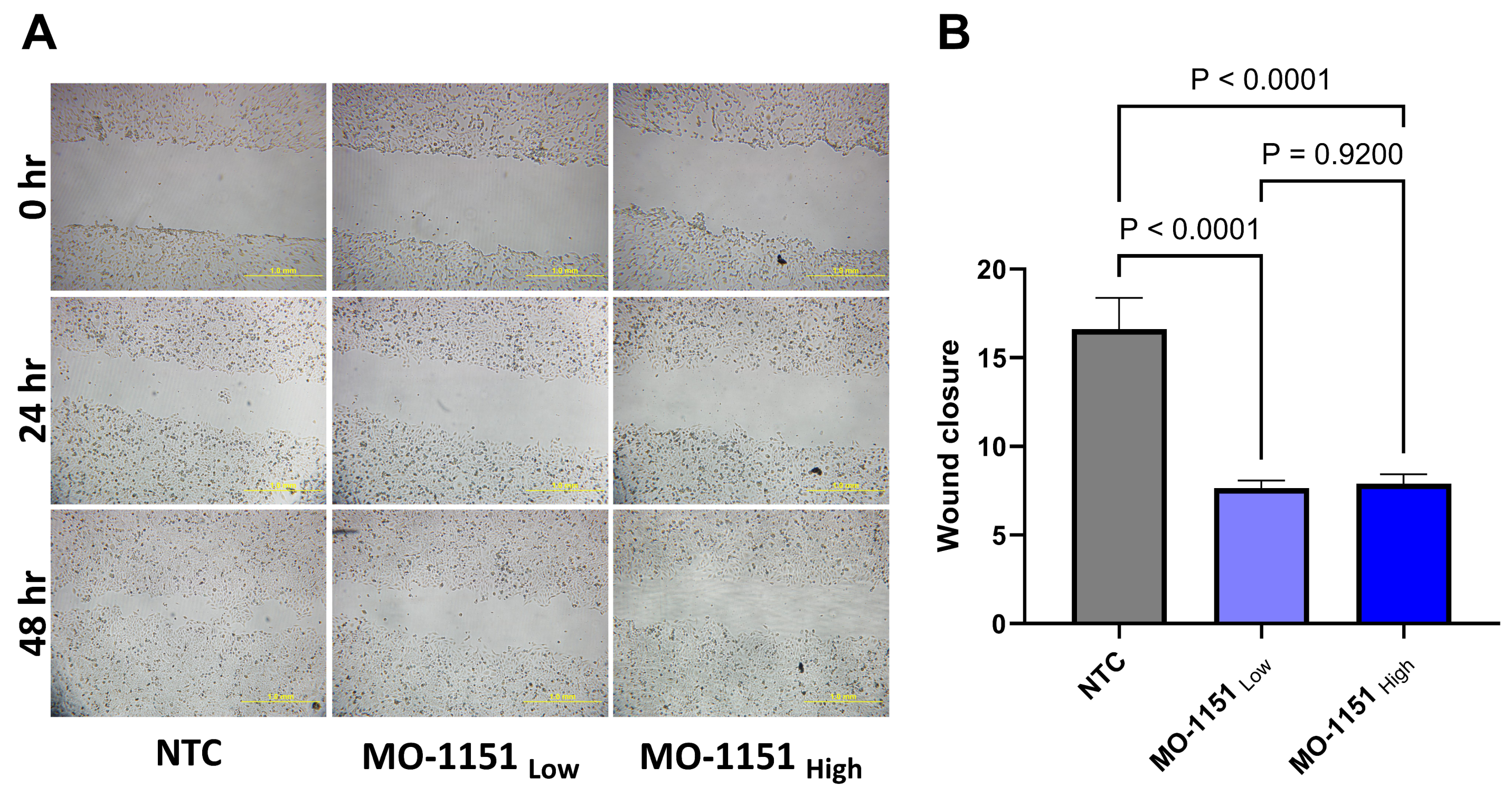
2.2. MO-1151 Inhibits Migration of HNSCC Cells
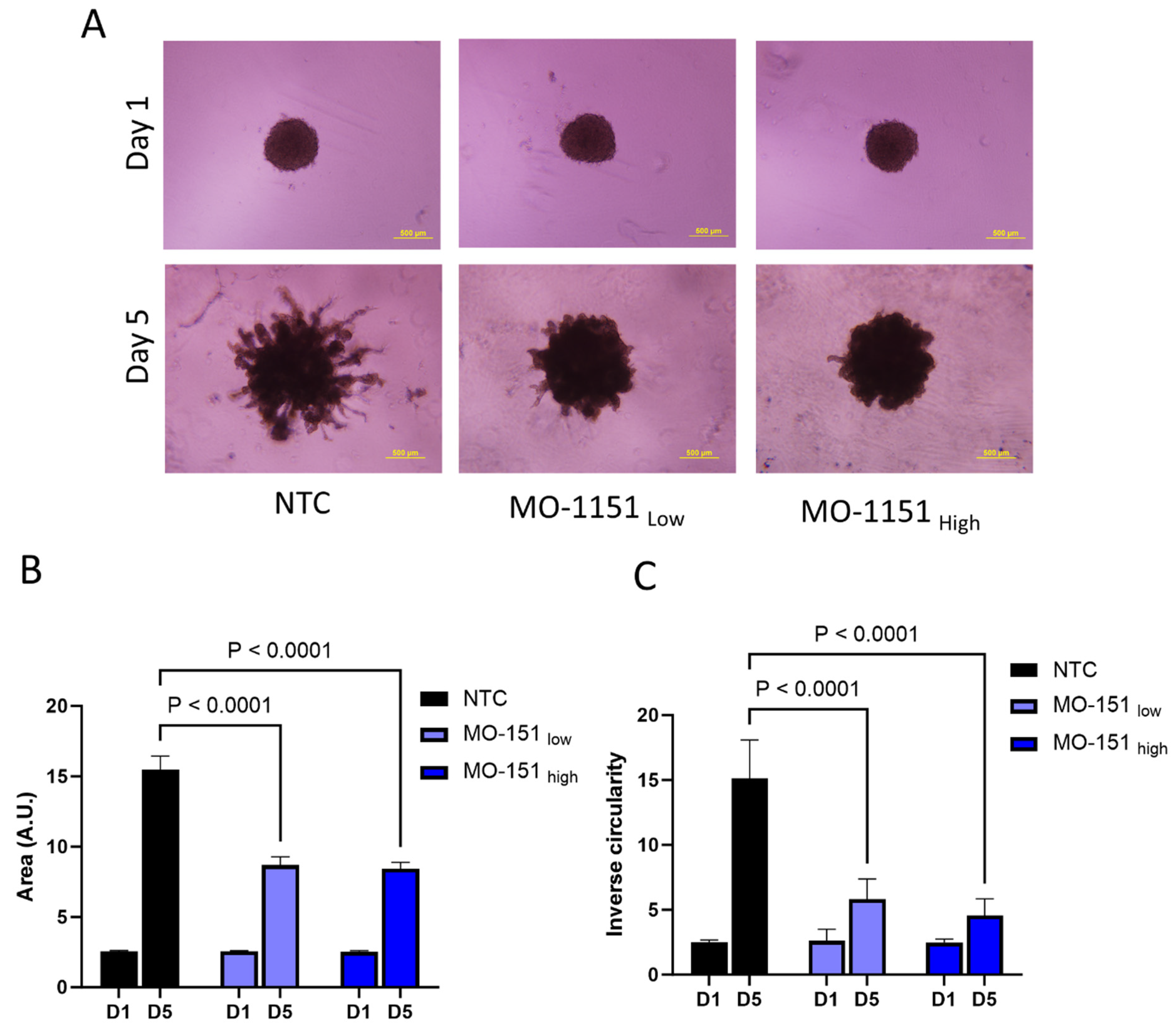
2.3. MO-1151 Inhibits CAF-Mediated Invasion of Spheroids in Matrigel
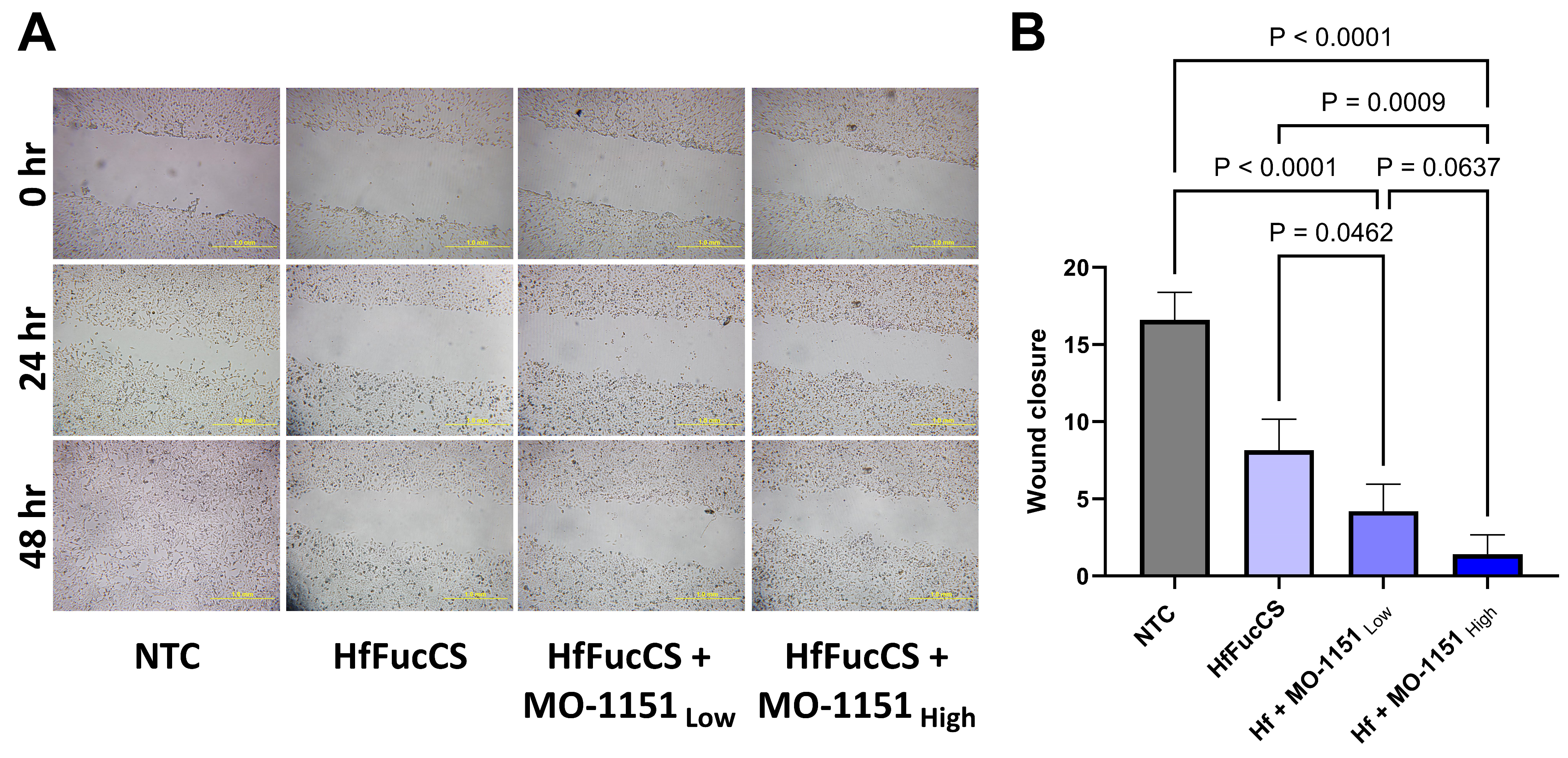
2.4. Combination of MO-I-1151 with HfFucCS Has an Additive Effect on Migration of HNSCC Cells
2.5. Combination of MO-I-1151 with HfFucCS Inhibitors Reduces the CAF-Mediated Invasion of Tumor Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Study Subjects and Samples
4.3. Tissue Microarray and Histological Sections
4.4. Immunohistochemistry and Pathology Scoring
4.5. Cell Culture
4.6. Wound Healing
4.7. Spheroid Invasion
4.8. Inhibitor Treatment of Spheroids
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Aihara, A.; Huang, C.-K.; Olsen, M.J.; Lin, Q.; Chung, W.; Tang, Q.; Dong, X.; Wands, J.R. A cell-surface β-hydroxylase is a biomarker and therapeutic target for hepatocellular carcinoma. J. Hepatol. 2014, 60, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Lin, Q.; Aihara, A.; Li, Y.; Huang, C.-K.; Chung, W.; Tang, Q.; Chen, X.; Carlson, R.; Nadolny, C.; et al. Aspartate β-hydroxylase expression promotes a malignant pancreatic cellular phenotype. Oncotarget 2014, 6, 1231–1248. [Google Scholar] [CrossRef]
- Huang, C.-K.; Iwagami, Y.; Aihara, A.; Chung, W.; de la Monte, S.; Thomas, J.-M.; Olsen, M.; Carlson, R.; Yu, T.; Dong, X.; et al. Anti-Tumor Effects of Second Generation β-Hydroxylase Inhibitors on Cholangiocarcinoma Development and Progression. PLoS ONE 2016, 11, e0150336. [Google Scholar] [CrossRef] [PubMed]
- Ince, N.; de la Monte, S.M.; Wands, J.R. Overexpression of Human Aspartyl (Asparaginyl) β-Hydroxylase Is Associated with Malignant Transformation1. Cancer Res. 2000, 60, 1261–1266. [Google Scholar] [PubMed]
- Kanwal, M.; Smahel, M.; Olsen, M.; Smahelova, J.; Tachezy, R. Aspartate β-hydroxylase as a target for cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 163. [Google Scholar] [CrossRef] [PubMed]
- Lavaissiere, L.; Jia, S.; Nishiyama, M.; de la Monte, S.; Stern, A.M.; Wands, J.R.; Friedman, P.A. Overexpression of human aspartyl(asparaginyl)beta-hydroxylase in hepatocellular carcinoma and cholangiocarcinoma. J. Clin. Investig. 1996, 98, 1313–1323. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, X.; Meng, F.; Ogawa, K.; Li, M.; Song, R.; Zhang, S.; Zhang, Z.; Kong, X.; Xu, Q.; et al. ASPH-notch Axis guided Exosomal delivery of Prometastatic Secretome renders breast Cancer multi-organ metastasis. Mol. Cancer 2019, 18, 156. [Google Scholar] [CrossRef]
- Silbermann, E.; Moskal, P.; Bowling, N.; Tong, M.; de la Monte, S.M. Role of aspartyl-(asparaginyl)-β-hydroxylase mediated notch signaling in cerebellar development and function. Behav. Brain Funct. 2010, 6, 68. [Google Scholar] [CrossRef]
- Yang, H.; Song, K.; Xue, T.; Xue, X.-P.; Huyan, T.; Wang, W.; Wang, H. The distribution and expression profiles of human Aspartyl/Asparaginyl beta-hydroxylase in tumor cell lines and human tissues. Oncol. Rep. 2010, 24, 1257–1264. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Tang, R.; Li, J.; Liu, Y.; Ye, L.; Shao, D.; Jin, M.; Huang, Q.; Shi, J. The aspartyl asparaginyl beta-hydroxylase in carcinomas. Front. Biosci. 2015, 20, 902–909. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, X.; Hu, J.; Bai, B.; Zhu, H. Diverse molecular functions of aspartate β-hydroxylase in cancer (Review). Oncol. Rep. 2020, 44, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Hou, Y.; Wang, H.; Wang, K.; Xing, X.; Xia, Y.; Wan, X.; Li, J.; Jiao, B.; Liu, J.; et al. Hydroxylase Activity of ASPH Promotes Hepatocellular Carcinoma Metastasis Through Epithelial-to-Mesenchymal Transition Pathway. EBioMedicine 2018, 31, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Brewitz, L.; Tumber, A.; Pfeffer, I.; McDonough, M.A.; Schofield, C.J. Aspartate/asparagine-β-hydroxylase: A high-throughput mass spectrometric assay for discovery of small molecule inhibitors. Sci. Rep. 2020, 10, 8650. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhou, Y.; Lin, Q.; Huang, C.-K.; Zhang, S.; Carlson, R.I.; Ghanbari, H.; Sun, B.; Wands, J.R.; Dong, X. Bio-nanoparticle based therapeutic vaccine induces immunogenic response against triple negative breast cancer. Am. J. Cancer Res. 2021, 11, 4141–4174. [Google Scholar] [PubMed]
- Nagaoka, K.; Bai, X.; Ogawa, K.; Dong, X.; Zhang, S.; Zhou, Y.; Carlson, R.I.; Jiang, Z.-G.; Fuller, S.; Lebowitz, M.S.; et al. Anti-tumor activity of antibody drug conjugate targeting aspartate-β-hydroxylase in pancreatic ductal adenocarcinoma. Cancer Lett. 2019, 449, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Ragothaman, M.; Yoo, S.Y. Engineered Phage-Based Cancer Vaccines: Current Advances and Future Directions. Vaccines 2023, 11, 919. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, C.; Shi, G.; Xu, X.; Luo, X.; Zhang, Y.; Fu, J.; Chen, L.; Zeng, A. Dendritic cell-based vaccine targeting aspartate-β-hydroxylas represents a promising therapeutic strategy for HCC. Immunotherapy 2019, 11, 1399–1407. [Google Scholar] [CrossRef]
- Dahn, H.; Lawendel, J.S.; Hoegger, E.F.; Fischer, R.; Schenker, E. Über eine neue Herstellung aromatisch substituierter Reduktone. Cell. Mol. Life Sci. 1954, 10, 245–246. [Google Scholar] [CrossRef]
- Zheng, G.; Cox, T.; Tribbey, L.; Wang, G.Z.; Iacoban, P.; Booher, M.E.; Gabriel, G.J.; Zhou, L.; Bae, N.; Rowles, J.; et al. Synthesis of a FTO Inhibitor with Anticonvulsant Activity. ACS Chem. Neurosci. 2014, 5, 658–665. [Google Scholar] [CrossRef]
- Ogawa, K.; Lin, Q.; Li, L.; Bai, X.; Chen, X.; Chen, H.; Kong, R.; Wang, Y.; Zhu, H.; He, F.; et al. Aspartate β-hydroxylase promotes pancreatic ductal adenocarcinoma metastasis through activation of SRC signaling pathway. J. Hematol. Oncol. 2019, 12, 144. [Google Scholar] [CrossRef]
- Huang, C.-K.; Iwagami, Y.; Zou, J.; Casulli, S.; Lu, S.; Nagaoka, K.; Ji, C.; Ogawa, K.; Cao, K.Y.; Gao, J.-S.; et al. Aspartate beta-hydroxylase promotes cholangiocarcinoma progression by modulating RB1 phosphorylation. Cancer Lett. 2018, 429, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Benelli, R.; Costa, D.; Mastracci, L.; Grillo, F.; Olsen, M.J.; Barboro, P.; Poggi, A.; Ferrari, N. Aspartate-β-Hydroxylase: A Promising Target to Limit the Local Invasiveness of Colorectal Cancer. Cancers 2020, 12, 971. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Lin, Q.; Li, L.; Bai, X.; Chen, X.; Chen, H.; Kong, R.; Wang, Y.; Zhu, H.; He, F.; et al. Prometastatic secretome trafficking via exosomes initiates pancreatic cancer pulmonary metastasis. Cancer Lett. 2020, 481, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Swarnali, A.; Matrisian, L.; Welch, D.R.; Massagué, J. 18—Invasion and Metastasis. In The Mo-Lecular Basis of Cancer, 4th ed.; Mendelsohn, J., Gray, J.W., Howley, P.M., Israel, M.A., Thompson, C.B., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 269–284.e2. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Asif, P.J.; Longobardi, C.; Hahne, M.; Medema, J.P. The Role of Cancer-Associated Fibroblasts in Cancer Invasion and Metastasis. Cancers 2021, 13, 4720. [Google Scholar] [CrossRef] [PubMed]
- Attieh, Y.; Vignjevic, D.M. The hallmarks of CAFs in cancer invasion. Eur. J. Cell Biol. 2016, 95, 493–502. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Wang, X.; Xu, M.; Sheng, W. The role of cancer-associated fibroblasts in tumorigenesis of gastric cancer. Cell Death Dis. 2022, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Bienkowska, K.J.; Hanley, C.J.; Thomas, G.J. Cancer-Associated Fibroblasts in Oral Cancer: A Current Perspective on Function and Potential for Therapeutic Targeting. Front. Oral Health 2021, 2, 686337. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D.; Suchak, K.; Moutasim, K.A.; Vallath, S.; Hopper, C.; Jerjes, W.; Upile, T.; Kalavrezos, N.; Violette, S.M.; Weinreb, P.H.; et al. Stromal features are predictive of disease mortality in oral cancer patients. J. Pathol. 2011, 223, 470–481. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Vipparthi, K.; Thatikonda, V.; Arun, I.; Bhattacharjee, S.; Sharan, R.; Arun, P.; Singh, S. A subtype of cancer-associated fibroblasts with lower expression of alpha-smooth muscle actin suppresses stemness through BMP4 in oral carcinoma. Oncogenesis 2018, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Zhou, X.; Benicky, J.; Panigrahi, A.; Aljuhani, R.; Liu, J.; Ailles, L.; Pomin, V.H.; Wang, Z.; Goldman, R. Heparan-6-O-Endosulfatase 2 Promotes Invasiveness of Head and Neck Squamous Carcinoma Cell Lines in Co-Cultures with Cancer-Associated Fibroblasts. Cancers 2023, 15, 5168. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Zizzari, I.G.; Scagnoli, S.; Pomati, G.; Strigari, L.; Cirillo, A.; Cerbelli, B.; Di Filippo, A.; Napoletano, C.; Scirocchi, F.; et al. The Role of Soluble LAG3 and Soluble Immune Checkpoints Profile in Advanced Head and Neck Cancer: A Pilot Study. J. Pers. Med. 2021, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Kim, S.S.; Pang, J.; Gold, K.A.; Gutkind, J.S.; Califano, J.A.; Mell, L.K.; Cohen, E.E.; Sharabi, A.B. Immune Modulation of Head and Neck Squamous Cell Carcinoma and the Tumor Microenvironment by Conventional Therapeutics. Clin. Cancer Res. 2019, 25, 4211–4223. [Google Scholar] [CrossRef]
- Shin, D.M.; Khuri, F.R. Advances in the management of recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck 2011, 35, 443–453. [Google Scholar] [CrossRef]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, J.W.; Steuer, C.E.; Owonikoko, T.K.; Saba, N.F. An update on the immune landscape in lung and head and neck cancers. CA A Cancer J. Clin. 2020, 70, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.-T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Kraus, D.H.; Ganly, I.; Lee, N.Y.; Shah, J.P.; Morris, L.G.T. Decision making in the management of recurrent head and neck cancer. Head Neck 2013, 36, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Farrag, M.; Dwivedi, R.; Sharma, P.; Kumar, D.; Tandon, R.; Pomin, V.H. Structural requirements of Holothuria floridana fucosylated chondroitin sulfate oligosaccharides in anti-SARS-CoV-2 and anticoagulant activities. PLoS ONE 2023, 18, e0285539. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.A.; Zhou, X.; Wu, J.; Wang, Y.; Makambi, K.; Kallakury, B.V.; Singer, M.S.; Rosen, S.D.; Davidson, B.; Goldman, R. Expression of the extracellular sulfatase SULF2 is associated with squamous cell carcinoma of the head and neck. Oncotarget 2016, 7, 43177–43187. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ahn, J.; Edwards, N.J.; Benicky, J.; Rozeboom, A.M.; Davidson, B.; Karamboulas, C.; Nixon, K.C.J.; Ailles, L.; Goldman, R. Extracellular Heparan 6-O-Endosulfatases SULF1 and SULF2 in Head and Neck Squamous Cell Carcinoma and Other Malignancies. Cancers 2022, 14, 5553. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ahn, J.; Raghunathan, R.; Kallakury, B.V.; Davidson, B.; Kennedy, Z.B.; Zaia, J.; Goldman, R. Expression of the Extracellular Sulfatase SULF2 Affects Survival of Head and Neck Squamous Cell Carcinoma Patients. Front. Oncol. 2021, 10, 582827. [Google Scholar] [CrossRef] [PubMed]
- Benicky, J.; Sanda, M.; Panigrahi, A.; Liu, J.; Wang, Z.; Pagadala, V.; Su, G.; Goldman, R. A 6-O-endosulfatase activity assay based on synthetic heparan sulfate oligomers. Glycobiology 2023, 33, 384–395. [Google Scholar] [CrossRef]
- Ihemelandu, C.; Naeem, A.; Parasido, E.; Berry, D.; Chaldekas, K.; Harris, B.T.; Rodriguez, O.; Albanese, C. Clinicopathologic and prognostic significance of LGR5, a cancer stem cell marker in patients with colorectal cancer. Color. Cancer 2019, 8, CRC11. [Google Scholar] [CrossRef]

| Variable | Parameter | No. of Cases | Percentage % |
|---|---|---|---|
| Gender | Male | 92 | 59 |
| Female | 63 | 41 | |
| Age | <60 y | 66 | 43 |
| ≥60 y | 86 | 56 | |
| Unknown | 3 | 2 | |
| Race | CA | 115 | 74 |
| AA | 11 | 7 | |
| Asian | 2 | 1 | |
| Other | 11 | 7 | |
| Unknown | 16 | 10 | |
| T-Stage | Early (T1, T2) | 107 | 69 |
| Late (T3, T4) | 46 | 30 | |
| Unknown | 2 | 1 | |
| Node | N+ | 74 | 48 |
| N- | 79 | 51 | |
| Unknown | 2 | 1 | |
| M-Stage | M+ | 1 | 1 |
| M- | 152 | 98 | |
| Unknown | 2 | 1 | |
| Disease Recurrence | Yes | 64 | 41 |
| No | 72 | 47 | |
| Unknown | 19 | 12 | |
| Margin | Positive | 18 | 11 |
| Negative | 69 | 45 | |
| Close | 58 | 37 | |
| Unknown | 10 | 7 | |
| Grade | w | 15 | 10 |
| m | 92 | 59 | |
| p | 48 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, P.; Zhou, X.; Galli, S.; Davidson, B.; Zhang, L.; Ahn, J.; Aljuhani, R.; Benicky, J.; Ailles, L.; Pomin, V.H.; et al. Aspartate β-Hydroxylase Is Upregulated in Head and Neck Squamous Cell Carcinoma and Regulates Invasiveness in Cancer Cell Models. Int. J. Mol. Sci. 2024, 25, 4998. https://doi.org/10.3390/ijms25094998
Mukherjee P, Zhou X, Galli S, Davidson B, Zhang L, Ahn J, Aljuhani R, Benicky J, Ailles L, Pomin VH, et al. Aspartate β-Hydroxylase Is Upregulated in Head and Neck Squamous Cell Carcinoma and Regulates Invasiveness in Cancer Cell Models. International Journal of Molecular Sciences. 2024; 25(9):4998. https://doi.org/10.3390/ijms25094998
Chicago/Turabian StyleMukherjee, Pritha, Xin Zhou, Susana Galli, Bruce Davidson, Lihua Zhang, Jaeil Ahn, Reem Aljuhani, Julius Benicky, Laurie Ailles, Vitor H. Pomin, and et al. 2024. "Aspartate β-Hydroxylase Is Upregulated in Head and Neck Squamous Cell Carcinoma and Regulates Invasiveness in Cancer Cell Models" International Journal of Molecular Sciences 25, no. 9: 4998. https://doi.org/10.3390/ijms25094998
APA StyleMukherjee, P., Zhou, X., Galli, S., Davidson, B., Zhang, L., Ahn, J., Aljuhani, R., Benicky, J., Ailles, L., Pomin, V. H., Olsen, M., & Goldman, R. (2024). Aspartate β-Hydroxylase Is Upregulated in Head and Neck Squamous Cell Carcinoma and Regulates Invasiveness in Cancer Cell Models. International Journal of Molecular Sciences, 25(9), 4998. https://doi.org/10.3390/ijms25094998






