Abstract
Alzheimer’s disease (AD) is characterized by amyloid beta (Aβ) buildup and neuronal degeneration. An association between low serum vitamin D levels and an increased risk of AD has been reported in several epidemiological studies. Calcitriol (1,25-dihydroxycholecalciferol) is the active form of vitamin D, and is generated in the kidney and many other tissues/organs, including the brain. It is a steroid hormone that regulates important functions like calcium/phosphorous levels, bone mineralization, and immunomodulation, indicating its broader systemic significance. In addition, calcitriol confers neuroprotection by mitigating oxidative stress and neuroinflammation, promoting the clearance of Aβ, myelin formation, neurogenesis, neurotransmission, and autophagy. The receptors to which calcitriol binds (vitamin D receptors; VDRs) to exert its effects are distributed over many organs and tissues, representing other significant roles of calcitriol beyond sustaining bone health. The biological effects of calcitriol are manifested through genomic (classical) and non-genomic actions through different pathways. The first is a slow genomic effect involving nuclear VDR directly affecting gene transcription. The association of AD with VDR gene polymorphisms relies on the changes in vitamin D consumption, which lowers VDR expression, protein stability, and binding affinity. It leads to the altered expression of genes involved in the neuroprotective effects of calcitriol. This review summarizes the neuroprotective mechanism of calcitriol and the role of VDR polymorphisms in AD, and might help develop potential therapeutic strategies and markers for AD in the future.
1. Introduction
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disease (ND) that results in progressive damage to the structure and function of neurons. Globally, over 400 million people are affected by AD and it is the main cause of dementia in the elderly population [1]. The pathogenesis of AD is multifactorial and is associated with the deposition of amyloid-β (Aβ) plaques and neurofibrillary tangles (NFTs), neuronal loss accompanied with increased oxidative stress, neuroinflammation, and synaptic changes [2,3]. The available Food and Drug Administration (FDA)-approved drugs (Donepezil, Galantamine, Rivastigmine, Memantine, Aduhelm) provide only symptomatic treatment [4] but a treatment to prevent the disease is lacking. The development of an effective, safe, and low-cost treatment would undoubtedly have a great global impact.
Various studies have shown an association of vitamin D deficiency with an increased risk of infections, including COVID-19; asthma; cardiovascular diseases; autoimmune diseases; hyperlipidemia; diabetes; and cancer [5,6,7,8,9]. The vitamin D status is evaluated from serum calcifediol levels (longer half-life compared to other vitamin D metabolites) and a value > 30 ng/mL is considered to be healthy. The recommended dietary allowance (RDA) of vitamin D for different age groups is 400 IU (0–12 months); 600 IU (up to 70 years), and 800 IU (over 70 years) [10]. Calcitriol, the bioactive form of vitamin D, is a fat-soluble vitamin with antioxidant activity, maintains calcium and phosphorous homeostasis, and strengthens immune function. Studies indicate a novel link between calcitriol and mitochondrial bioenergetics involving calcium channels [11]. Calcitriol displays its effect by binding to vitamin D receptors (VDRs), which are present in various tissues and organs such as the skin, brain, parathyroid, skeletal muscles, heart muscles, pancreas, pituitary, ovaries, testes, and blood cells, but not the kidney and bones [12]. The diverse locations of VDRs indicate other important functions of calcitriol in the body. The research in the past two decades has identified calcitriol as a likely neurosteroid [13] and its possible link with psychiatric disorders and [14,15] NDs was explored [16,17,18]. The brain is capable of synthesizing and receiving calcitriol, which is speculated to support synaptic plasticity and neurotransmission [19,20], and its role in neurogenesis, preventing neuroinflammation, improving cognition, and the clearance of amyloid plaques has been studied [21,22,23,24]. It is also known to protect the CNS from immunopathogenic diseases and inflammasome activation. Early intervention with calcitriol ameliorates the activation of local microglia/macrophage, preventing neuroinflammation [24]. Calcitriol improves Aβ clearance by stimulating phagocytosis along with reduced monoamine oxidase B (MAO-B) expression [25]. Calcitriol is known to be present after brain injury and protects the integrity of the blood–brain barrier (BBB) following acute ischemic stroke [26]. Calcitriol possesses good antioxidant activity, reduces cell apoptosis, and increases the production of neurotrophic factors [nerve growth factors (NGFs) and glial cell line derived neurotrophic factors (GDNFs), neurotrophin-3, Brain-derived neurotrophic factors (BDNFs)] required for the survival and growth of neurons, which ultimately improves cognition [26,27].
In the present review, we provide an overview of the neuroprotective mechanism of calcitriol and VDR polymorphisms in AD. Understanding the molecular mechanisms and the role of genetic variation could be important for developing therapies for NDs in the future.
2. Methods
The present work gives an inclusive overview of the published scientific research available on various databases (PubMed, Google Scholar, and Science Direct) up until January 2024. The search terms used were “calcitriol” or “vitamin D” or “vitamin D receptor” or “vitamin D transport” with the filter “brain”, “neuroprotection”, “Alzheimer’s disease”, “VDR polymorphism”, and “English”. Exclusion criteria: the papers not in the English language were not included.
3. Synthesis and Activation of Vitamin D
Vitamin D prohormone (calciferol; sunshine vitamin) is a collective term for vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D3 is mainly synthesized in the skin through the photochemical action of solar ultraviolet type B (UVB) radiation on 7-dehydrocholesterol (7-DHC) (Figure 1). In contrast, the body is unable to produce vitamin D2 and can only be consumed through the consumption of plants in the diet. Vitamin D2 has a double bond between C22 and C23 and a methyl group on C24, which is absent in vitamin D3. This structural difference affects metabolic activity and thus vitamin D3 exhibits a better affinity for vitamin D binding protein (VDBP) and vitamin D receptor (VDR) [28]. Vitamin D is biologically inert and requires two hydroxylation steps for its activation. Initially, it is hydroxylated in the liver by cytochrome P450 enzyme (CYP2R1; 25-Hydroxylase) to calcifediol (25-hydroxy vitamin D3; [25(OH)D3]) and further metabolized to calcitriol (1,25-dihydroxy vitamin D3; 1,25(OH)2D3) by CYP27B1 (1α-Hydroxylase) in the kidneys and other tissues/organs, including the brain.
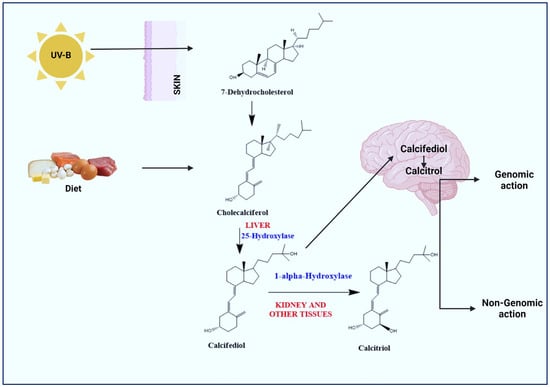
Figure 1.
Activation of vitamin D to calcitriol. Exposure of skin to sunlight (UV-B) results in conversion of pro-vitamin D3 (7-dehydrocholesterol) to pre-vitamin D3 (Cholecalciferol). Diet/vitamin D supplements bind to vitamin D-binding proteins and are transported to liver and hydroxylated by 25-Hydroxylase to calcifediol. In the kidneys and other tissues/organs including the brain, calcifediol is hydroxylated to calcitriol (active form of vitamin D) by 1α-hydroxylase. Calcitriol affects different targets through genomic and non-genomic pathways.
4. Transport of Vitamin D
The major circulating form of vitamin D is serum calcifediol (half-life of 15–20 days), which is in equilibrium with the level of vitamin D stored in muscle and adipose tissues. The plasma or serum calcifediol concentration is inversely related to the risk of vascular dementia and AD in the elderly [29]. Patients with calcifediol (<10 ng/mL) tend to score lower in the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA), and have an increased risk of developing cognitive impairment calculated by the Clinical Dementia Rating (CDR) scale [30]. A meta-analysis on cohort studies using the prediction interval (PI) to describe the heterogeneity described an inverse relation between the calcifediol level and the risk of dementia and AD, consistent with a linear dose–response relationship. Additionally, the data indicated a decrease in dementia (5%) and AD (7%) with each increase of 10 nmol/L of calcifediol [31]. In the past, few other similar studies have shown an inverse relation between AD and low calcifediol but a causal association could not be established either due to the small sample groups and the method of quantifying the heterogeneity or restricting the categories of the calcifediol level [32,33,34].
As a result, the serum calcifediol is considered a marker to evaluate the vitamin D status in the body. Albumin helps in the transport of some vitamin D metabolites but the majority (~85%) are transported to various tissues by binding to VDBP, and among them, calcifediol has a stronger binding affinity to VDBP compared to the others [35]. When the levels of vitamin D exceed the tolerated levels in the body, it is metabolized by CYP24A1 to inactive metabolites, i.e., 1,24,25(OH)D3, calcitroic acid, and 24,25(OH)2D3. Bioavailable vitamin D is the sum of the free and albumin-bound forms, which constitute around 15% in a healthy person [36]. VDBP is a highly polymorphic serum α2-globulin (52–59 kDa) widely distributed in various tissues. It performs diverse functions besides binding to vitamin D, like scavenging endotoxins and actin, binding fatty acids, and mediating the immune system [37]. The VDBP levels were reported to be altered in disease conditions [38,39,40] that affect the total level of vitamin D.
For most cells, it is the unbound calcifediol that enters cells (free hormone hypothe-sis); however, in some cases (kidney, parathyroid gland, and placenta), VDBP-bound calcifediol is transported in the cell via a megalin/cubilin complex [41]. An association of single nucleotide polymorphisms (SNPs) of megalin and VDBP with AD and PD has been reported, respectively [42,43].
5. Distribution of VDR in Brain
VDR belongs to the zinc finger steroid hormone nuclear receptor family and is widely distributed in the body [44]. After Sutherland et al. [45] reported the expression of VDR in AD brains, various studies reported VDR expression in neuroblastoma cell lines [46] and in the developing and adult brain in various mammalian species [47,48,49]. The increased expression of VDR with gestational age indicates the importance of vitamin D in maintaining normal brain development [47]. Vitamin D deficiency in pregnancy has been associated with various neurological issues like autism, attention deficit hyperactivity disorder (ADHD), and schizophrenia in infants [15,50,51,52,53]. Studies have established the presence of VDR and CYP27B1 in neurons and glial cells and CYP24A1 in astrocytes was established [13]. In the hippocampus, VDR is present exclusively in the CA1 and CA2 regions while a minor amount is present in CA3. VDR is concentrated in the nucleus whereas CYP27B1 is dispersed all over the cytoplasm. Both VDR and CYP27B1 are present in equivalent amounts in most of the brain regions, with the maximum amount reported in the hypothalamus and substantia nigra. However, CYP27B1 is exclusively present in Purkinje neurons (cerebellum) and substantia innominata neurons (basal forebrain) [13].
The key forms of vitamin D [calcifediol, calcitriol and 24,25(OH)2D3] have the ability to cross the BBB and exist in human cerebrospinal fluid (CSF) [54]. Moreover, the enzymes for synthesizing (CYP27B1) and catabolizing (CYP24A1) calcifediol are present in the brain, suggesting in situ synthesis of calcitriol and autoregulated elimination in the brain. The calcitriol levels in the brain correlate with the plasma calcifediol [55] and remain unaffected by vitamin D supplementation, again proving the local synthesis of calcitriol in the brain [56]. Recently, the Ultra-Pressure LC-Tandem Mass Spectra (UPLC-MS) technique was used to evaluate vitamin D metabolites in the human brain. All the examined regions contained calcifediol, with the corpus callosum being the richest in calcifediol (334 pg/g). On the other hand, low levels of calcitriol were spotted in the prefrontal (30 pg/g) and middle frontal cortices (35 pg/g) only [57].
6. Molecular Mechanism of Action of Calcitriol
The active form of vitamin D is calcitriol, which has a crucial role in brain development and neuroprotection. Calcitriol exerts its action by binding to VDR through genomic (classical) pathways (slow response) or non-genomic pathways (rapid response) (Figure 2).
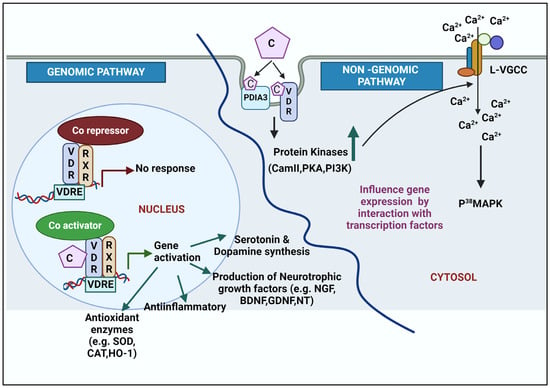
Figure 2.
A schematic for genomic and non-genomic action of calcitriol in brain. Genomic pathways occur in the nucleus. Binding of calcitriol to the VDR/RXR complex removes the co-repressors and interacts with co-activators on the VDREs located on regulatory regions to promote gene expression. The non-genomic action takes place in the cytosol and is initiated by the binding of calcitriol to VDR, PDIA3, or both. The binding activates protein kinases, which facilitates influx of calcium through L-VGCC. Intracellular calcium activates p38MAPK to further modulate downstream signaling. Abbreviations: VDR: vitamin D receptor; RXR: retinoic acid receptor; VDRE: vitamin D response element; L-VGCC: L-type voltage-gated calcium channel; PDIA3: protein disulphide isomerase family member 3; CaM II: Ca2+/calmodulin-dependent protein kinase; PKA: protein kinase A; PI3K: phosphatidylinositol-3-kinase, p38MAPK: mitogen-associated kinase; C: calcitriol; Ca2+: calcium; SOD: superoxide dismutase; CAT: catalase; HO-1: heme oxygenase; NGF: neurotrophic growth factors; BDNF: brain-derived neurotrophic factor; GDNF: glial cell-derived neurotrophic factor; NT: neurotrophin.
6.1. Genomic Action of Calcitriol
The endocrine action of calcitriol is mediated through VDR. Calcitriol has a high binding affinity (~1000 times) for VDR as compared to calcifediol [58] due to the interactions of its three hydroxyl groups with polar amino acids in the ligand binding pocket. Yet, the 1000-fold higher serum concentrations of calcifediol (50–250 nM) compared to calcitriol compensate for its effective binding to VDR [59].
The binding of calcitriol to the VDR leads to the formation of a heterodimeric complex with retinoic acid X receptor (RXR). The protein importin-β, and its ligand, vitamin A, help in the transport of RXR to the nucleus, while importin-α assists with the transport of VDR to the nucleus, and this process is significantly improved by the mediation of calcitriol. The complex is then transported into the nucleus where it binds to vitamin D response elements (VDREs) [60] with the release of co-repressors (nuclear receptor co-repressor 2/silencing mediator of retinoic acid and thyroid hormone receptor: NcoR2/SMART) and recruitment of coactivators, which increase histone acetylation (steroid receptor co-activator: SRC1) and modify chromatin (lysine demethylase 6B) to promote the expression of several genes involved in bone calcium homeostasis [alkaline phosphatase (ALP), osteopontin (SPP1), stanniocalcin 1 (STC1), Transient receptor potential vanilloid type 6 (TRPV6)], parathyroid hormone (PTH), vitamin D hydroxylases (CYP27A, CYP27B1, CYP24), calcitriol-responsive endobiotic/xenobiotic metabolizing enzyme (CYP3A4), and metallothionein 2 (MT2) [61,62,63]. Such activated genes may further modulate the action of other genes as a secondary genomic response [64]. Genomic regulation has epigenetic effects as it modifies the expression of enzymes involved in methylation and acetylation [65]. Additionally, micro-RNA (miR) expression that controls post-transcriptional gene expression and silencing is also controlled by this mechanism [66]. In short, calcitriol, VDREs, and modulators bound to the heterodimer-RXR are responsible for the physiological, genetic, and cell/tissue specificities, respectively. Lastly, the VDR gene product exerts a biological response [67].
In the brain, calcitriol has been reported to exert multifaceted neuroprotection by increasing the expression of glial-derived neurotrophic factor (GDNF) in the cortex [68] and striatum [69], and the enzyme for the synthesis of dopamine, i.e., tyrosine hydroxylase (TH), and N-cadherin (neural adhesion molecule with role in neurogenesis), which in turn increase dopaminergic (DA) neurons [70,71]. To maintain ideal neurotransmission, DA neurons stimulate the expression of DA-catabolizing enzymes (catechol-O-methyltransferase: COMT and monoamine oxidase A (MAOA) by negative feedback [72]. Hence, calcitriol could be a promising therapy in Parkinson’s disease (PD). Calcitriol was reported to increase serotonin neuronal cells by increasing the expression of the enzyme (tryptophan hydroxylase-2: TPH2) involved in its synthesis, and suppressing the enzyme involved in its catabolism (MAOA) and the serotonin reuptake transporter (SERT) [73]. Calcitriol also downregulated MerTK expression [74], reducing the phagocytosis of myelin and apoptotic T cells, which is beneficial in the treatment of multiple sclerosis. Amyloid beta has been known to overturn the expression of VDR in cortical neurons by modulating nerve growth factor (NGF) synthesis and Ca2+ homeostasis [75].
6.2. Non-Genomic Action of Calcitriol
The non-genomic pathways are rapid compared to the genomic response and are mediated through specific membrane receptors like VDR and PDIA3 (protein disulfide isomerase family A member 3) (Figure 2). VDR interacts with various target proteins such as β-catenin, c-Jun, STAT1, inhibitor of nuclear factor-κB (IκB) kinase (IKK), cAMP response element-binding protein, and Runt-related transcription factor 1 (RunX 1) in the non-genomic response in vitro [76,77].
The regulation of Ca2+ channels, activation of protein kinases (protein kinase A: PKA; protein kinase C: PKC; mitogen-activated protein kinase: MAPK; Phosphoinositide 3- kinase: PI3K; Ca2+/calmodulin-dependent kinase II: CaMKII) and phospholipase (Phospholipase A2: PLA2; Phospholipase C; PLC) are some of the non-genomic signal transduction events triggered by calcitriol. These actions do not require gene transcription. Additionally, calcitriol, via the non-genomic pathway, indirectly influences the expression of various genes by interacting with multiple transcription factors (like Aryl hydrocarbon Receptor: AhR; Nuclear factor kappa: NF-κB; Nuclear factor erythroid-2-related factor 2: Nrf2, RAR-related orphan receptor alpha and gamma: RORα, RORγ; Signal transducer and activator of transcription 3: STAT3) [78].
In the microglia, calcitriol exerts the neuroinflammatory response mostly through a non-genomic mechanism by inhibiting the translocation of NF-κB and phosphorylation of extracellular signal-related kinase (ERK) [79]. Calcitriol and calcifediol also upregulate the expression of the anti-inflammatory cytokine (IL-10), which in turn induces suppressor of cytokine signaling (SOCS) to downregulate pro-inflammatory cytokines [80]. Calcitriol supplementation promoted cell proliferation and reduced senescence in [1α(OH)ase−/−] mice by upregulating Nrf2, reducing oxidative stress, and inactivating senescence-promoting genes (p53/p21 and p16/pRb) [81].
7. Genetic Variants in VDR and Risk of Late-Onset AD (LOAD)
The apolipoprotein E (APOE) gene, present on chromosome 19, has been recognized as the major risk gene for LOAD, as ~50% of patients diagnosed with LOAD have the APOEε4 gene [82]. This gene causes neurodegeneration by affecting the neuronal cytoskeleton, inhibiting neurite outgrowth, and stimulating tau phosphorylation [83]. Other genes, related to cholesterol biosynthesis (CLU, ABCA7), endocytosis, synaptic function (CD2AP, BIN1, PICALM), and inflammation and the immune response (CR1, CD33, EPHA1, TREM2, MS4A) have been identified through genome-wide association studies (GWASs) [84].
In the search for additional genetic risk factors for LOAD, studies have reported the association of VDR gene polymorphisms and cognitive decline in NDs [85,86,87]. The VDR gene (100 kb) is located on chromosome 12 (12q13–12q14) and consists of nine exons and eight introns. Exons 2 and 3 encode the DNA binding site, while exons 4 to 9 encode the ligand binding site. This locus was reported to be a vulnerable locus linked to LOAD [88]. According to GWASs, VDR is among the most likely risk genes for developing AD [89]. The association of AD with VDR gene polymorphisms suggests a potential negative impact on the neuroprotective effect of calcitriol. Changes in vitamin D consumption are said to lower VDR expression, protein stability, and binding affinity, leading to the altered expression of genes involved in neuroprotection.
Several single nucleotide polymorphisms (SNPs) have been identified in the VDR gene with roles in various disease conditions. But VDR gene polymorphisms in most cases do not affect VDR function, as they do not result in an amino acid change. The main VDR SNPs studied in relation to AD are ApaI, TaqI, BsmI, and FokI, which lead to changes in vitamin D utilization, leading to increased neurodegeneration [90]. ApaI (rs7975232; intron 8; G to T polymorphism), TaqI (rs731236; exon 9; T to C polymorphism), and BsmI (rs1544410; intron 8; A to G polymorphism) are 3′ UTR polymorphisms that increase mRNA stability. The FokI (rs2228570; exon 2; C to T polymorphism) polymorphism alters the gene’s initiation sites and modifies the protein’s structure by extending its length by three amino acids. As a result, two VDR variants exist, and the long version of VDR is called M1 (methionine at first position; T-allele or the f allele), while the short form is referred to as M4 (methionine at fourth position; C-allele or F allele). The Cdx2 (rs11568820; promoter region; A to G polymorphism) polymorphism might affect the transcriptional activity [91], whereas Tru9I (rs757343; intron 8; G to A polymorphism) is associated with serum vitamin D levels [92] (Figure 3).
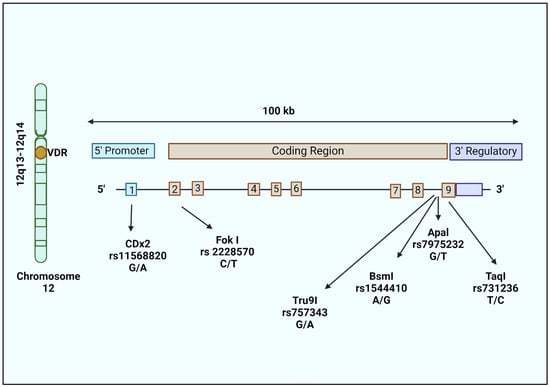
Figure 3.
Structure of the VDR gene and position of polymorphisms studied in AD. The VDR gene is located on chromosome 12 (12q13–12q14). The VDR gene is 100 kb and divided into 8 introns and 9 exons. The first exon contains the gene promoter, exon 2–3 code for the DNA binding domain, and exon 6–9 for the ligand binding domain. Widely studied polymorphisms in AD include Fok-I (rs10735810), Apa-I (rs7975232), Bsm-I (rs1544410), Taq-I (rs731236), and Tru9I (rs757343).
In the aged population (over 75 years old) Apa1 polymorphisms (T allele) and Taq1 polymorphisms (G allele), were found to be associated with AD and also displayed interactions with genes regulating inflammation (interleukin 10: IL-10; dopamine-β hydroxylase: DBH) [93]. The ApaI polymorphism (A allele) was associated with a 30% lower risk of AD in Polish and British populations [94], while only the TaqI polymorphism (T allele) was significantly associated with the risk of AD in a Korean population. However, in an Iranian population, the ApaI and TaqI polymorphisms were not associated with the risk of LOAD [95]. ApaI (C allele) and TaqI (T allele) were more frequent in individuals with mild cognitive impairment (MCI) in a Chilean population. In addition, these polymorphisms decreased the expression of p-glycoprotein (p-gp), the transporter of β-amyloid peptide (Aβ) [96]. Another contrasting result was obtained for the TaqI C allele, which increased the AD risk in Northwestern European Caucasians (NECs) [93], but exerted a protective effect in a Southeastern European Caucasian (SEC) population by decreasing the AD risk by 46% [97], and caused no effect in an elderly Brazilian population [98]. Another study’s results showed that the TaqI polymorphism (dominant and homozygous) and BsmI (recessive) and FokI polymorphisms (heterozygous) were linked with increased AD risk in Caucasian and Asian populations, respectively [87]. A recent study provided a statistical indication that the ApaI and BsmI polymorphisms were connected to a risk of MCI, while the TaqI polymorphisms may have been associated with AD risk [87]. In another study, the risk allele of CDX2 lowered VDR promoter activity in Neuro2A cells [99] and a strong association between SNP CDX2 and an LOAD patient group was observed. The contradictory results obtained from different populations indicate that both genetic and non-genetic factors such as ethnicity, climate, and environment modify the expression and response of VDR [87]. This specifies that external factors impacting vitamin D intake could potentially compromise the efficacy of its neuroprotective mechanism.
The studies to identify a potential link between VDR SNP haplotypes are limited. Strong linkage disequilibrium (LD) was observed in BsmI, ApaI, and TaqI polymorphisms that described five haplotypes (Table 1). The frequency of haplotype 1 (CCA) and haplotype 2 (TAG) was the highest (45.5% and 42.4%, respectively) of the other three in an elderly (over 85 years) population [85]. Gezen-Ak et al. [89] found no significant difference between AD vs. controls when the BsmI, Tru9I, and FokI polymorphic sites were compared. A haplotype analysis carried out with additional SNPs indicated a significantly higher frequency of the “TaubF” haplotype (corresponds to alleles of TaqI, ApaI, Tru9I, BsmI, and FokI, respectively) in the AD patient group, suggesting that this haplotype is a risk factor for AD. The BsmI, ApaI, and TaqI polymorphisms, located in the 3′-UTR, were identified as risk haplotypes associated with age-related cognitive decline [85]. In yet another cohort study on SECs, the TAC (TaqI, BsmI, FokI) and TA (TaqI, BsmI) haplotypes were associated with a nearly 6% and 8% increase in the LOAD risk, respectively. On the other hand, the CAC (TaqI, BsmI and FokI) haplotype was protective with a 53% lesser risk of developing AD. Additionally, SEC female TAC/TC carriers carry a greater risk (approximately 9 to 14%) of developing AD, suggesting that this haplotype affects vitamin D utilization [97].

Table 1.
VDR haplotypes associated with AD.
8. Conclusions
Various in vitro, in vivo, and epidemiological studies have established that vitamin D (calcitriol) is required for healthy brain function and its deficiency during pregnancy might result in several neurological conditions in newborns. The association of circulating vitamin D levels and AD risk has been proven by Mendelian randomization studies in large datasets [100], which provide a potential avenue for understanding and preventing the disease. In AD, calcitriol exerts multi-targeted neuroprotection via both genomic and non-genomic actions, resulting in Aβ clearance, the promotion of neurogenesis and production of neurotrophic factors, a decrease in neuroinflammation and oxidative stress, the downregulation of the expression of L-type calcium channels, and improved cognition [25,101,102]. It is possible that calcitriol’s therapeutic effects will take longer to manifest because of the slow effects of the genomic pathways; thus, it could be used as a preventive approach in AD. Nevertheless, controversial outcomes have also been reported in some studies [103,104,105]. The contradictory results might stem from diverse dosages, the duration of treatment, the age/sex of the patient, the population size, and the vitamin D level of each patient under study. Thus, clinical trials need to be planned intricately, considering these variables.
Vitamin D from foods and exposure to sunlight is not adequate in the majority of cases, and hence, vitamin supplementation is required. Studies have suggested a positive effect of vitamin D supplementation, especially in people with severe vitamin D deficiency compared to vitamin D-replete people, and that a vitamin D intake ≥ 4000 IU/day conveys some health risks [106]. Clinical trials on vitamin D supplementation and cognition have resulted in conflicting conclusions [107,108]. Clinical trials have varied in terms of the formulation (cholecalciferol/ergocalciferol/calcium–vitamin D) of vitamin D [109,110,111]. Cholecalciferol supplementation was found to be more effective [112] and the use of calcium in the formulation increased intestinal calcium absorption [113]. Moreover, it is important to study whether low serum calcifediol is the result of the disease or is causing the disease.
The VDR gene is regulated by genetic and environmental factors and the response to vitamin D supplementation could be affected by VDR polymorphisms, e.g., the FF genotype of VDR Fok I is associated with a better response to vitamin D supplementation [114]. Association studies on VDR polymorphisms and the risk of AD might be helpful in designing custom-made methods for treating NDs like AD. The VDR polymorphisms ApaI and BsmI predict MCI, while TaqI indicates a risk of AD, with population differences. As the vitamin D supplementation requirement varies based on the VDR polymorphism, this presents a possible limitation, as the effectiveness of the treatment may depend on genetic factors, potentially limiting its applicability across different individuals. Therefore, further research is needed to confirm these associations, particularly gene-gene interactions and gene-environment interactions, and interactions with other confounding factors should be considered.
Author Contributions
Conceptualization, N.S.; writing—original draft preparation, N.S.; writing—review and editing, N.S., S.P.J. and S.S.A.A.; funding acquisition, S.S.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: RS-2023-00251396 and 2021R1A6A1A03038996) and GCU-202008480012.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimer’s Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.A.; Sharma, N.; An, S.S.A. Phyto-carbazole alkaloids from the rutaceae family as potential protective agents against neurodegenerative diseases. Antioxidants 2022, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M.; Shin, E.-A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, M.; Wang, C.; Xiao, Y.; An, T.; Zou, M.; Cheng, G. Association between vitamin D status and asthma control: A meta-analysis of randomized trials. Respir. Med. 2019, 150, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Demir, F.; Aygun, H. Vitamin D deficiency is associated with COVID-19 positivity and severity of the disease. J. Med. Virol. 2021, 93, 2992–2999. [Google Scholar] [CrossRef] [PubMed]
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef]
- Dietary Supplement Fact Sheets-Vitamin D. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 5 January 2024).
- Olszewska, A.M.; Sieradzan, A.K.; Bednarczyk, P.; Szewczyk, A.; Żmijewski, M.A. Mitochondrial potassium channels: A novel calcitriol target. Cell. Mol. Biol. Lett. 2022, 27, 3. [Google Scholar] [CrossRef]
- Simpson, R.U.; Weishaar, R. Newly discovered activities for calcitriol (1,25-dihydroxyvitamin D3): Implications for future pharmacological use. Bioessays 1986, 4, 65–70. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Cannell, J.J. Autism and vitamin D. Med. Hypotheses 2008, 70, 750–759. [Google Scholar] [CrossRef]
- McGrath, J. Hypothesis: Is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr. Res. 1999, 40, 173–177. [Google Scholar] [CrossRef]
- Darwish, H.; Haddad, R.; Osman, S.; Ghassan, S.; Yamout, B.; Tamim, H.; Khoury, S. Effect of Vitamin D Replacement on Cognition in Multiple Sclerosis Patients. Sci. Rep. 2017, 7, 45926. [Google Scholar] [CrossRef]
- Hongell, K.; Silva, D.G.; Ritter, S.; Meier, D.P.; Soilu-Hänninen, M. Efficacy and safety outcomes in vitamin D supplement users in the fingolimod phase 3 trials. J. Neurol. 2018, 265, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Nakajima-Ohyama, K.C.; Tansho, K.; Tanimukai, H. An alarm on vitamin D therapy for Alzheimer’s disease patients: A case with Alzheimer’s disease whose symptoms were exacerbated under chronic use of eldecalcitol. Psychogeriatrics 2022, 22, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Agnello, L.; Bellia, C.; Iacolino, G.; Scazzone, C.; Lo Sasso, B.; Ciaccio, M. Non-skeletal activities of vitamin D: From physiology to brain pathology. Medicina 2019, 55, 341. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Pelekanos, M.; Liu, P.-Y.; Burne, T.; McGrath, J.; Eyles, D. The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience 2013, 236, 77–87. [Google Scholar] [CrossRef]
- Annweiler, C. Vitamin D in dementia prevention. Ann. N. Y. Acad. Sci. 2016, 1367, 57–63. [Google Scholar] [CrossRef]
- Di Somma, C.; Scarano, E.; Barrea, L.; Zhukouskaya, V.V.; Savastano, S.; Mele, C.; Scacchi, M.; Aimaretti, G.; Colao, A.; Marzullo, P. Vitamin D and Neurological Diseases: An Endocrine View. Int. J. Mol. Sci. 2017, 18, 2482. [Google Scholar] [CrossRef]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J. Neuroimmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef]
- De Oliveira, L.R.C.; Mimura, L.A.N.; Fraga-Silva, T.F.D.C.; Ishikawa, L.L.W.; Fernandes, A.A.H.; Zorzella-Pezavento, S.F.G.; Sartori, A. Calcitriol prevents neuroinflammation and reduces blood-brain barrier disruption and local macrophage/microglia activation. Front. Pharmacol. 2020, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, M.; Lee, E.; Jung, J.; Kim, T. The Role of Vitamin D in Alzheimer’s Disease: A Transcriptional Regulator of Amyloidopathy and Gliopathy. Biomedicines 2022, 10, 1824. [Google Scholar] [CrossRef]
- Sadeghian, N.; Shadman, J.; Moradi, A.; ghasem Golmohammadi, M.; Panahpour, H. Calcitriol protects the Blood-Brain Barrier integrity against ischemic stroke and reduces vasogenic brain edema via antioxidant and antiapoptotic actions in rats. Brain Res. Bull. 2019, 150, 281–289. [Google Scholar] [CrossRef]
- Scandinavian Society for Clinical Chemistry, Clinical Physiology. Scandinavian Journal of Clinical & Laboratory Investigation; Blackwell Scientific Publications: Hoboken, NJ, USA, 1987; Volume 47. [Google Scholar]
- Hollis, B.W. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J. Steroid Biochem. 1984, 21, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimer’s Dement. 2014, 10, 296–302. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Hu, T.; Huang, G. Serum 25-hydroxy vitamin D level is associated with cognitive impairment in people aged 65 years and older. Ann. Palliat. Med. 2021, 10, 7479–7485. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xue, W.; Li, J.; Fu, K.; Shi, H.; Zhang, B.; Teng, W.; Tian, L. 25-Hydroxyvitamin D Levels and the Risk of Dementia and Alzheimer’s Disease: A Dose-Response Meta-Analysis. Front. Aging Neurosci. 2018, 10, 368. [Google Scholar] [CrossRef]
- Jayedi, A.; Rashidy-Pour, A.; Shab-Bidar, S. Vitamin D status and risk of dementia and Alzheimer’s disease: A meta-analysis of dose-response. Nutr. Neurosci. 2019, 22, 750–759. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. Vitamin D deficiency is associated with increased risk of Alzheimer’s disease and dementia: Evidence from meta-analysis. Nutr. J. 2015, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Sommer, I.; Griebler, U.; Kien, C.; Auer, S.; Klerings, I.; Hammer, R.; Holzer, P.; Gartlehner, G. Vitamin D deficiency as a risk factor for dementia: A systematic review and meta-analysis. BMC Geriatr. 2017, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Acar, S.; Özkan, B. Vitamin D Metabolism; Özdemir, Ö., Ed.; IntechOpen: London, UK, 2021; Volume 2021, pp. 1–23. [Google Scholar]
- Bikle, D.D.; Malmstroem, S.; Schwartz, J. Current controversies: Are free vitamin metabolite levels a more accurate assessment of vitamin D status than total levels? Endocrinol. Metab. Clin. 2017, 46, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; de Leeuw, P.W. Chapter 16—Vitamin D in health and disease. In Reciprocal Translation Between Pathophysiology and Practice in Health and Disease; Soeters, P.B., de Leeuw, P.W., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 201–219. [Google Scholar] [CrossRef]
- Zhang, G.; Li, L.; Kong, Y.; Xu, D.; Bao, Y.; Zhang, Z.; Liao, Z.; Jiao, J.; Fan, D.; Long, X.; et al. Vitamin D-binding protein in plasma microglia-derived extracellular vesicles as a potential biomarker for major depressive disorder. Genes Dis. 2024, 11, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Halloran, B.; Gee, E.; Ryzen, E.; Haddad, J. Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. J. Clin. Investig. 1986, 78, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Omidifar, A.; Varzandi, T.; Salehnezhad, T.; Sahraian, M.A. Reduction in circulating vitamin D binding protein in patients with multiple sclerosis. BMC Neurol. 2021, 21, 168. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Schwartz, J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Gezen-Ak, D.; Alaylıoğlu, M.; Genç, G.; Gündüz, A.; Candaş, E.; Bilgiç, B.; Atasoy, I.L.; Apaydın, H.; Kızıltan, G.; Gürvit, H. GC and VDR SNPs and vitamin D levels in Parkinson’s disease: The relevance to clinical features. Neuromolecular Med. 2017, 19, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Vargas, T.; Bullido, M.J.; Martinez-Garcia, A.; Antequera, D.; Clarimon, J.; Rosich-Estrago, M.; Martin-Requero, A.; Mateo, I.; Rodriguez-Rodriguez, E.; Vilella-Cuadrada, E. A megalin polymorphism associated with promoter activity and Alzheimer’s disease risk. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2010, 153, 895–902. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef]
- Sutherland, M.K.; Somerville, M.J.; Yoong, L.K.; Bergeron, C.; Haussler, M.R.; McLachlan, D.R.C. Reduction of vitamin D hormone receptor mRNA levels in Alzheimer as compared to Huntington hippocampus: Correlation with calbindin-28k mRNA levels. Mol. Brain Res. 1992, 13, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.B.; Koeffler, H.P.; Yamashiro, J.M.; Wada, R.K. Vitamin D 3 analogs inhibit growth and induce differentiation in LA-N-5 human neuroblastoma cells. Clin. Exp. Metastasis 1996, 14, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Burkert, R.; McGrath, J.; Eyles, D. Vitamin D receptor expression in the embryonic rat brain. Neurosci. Res. Commun. 2003, 33, 63–71. [Google Scholar] [CrossRef]
- Musiol, I.; Stumpf, W.; Bidmon, H.-J.; Heiss, C.; Mayerhofer, A.; Bartke, A. Vitamin D nuclear binding to neurons of the septal, substriatal and amygdaloid area in the Siberian hamster (Phodopus sungorus) brain. Neuroscience 1992, 48, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Prüfer, K.; Veenstra, T.D.; Jirikowski, G.F.; Kumar, R. Distribution of 1, 25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J. Chem. Neuroanat. 1999, 16, 135–145. [Google Scholar] [CrossRef] [PubMed]
- López-Vicente, M.; Sunyer, J.; Lertxundi, N.; González, L.; Rodríguez-Dehli, C.; Espada Sáenz-Torre, M.; Vrijheid, M.; Tardón, A.; Llop, S.; Torrent, M.; et al. Maternal circulating Vitamin D3 levels during pregnancy and behaviour across childhood. Sci. Rep. 2019, 9, 14792. [Google Scholar] [CrossRef]
- Mossin, M.H.; Aaby, J.B.; Dalgård, C.; Lykkedegn, S.; Christesen, H.T.; Bilenberg, N. Inverse associations between cord vitamin D and attention deficit hyperactivity disorder symptoms: A child cohort study. Aust. New Zealand J. Psychiatry 2017, 51, 703–710. [Google Scholar] [CrossRef]
- Schmidt, R.J. Gestational vitamin D and Autism Spectrum Disorder. Biol. Psychiatry 2021, 90, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Windham, G.C.; Pearl, M.; Anderson, M.C.; Poon, V.; Eyles, D.; Jones, K.L.; Lyall, K.; Kharrazi, M.; Croen, L.A. Newborn vitamin D levels in relation to autism spectrum disorders and intellectual disability: A case–control study in California. Autism Res. 2019, 12, 989–998. [Google Scholar] [CrossRef]
- Harms, L.R.; Burne, T.H.J.; Eyles, D.W.; McGrath, J.J. Vitamin D and the brain. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 657–669. [Google Scholar] [CrossRef]
- Xue, Y.; He, X.; Li, H.-D.; Deng, Y.; Yan, M.; Cai, H.-L.; Tang, M.-M.; Dang, R.-L.; Jiang, P. Simultaneous quantification of 25-hydroxyvitamin D 3 and 24, 25-dihydroxyvitamin D 3 in rats shows strong correlations between serum and brain tissue levels. Int. J. Endocrinol. 2015, 2015, 296531. [Google Scholar] [CrossRef] [PubMed]
- Spach, K.M.; Hayes, C.E. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J. Immunol. 2005, 175, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Dolnikowski, G.G.; Patterson, W.B.; Dawson-Hughes, B.; Zheng, T.; Morris, M.C.; Holland, T.M.; Booth, S.L. Determination of vitamin D and its metabolites in human brain using an Ultra-Pressure LC–Tandem Mass Spectra method. Curr. Dev. Nutr. 2019, 3, nzz074. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.-R.; Molnár, F.; Peräkylä, M.; Qiao, S.; Kalueff, A.V.; St-Arnaud, R.; Carlberg, C.; Tuohimaa, P. 25-Hydroxyvitamin D3 is an agonistic vitamin D receptor ligand. J. Steroid Biochem. Mol. Biol. 2010, 118, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, F.; Mayer, E.; Norman, A.W. Biological activity assessment of the 26, 23-lactones of 1, 25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 and their binding properties to chick intestinal receptor and plasma vitamin D binding protein. Arch. Biochem. Biophys. 1984, 233, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Malloy, P.J.; Gross, C. Vitamin D: Biology, action, and clinical implications. In Osteoporosis; Elsevier: Amsterdam, The Netherlands, 2001; pp. 257–303. [Google Scholar]
- Morris, H.A. Vitamin D metabolism and molecular modes of action: New insights into vitamin D activities. Med. Res. J. 2014, 2, 1–5. [Google Scholar]
- Theodoropoulos, C.; Demers, C.; Delvin, E.; Ménard, D.; Gascon-Barré, M. Calcitriol regulates the expression of the genes encoding the three key vitamin D3 hydroxylases and the drug-metabolizing enzyme CYP3A4 in the human fetal intestine. Clin. Endocrinol. 2003, 58, 489–499. [Google Scholar] [CrossRef]
- Liu, H.L.; Chuang, H.Y.; Hsu, C.N.; Lee, S.S.; Yang, C.C.; Liu, K.T. Effects of Vitamin D Receptor, Metallothionein 1A, and 2A Gene Polymorphisms on Toxicity of the Peripheral Nervous System in Chronically Lead-Exposed Workers. Int. J. Env. Res. Public Health 2020, 17, 2909. [Google Scholar] [CrossRef] [PubMed]
- Warwick, T.; Schulz, M.H.; Günther, S.; Gilsbach, R.; Neme, A.; Carlberg, C.; Brandes, R.P.; Seuter, S. A hierarchical regulatory network analysis of the vitamin D induced transcriptome reveals novel regulators and complete VDR dependency in monocytes. Sci. Rep. 2021, 11, 6518. [Google Scholar] [CrossRef]
- Ideraabdullah, F.Y.; Belenchia, A.M.; Rosenfeld, C.S.; Kullman, S.W.; Knuth, M.; Mahapatra, D.; Bereman, M.; Levin, E.D.; Peterson, C.A. Maternal vitamin D deficiency and developmental origins of health and disease (DOHaD). J. Endocrinol. 2019, 241, R65–R80. [Google Scholar] [CrossRef]
- Pertile, R.A.N.; Kiltschewskij, D.; Geaghan, M.; Barnett, M.; Cui, X.; Cairns, M.J.; Eyles, D. Developmental vitamin D-deficiency increases the expression of microRNAs involved in dopamine neuron development. Brain Res. 2022, 1789, 147953. [Google Scholar] [CrossRef]
- Donati, S.; Palmini, G.; Aurilia, C.; Falsetti, I.; Marini, F.; Giusti, F.; Iantomasi, T.; Brandi, M.L. Calcifediol: Mechanisms of Action. Nutrients 2023, 15, 4409. [Google Scholar] [CrossRef]
- Wang, Y.; Chiang, Y.-H.; Su, T.-P.; Hayashi, T.; Morales, M.; Hoffer, B.; Lin, S.-Z. Vitamin D3 attenuates cortical infarction induced by middle cerebral arterial ligation in rats. Neuropharmacology 2000, 39, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.; Lopez-Martin, E.; Segura, C.; Labandeira-Garcia, J.L.; Perez-Fernandez, R. 1,25-Dihydroxyvitamin D3 increases striatal GDNF mRNA and protein expression in adult rats. Mol. Brain Res. 2002, 108, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Roussa, E.; Krieglstein, K. GDNF promotes neuronal differentiation and dopaminergic development of mouse mesencephalic neurospheres. Neurosci. Lett. 2004, 361, 52–55. [Google Scholar] [CrossRef]
- Cui, X.; Pertile, R.; Liu, P.; Eyles, D.W. Vitamin D regulates tyrosine hydroxylase expression: N-cadherin a possible mediator. Neuroscience 2015, 304, 90–100. [Google Scholar] [CrossRef]
- Cass, W.A.; Smith, M.P.; Peters, L.E. Calcitriol protects against the dopamine-and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann. New York Acad. Sci. 2006, 1074, 261–271. [Google Scholar] [CrossRef]
- Sabir, M.S.; Haussler, M.R.; Mallick, S.; Kaneko, I.; Lucas, D.A.; Haussler, C.A.; Whitfield, G.K.; Jurutka, P.W. Optimal vitamin D spurs serotonin: 1,25-dihydroxyvitamin D represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes Nutr. 2018, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.; Yaqubi, M.; Futhey, N.C.; Sedaghat, S.; Baufeld, C.; Blain, M.; Baranzini, S.; Butovsky, O.; Antel, J.; White, J.H. Vitamin D regulates MerTK-dependent phagocytosis in human myeloid cells. J. Immunol. 2020, 205, 398–406. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Dursun, E.; Yilmazer, S. The effects of vitamin D receptor silencing on the expression of LVSCC-A1C and LVSCC-A1D and the release of NGF in cortical neurons. PLoS ONE 2011, 6, e17553. [Google Scholar] [CrossRef]
- Lasoń, W.; Jantas, D.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. Vitamin D3 and Ischemic Stroke: A Narrative Review. Antioxidants 2022, 11, 2120. [Google Scholar] [CrossRef] [PubMed]
- Mirarchi, A.; Albi, E.; Beccari, T.; Arcuri, C. Microglia and Brain Disorders: The Role of Vitamin D and Its Receptor. Int. J. Mol. Sci. 2023, 24, 11892. [Google Scholar] [CrossRef] [PubMed]
- Lasoń, W.; Jantas, D.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer’s and Parkinson’s Diseases: A Narrative Review. Cells 2023, 12, 660. [Google Scholar] [CrossRef] [PubMed]
- Dulla, Y.A.T.; Kurauchi, Y.; Hisatsune, A.; Seki, T.; Shudo, K.; Katsuki, H. Regulatory mechanisms of vitamin D 3 on production of nitric oxide and pro-inflammatory cytokines in microglial BV-2 cells. Neurochem. Res. 2016, 41, 2848–2858. [Google Scholar] [CrossRef] [PubMed]
- Boontanrart, M.; Hall, S.D.; Spanier, J.A.; Hayes, C.E.; Olson, J.K. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J. Neuroimmunol. 2016, 292, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, R.; Qiao, W.; Zhang, W.; Chen, J.; Mao, L.; Goltzman, D.; Miao, D. 1,25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell 2019, 18, e12951. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Crean, S.; Mercaldi, C.J.; Collins, J.M.; Boyd, D.; Cook, M.N.; Arrighi, H.M. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: A systematic review and meta-analysis. Neuroepidemiology 2012, 38, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Bien-Ly, N.; Andrews-Zwilling, Y.; Xu, Q.; Bernardo, A.; Ring, K.; Halabisky, B.; Deng, C.; Mahley, R.W.; Huang, Y. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell 2009, 5, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Chakrabarti, S.S.; Gambhir, I.S. New genetic players in late-onset Alzheimer’s disease: Findings of genome-wide association studies. Indian J. Med. Res. 2018, 148, 135–144. [Google Scholar] [CrossRef]
- Kuningas, M.; Mooijaart, S.P.; Jolles, J.; Slagboom, P.E.; Westendorp, R.G.; van Heemst, D. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol. Aging 2009, 30, 466–473. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Ding, E.L.; Beydoun, H.A.; Tanaka, T.; Ferrucci, L.; Zonderman, A.B. Vitamin D receptor and megalin gene polymorphisms and their associations with longitudinal cognitive change in US adults. Am. J. Clin. Nutr. 2012, 95, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, T.; Ma, L.; Wei, W.; Li, Z.; Jiang, X.; Sun, J.; Pei, H.; Li, H. Vitamin D Receptor Gene Polymorphisms and Risk of Alzheimer Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Poduslo, S.; Yin, X. Chromosome 12 and late-onset Alzheimer’s disease. Neurosci. Lett. 2001, 310, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Gezen-Ak, D.; Dursun, E.; Bilgic, B.; Hanagasi, H.; Ertan, T.; Gürvit, H.; Emre, M.; Eker, E.; Ulutin, T.; Uysal, Ö. Vitamin D receptor gene haplotype is associated with late-onset Alzheimer’s disease. Tohoku J. Exp. Med. 2012, 228, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Janjusevic, M.; Gagno, G.; Fluca, A.L.; Padoan, L.; Beltrami, A.P.; Sinagra, G.; Moretti, R.; Aleksova, A. The peculiar role of vitamin D in the pathophysiology of cardiovascular and neurodegenerative diseases. Life Sci. 2022, 289, 120193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Yu, L.; Liu, J. Polymorphisms in Vitamin D Receptor Genes in Association with Childhood Autism Spectrum Disorder. Dis. Markers 2018, 2018, 7862892. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, E.; Jansen, T.; Ivaskevicius, V.; Kahles, H.; Klepzig, C.; Oldenburg, J.; Badenhoop, K. Protection from type 1 diabetes by vitamin D receptor haplotypes. Ann. New York Acad. Sci. 2006, 1079, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.J.; Refsum, H.; Warden, D.R.; Medway, C.; Wilcock, G.K.; Smith, A.D. The vitamin D receptor gene is associated with Alzheimer’s disease. Neurosci. Lett. 2011, 504, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Łaczmański, Ł.; Jakubik, M.; Bednarek-Tupikowska, G.; Rymaszewska, J.; Słoka, N.; Lwow, F. Vitamin D receptor gene polymorphisms in Alzheimer’s disease patients. Exp. Gerontol. 2015, 69, 142–147. [Google Scholar] [CrossRef]
- Khorram Khorshid, H.R.; Gozalpour, E.; Saliminejad, K.; Karimloo, M.; Ohadi, M.; Kamali, K. Vitamin D Receptor (VDR) Polymorphisms and Late-Onset Alzheimer’s Disease: An Association Study. Iran. J. Public Health 2013, 42, 1253–1258. [Google Scholar]
- Arevalo, N.; Castillo, D.; Rogers, N.; Delgado, C.; Farías, G.; Behrens, M.I.; SanMartín, C.D. Apa I, Taq I and Fok I VDR polymorphisms: Functional effect on mRNA levels of amyloid beta transporters LRP1 and P-gp in lymphocytes of mild cognitive impairment patients: Genetics/genetic factors of Alzheimer’s disease. Alzheimer’s Dement. 2020, 16, e045564. [Google Scholar] [CrossRef]
- Dimitrakis, E.; Katsarou, M.-S.; Lagiou, M.; Papastefanopoulou, V.; Stanitsa, E.; Spandidos, D.A.; Tsatsakis, A.; Papageorgiou, S.; Moutsatsou, P.; Antoniou, K. Association of vitamin D receptor gene TaqI polymorphism with Alzheimer’s disease in a Southeastern European Caucasian population. Exp. Ther. Med. 2022, 23, 341. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.d.S.; Fratelli, C.F.; Silva, C.M.d.S.; Lima, L.R.d.; Stival, M.M.; Silva, I.C.R.d.; Funghetto, S.S. Association of vitamin D with the TaqI polymorphism of the VDR gene in older women attending the basic health unit of the Federal District, DF (Brazil). J. Aging Res. 2020, 2020, 7145193. [Google Scholar]
- Wang, L.; Hara, K.; Van Baaren, J.M.; Price, J.C.; Beecham, G.W.; Gallins, P.J.; Whitehead, P.L.; Wang, G.; Lu, C.; Slifer, M.A.; et al. Vitamin D receptor and Alzheimer’s disease: A genetic and functional study. Neurobiol. Aging 2012, 33, e1841–e1844. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiao, Y.; Zhang, H.; Zhang, Y.; Hua, J.; Jin, S.; Liu, G. Circulating Vitamin D Levels and Alzheimer’s Disease: A Mendelian Randomization Study in the IGAP and UK Biobank. J. Alzheimer’s Dis. 2020, 73, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Lu’o’ng, K.V.Q.; Nguyễn, L.T.H. The Beneficial Role of Vitamin D in Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement.® 2011, 26, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Mizwicki, M.T.; Menegaz, D.; Zhang, J.; Barrientos-Durán, A.; Tse, S.; Cashman, J.R.; Griffin, P.R.; Fiala, M. Genomic and nongenomic signaling induced by 1α, 25(OH)2-vitamin D3 promotes the recovery of amyloid-β phagocytosis by Alzheimer’s disease macrophages. J. Alzheimer’s Dis. 2012, 29, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.H.; Hsu, C.C.; Yu, B.H.; Lo, Y.R.; Hsu, Y.Y.; Chen, M.H.; Juang, J.L. Vitamin D supplementation worsens Alzheimer’s progression: Animal model and human cohort studies. Aging Cell 2022, 21, e13670. [Google Scholar] [CrossRef]
- Landel, V.; Annweiler, C.; Millet, P.; Morello, M.; Féron, F. Vitamin D, cognition and Alzheimer’s disease: The therapeutic benefit is in the D-tails. J. Alzheimer’s Dis. 2016, 53, 419–444. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Lo Sasso, B.; Gambino, C.M.; Giglio, R.V.; Scazzone, C.; Agnello, L.; Ciaccio, M. The Role of Vitamin D as a Biomarker in Alzheimer’s Disease. Brain Sci. 2021, 11, 334. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011.
- Beauchet, O.; Cooper-Brown, L.A.; Allali, G. Vitamin D supplementation and cognition in adults: A systematic review of randomized controlled trials. CNS Drugs 2021, 35, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liang, F.; Zhang, L.; Liu, J.; Dou, H. Vitamin D Supplement for Prevention of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Am. J. Ther. 2021, 28, e638–e648. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Kubiak, J.; Svartberg, J.; Fuskevåg, O.M.; Figenschau, Y.; Martinaityte, I.; Grimnes, G. Vitamin D supplementation has no effect on cognitive performance after four months in mid-aged and older subjects. J. Neurol. Sci. 2019, 396, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jia, J.; Zhang, Y.; Miao, R.; Huo, X.; Ma, F. Effects of vitamin D3 supplementation on cognition and blood lipids: A 12-month randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.S.; Scherer, S.C.; Ladd, K.S.; Harrison, L.C. A randomized controlled trial of high-dose vitamin D2 followed by intranasal insulin in Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 26, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef]
- Norman, A. Intestinal calcium absorption: A vitamin D-hormone-mediated adaptive response. Am. J. Clin. Nutr. 1990, 51, 290–300. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; De Luis-Román, D.-A.; Fernández-Gómez, J.M.; Ruiz-Mambrilla, M.; Pérez-Castrillón, J.-L. Vitamin D receptor (VDR) gene polymorphisms modify the response to vitamin D supplementation: A systematic review and meta-analysis. Nutrients 2022, 14, 360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).