Serum Galectin-3 as a Non-Invasive Marker for Primary Sclerosing Cholangitis
Abstract
1. Introduction
2. Results
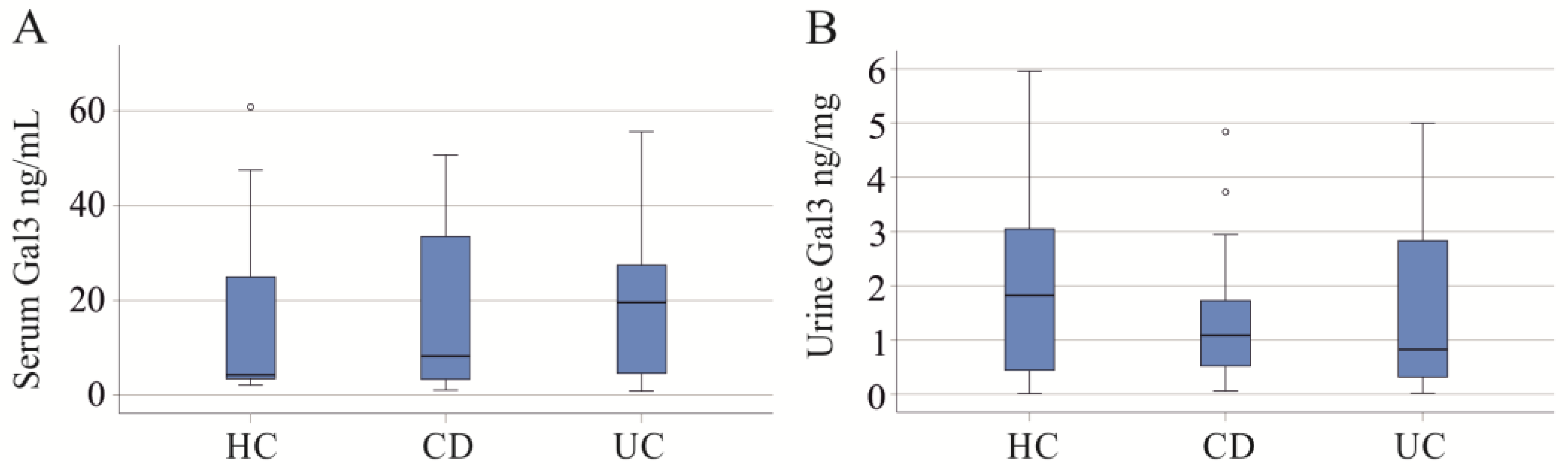
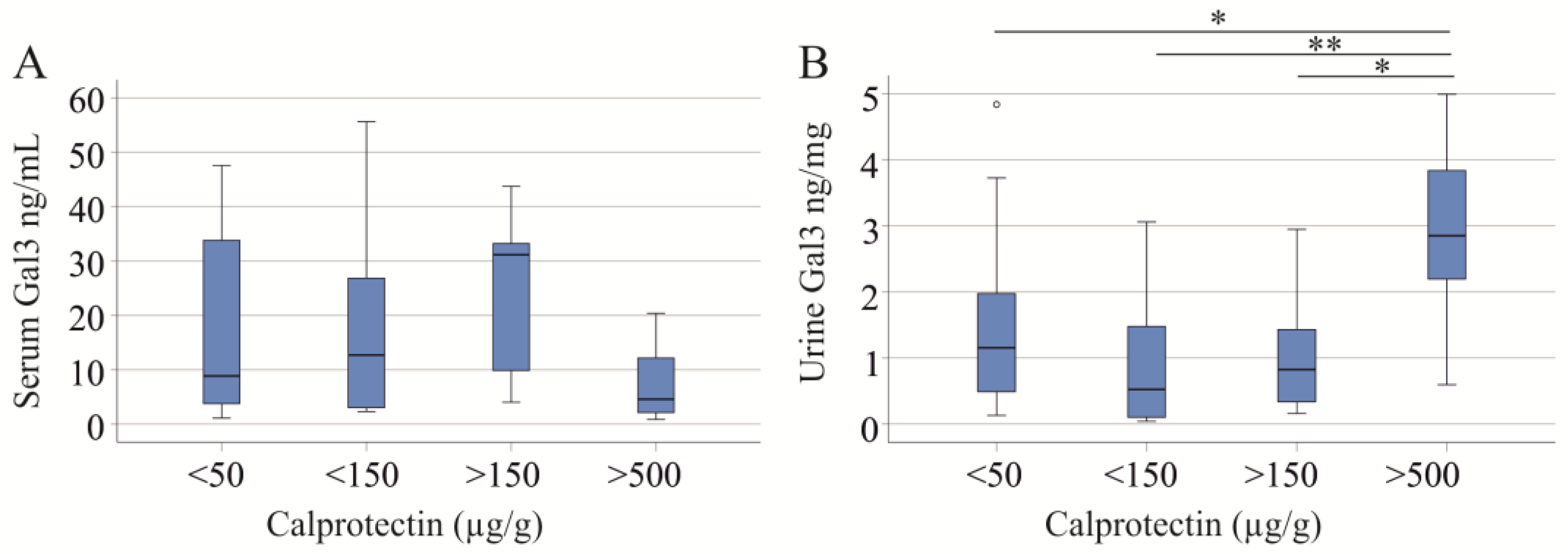
2.1. Serum and Urinary Galectin-3 of IBD Patients
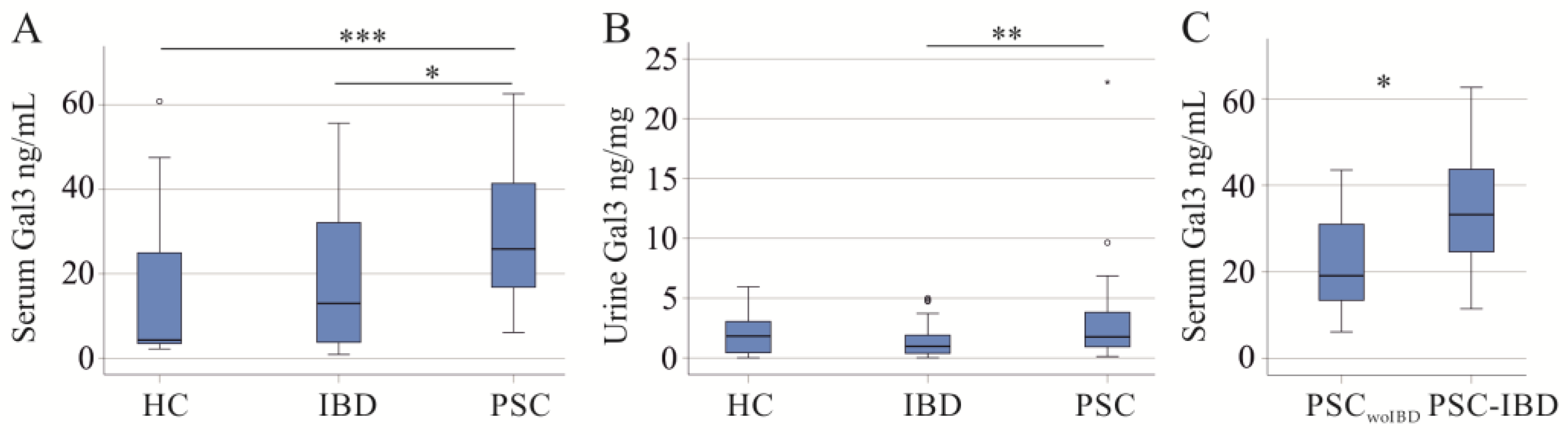
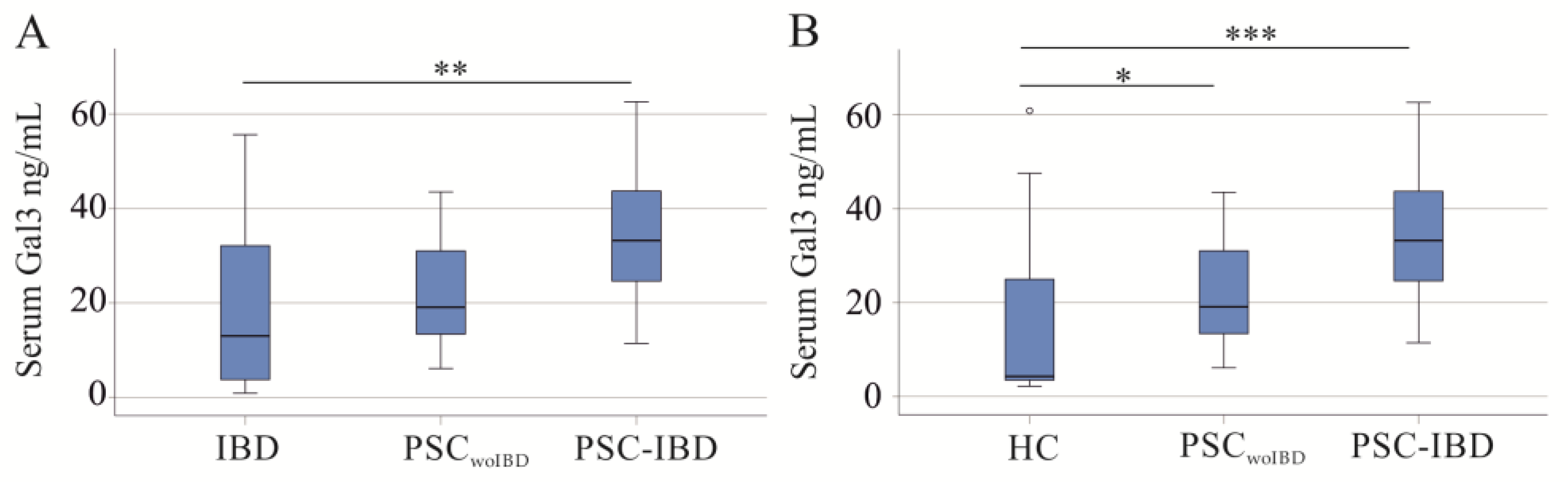
2.2. Serum and Urinary Galectin-3 of PSC Patients
3. Discussion
4. Materials and Methods
4.1. Patients and Control Cohorts
4.2. Measurement of Galectin-3, Urinary Creatinine, and Urinary Protein
4.3. Collection of Urine
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, H.; Manne, S.; Shick, J.; Lissoos, T.; Dolin, P. Incidence, prevalence, and natural history of primary sclerosing cholangitis in the United Kingdom. Medicine 2017, 96, e7116. [Google Scholar] [CrossRef] [PubMed]
- Pria, H.D.; Torres, U.S.; Faria, S.C.; Velloni, F.G.; Caiado, A.H.M.; Tiferes, D.A.; D’Ippolito, G. Practical Guide for Radiological Diagnosis of Primary and Secondary Sclerosing Cholangitis. Semin. Ultrasound CT MR 2022, 43, 490–509. [Google Scholar] [CrossRef] [PubMed]
- Schramm, C.; Eaton, J.; Ringe, K.I.; Venkatesh, S.; Yamamura, J.; MRI Working Group of the IPSCSG. Recommendations on the use of magnetic resonance imaging in PSC-A position statement from the International PSC Study Group. Hepatology 2017, 66, 1675–1688. [Google Scholar] [CrossRef]
- Dave, M.; Elmunzer, B.J.; Dwamena, B.A.; Higgins, P.D. Primary sclerosing cholangitis: Meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology 2010, 256, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Mertz, A.; Nguyen, N.A.; Katsanos, K.H.; Kwok, R.M. Primary sclerosing cholangitis and inflammatory bowel disease comorbidity: An update of the evidence. Ann. Gastroenterol. 2019, 32, 124–133. [Google Scholar] [CrossRef]
- Brown, S.J.; Mayer, L. The immune response in inflammatory bowel disease. Am. J. Gastroenterol. 2007, 102, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Dis. Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis. Mon. 2019, 65, 100851. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kwon, J.E.; Cho, M.L. Immunological pathogenesis of inflammatory bowel disease. Intest. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Fritzler, M.J.; Swain, M.G. A Review on Biomarkers for the Evaluation of Autoimmune Cholestatic Liver Diseases and Their Overlap Syndromes. Front. Mol. Med. 2022, 2, 914505. [Google Scholar] [CrossRef]
- Lee, W.I.; Subramaniam, K.; Hawkins, C.A.; Randall, K.L. The significance of ANCA positivity in patients with inflammatory bowel disease. Pathology 2019, 51, 634–639. [Google Scholar] [CrossRef]
- Wunsch, E.; Norman, G.L.; Milkiewicz, M.; Krawczyk, M.; Bentow, C.; Shums, Z.; Mahler, M.; Lopens, S.; Reinhold, D.; Franke, A.; et al. Anti-glycoprotein 2 (anti-GP2) IgA and anti-neutrophil cytoplasmic antibodies to serine proteinase 3 (PR3-ANCA): Antibodies to predict severe disease, poor survival and cholangiocarcinoma in primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 2021, 53, 302–313. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Krautbauer, S.; Eisinger, K.; Hader, Y.; Buechler, C. Free fatty acids and IL-6 induce adipocyte galectin-3 which is increased in white and brown adipose tissues of obese mice. Cytokine 2014, 69, 263–271. [Google Scholar] [CrossRef]
- Weigert, J.; Neumeier, M.; Wanninger, J.; Bauer, S.; Farkas, S.; Scherer, M.N.; Schnitzbauer, A.; Schaffler, A.; Aslanidis, C.; Scholmerich, J.; et al. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J. Clin. Endocrinol. Metab. 2010, 95, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Anwar, S.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H. 6-Gingerol, a Major Ingredient of Ginger Attenuates Diethylnitrosamine-Induced Liver Injury in Rats through the Modulation of Oxidative Stress and Anti-Inflammatory Activity. Mediat. Inflamm. 2021, 2021, 6661937. [Google Scholar] [CrossRef]
- Wang, W.; Gao, W.; Zhu, Q.; Alasbahi, A.; Seki, E.; Yang, L. TAK1: A Molecular Link Between Liver Inflammation, Fibrosis, Steatosis, and Carcinogenesis. Front. Cell Dev. Biol. 2021, 9, 734749. [Google Scholar] [CrossRef]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Poirier, F.; Russo, F.P.; Iredale, J.P.; Haslett, C.; Simpson, K.J.; Sethi, T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5060–5065. [Google Scholar] [CrossRef] [PubMed]
- Jeftic, I.; Jovicic, N.; Pantic, J.; Arsenijevic, N.; Lukic, M.L.; Pejnovic, N. Galectin-3 Ablation Enhances Liver Steatosis, but Attenuates Inflammation and IL-33-Dependent Fibrosis in Obesogenic Mouse Model of Nonalcoholic Steatohepatitis. Mol. Med. 2015, 21, 453–465. [Google Scholar] [CrossRef]
- Gudowska, M.; Gruszewska, E.; Cylwik, B.; Panasiuk, A.; Rogalska, M.; Flisiak, R.; Szmitkowski, M.; Chrostek, L. Galectin-3 Concentration in Liver Diseases. Ann. Clin. Lab. Sci. 2015, 45, 669–673. [Google Scholar]
- Wanninger, J.; Weigert, J.; Wiest, R.; Bauer, S.; Karrasch, T.; Farkas, S.; Scherer, M.N.; Walter, R.; Weiss, T.S.; Hellerbrand, C.; et al. Systemic and hepatic vein galectin-3 are increased in patients with alcoholic liver cirrhosis and negatively correlate with liver function. Cytokine 2011, 55, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.K.; Dowling, C.A.; Jeng, K.C.; Chen, J.T.; Yang, R.Y.; Liu, F.T. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int. J. Cancer 1999, 81, 519–526. [Google Scholar] [CrossRef]
- Cervantes-Alvarez, E.; Limon-de la Rosa, N.; Vilatoba, M.; Perez-Monter, C.; Hurtado-Gomez, S.; Martinez-Cabrera, C.; Argemi, J.; Alatorre-Arenas, E.; Yarza-Regalado, S.; Tejeda-Dominguez, F.; et al. Galectin-3 is overexpressed in advanced cirrhosis and predicts post-liver transplant infectious complications. Liver Int. 2022, 42, 2260–2273. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, M.L.; Manzi, M.; Rabinovich, G.A.; Troncoso, M.F. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 8831–8849. [Google Scholar] [CrossRef]
- An, Y.; Xu, S.; Liu, Y.; Xu, X.; Philips, C.A.; Chen, J.; Mendez-Sanchez, N.; Guo, X.; Qi, X. Role of Galectins in the Liver Diseases: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 744518. [Google Scholar] [CrossRef]
- Butscheid, M.; Hauptvogel, P.; Fritz, P.; Klotz, U.; Alscher, D.M. Hepatic expression of galectin-3 and receptor for advanced glycation end products in patients with liver disease. J. Clin. Pathol. 2007, 60, 415–418. [Google Scholar] [CrossRef]
- Shimonishi, T.; Miyazaki, K.; Kono, N.; Sabit, H.; Tuneyama, K.; Harada, K.; Hirabayashi, J.; Kasai, K.; Nakanuma, Y. Expression of endogenous galectin-1 and galectin-3 in intrahepatic cholangiocarcinoma. Hum. Pathol. 2001, 32, 302–310. [Google Scholar] [CrossRef]
- Slack, R.J.; Mills, R.; Mackinnon, A.C. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int. J. Biochem. Cell Biol. 2021, 130, 105881. [Google Scholar] [CrossRef]
- Kim, S.I.; Choi, M.E. TGF-beta-activated kinase-1: New insights into the mechanism of TGF-beta signaling and kidney disease. Kidney Res. Clin. Pract. 2012, 31, 94–105. [Google Scholar] [CrossRef]
- Yang, C.; Merlin, D. Unveiling Colitis: A Journey through the Dextran Sodium Sulfate-induced Model. Inflamm. Bowel Dis. 2024, izad312. [Google Scholar] [CrossRef]
- Volarevic, V.; Zdravkovic, N.; Harrell, C.R.; Arsenijevic, N.; Fellabaum, C.; Djonov, V.; Lukic, M.L.; Simovic Markovic, B. Galectin-3 Regulates Indoleamine-2,3-dioxygenase-Dependent Cross-Talk between Colon-Infiltrating Dendritic Cells and T Regulatory Cells and May Represent a Valuable Biomarker for Monitoring the Progression of Ulcerative Colitis. Cells 2019, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Lippert, E.; Stieber-Gunckel, M.; Dunger, N.; Falk, W.; Obermeier, F.; Kunst, C. Galectin-3 Modulates Experimental Colitis. Digestion 2015, 92, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Frol’ova, L.; Smetana, K., Jr.; Borovska, D.; Kitanovicova, A.; Klimesova, K.; Janatkova, I.; Malickova, K.; Lukas, M.; Drastich, P.; Benes, Z.; et al. Detection of galectin-3 in patients with inflammatory bowel diseases: New serum marker of active forms of IBD? Inflamm. Res. 2009, 58, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.B.; Dodd, S.; Yu, L.G.; Subramanian, S. Serum galectins as potential biomarkers of inflammatory bowel diseases. PLoS ONE 2020, 15, e0227306. [Google Scholar] [CrossRef] [PubMed]
- Cibor, D.; Szczeklik, K.; Brzozowski, B.; Mach, T.; Owczarek, D. Serum galectin 3, galectin 9 and galectin 3-binding proteins in patients with active and inactive inflammatory bowel disease. J. Physiol. Pharmacol. 2019, 70, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Tews, H.C.; Elger, T.; Grewal, T.; Weidlich, S.; Vitali, F.; Buechler, C. Fecal and Urinary Adipokines as Disease Biomarkers. Biomedicines 2023, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Rao, V.; Chunara, Z.; Mahoney, D.; Jackson, K.; Hodson, D.; Tarleton, C.; Thomas, D.; Chen, M.; Jacoby, D.; et al. Urine Galectin-3 Levels Identify High Risk Renal Dysfunction in Patients with Heart Failure. J. Card. Fail. 2017, 23, S32. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Vasudevamurthy, R.; Venkateshaiah, S.U.; Thomas, A.; Vishweshwara, A.; Dharmesh, S.M. Galectin-3 in urine of cancer patients: Stage and tissue specificity. J. Cancer Res. Clin. Oncol. 2009, 135, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Ambruzs, J.M.; Larsen, C.P. Renal Manifestations of Inflammatory Bowel Disease. Rheum. Dis. Clin. N. Am. 2018, 44, 699–714. [Google Scholar] [CrossRef]
- Dincer, M.T.; Dincer, Z.T.; Bakkaloglu, O.K.; Yalin, S.F.; Trabulus, S.; Celik, A.F.; Seyahi, N.; Altiparmak, M.R. Renal Manifestations in Inflammatory Bowel Disease: A Cohort Study During the Biologic Era. Med. Sci. Monit. 2022, 28, e936497. [Google Scholar] [CrossRef] [PubMed]
- Porter, I.E., 2nd; Palmer, W.C.; Parker, A.S.; Hodge, D.O.; Diehl, N.N.; Haley, W.E. Prevalence of Nephrolithiasis in Patients with Chronic Liver Disease: A Case-Control Study. J. Clin. Exp. Hepatol. 2018, 8, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, J.; Dymicka-Piekarska, V.; Tomaszewska, J.; Matowicka-Karna, J.; Koper-Lenkiewicz, O.M. Diagnostic utility of protein to creatinine ratio (P/C ratio) in spot urine sample within routine clinical practice. Crit. Rev. Clin. Lab. Sci. 2020, 57, 345–364. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shen, J.; Ran, Z. Epithelial-mesenchymal transition in Crohn’s disease. Mucosal Immunol. 2018, 11, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.F.; Wu, C.S.; Chen, Y.L.; Liao, H.J.; Chyuan, I.T.; Hsu, P.N. Galectin-3 suppresses mucosal inflammation and reduces disease severity in experimental colitis. J. Mol. Med. 2016, 94, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, A.; Silveira, M.G. Primary sclerosing cholangitis. Transl. Gastroenterol. Hepatol. 2021, 6, 29. [Google Scholar] [CrossRef]
- Banales, J.M.; Inarrairaegui, M.; Arbelaiz, A.; Milkiewicz, P.; Muntane, J.; Munoz-Bellvis, L.; La Casta, A.; Gonzalez, L.M.; Arretxe, E.; Alonso, C.; et al. Serum Metabolites as Diagnostic Biomarkers for Cholangiocarcinoma, Hepatocellular Carcinoma, and Primary Sclerosing Cholangitis. Hepatology 2019, 70, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Simovic Markovic, B.; Gajovic, N.; Jurisevic, M.; Djukic, A.; Jovanovic, I.; Arsenijevic, N.; Lukic, A.; Zdravkovic, N. Metabolic syndrome attenuates ulcerative colitis: Correlation with interleukin-10 and galectin-3 expression. World J. Gastroenterol. 2019, 25, 6465–6482. [Google Scholar] [CrossRef]
- Rennebaum, F.; Demmig, C.; Schmidt, H.H.; Vollenberg, R.; Tepasse, P.R.; Trebicka, J.; Gu, W.; Ullerich, H.; Kabar, I.; Cordes, F. Elevated Liver Fibrosis Progression in Isolated PSC Patients and Increased Malignancy Risk in a PSC-IBD Cohort: A Retrospective Study. Int. J. Mol. Sci. 2023, 24, 15431. [Google Scholar] [CrossRef]
- Amirzada, M.; Buczak-Stec, E.; Konig, H.H.; Hajek, A. Multimorbidity patterns in the German general population aged 40 years and over. Arch. Gerontol. Geriatr. 2023, 114, 105067. [Google Scholar] [CrossRef]
- Chen, B.; Collen, L.V.; Mowat, C.; Isaacs, K.L.; Singh, S.; Kane, S.V.; Farraye, F.A.; Snapper, S.; Jneid, H.; Lavie, C.J.; et al. Inflammatory Bowel Disease and Cardiovascular Diseases. Am. J. Med. 2022, 135, 1453–1460. [Google Scholar] [CrossRef]
- de Boer, R.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.; van der Harst, P. The fibrosis marker galectin-3 and outcome in the general population. J. Intern. Med. 2012, 272, 55–64. [Google Scholar] [CrossRef]
- Meijers, W.C.; Schroten, N.F.; Ruifrok, W.P.; Assa, S.; Dokter, M.M.; Damman, K.; Gansevoort, R.T.; Van Gilst, W.H.; Sillje, H.H.; De Boer, R.A. Urinary and plasma galectin-3 in heart failure—Insights in renal handling. Eur. Heart J. 2013, 34, P4243. [Google Scholar] [CrossRef]
- Kucharzik, T.; Dignass, A.; Siegmund, B. Aktualisierung der S3-Leitlinie Colitis ulcerosa 2019. Z. Gastroenterol. 2019; 57, 1279–1280. [Google Scholar] [CrossRef]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J. Crohns Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef] [PubMed]
- EASL Clinical Practice Guidelines on sclerosing cholangitis. J. Hepatol. 2022, 77, 761–806. [CrossRef] [PubMed]
- Tornai, D.; Ven, P.L.; Lakatos, P.L.; Papp, M. Serological biomarkers for management of primary sclerosing cholangitis. World J. Gastroenterol. 2022, 28, 2291–2301. [Google Scholar] [CrossRef]
- Morgan, M.A.; Khot, R.; Sundaram, K.M.; Ludwig, D.R.; Nair, R.T.; Mittal, P.K.; Ganeshan, D.M.; Venkatesh, S.K. Primary sclerosing cholangitis: Review for radiologists. Abdom. Radiol. 2023, 48, 136–150. [Google Scholar] [CrossRef]
| Characteristics | IBD | PSC | Controls |
|---|---|---|---|
| Number (females/males) | 55 (23/32) | 22 (6/16) | 38 (20/18) |
| Age (years) | 47 (19–70) | 52 (18–70) | 55 (23–78) |
| BMI (kg/m2) | 25.1 (15.5–40.4) | 24.8 (18.0–31.8) | n.d. |
| C-reactive protein (mg/L) | 3 (0–144) | 2 (0–51) | n.d. |
| Creatinine (mg/dL) | 0.83 (0.51–1.25) * | 1.02 (0.68–3.94) * | n.d. |
| GFR (mL/min) | 100 (61–136) * | 86 (12–135) * | n.d. |
| Fecal calprotectin (µg/g) | 49 (0–3889) | 35 (0–999) | n.d. |
| AST (U/L) | 25 (10–41) * | 27 (15–177) * | n.d. |
| ALT (U/L) | 20 (7–63) | 27 (5–89) | n.d. |
| Gamma GT (U/L) | 26 (11–74) * | 54 (10–345) * | n.d. |
| AP (U/L) | 64 (43–142) *** | 117 (57–537) *** | n.d. |
| Bilirubin (mg/dL) | 0.50 (0.15–1.90) ** | 0.70 (0.20–14.00) ** | n.d. |
| MELD Score | n.d. | 6 (6–20) | n.d. |
| Urine protein/creatinine | 0.05 (0–14.89) | 0.08 (0–4.60) | 0.05 (0 –0.20) |
| Creatinine | Glomerular Filtration Rate | C-Reactive Protein | Fecal Calprotectin |
|---|---|---|---|
| Serum Galectin-3 | |||
| r = 0.049 | r = 0.002 | r = −0.284 | r = −0.102 |
| p = 0.727 | p = 0.989 | p = 0.043 | p = 0.463 |
| Urinary Galectin-3 | |||
| r = −0.203 | r = 0.126 | r = 0.107 | r = 0.090 |
| p = 0.161 | p = 0.387 | p = 0.474 | p = 0.539 |
| Creatinine | Glomerular Filtration Rate | C-Reactive Protein | Fecal Calprotectin |
|---|---|---|---|
| Serum Galectin-3 | |||
| r = 0.091 | r = 0.035 | r = −0.335 | r = 0.105 |
| p = 0.803 | p = 0.914 | p = 0.287 | p = 0.759 |
| Urinary Galectin-3 | |||
| r = −0.145 | r = −0.049 | r = −0.145 | r = −0.138 |
| p = 0.592 | p = 0.858 | p = 0.592 | p = 0.623 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajraktari, G.; Elger, T.; Huss, M.; Loibl, J.; Albert, A.; Kandulski, A.; Müller, M.; Tews, H.C.; Buechler, C. Serum Galectin-3 as a Non-Invasive Marker for Primary Sclerosing Cholangitis. Int. J. Mol. Sci. 2024, 25, 4765. https://doi.org/10.3390/ijms25094765
Bajraktari G, Elger T, Huss M, Loibl J, Albert A, Kandulski A, Müller M, Tews HC, Buechler C. Serum Galectin-3 as a Non-Invasive Marker for Primary Sclerosing Cholangitis. International Journal of Molecular Sciences. 2024; 25(9):4765. https://doi.org/10.3390/ijms25094765
Chicago/Turabian StyleBajraktari, Ganimete, Tanja Elger, Muriel Huss, Johanna Loibl, Andreas Albert, Arne Kandulski, Martina Müller, Hauke Christian Tews, and Christa Buechler. 2024. "Serum Galectin-3 as a Non-Invasive Marker for Primary Sclerosing Cholangitis" International Journal of Molecular Sciences 25, no. 9: 4765. https://doi.org/10.3390/ijms25094765
APA StyleBajraktari, G., Elger, T., Huss, M., Loibl, J., Albert, A., Kandulski, A., Müller, M., Tews, H. C., & Buechler, C. (2024). Serum Galectin-3 as a Non-Invasive Marker for Primary Sclerosing Cholangitis. International Journal of Molecular Sciences, 25(9), 4765. https://doi.org/10.3390/ijms25094765







