Differential Response of Human Dendritic Cells upon Stimulation with Encapsulated or Non-Encapsulated Isogenic Strains of Porphyromonas gingivalis
Abstract
1. Introduction
2. Results
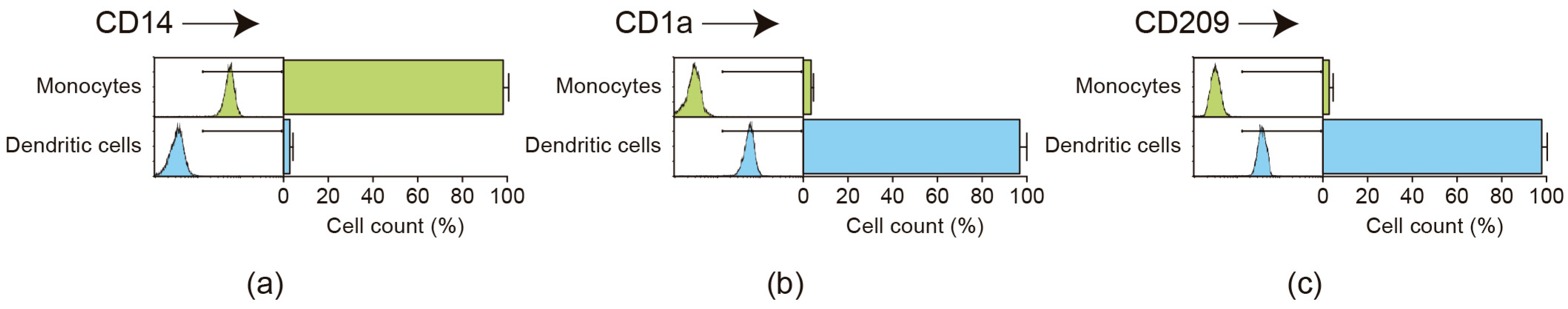
2.1. Flow Cytometric Analysis of Monocyte Purification and Dendritic Cell Differentiation
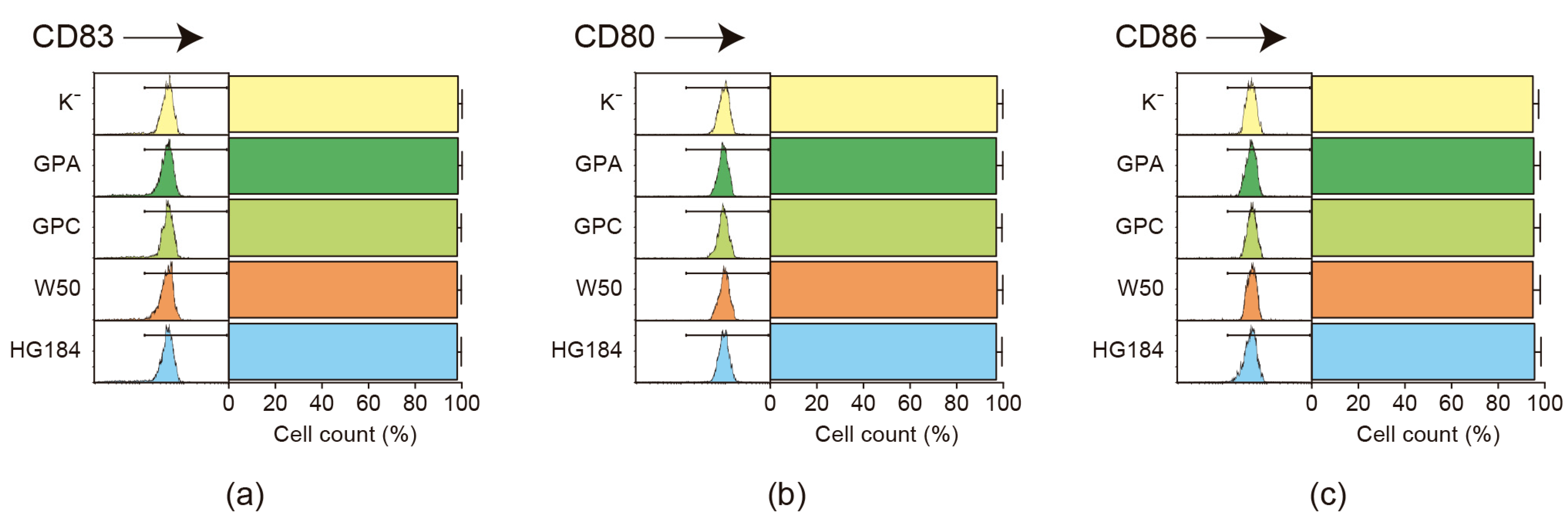
2.2. Flow Cytometric Analysis of Dendritic Cell Maturation
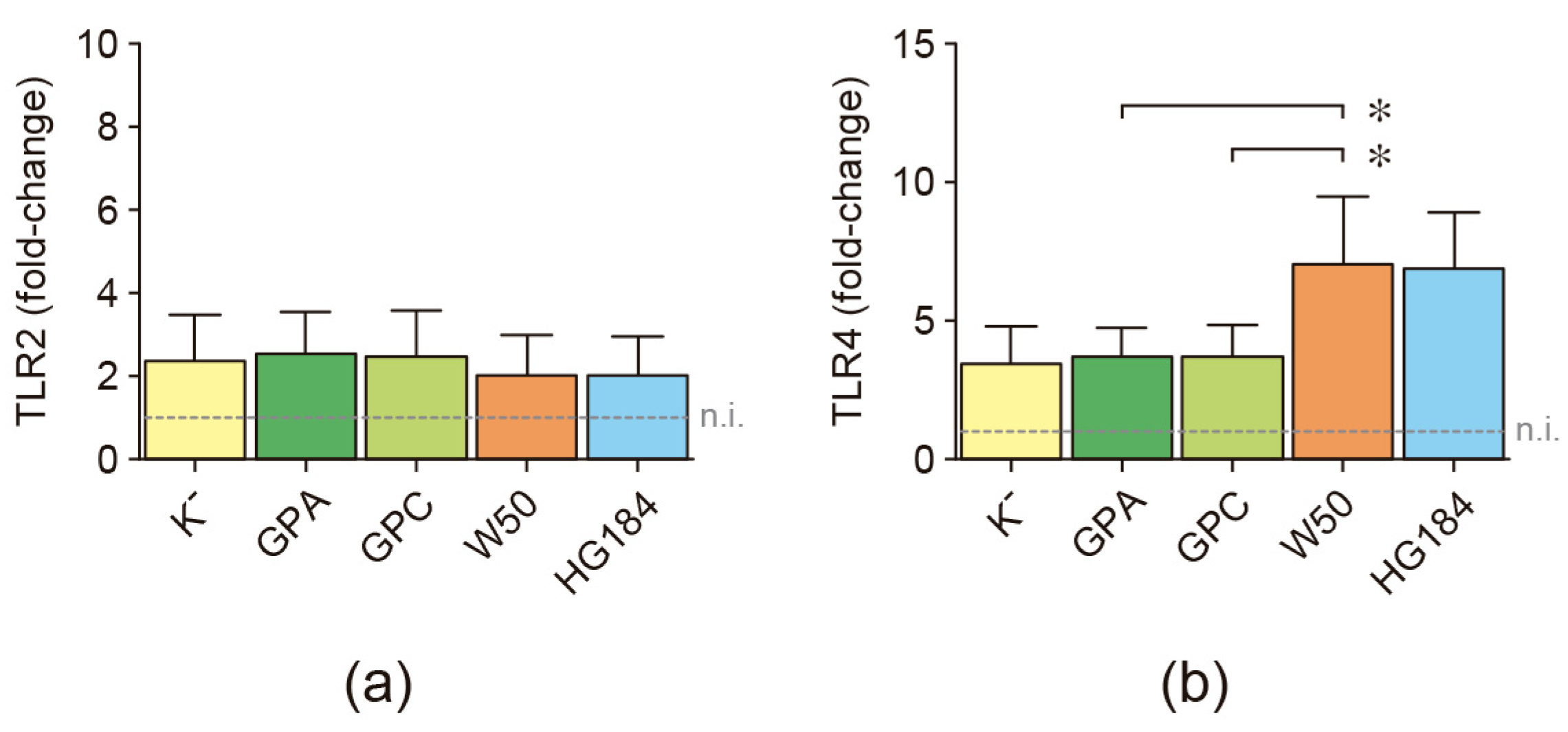
2.3. Toll-like Receptor Expression in P. gingivalis-Infected Dendritic Cells
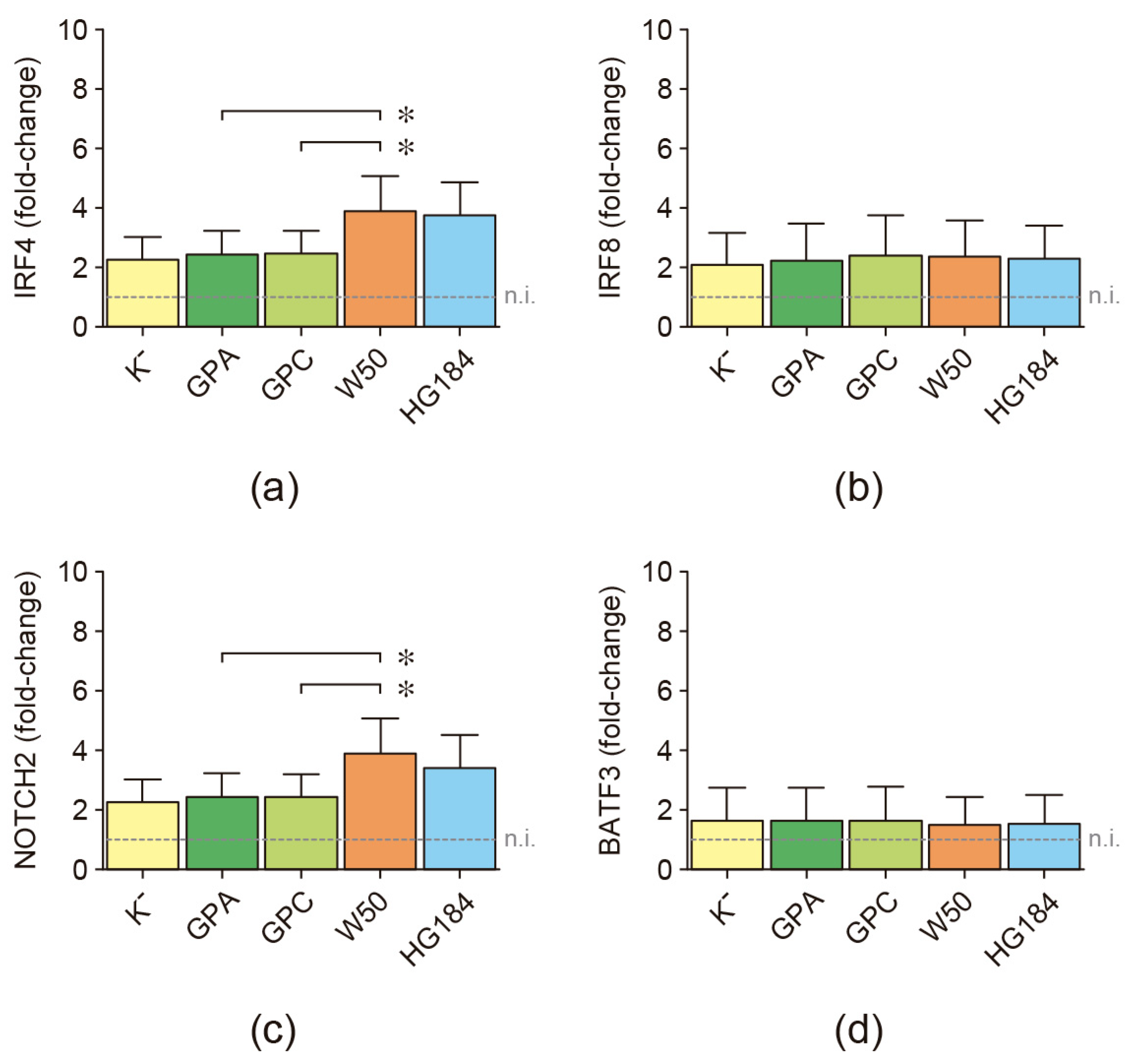
2.4. Transcription Factor Expression in P. gingivalis-Infected Dendritic Cells
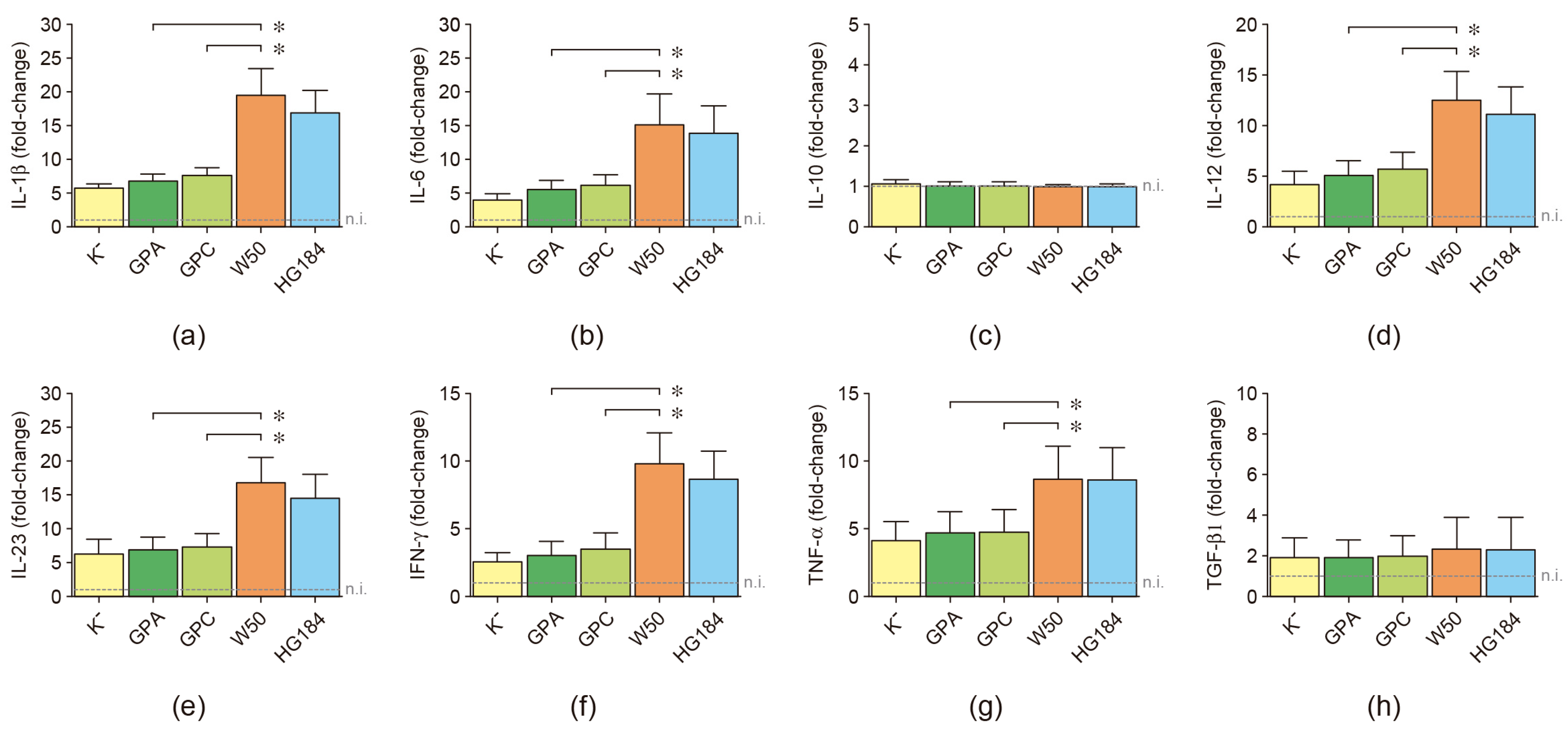
2.5. Cytokine Expression in P. gingivalis-Infected Dendritic Cells
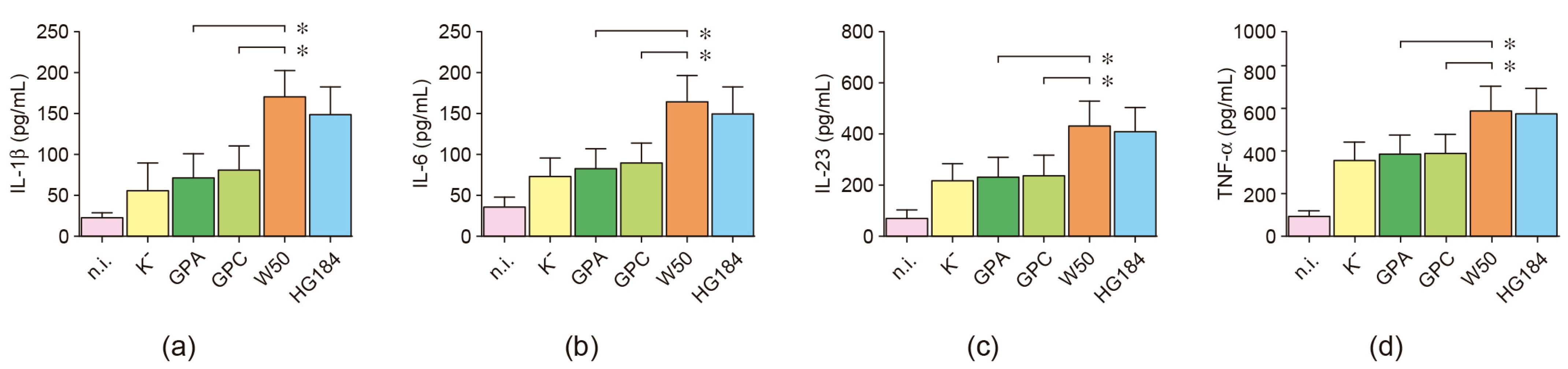
2.6. Cytokine Secretion in P. gingivalis-Infected Dendritic Cells
3. Discussion
4. Materials and Methods
4.1. Porphyromonas gingivalis Strains
4.2. Individuals
4.3. Monocyte-Derived Dendritic Cell Generation
4.4. Dendritic Cell Infection
4.5. Flow Cytometry Analysis
4.6. Expression of Cytokines, Transcription Factors, and Toll-like Receptors
4.7. Secretion of Cytokines
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreno, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabe, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; Collaborators, G.B.D.O.H. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: A systematic analysis for the global burden of diseases, injuries, and risk factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Papapanou, P.N.; Philips, K.H.; Offenbacher, S. Periodontal Medicine: 100 Years of Progress. J. Dent. Res. 2019, 98, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, S.Q.; Zhao, L.; Ren, Z.H.; Hu, C.Y. Global, regional, and national burden of periodontitis from 1990 to 2019: Results from the Global Burden of Disease study 2019. J. Periodontol. 2022, 93, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Polymicrobial communities in periodontal disease: Their quasi-organismal nature and dialogue with the host. Periodontology 2000 2021, 86, 210–230. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Monasterio, G.; Cavalla, F.; Córdova, L.A.; Hernández, M.; Heymann, D.; Garlet, G.P.; Sorsa, T.; Pärnänen, P.; Lee, H.M.; et al. Osteoimmunology of oral and maxillofacial diseases: Translational applications based on biological mechanisms. Front. Immunol. 2019, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Oates, T.; Garlet, G.P. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J. Oral Microbiol. 2011, 3, 5304. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontology 2000 2022, 89, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Garlet, G.P. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 2010, 89, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Michea, M.A.; Vegvari, A.; Arce, M.; Morales, A.; Lanyon, E.; Alcota, M.; Fuentes, C.; Vernal, R.; Budini, M.; et al. Proteomic profile of human gingival crevicular fluid reveals specific biological and molecular processes during clinical progression of periodontitis. J. Periodontal Res. 2023, 58, 1061–1081. [Google Scholar] [CrossRef] [PubMed]
- Buetas, E.; Jordan-Lopez, M.; Lopez-Roldan, A.; D’Auria, G.; Martinez-Priego, L.; De Marco, G.; Carda-Dieguez, M.; Mira, A. Full-length 16S rRNA gene sequencing by PacBio improves taxonomic resolution in human microbiome samples. BMC Genom. 2024, 25, 310. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Dongari-Bagtzoglou, A. Dysbiosis revisited: Understanding the role of the oral microbiome in the pathogenesis of gingivitis and periodontitis: A critical assessment. J. Periodontol. 2021, 92, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- de Jongh, C.A.; de Vries, T.J.; Bikker, F.J.; Gibbs, S.; Krom, B.P. Mechanisms of Porphyromonas gingivalis to translocate over the oral mucosa and other tissue barriers. J. Oral Microbiol. 2023, 15, 2205291. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.A.; Dou, Y.; Fletcher, H.M.; Boskovic, D.S. Local and systemic effects of Porphyromonas gingivalis infection. Microorganisms 2023, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Belmar, J.; Morales-Olavarría, M.; Vicencio, E.; Vernal, R.; Cárdenas, J.P.; Cortez, C. Contribution of -omics technologies in the study of Porphyromonas gingivalis during periodontitis pathogenesis: A minireview. Int. J. Mol. Sci. 2023, 24, 620. [Google Scholar] [CrossRef] [PubMed]
- Lunar Silva, I.; Cascales, E. Molecular strategies underlying Porphyromonas gingivalis virulence. J. Mol. Biol. 2021, 433, 166836. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.G.; Berges, A.; Sedra, A.; Ghods, S.; Kapoor, N.; Pill, L.; Davey, M.E.; Fairman, J.; Gibson, F.C., 3rd. A Porphyromonas gingivalis capsule-conjugate vaccine protects from experimental oral bone loss. Front. Oral Health 2021, 2, 686402. [Google Scholar] [CrossRef] [PubMed]
- Bregaint, S.; Boyer, E.; Fong, S.B.; Meuric, V.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Porphyromonas gingivalis outside the oral cavity. Odontology 2022, 110, 1–19. [Google Scholar] [CrossRef]
- Boyer, E.; Leroyer, P.; Malherbe, L.; Fong, S.B.; Loreal, O.; Bonnaure Mallet, M.; Meuric, V. Oral dysbiosis induced by Porphyromonas gingivalis is strain-dependent in mice. J. Oral Microbiol. 2020, 12, 1832837. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, G.; Fernández, B.; Castillo, F.; Rojas, C.; Cafferata, E.A.; Rojas, L.; Alvarez, C.; Fernández, A.; Hernández, M.; Bravo, D.; et al. Capsular-defective Porphyromonas gingivalis mutant strains induce less alveolar bone resorption than W50 wild-type strain due to a decreased Th1/Th17 immune response and less osteoclast activity. J. Periodontol. 2019, 90, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Ferdman, O.; Houri-Haddad, Y. Porphyromonas gingivalis capsule-mediated coaggregation as a virulence factor in mixed infection with Fusobacterium nucleatum. J. Periodontol. 2017, 88, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Vernal, R.; Diaz-Guerra, E.; Silva, A.; Sanz, M.; Garcia-Sanz, J.A. Distinct human T-lymphocyte responses triggered by Porphyromonas gingivalis capsular serotypes. J. Clin. Periodontol. 2014, 41, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Vernal, R.; Díaz-Zúñiga, J.; Melgar-Rodríguez, S.; Pujol, M.; Díaz-Guerra, E.; Silva, A.; Sanz, M.; Garcia-Sanz, J.A. Activation of RANKL-induced osteoclasts and memory T lymphocytes by Porphyromonas gingivalis is serotype dependant. J. Clin. Periodontol. 2014, 41, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, C.M.F.; Maltos, K.L.M.; Shehabeldin, M.S.; Thomas, L.L.; Zhuang, Z.; Yoshizawa, S.; Verdelis, K.; Gaffen, S.L.; Garlet, G.P.; Little, S.R.; et al. Local sustained delivery of anti-IL-17A antibodies limits inflammatory bone loss in murine experimental periodontitis. J. Immunol. 2021, 206, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Sosa Cuevas, E.; Saas, P.; Aspord, C. Dendritic cell subsets in melanoma: Pathophysiology, clinical prognosis and therapeutic exploitation. Cancers 2023, 15, 2206. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, A.; Salvi, V.; Soriani, A.; Laffranchi, M.; Sozio, F.; Bosisio, D.; Sozzani, S. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol. Immunol. 2023, 20, 432–447. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Dong, G.; Guo, L.; Graves, D.T. The function of dendritic cells in modulating the host response. Mol. Oral Microbiol. 2018, 33, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, N.; Kumamoto, Y. Role of mouse dendritic cell subsets in priming naive CD4 T cells. Curr. Opin. Immunol. 2023, 83, 102352. [Google Scholar] [CrossRef] [PubMed]
- Schlitzer, A.; McGovern, N.; Teo, P.; Zelante, T.; Atarashi, K.; Low, D.; Ho, A.W.; See, P.; Shin, A.; Wasan, P.S.; et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 2013, 38, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Mildner, A.; Jung, S. Development and function of dendritic cell subsets. Immunity 2014, 40, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, A.T.; Wu, X.; Albring, J.C.; Murphy, K.M. Re(de)fining the dendritic cell lineage. Nat. Immunol. 2012, 13, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, G.; Guevara, J.; Ibarra, J.P.; Castillo, F.; Díaz-Zúñiga, J.; Alvarez, C.; Cafferata, E.A.; Vernal, R. Immunostimulatory activity of low-molecular-weight hyaluronan on dendritic cells stimulated with Aggregatibacter actinomycetemcomitans or Porphyromonas gingivalis. Clin. Oral Investig. 2019, 23, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, S.; Heath, W.R. Krüppel-ling of IRF4-dependent DCs into two functionally distinct DC subsets. Immunity 2015, 42, 785–787. [Google Scholar] [CrossRef][Green Version]
- Chandra, J.; Kuo, P.T.; Hahn, A.M.; Belz, G.T.; Frazer, I.H. Batf3 selectively determines acquisition of CD8+ dendritic cell phenotype and function. Immunol. Cell Biol. 2017, 95, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Nizzoli, G.; Krietsch, J.; Weick, A.; Steinfelder, S.; Facciotti, F.; Gruarin, P.; Bianco, A.; Steckel, B.; Moro, M.; Crosti, M.; et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 2013, 122, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Persson, E.K.; Uronen-Hansson, H.; Semmrich, M.; Rivollier, A.; Hägerbrand, K.; Marsal, J.; Gudjonsson, S.; Håkansson, U.; Reizis, B.; Kotarsky, K.; et al. IRF4 transcription-factor-dependent CD103+CD11b+ dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 2013, 38, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Tussiwand, R.; Gautier, E.L. Transcriptional regulation of mononuclear phagocyte development. Front. Immunol. 2015, 6, 533. [Google Scholar] [CrossRef] [PubMed]
- Geginat, J.; Nizzoli, G.; Paroni, M.; Maglie, S.; Larghi, P.; Pascolo, S.; Abrignani, S. Immunity to pathogens taught by specialized human dendritic cell subsets. Front. Immunol. 2015, 6, 527. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Zúñiga, J.; Monasterio, G.; Alvarez, C.; Melgar-Rodríguez, S.; Benítez, A.; Ciuchi, P.; García, M.; Arias, J.; Sanz, M.; Vernal, R. Variability of the dendritic cell response triggered by different serotypes of Aggregatibacter actinomycetemcomitans or Porphyromonas gingivalis is toll-like receptor 2 (TLR2) or TLR4 dependent. J. Periodontol. 2015, 86, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Vernal, R.; León, R.; Silva, A.; van Winkelhoff, A.J.; García-Sanz, J.A.; Sanz, M. Differential cytokine expression by human dendritic cells in response to different Porphyromonas gingivalis capsular serotypes. J. Clin. Periodontol. 2009, 36, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.R.; D’Epiro, T.T.; Pinheiro, E.T.; Simionato, M.R.; Taniwaki, N.N.; Kisielius, J.J.; Mayer, M.P. Lineage variability in surface components expression within Porphyromonas gingivalis. Microb. Pathog. 2014, 77, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Ranjit, D.K.; Walker, A.R.; Getachew, H.; Progulske-Fox, A.; Davey, M.E. A novel regulation of K-antigen capsule synthesis in Porphyromonas gingivalis is driven by the response regulator PG0720-directed antisense RNA. Front. Oral Health 2021, 2, 701659. [Google Scholar] [CrossRef] [PubMed]
- Aduse-Opoku, J.; Slaney, J.M.; Hashim, A.; Gallagher, A.; Gallagher, R.P.; Rangarajan, M.; Boutaga, K.; Laine, M.L.; Van Winkelhoff, A.J.; Curtis, M.A. Identification and characterization of the capsular polysaccharide (K-antigen) locus of Porphyromonas gingivalis. Infect. Immun. 2006, 74, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Laine, M.L.; van Winkelhoff, A.J.; Dahlen, G. Genotype variation and capsular serotypes of Porphyromonas gingivalis from chronic periodontitis and periodontal abscesses. FEMS Microbiol. Lett. 2007, 270, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Breaking bad: Manipulation of the host response by Porphyromonas gingivalis. Eur. J. Immunol. 2014, 44, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, F.; Murakami, Y.; Nishikawa, K.; Hasegawa, Y.; Kawaminami, S. Surface components of Porphyromonas gingivalis. J. Periodontal Res. 2009, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, K.; Pauwels, M.; Laine, M.L.; Van Eldere, J.; Cassiman, J.J.; van Winkelhoff, A.J.; van Steenberghe, D.; Quirynen, M. Adhesion of Porphyromonas gingivalis serotypes to pocket epithelium. J. Periodontol. 2003, 74, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.; Sela, M.N. Coaggregation of Porphyromonas gingivalis and Fusobacterium nucleatum PK 1594 is mediated by capsular polysaccharide and lipopolysaccharide. FEMS Microbiol. Lett. 2006, 256, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, V.; Utreja, S.; Hovey, K.M.; Sun, Y.; LaMonte, M.J.; Wactawski-Wende, J.; Diaz, P.I.; Buck, M.J. Defining Porphyromonas gingivalis strains associated with periodontal disease. Sci. Rep. 2024, 14, 6222. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.L.; Appelmelk, B.J.; van Winkelhoff, A.J. Novel polysaccharide capsular serotypes in Porphyromonas gingivalis. J. Periodontal Res. 1996, 31, 278–284. [Google Scholar] [CrossRef] [PubMed]
- van Winkelhoff, A.J.; Appelmelk, B.J.; Kippuw, N.; de Graaff, J. K-antigens in Porphyromonas gingivalis are associated with virulence. Oral Microbiol. Immunol. 1993, 8, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Igboin, C.O.; Griffen, A.L.; Leys, E.J. Porphyromonas gingivalis strain diversity. J. Clin. Microbiol. 2009, 47, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Hirakawa, H.; Yamashita, A.; Ohara, N.; Shoji, M.; Yukitake, H.; Nakayama, K.; Toh, H.; Yoshimura, F.; Kuhara, S.; et al. Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P. gingivalis. DNA Res. 2008, 15, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cortegana, C.; Palomares, F.; Alba, G.; Santa-María, C.; de la Cruz-Merino, L.; Sánchez-Margalet, V.; López-Enriquez, S. Dendritic cells: The yin and yang in disease progression. Front. Immunol. 2023, 14, 1321051. [Google Scholar] [CrossRef] [PubMed]
- Acuto, O. T-cell virtuosity in “knowing thyself”. Front. Immunol. 2024, 15, 1343575. [Google Scholar] [CrossRef]
- Behzadi, P.; Garcia-Perdomo, H.A.; Karpinski, T.M. Toll-Like receptors: General molecular and structural biology. J. Immunol. Res. 2021, 2021, 9914854. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Zúñiga, J.; Yánez, J.P.; Alvarez, C.; Melgar-Rodríguez, S.; Hernández, M.; Sanz, M.; Vernal, R. Serotype-dependent response of human dendritic cells stimulated with Aggregatibacter actinomycetemcomitans. J. Clin. Periodontol. 2014, 41, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Hahne, F.; Gopalakrishnan, N.; Khodabakhshi, A.; Wong, C.; Lee, K. flowStats: Statistical Methods for the Analysis of Flow Cytometry Data. 2023. Available online: http://www.github.com/RGLab/flowStatsBioinformatics (accessed on 20 April 2023).
- Van, P.; Jiang, W.; Gottardo, R.; Finak, G. ggcyto: Next-generation open-source visualization software for cytometry. Bioinformatics 2018, 34, 3951–3953. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Clone | Fluorochrome | Dilution | Supplier | Code |
|---|---|---|---|---|---|
| anti-CD1a | HI149 | APC-Cy7 2 | 1:400 | Biolegend 7 | 300126 |
| anti-CD3 | OKT3 | eFluor 660 | 1:800 | eBioscience 8 | 50003741 |
| anti-CD4 | RPA-T4 | BUV395 3 | 1:200 | BD Biosciences 9 | 564724 |
| anti-CD8 | SK1 | BV510 4 | 1:200 | Biolegend | 344732 |
| anti-CD14 | M5E2 | BV650 | 1:200 | Biolegend | 301835 |
| anti-CD45 | 2D1 | Alexa Fluor 700 | 1:800 | eBioscience | 56945941 |
| anti-CD80 | W17149D | PerCP-Cy5.5 5 | 1:400 | Biolegend | 375412 |
| anti-CD83 | HB15e | BV711 | 1:400 | Biolegend | 305333 |
| anti-CD86 | IT2.2 | PE-Cy7 6 | 1:400 | eBioscience | 25086941 |
| anti-CD209 | 9E9A8 | BV421 | 1:400 | Biolegend | 330118 |
| Cell viability kit 1 | -- | BUV496 | 1:1000 | Biolegend | 423108 |
| mRNA | Forward Primer | Reverse Primer |
|---|---|---|
| IL-1β | ctgtcctgcgtgttgaaaga | ttgggtaatttttgggatctaca |
| IL-6 | gcccagctatgaactccttct | gaaggcagcaggcaacac |
| IL-10 | tgggggagaacctgaagac | ccttgctcttgttttcacagg |
| IL-12p35 | cactcccaaaacctgctgag | tctcttcagaagtgcaagggta |
| IL-23 | agcttcatgcctccctactg | ctgctgagtctcccagtggt |
| IFN-γ | ggcattttgaagaattggaaag | tttggatgctctggtcatctt |
| TNF-α | cagcctcttctccttcctgat | gccagagggctgattagaga |
| TGF-β1 | cacgtggagctgtaccagaa | cagccggttgctgaggta |
| IRF4 1 | gacaacgccttacccttcg | aggggtggcatcatgtagtt |
| IRF8 2 | tggggatgatcaaaaggagcc | aactggctggtgtcgaagac |
| NOTCH2 3 | cagttacccacccacaggtc | ccatacaggcagtcaatggaa |
| BATF3 4 | agacccagaaggctgacaag | ctccgcagcatggtgttt |
| TLR2 | ctctcggtgtcggaatgtc | aggatcagcaggaacagagc |
| TLR4 | ccctcccctgtaccttct | tccctgccttgaataccttc |
| 18S rRNA | ctcaacacgggaaacctcac | cgctccaccaactaagaacg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melgar-Rodríguez, S.; Polanco, A.; Ríos-Muñoz, J.; García, M.; Sierra-Cristancho, A.; González-Osuna, L.; Díaz-Zúñiga, J.; Carvajal, P.; Vernal, R.; Bravo, D. Differential Response of Human Dendritic Cells upon Stimulation with Encapsulated or Non-Encapsulated Isogenic Strains of Porphyromonas gingivalis. Int. J. Mol. Sci. 2024, 25, 4510. https://doi.org/10.3390/ijms25084510
Melgar-Rodríguez S, Polanco A, Ríos-Muñoz J, García M, Sierra-Cristancho A, González-Osuna L, Díaz-Zúñiga J, Carvajal P, Vernal R, Bravo D. Differential Response of Human Dendritic Cells upon Stimulation with Encapsulated or Non-Encapsulated Isogenic Strains of Porphyromonas gingivalis. International Journal of Molecular Sciences. 2024; 25(8):4510. https://doi.org/10.3390/ijms25084510
Chicago/Turabian StyleMelgar-Rodríguez, Samanta, Alan Polanco, Jearitza Ríos-Muñoz, Michelle García, Alfredo Sierra-Cristancho, Luis González-Osuna, Jaime Díaz-Zúñiga, Paola Carvajal, Rolando Vernal, and Denisse Bravo. 2024. "Differential Response of Human Dendritic Cells upon Stimulation with Encapsulated or Non-Encapsulated Isogenic Strains of Porphyromonas gingivalis" International Journal of Molecular Sciences 25, no. 8: 4510. https://doi.org/10.3390/ijms25084510
APA StyleMelgar-Rodríguez, S., Polanco, A., Ríos-Muñoz, J., García, M., Sierra-Cristancho, A., González-Osuna, L., Díaz-Zúñiga, J., Carvajal, P., Vernal, R., & Bravo, D. (2024). Differential Response of Human Dendritic Cells upon Stimulation with Encapsulated or Non-Encapsulated Isogenic Strains of Porphyromonas gingivalis. International Journal of Molecular Sciences, 25(8), 4510. https://doi.org/10.3390/ijms25084510







