Mitochondrial Kinase Signaling for Cardioprotection
Abstract
1. Introduction
2. Cardioprotective Signaling to and within Mitochondria
3. Protein Kinases of the NO/PKG Pathway
3.1. Protein Kinase A (PKA)
3.2. Protein Kinase C (PKC)
4. Protein Kinases of the RISK Pathway
4.1. Glycogen Synthase Kinase 3β (GSK3β)
4.2. Hexokinase II (HKII)
4.3. Adenosine Monophosphate-Activated Protein Kinase (AMPK)
5. Protein Kinases Not Assigned to the RISK or NO/PKG Pathways
5.1. C-Jun N-Terminal Kinase (JNK)
5.2. Src-Family Protein Tyrosine Kinases (SFKs)
5.3. p38 Mitogen-Activated Protein Kinases (p38 MAPK)
5.4. PTEN-Induced Putative Kinase 1 (PINK1)
6. Conclusions
| Protein Kinase | Experimental Model | Functional Effects | |
|---|---|---|---|
| Preparation | Stimulus | ||
| PKA | adult guinea pig cardiomyocytes without vs. with pharmacological PKA activation | - | prevention of mitochondrial Ca2+ overload [77] |
| mitochondria, mitoplasts from cattle heart | - | activation of mitochondrial complex I respiration [37] | |
| permeabilized adult rat cardiomyocytes | - | increased mitochondrial ROS generation [78] | |
| H9c2 rat cardiomyocytes with in vitro H/R, rat myocardium and mitochondria from WT mice myocardium vs. myocardium of mice expressing activated PKCε with in vitro I/R | without vs. with NO | Drp1-dependent reduction of mitochondrial fission [39] | |
| adult and neonatal rat cardiomyocytes with in vitro H/R | without vs. with HC | GSK3β-dependent inhibition of MPTP opening [74] | |
| PKCε | mitochondria from myocardium of WT mice vs. of mice with transgene expression of activated PKCε | - | PKCε/VDAC-dependent reduction of apoptosis [43] and inhibition of MPTP opening [45] |
| neonatal rat cardiomyocytes with in vitro H/R and without and with pharmacological PKCε specific translocation inhibitor | without vs. with HC | cytochrome c oxidase dependent PKCε translocation, improved mitochondrial respiration [44] | |
| mitochondria from rabbit hearts with in vitro I/R | without vs. with IC | increased KATP opening [97] | |
| mitochondria, mitoplasts from rat hearts without vs. with pharmacological PKCε activation | - | increased KATP opening and reduced ROS formation [98] | |
| PKCδ | mitochondria from rat hearts with in vitro I/R, without vs. with pharmacological inhibition of PKCδ translocation | - | decreased mitochondrial ROS formation and improved mitochondrial respiration [108] |
| rat hearts with in vitro I/R, without vs. with pharmacological inhibition of PKCδ translocation | - | reduction of apoptosis via release of cytochrome c [107] | |
| GSK3β | mitochondria from WT mice and mice with permanently activated GSK3β with in vivo I/R, without and with pharmacological GSK3β inhibition | without vs. with IC | increased respiration, inhibition of MPTP opening [128] |
| adult and neonatal rat cardiomyocytes with in vitro H/R | without vs. with HC | inhibition of MPTP opening and apoptosis, enhanced mitochondrial biogenesis [74] | |
| adult cardiomyocytes and mitochondria from mouse hearts without vs. with pharmacological GSK3β inhibition and neonatal rat cardiomyocytes | - | increased mitochondrial respiration and ATP production [130] | |
| HKII | neonatal rat cardiomyocytes with Ca2+ and H2O2-treatment without vs. with pharmacological AKT activation and mouse mitochondria with Ca2+-treatment without vs. with recombinant kinase active AKT | - | inhibition of MPTP opening—decreased release of cytochrome c [50] |
| adult rat cardiomyocytes without vs. with pharmacological enhancement of mitochondrial HKII binding with recombinant GSK3β | - | inhibition of MPTP opening [126] | |
| HeLa cells and adult rat cardiomyocytes without vs. with peptide displacing HKII from mitochondria | - | enhanced MPTP susceptibility to ROS [152] | |
| mitochondria from rat hearts without vs. with the cAMP analogue 8-Br-cAMP-AM | - | inhibition of MPTP opening [172] | |
| mitochondria from neonatal rat cardiomyocytes with in vitro H and mitochondria from mouse hearts with in vivo I without vs. with AAV9-mediated expression of mitochondrial HKII dissociating peptide | - | increased Parkin-mediated mitophagy [155] | |
| mouse hearts in vitro perfused without vs. with HKII peptide reducing mitochondrial HKII | - | increased mitochondrial respiration [166] | |
| AMPK | H9c2 rat cardiomyocytes with in vitro H | without vs. with pharmacological AMPK activation | induced mitophagy [178] |
| adult rat cardiomyocytes with mechanical stress | without vs. with pharmacological AMPK activation | inhibition of mPTP opening [181] | |
| JNK | mitochondria from H9c2 rat cardiomyocytes and primary human cardiomyocytes without vs. with in vitro H2O2/FeSO4-treatment, neonatal rat cardiomyocytes without vs. with in vitro H2O2/FeSO4-treatment, mitochondria from rat hearts without vs. with in vivo I/R | - | increased ROS formation [200] |
| mitochondria from rat hearts without vs. with in vitro JNK-activation | - | increased cytochrome c release [206] | |
| adult cardiomyocytes from hearts of WT mice and Mst KO mice without vs. with in vitro H and without vs. with in vivo I | - | enhanced fission [217] | |
| hearts of WT mice vs. DUSP1 KO mice with in vivo I/R | - | enhanced fission [218] | |
| SFKs (Src) | mitochondria from adult rat cardiomyocytes with in vivo I/R | without vs. with IC | decreased mitochondrial respiration during IC, reduced ROS generation [55] |
| adult rat cardiomyocytes with in vitro H/R | without vs. with NO | decreased complex I activity, reduced ROS generation [225] | |
| p38 MAPK | mitochondria from hearts of WT and Ovx mice/ER null mice with in vivo I/R | without vs. with 17β-estradiol | p38 MAPKβ decreased ROS formation [61] |
| mitochondria from rat hearts with in vivo I/R | pharmacological p38 MAPK inhibition | attenuated mitochondrial swelling, mitochondrial ROS generation, and mitochondrial membrane potential depolarization [237] | |
| PINK1 | HL-1 mouse cardiomyocytes, WT vs. with PINK1 over-expression with mechanical stress and adult cardiomyocytes from WT vs. PINK1-deficient mice | - | inhibition of MPTP opening, decreased mitochondrial membrane potential, reduced mitochondrial respiration, increased ROS [245] |
| adult and H9c2 rat cardiomyocytes with in vitro H/R mitochondria from WT vs. PINK1-deficient mice | without vs. with acetylcholine at reoxygenation | increased mitophagy [255] | |
Funding
Conflicts of Interest
References
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in health, disease, and ageing. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Barrabes, J.A.; Bøtker, H.E.; Davidson, S.M.; Di Lisa, F.; Downey, J.; Engstrom, T.; Ferdinandy, P.; Carbrera-Fuentes, H.A.; Heusch, G.; et al. Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery. Basic Res. Cardiol. 2016, 111, 70. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Gersh, B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur. Heart J. 2017, 38, 774–784. [Google Scholar] [CrossRef]
- Bell, R.M.; Bøtker, H.E.; Carr, R.D.; Davidson, S.M.; Downey, J.M.; Dutka, D.P.; Heusch, G.; Ibanez, B.; Macallister, R.; Stoppe, C.; et al. 9th Hatter Biannual Meeting: Position document on ischaemia/reperfusion injury, conditioning and the ten commandments of cardioprotection. Basic Res. Cardiol. 2016, 111, 41. [Google Scholar] [CrossRef]
- Hoshida, S.; Yamashita, N.; Otsu, K.; Hori, M. The importance of manganese superoxide dismutase in delayed preconditioning: Involvement of reactive oxygen species and cytokines. Cardiovasc. Res. 2002, 55, 495–505. [Google Scholar] [CrossRef][Green Version]
- Bolli, R.; Li, Q.H.; Tang, X.L.; Guo, Y.; Xuan, Y.T.; Rokosh, G.; Dawn, B. The late phase of preconditioning and its natural clinical application-gene therapy. Heart Fail. Rev. 2007, 12, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Baxter, G.F.; Goma, F.M.; Yellon, D.M. Involvement of protein kinase C in the delayed cytoprotection following sublethal ischaemia in rabbit myocardium. Br. J. Pharmacol. 1995, 115, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Torregroza, C.; Feige, K.; Preckel, B.; Hollmann, M.W.; Weber, N.C.; Huhn, R. Pharmacological conditioning of the heart: An update on experimental developments and clinical implications. Int. J. Mol. Sci. 2021, 22, 2519. [Google Scholar] [CrossRef]
- Heusch, G.; Rassaf, T. Time to give up on cardioprotection? A critical appraisal of clinical studies on ischemic pre-, post-, and remote conditioning. Circ. Res. 2016, 119, 676–695. [Google Scholar] [CrossRef]
- Gedik, N.; Maciel, L.; Schulte, C.; Skyschally, A.; Heusch, G.; Kleinbongard, P. Cardiomyocyte mitochondria as targets of humoral factors released by remote ischemic preconditioning. Arch. Med. Sci. 2017, 13, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Lochnit, G.; Schulz, R. Mitochondria “THE” target of myocardial conditioning. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1215–H1231. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Javadov, S.; Margreiter, R.; Grimm, M.; Hagenbuchner, J.; Ausserlechner, M.J. The role of mitochondria in the mechanisms of cardiac ischemia-reperfusion injury. Antioxidants 2019, 8, 454. [Google Scholar] [CrossRef]
- Titus, A.S.; Sung, E.A.; Zablocki, D.; Sadoshima, J. Mitophagy for cardioprotection. Basic Res. Cardiol. 2023, 118, 42. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, W.; Valencia, R.; Mahmud, Z.; Seubert, J.M.; Schulz, R. Matrix metalloproteinase-2 proteolyzes mitofusin-2 and impairs mitochondrial function during myocardial ischemia-reperfusion injury. Basic Res. Cardiol. 2023, 118, 29. [Google Scholar] [CrossRef]
- Prag, H.A.; Murphy, M.P.; Krieg, T. Preventing mitochondrial reverse electron transport as a strategy for cardioprotection. Basic Res. Cardiol. 2023, 118, 34. [Google Scholar] [CrossRef]
- Li, A.; Shami, G.J.; Griffiths, L.; Lal, S.; Irving, H.; Braet, F. Giant mitochondria in cardiomyocytes: Cellular architecture in health and disease. Basic Res. Cardiol. 2023, 118, 39. [Google Scholar] [CrossRef]
- Kaludercic, N.; Arusei, R.J.; Di Lisa, F. Recent advances on the role of monoamine oxidases in cardiac pathophysiology. Basic Res. Cardiol. 2023, 118, 41. [Google Scholar] [CrossRef]
- Saito, T.; Hamano, K.; Sadoshima, J. Molecular mechanisms and clinical implications of multiple forms of mitophagy in the heart. Cardiovasc. Res. 2021, 117, 2730–2741. [Google Scholar] [CrossRef]
- Niemi, N.M.; Pagliarini, D.J. The extensive and functionally uncharacterized mitochondrial phosphoproteome. J. Biol. Chem. 2021, 297, 100880. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Leon, I.R.; Bak, S.; Mogensen, M.; Wrzesinski, K.; Hojlund, K.; Jensen, O.N. Phosphoproteome analysis of functional mitochondria isolated from resting human muscle reveals extensive phosphorylation of inner membrane protein complexes and enzymes. Mol. Cell Proteom. 2011, 10, M110.000299. [Google Scholar] [CrossRef]
- Padrao, A.I.; Vitorino, R.; Duarte, J.A.; Ferreira, R.; Amado, F. Unraveling the phosphoproteome dynamics in mammal mitochondria from a network perspective. J. Proteome Res. 2013, 12, 4257–4267. [Google Scholar] [CrossRef]
- Deng, N.; Zhang, J.; Zong, C.; Wang, Y.; Lu, H.; Yang, P.; Wang, W.; Young, G.W.; Wang, Y.; Korge, P.; et al. Phosphoproteome analysis reveals regulatory sites in major pathways of cardiac mitochondria. Mol. Cell Proteom. 2011, 10, M110.000117. [Google Scholar] [CrossRef]
- Bak, S.; Leon, I.R.; Jensen, O.N.; Hojlund, K. Tissue specific phosphorylation of mitochondrial proteins isolated from rat liver, heart muscle, and skeletal muscle. J. Proteome Res. 2013, 12, 4327–4339. [Google Scholar] [CrossRef]
- Cui, Z.; Hou, J.; Chen, X.; Li, J.; Xie, Z.; Xue, P.; Cai, T.; Wu, P.; Xu, T.; Yang, F. The profile of mitochondrial proteins and their phosphorylation signaling network in INS-1 beta cells. J. Proteome Res. 2010, 9, 2898–2908. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Boengler, K.; Schulz, R. Inhibition of mitochondrial permeability transition pore opening: The holy grail of cardioprotection. Basic Res. Cardiol. 2010, 105, 151–154. [Google Scholar] [CrossRef]
- Rossello, X.; Yellon, D.M. The RISK pathway and beyond. Basic Res. Cardiol. 2018, 113, 2. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. New directions for protecting the heart against ischaemia-reperfusion injury: Targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc. Res. 2004, 61, 448–460. [Google Scholar] [CrossRef]
- Yellon, D.M.; Beikoghli Kalkhoran, S.; Davidson, S.M. The RISK pathway leading to mitochondria and cardioprotection: How everything started. Basic Res. Cardiol. 2023, 118, 22. [Google Scholar] [CrossRef]
- Hadebe, N.; Cour, M.; Lecour, S. The SAFE pathway for cardioprotection: Is this a promising target? Basic Res. Cardiol. 2018, 113, 9. [Google Scholar] [CrossRef] [PubMed]
- Comita, S.; Femmino, S.; Thairi, C.; Alloatti, G.; Boengler, K.; Pagliaro, P.; Penna, C. Regulation of STAT3 and its role in cardioprotection by conditioning: Focus on non-genomic roles targeting mitochondrial function. Basic Res. Cardiol. 2021, 116, 56. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P. Perspective: Mitochondrial STAT3 in cardioprotection. Basic Res. Cardiol. 2023, 118, 32. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.V.; Downey, J.M. Cardioprotection: Spotlight on PKG. Br. J. Pharmacol. 2007, 152, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L., Jr.; Loson, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sunahara, R.K.; Krumins, A.; Perkins, G.; Crochiere, M.L.; Mackey, M.; Bell, S.; Ellisman, M.H.; Taylor, S.S. Cloning and mitochondrial localization of full-length D-AKAP2, a protein kinase A anchoring protein. Proc. Natl. Acad. Sci. USA 2001, 98, 3220–3225. [Google Scholar] [CrossRef] [PubMed]
- Technikova-Dobrova, Z.; Sardanelli, A.M.; Speranza, F.; Scacco, S.; Signorile, A.; Lorusso, V.; Papa, S. Cyclic adenosine monophosphate-dependent phosphorylation of mammalian mitochondrial proteins: Enzyme and substrate characterization and functional role. Biochemistry 2001, 40, 13941–13947. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, A.M.; Signorile, A.; Nuzzi, R.; Rasmo, D.D.; Technikova-Dobrova, Z.; Drahota, Z.; Occhiello, A.; Pica, A.; Papa, S. Occurrence of A-kinase anchor protein and associated cAMP-dependent protein kinase in the inner compartment of mammalian mitochondria. FEBS Lett. 2006, 580, 5690–5696. [Google Scholar] [CrossRef] [PubMed]
- Pride, C.K.; Mo, L.; Quesnelle, K.; Dagda, R.K.; Murillo, D.; Geary, L.; Corey, C.; Portella, R.; Zharikov, S.; St, C.C.; et al. Nitrite activates protein kinase A in normoxia to mediate mitochondrial fusion and tolerance to ischaemia/reperfusion. Cardiovasc. Res. 2014, 101, 57–68. [Google Scholar] [CrossRef]
- Budas, G.R.; Churchill, E.N.; Disatnik, M.-H.; Sun, L.; Mochly-Rosen, D. Mitochondrial import of PKCe is mediated by HSP90: A role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc. Res. 2010, 88, 83–92. [Google Scholar] [CrossRef]
- Costa, A.D.; Jakob, R.; Costa, C.L.; Andrukhiv, K.; West, I.C.; Garlid, K.D. The mechanism by which the mitochondrial ATP-sensitive K+ channel opening and H2O2 inhibit the mitochondrial permeability transition. J. Biol. Chem. 2006, 281, 20801–20808. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, W.; Hu, K. Molecular mechanism underlying adenosine receptor-mediated mitochondrial targeting of protein kinase C. Biochim. Biophys. Acta 2012, 1823, 950–958. [Google Scholar] [CrossRef]
- Baines, C.P.; Zhang, J.; Wang, G.-W.; Zheng, Y.-T.; Xiu, J.X.; Cardwell, E.M.; Bolli, R.; Ping, P. Mitochondrial PKCe and MAPK form signaling modules in the murine heart. Circ. Res. 2002, 90, 390–397. [Google Scholar] [CrossRef]
- Ogbi, M.; Johnson, J.A. Protein kinase Cepsilon interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning. Biochem. J. 2006, 393, 191–199. [Google Scholar] [CrossRef]
- Baines, C.P.; Song, C.-X.; Zheng, Y.-T.; Wang, G.-W.; Zhang, J.; Wang, O.-L.; Guo, Y.; Bolli, R.; Cardwell, E.M.; Ping, P. Protein kinase Ce interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ. Res. 2003, 92, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Wong, R.; Rajapakse, N.; Murphy, E.; Steenbergen, C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ. Res. 2008, 103, 983–991. [Google Scholar] [CrossRef]
- Tanno, M.; Kuno, A.; Ishikawa, S.; Miki, T.; Kouzu, H.; Yano, T.; Murase, H.; Tobisawa, T.; Ogasawara, M.; Horio, Y.; et al. Translocation of glycogen synthase kinase-3β (GSK-3β), a trigger of permeability transition, is kinase activity-dependent and mediated by interaction with voltage-dependent anion channel 2 (VDAC2). J. Biol. Chem. 2014, 289, 29285–29296. [Google Scholar] [CrossRef]
- Nishihara, M.; Miura, T.; Miki, T.; Tanno, M.; Yano, T.; Naitoh, K.; Ohori, K.; Hotta, H.; Terashima, Y.; Shimamoto, K. Modulation of the mitochondrial permeability transition pore complex in GSK-3beta-mediated myocardial protection. J. Mol. Cell Cardiol. 2007, 43, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, J.G.; Hoek, J.B. Regulation of hexokinase binding to VDAC. J. Bioenerg. Biomembr. 2008, 40, 171–182. [Google Scholar] [CrossRef]
- Miyamoto, S.; Murphy, A.N.; Brown, J.H. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008, 15, 521–529. [Google Scholar] [CrossRef]
- Roberts, D.J.; Tan-Sah, V.P.; Smith, J.M.; Miyamoto, S. Akt phosphorylates HK-II at Thr-473 and increases mitochondrial HK-II association to protect cardiomyocytes. J. Biol. Chem. 2013, 288, 23798–23806. [Google Scholar] [CrossRef] [PubMed]
- Klepinin, A.; Ounpuu, L.; Mado, K.; Truu, L.; Chekulayev, V.; Puurand, M.; Shevchuk, I.; Tepp, K.; Planken, A.; Kaambre, T. The complexity of mitochondrial outer membrane permeability and VDAC regulation by associated proteins. J. Bioenerg. Biomembr. 2018, 50, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.C.; Wilson, R.J.; Laker, R.C.; Guan, Y.; Spaulding, H.R.; Nichenko, A.S.; Shen, W.; Shang, H.; Dorn, M.V.; Huang, K.; et al. Mitochondria-localized AMPK responds to local energetics and contributes to exercise and energetic stress-induced mitophagy. Proc. Natl. Acad. Sci. USA 2021, 118, e2025932118. [Google Scholar] [CrossRef] [PubMed]
- Win, S.; Than, T.A.; Han, D.; Petrovic, L.M.; Kaplowitz, N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J. Biol. Chem. 2011, 286, 35071–35078. [Google Scholar] [CrossRef]
- Ge, H.; Zhao, M.; Lee, S.; Xu, Z. Mitochondrial Src tyrosine kinase plays a role in the cardioprotective effect of ischemic preconditioning by modulating complex I activity and mitochondrial ROS generation. Free Radic. Res. 2015, 49, 1210–1217. [Google Scholar] [CrossRef]
- Guedouari, H.; Ould Amer, Y.; Pichaud, N.; Hebert-Chatelain, E. Characterization of the interactome of c-Src within the mitochondrial matrix by proximity-dependent biotin identification. Mitochondrion 2021, 57, 257–269. [Google Scholar] [CrossRef]
- Livigni, A.; Scorziello, A.; Agnese, S.; Adornetto, A.; Carlucci, A.; Garbi, C.; Castaldo, I.; Annunziato, L.; Avvedimento, E.V.; Feliciello, A. Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Mol. Biol. Cell 2006, 17, 263–271. [Google Scholar] [CrossRef]
- Feng, J.; Lucchinetti, E.; Enkavi, G.; Wang, Y.; Gehrig, P.; Roschitzki, B.; Schaub, M.C.; Tajkhorshid, E.; Zaugg, K.; Zaugg, M. Tyrosine phosphorylation by Src within the cavity of the adenine nucleotide translocase 1 regulates ADP/ATP exchange in mitochondria. Am. J. Physiol. Cell Physiol. 2010, 298, C740–C748. [Google Scholar] [CrossRef]
- Zang, Q.S.; Martinez, B.; Yao, X.; Maass, D.L.; Ma, L.; Wolf, S.E.; Minei, J.P. Sepsis-induced cardiac mitochondrial dysfunction involves altered mitochondrial-localization of tyrosine kinase Src and tyrosine phosphatase SHP2. PLoS ONE 2012, 7, e43424. [Google Scholar] [CrossRef]
- Itoh, S.; Lemay, S.; Osawa, M.; Che, W.; Duan, Y.; Tompkins, A.; Brookes, P.S.; Sheu, S.S.; Abe, J. Mitochondrial Dok-4 recruits Src kinase and regulates NF-kappaB activation in endothelial cells. J. Biol. Chem. 2005, 280, 26383–26396. [Google Scholar] [CrossRef]
- Luo, T.; Liu, H.; Kim, J.K. Estrogen protects the female heart from ischemia/reperfusion injury through manganese superoxide dismutase phosphorylation by mitochondrial p38β at threonine 79 and serine 106. PLoS ONE 2016, 11, e0167761. [Google Scholar] [CrossRef]
- Liu, H.; Yanamandala, M.; Lee, T.C.; Kim, J.K. Mitochondrial p38β and manganese superoxide dismutase interaction mediated by estrogen in cardiomyocytes. PLoS ONE 2014, 9, e85272. [Google Scholar] [CrossRef]
- Lazarou, M.; Jin, S.M.; Kane, L.A.; Youle, R.J. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev. Cell 2012, 22, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Reichert, A.S. How to get rid of mitochondria: Crosstalk and regulation of multiple mitophagy pathways. Biol. Chem. 2017, 399, 29–45. [Google Scholar] [CrossRef]
- Steer, E.K.; Dail, M.K.; Chu, C.T. Beyond mitophagy: Cytosolic PINK1 as a messenger of mitochondrial health. Antioxid. Redox Signal 2015, 22, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Kondapalli, C.; Kazlauskaite, A.; Zhang, N.; Woodroof, H.I.; Campbell, D.G.; Gourlay, R.; Burchell, L.; Walden, H.; Macartney, T.J.; Deak, M.; et al. PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2012, 2, 120080. [Google Scholar] [CrossRef]
- Inserte, J.; Garcia-Dorado, D.; Ruiz-Maena, M.; Agulló, J.; Pina, P.; Soler-Soler, J. Ischemic preconditioning attenuates calpain-mediated degradation of structural proteins through a protein kinase A-dependent mechanism. Cardiovasc. Res. 2004, 64, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Marais, E.; Genade, S.; Lochner, A. CREB activation and ischaemic preconditioning. Cardiovasc. Drugs Ther. 2008, 22, 3–17. [Google Scholar] [CrossRef]
- Yang, C.; Talukder, M.A.; Varadharaj, S.; Velayutham, M.; Zweier, J.L. Early ischaemic preconditioning requires Akt- and PKA-mediated activation of eNOS via serine1176 phosphorylation. Cardiovasc. Res. 2013, 97, 33–43. [Google Scholar] [CrossRef]
- Heusch, G.; Boengler, K.; Schulz, R. Cardioprotection: Nitric oxide, protein kinases, and mitochondria. Circulation 2008, 118, 1915–1919. [Google Scholar] [CrossRef]
- Simon, J.N.; Vrellaku, B.; Monterisi, S.; Chu, S.M.; Rawlings, N.; Lomas, O.; Marchal, G.A.; Waithe, D.; Syeda, F.; Gajendragadkar, P.R.; et al. Oxidation of protein kinase A regulatory subunit PKARIα protects against myocardial ischemia-reperfusion injury by inhibiting lysosomal-triggered calcium release. Circulation 2021, 143, 449–465. [Google Scholar] [CrossRef]
- Ould Amer, Y.; Hebert-Chatelain, E. Mitochondrial cAMP-PKA signaling: What do we really know? Biochim. Biophys. Acta Bioenerg. 2018, 1859, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Haushalter, K.J.; Schilling, J.M.; Song, Y.; Sastri, M.; Perkins, G.A.; Strack, S.; Taylor, S.S.; Patel, H.H. Cardiac ischemia-reperfusion injury induces ROS-dependent loss of PKA regulatory subunit RIα. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1231–H1242. [Google Scholar] [CrossRef] [PubMed]
- Juhaszova, M.; Zorov, D.B.; Kim, S.-H.; Pepe, S.; Fu, Q.; Fishbein, K.W.; Ziman, B.D.; Wang, S.; Ytrehus, K.; Antos, C.L.; et al. Glycogen synthase kinase-3ß mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Investig. 2004, 113, 1535–1549. [Google Scholar] [CrossRef] [PubMed]
- Juhaszova, M.; Zorov, D.B.; Yaniv, Y.; Nuss, H.B.; Wang, S.; Sollott, S.J. Role of glycogen synthase kinase-3β in cardioprotection. Circ. Res. 2009, 104, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, Y.; Wang, S.; McDonald, T.; van Eyk, J.E.; Sidor, A.; O’Rourke, B. Cytoprotective role of Ca2+ -activated K+ channels in the cardiac inner mitochondrial membrane. Science 2002, 298, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Saito, T.; Saegusa, N.; Nakaya, H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes. A mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation 2005, 111, 198–203. [Google Scholar] [CrossRef]
- Nagasaka, S.; Katoh, H.; Niu, C.F.; Matsui, S.; Urushida, T.; Satoh, H.; Watanabe, Y.; Hayashi, H. Protein kinase A catalytic subunit alters cardiac mitochondrial redox state and membrane potential via the formation of reactive oxygen species. Circ. J. 2007, 71, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.A.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Lin, Y.H.; Hausenloy, D.J. Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine 2020, 57, 102884. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Cattaneo, F.; Pironti, G.; Magliulo, F.; Carotenuto, G.; Pirozzi, M.; Polishchuk, R.; Borzacchiello, D.; Paolillo, R.; Oliveti, M.; et al. Akap1 deficiency promotes mitochondrial aberrations and exacerbates cardiac injury following permanent coronary ligation via enhanced mitophagy and apoptosis. PLoS ONE 2016, 11, e0154076. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Fontes, S.K.; Bautista, E.N.; Cheng, Z. Physiological and pathological roles of protein kinase A in the heart. Cardiovasc. Res. 2022, 118, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Ytrehus, K.; Liu, Y.; Downey, J.M. Preconditioning protects ischemic rabbit heart by protein C activation. Am. J. Physiol. Heart Circ. Physiol. 1994, 266, H1145–H1152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cohen, M.V.; Downey, J.M. Chelerythrine, a highly selective protein kinase C inhibitor, blocks the antiinfarct effect of ischemic preconditioning in rabbit hearts. Cardiovasc. Drugs Ther. 1994, 8, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Rastaldo, R.; Mancardi, D.; Raimondo, S.; Cappello, S.; Gattullo, D.; Losano, G.; Pagliario, P. Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res. Cardiol. 2006, 101, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Zatta, A.J.; Kin, H.; Lee, G.; Wang, N.; Jiang, R.; Lust, R.; Reeves, J.G.; Mykytenko, J.; Guyton, R.A.; Zhao, Z.Q.; et al. Infarct-sparing effect of myocardial postconditioning is dependent on protein kinase C signalling. Cardiovasc. Res. 2006, 70, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.R.; Hsu, A.K.; Urban, T.J.; Mochly-Rosen, D.; Gross, G.J. Nociceptive-induced myocardial remote conditioning is mediated by neuronal gamma protein kinase C. Basic Res. Cardiol. 2013, 108, 381. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Skyschally, A.; Heusch, G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflügers Arch-Eur. J. Physiol. 2017, 469, 159–181. [Google Scholar] [CrossRef] [PubMed]
- Vahlhaus, C.; Schulz, R.; Post, H.; Onallah, R.; Heusch, G. No prevention of ischemic preconditioning by the protein kinase C inhibitor staurosporine in swine. Circ. Res. 1996, 79, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Vahlhaus, C.; Schulz, R.; Post, H.; Rose, J.; Heusch, G. Prevention of ischemic preconditioning only by combined inhibition of protein kinase C and protein tyrosine kinase in pigs. J. Mol. Cell Cardiol. 1998, 30, 197–209. [Google Scholar] [CrossRef]
- Kitakaze, M.; Funaya, H.; Minamino, T.; Node, K.; Sato, H.; Ueda, Y.; Okuyama, Y.; Kuzuya, T.; Hori, M.; Yoshida, K.-I. Role of protein kinase C-a in activation of Ecto-5′-nucleotidase in the preconditioned canine myocardium. Biochem. Biophys. Res. Commun. 1997, 239, 171–175. [Google Scholar] [CrossRef]
- Schulz, R.; Gres, P.; Skyschally, A.; Duschin, A.; Belosjorow, S.; Konietzka, I.; Heusch, G. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. Faseb J. 2003, 17, 1355–1357. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.O.; Karliner, J.S.; Mochly-Rosen, D. A selective e-protein kinase c antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J. Biol. Chem. 1997, 272, 30945–30951. [Google Scholar] [CrossRef]
- Ping, P.; Song, C.; Zhang, J.; Guo, Y.; Cao, X.; Li, R.C.X.; Wu, W.; Vondriska, T.M.; Pass, J.M.; Tang, X.-L.; et al. Formation of protein kinase Ce-Lck signaling modules confers cardioprotection. J. Clin. Investig. 2002, 109, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Mayr, M.; Metzler, B.; Chung, Y.-L.; McGregor, E.; Mayr, U.; Troy, H.; Hu, Y.; Leitges, M.; Pachinger, O.; Griffiths, J.R.; et al. Ischemic preconditioning exaggerates cardiac damage in PKC-d null mice. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H946–H956. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, K.; Chen, L.; Ikeno, F.; Lee, F.H.; Imahashi, K.; Bouley, D.M.; Rezaee, M.; Yock, P.G.; Murphy, E.; Mochly-Rosen, D. Inhibition of d-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation 2003, 108, 2304–2307. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.M.; Townsend, P.A.; Davidson, S.M.; Carroll, C.J.; Eaton, S.; Hubank, M.; Knight, R.A.; Stephanou, A.; Latchman, D.S. The cardioprotective effect of urocortin during ischaemia/reperfusion involves the prevention of mitochondrial damage. Biochem. Biophys. Res. Commun. 2004, 321, 479–486. [Google Scholar] [CrossRef]
- Ohnuma, Y.; Miura, T.; Miki, T.; Tanno, M.; Kuno, A.; Tsuchida, A.; Shimamoto, K. Opening of mitochondrial KATP channel occurs downstream of PKC-e activation in the mechanism of preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H440–H447. [Google Scholar] [CrossRef]
- Costa, A.D.; Garlid, K.D. Intramitochondrial signaling—Interactions among mitoKATP, PKCe, ROS, and MPT. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H874–H882. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Korge, P.; Honda, H.M.; Ping, P. Role of the mitochondrial permeability transition in myocardial disease. Circ. Res. 2003, 93, 292–301. [Google Scholar] [CrossRef]
- Heinzel, F.R.; Luo, Y.; Li, X.; Boengler, K.; Buechert, A.; García-Dorado, D.; Di Lisa, F.; Schulz, R.; Heusch, G. Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ. Res. 2005, 97, 583–586. [Google Scholar] [CrossRef]
- Schwanke, U.; Konietzka, I.; Duschin, A.; Li, X.; Schulz, R.; Heusch, G. No ischemic preconditioning in heterozygous connexin43-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1740–H1742. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Leybaert, L.; Ruiz-Meana, M.; Schulz, R. Connexin 43 in mitochondria: What do we really know about its function? Front. Physiol. 2022, 13, 928934. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Stahlhofen, S.; van de Sand, A.; Gres, P.; Ruiz-Meana, M.; Garcia-Dorado, D.; Heusch, G.; Schulz, R. Presence of connexin 43 in subsarcolemmal but not in interfibrillar cardiomyocyte mitochondria. Basic Res. Cardiol. 2009, 104, 141–147. [Google Scholar] [CrossRef]
- Hirschhauser, C.; Lissoni, A.; Gorge, P.M.; Lampe, P.D.; Heger, J.; Schluter, K.D.; Leybaert, L.; Schulz, R.; Boengler, K. Connexin 43 phosphorylation by casein kinase 1 is essential for the cardioprotection by ischemic preconditioning. Basic Res. Cardiol. 2021, 116, 21. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.H.; Nguyen, T.; Ogbi, M.; Tawfik, H.; Ma, G.C.; Yu, Q.L.; Caldwell, R.W.; Johnson, J.A. Protein kinase C-ε coimmunoprecipitates with cytochrome oxidase subunit IV and is associated with improved cytochrome-c oxidase activity and cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2219–H2230. [Google Scholar] [CrossRef] [PubMed]
- Pravdic, D.; Sedlic, F.; Mio, Y.; Vladic, N.; Bienengraeber, M.; Bosnjak, Z.J. Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein kinase Cϵ–mediated pathway. Anesthesiology 2009, 111, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Murriel, C.L.; Churchill, E.; Inagaki, K.; Szweda, L.I.; Mochly-Rosen, D. Protein kinase Cδ activation induces apoptosis in response to cardiac ischemia and reperfusion damage: A mechanism involving BAD and the mitochondria. J. Biol. Chem. 2004, 279, 47985–47991. [Google Scholar] [CrossRef] [PubMed]
- Churchill, E.N.; Szweda, L.I. Translocation of δPKC to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Arch. Biochem. Biophys. 2005, 439, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, K.; Hahn, H.S.; Dorn II, G.W.; Mochly-Rosen, D. Additive protection of the ischemic heart ex vivo by combined treatment with d-protein kinase C inhibitor and e-protein kinase C activator. Circulation 2003, 108, 369–875. [Google Scholar] [CrossRef]
- Churchill, E.N.; Ferreira, J.C.; Brum, P.C.; Szweda, L.I.; Mochly-Rosen, D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of δPKC during reperfusion. Cardiovasc. Res. 2010, 85, 385–394. [Google Scholar] [CrossRef]
- Speechly-Dick, M.E.; Grover, G.J.; Yellon, D.M. Does ischemic preconditioning in the human involve protein kinase C and the ATP-dependent K+ channel? Studies of contractile function after simulated ischemia in an atrial in vitro model. Circ. Res. 1995, 77, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Hassouna, A.; Matata, B.M.; Galinanes, M. PKC-e is upstream and PKC-a is downstream of mitoKATP channels in the signal transduction pathway of ischemic preconditioning of human myocardium. Am. J. Physiol. Cell Physiol. 2004, 287, C1418–C1425. [Google Scholar] [CrossRef] [PubMed]
- Ikeno, F.; Inagaki, K.; Rezaee, M.; Mochly-Rosen, D. Impaired perfusion after myocardial infarction is due to reperfusion-induced δPKC-mediated myocardial damage. Cardiovasc. Res. 2007, 73, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Roe, M.; Aylward, P.; Galla, J.; Rynkiewicz, A.; Guetta, V.; Zelizko, M.; Kleiman, N.; White, H.; McErlean, E.; et al. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: Results of the PROTECTION AMI Randomized Controlled Trial. Eur. Heart J. 2014, 35, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Linding, R.; Jensen, L.J.; Ostheimer, G.J.; van Vugt, M.A.; Jorgensen, C.; Miron, I.M.; Diella, F.; Colwill, K.; Taylor, L.; Elder, K.; et al. Systematic discovery of in vivo phosphorylation networks. Cell 2007, 129, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yang, Z.; Xin, Z.; Yang, Y.; Yu, Y.; Cui, J.; Liu, H.; Chen, F. Glycogen synthase kinase-3β: A promising candidate in the fight against fibrosis. Theranostics 2020, 10, 11737–11753. [Google Scholar] [CrossRef] [PubMed]
- Antos, C.L.; McKinsey, T.A.; Frey, N.; Kutschke, W.; McAnally, J.; Shelton, J.M.; Richardson, J.A.; Hill, J.A.; Olson, E.N. Activated glycogen synthase-3β suppresses cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, S.; Zhai, P.; Tomita, H.; Galeotti, J.; Marquez, J.P.; Gao, S.; Hong, C.; Yatani, A.; Avila, J.; Sadoshima, J. Inhibition of glycogen synthase kinase 3beta during heart failure is protective. Circ. Res. 2007, 101, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Stachowski-Doll, M.J.; Papadaki, M.; Martin, T.G.; Ma, W.; Gong, H.M.; Shao, S.; Shen, S.; Muntu, N.A.; Kumar, M.; Perez, E.; et al. GSK-3β localizes to the cardiac Z-disc to maintain length dependent activation. Circ. Res. 2022, 130, 871–886. [Google Scholar] [CrossRef]
- Lal, H.; Zhou, J.; Ahmad, F.; Zaka, R.; Vagnozzi, R.J.; DeCaul, M.; Woodgett, J.; Gao, E.; Force, T. Glycogen synthase kinase-3α limits ischemic injury, cardiac rupture, post-myocardial infarction remodeling and death. Circulation 2012, 125, 65–75. [Google Scholar] [CrossRef]
- Cheng, H.; Woodgett, J.; Maamari, M.; Force, T. Targeting GSK-3 family members in the heart: A very sharp double-edged sword. J. Mol. Cell Cardiol. 2011, 51, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Force, T.; Woodgett, J.R. Unique and overlapping functions of GSK-3 isoforms in cell differentiation and proliferation and cardiovascular development. J. Biol. Chem. 2009, 284, 9643–9647. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.; Nikolakaki, E.; Plyte, S.E.; Totty, N.F.; Woodgett, J.R. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993, 12, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ge, C.; Chen, J.; Tang, K.; Liu, J. GSK-3beta inhibition confers cardioprotection associated with the restoration of mitochondrial function and suppression of endoplasmic reticulum stress in sevoflurane preconditioned rats following ischemia/reperfusion injury. Perfusion 2018, 33, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, X.; Guan, Y.; Shu, W.; Jia, P.; Jia, D. Hypercholesterolemia abrogates the cardioprotection of ischemic postconditioning in isolated rat heart: Roles of glycogen synthase kinase-3beta and the mitochondrial permeability transition pore. Cell Biochem. Biophys. 2014, 69, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Saotome, M.; Katoh, H.; Satoh, T.; Hasan, P.; Ohtani, H.; Satoh, H.; Hayashi, H.; Maekawa, Y. Glycogen synthase kinase-3β opens mitochondrial permeability transition pore through mitochondrial hexokinase II dissociation. J. Physiol. Sci. 2018, 68, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Terashima, Y.; Sato, T.; Yano, T.; Maas, O.; Itoh, T.; Miki, T.; Tanno, M.; Kuno, A.; Shimamoto, K.; Miura, T. Roles of phospho-GSK-3β in myocardial protection afforded by activation of the mitochondrial KATP channel. J. Mol. Cell Cardiol. 2010, 49, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Paillard, M.; Thibault, H.; Derumeaux, G.; Ovize, M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation 2008, 117, 2761–2768. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Imahashi, K.; Steenbergen, C.; Murphy, E. Phosphorylation of glycogen synthase kinase-3ß during preconditioning through a phosphatidylinositol-3-kinase-dependent pathway is cardioprotective. Circ. Res. 2002, 90, 374–376. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, M.; Marquez, J.C.; Jeong, S.H.; Ko, T.H.; Noh, Y.H.; Kha, P.T.; Choi, H.M.; Kim, D.H.; Kim, J.T.; et al. Novel GSK-3β inhibitor neopetroside A protects against murine myocardial ischemia/reperfusion injury. JACC Basic Transl. Sci. 2022, 7, 1102–1116. [Google Scholar] [CrossRef]
- Zhai, P.; Sciarretta, S.; Galeotti, J.; Volpe, M.; Sadoshima, J. Differential roles of GSK-3β during myocardial ischemia and ischemia/reperfusion. Circ. Res. 2011, 109, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, F.; Dos Santos, P.; Lemoine, S.; Bonnet, M.; Tariosse, L.; Couffinhal, T.; Duplaa, C.; Jaspard-Vinassa, B. GSK-3β at the crossroads in the signalling of heart preconditioning: Implication of mTOR and Wnt pathways. Cardiovasc. Res. 2011, 90, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, L.L.; Zhou, C.; Zhang, F.J.; Kong, F.J.; Wang, W.N.; Qian, L.B.; Wang, C.C.; Liu, X.B.; Yan, M.; et al. Hypercholesterolemic myocardium is vulnerable to ischemia-reperfusion injury and refractory to sevoflurane-induced protection. PLoS ONE 2013, 8, e76652. [Google Scholar] [CrossRef] [PubMed]
- Kindernay, L.; Farkasova, V.; Neckar, J.; Hrdlicka, J.; Ytrehus, K.; Ravingerova, T. Impact of maturation on myocardial response to ischemia and the effectiveness of remote preconditioning in male rats. Int. J. Mol. Sci. 2021, 22, 11009. [Google Scholar] [CrossRef] [PubMed]
- Nishino, Y.; Webb, I.G.; Davidson, S.M.; Ahmed, A.I.; Clark, J.E.; Jacquet, S.; Shah, A.M.; Miura, T.; Yellon, D.M.; Avkiran, M.; et al. Glycogen synthase kinase-3 inactivation is not required for ischemic preconditioning or postconditioning in the mouse. Circ. Res. 2008, 103, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Velez, D.E.; Mestre-Cordero, V.E.; Hermann, R.; Perego, J.; Harriet, S.; Fernandez-Pazos, M.L.M.; Mourglia, J.; Marina-Prendes, M.G. Rosuvastatin protects isolated hearts against ischemia-reperfusion injury: Role of Akt-GSK-3β, metabolic environment, and mitochondrial permeability transition pore. J. Physiol. Biochem. 2020, 76, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.; Mestre Cordero, V.E.; Fernandez Pazos, M.L.M.; Cordoba, M.F.; Reznik, F.J.; Velez, D.E.; Fellet, A.L.; Marina Prendes, M.G. Role of AMPK in the protective effects exerted by triiodothyronine in ischemic-reperfused myocardium. J. Mol. Endocrinol. 2021, 66, 207–221. [Google Scholar] [CrossRef]
- Murphy, E.; Steenbergen, C. Does inhibition of glycogen synthase kinase protect in mice? Circ. Res. 2008, 103, 226–228. [Google Scholar] [CrossRef]
- Nikolaou, P.E.; Boengler, K.; Efentakis, P.; Vouvogiannopoulou, K.; Zoga, A.; Gaboriaud-Kolar, N.; Myrianthopoulos, V.; Alexakos, P.; Kostomitsopoulos, N.; Rerras, I.; et al. Investigating and re-evaluating the role of glycogen synthase kinase 3 beta kinase as a molecular target for cardioprotection by using novel pharmacological inhibitors. Cardiovasc. Res. 2019, 115, 1228–1243. [Google Scholar] [CrossRef]
- Palmer, J.W.; Tandler, B.; Hoppel, C.L. Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: Effects of procedural manipulations. Arch. Biochem. Biophys. 1985, 236, 691–702. [Google Scholar] [CrossRef]
- Palmer, J.; Tandler, B.; Hoppel, C. Heterogeneous response of subsarcolemmal heart mitochondria to calcium. Am. J. Physiol. 1986, 250, H741–H748. [Google Scholar] [CrossRef] [PubMed]
- Kostyak, J.C.; Hunter, J.C.; Korzick, D.H. Acute PKCδ inhibition limits ischaemia–reperfusion injury in the aged rat heart: Role of GSK-3β. Cardiovasc. Res. 2006, 70, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, D.; Tanno, M.; Kuno, A.; Ishikawa, S.; Ogasawara, M.; Yano, T.; Miki, T.; Miura, T. Accelerated recovery of mitochondrial membrane potential by GSK-3β inactivation affords cardiomyocytes protection from oxidant-induced necrosis. PLoS ONE 2014, 9, e112529. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, Z.; Gao, J.; Shi, W.; Li, L.; Jiang, S.; Hu, H.; Liu, Z.; Xu, D.; Wu, L. The key roles of GSK-3β in regulating mitochondrial activity. Cell Physiol. Biochem. 2017, 44, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Miki, T. GSK-3β, a therapeutic target for cardiomyocyte protection. Circ. J. 2009, 73, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Endlicher, R.; Drahota, Z.; Stefkova, K.; Cervinkova, Z.; Kucera, O. The mitochondrial permeability transition pore-current knowledge of its structure, function, and regulation, and optimized methods for evaluating its functional state. Cells 2023, 12, 1273. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Miyamoto, S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015, 22, 248–257. [Google Scholar] [CrossRef]
- Calmettes, G.; John, S.A.; Weiss, J.N.; Ribalet, B. Hexokinase-mitochondrial interactions regulate glucose metabolism differentially in adult and neonatal cardiac myocytes. J. Gen. Physiol. 2013, 142, 425–436. [Google Scholar] [CrossRef]
- John, S.; Weiss, J.N.; Ribalet, B. Subcellular localization of hexokinases I and II directs the metabolic fate of glucose. PLoS ONE 2011, 6, e17674. [Google Scholar] [CrossRef]
- Sui, D.; Wilson, J.E. Structural determinants for the intracellular localization of the isozymes of mammalian hexokinase: Intracellular localization of fusion constructs incorporating structural elements from the hexokinase isozymes and the green fluorescent protein. Arch. Biochem. Biophys. 1997, 345, 111–125. [Google Scholar] [CrossRef]
- Yang, M.; Sun, J.; Stowe, D.F.; Tajkhorshid, E.; Kwok, W.M.; Camara, A.K.S. Knockout of VDAC1 in H9c2 cells promotes oxidative stress-induced cell apoptosis through decreased mitochondrial hexokinase II binding and enhanced glycolytic stress. Cell Physiol. Biochem. 2020, 54, 853–874. [Google Scholar] [CrossRef] [PubMed]
- Chiara, F.; Castellaro, D.; Marin, O.; Petronilli, V.; Brusilow, W.S.; Juhaszova, M.; Sollott, S.J.; Forte, M.; Bernardi, P.; Rasola, A. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS ONE 2008, 3, e1852. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.S.; Soukas, A.A. Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef] [PubMed]
- Nederlof, R.; van den Elshout, M.A.M.; Koeman, A.; Uthman, L.; Koning, I.; Eerbeek, O.; Weber, N.C.; Hollmann, M.W.; Zuurbier, C.J. Cyclophilin D ablation is associated with increased end-ischemic mitochondrial hexokinase activity. Sci. Rep. 2017, 7, 12749. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.P.; Smith, J.M.; Tu, M.; Yu, J.D.; Ding, E.Y.; Miyamoto, S. Dissociation of mitochondrial HK-II elicits mitophagy and confers cardioprotection against ischemia. Cell Death Dis. 2019, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Southworth, R.; Davey, K.A.; Warley, A.; Garlick, P.B. A reevaluation of the roles of hexokinase I and II in the heart. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H378–H386. [Google Scholar] [CrossRef] [PubMed]
- Pasdois, P.; Parker, J.E.; Griffiths, E.J.; Halestrap, A.P. Hexokinase II and reperfusion injury: TAT-HK2 peptide impairs vascular function in Langendorff-perfused rat hearts. Circ. Res. 2013, 112, e3–e7. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Smeele, K.M.; Wyatt, E.; Ichikawa, Y.; Eerbeek, O.; Sun, L.; Chawla, K.; Hollmann, M.W.; Nagpal, V.; Heikkinen, S.; et al. Reduction in hexokinase II levels results in decreased cardiac function and altered remodeling after ischemia/reperfusion injury. Circ. Res. 2011, 108, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Kolar, D.; Gresikova, M.; Waskova-Arnostova, P.; Elsnicova, B.; Kohutova, J.; Hornikova, D.; Vebr, P.; Neckar, J.; Blahova, T.; Kasparova, D.; et al. Adaptation to chronic continuous hypoxia potentiates Akt/HK2 anti-apoptotic pathway during brief myocardial ischemia/reperfusion insult. Mol. Cell Biochem. 2017, 432, 99–108. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Hu, S.; Shi, C.; Zhu, P.; Ma, Q.; Jin, Q.; Cao, F.; Tian, F.; Chen, Y. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal Res. 2017, 63, e12413. [Google Scholar] [CrossRef]
- Nederlof, R.; Gurel-Gurevin, E.; Eerbeek, O.; Xie, C.; Deijs, G.S.; Konkel, M.; Hu, J.; Weber, N.C.; Schumacher, C.A.; Baartscheer, A.; et al. Reducing mitochondrial bound hexokinase II mediates transition from non-injurious into injurious ischemia/reperfusion of the intact heart. J. Physiol. Biochem. 2016, 73, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Pasdois, P.; Parker, J.E.; Halestrap, A.P. Extent of mitochondrial hexokinase II dissociation during ischemia correlates with mitochondrial cytochrome c release, reactive oxygen species production, and infarct size on reperfusion. J. Am. Heart Assoc. 2012, 2, e005645. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, J.G.; Shulga, N.; Hoek, J.B. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 2002, 277, 7610–7618. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P.; Pereira, G.C.; Pasdois, P. The role of hexokinase in cardioprotection—Mechanism and potential for translation. Br. J. Pharmacol. 2014, 172, 2085–2100. [Google Scholar] [CrossRef] [PubMed]
- Calmettes, G.; Ribalet, B.; John, S.; Korge, P.; Ping, P.; Weiss, J.N. Hexokinases and cardioprotection. J. Mol. Cell Cardiol. 2014, 78, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Nederlof, R.; Denis, S.; Lauzier, B.; Rosiers, C.D.; Laakso, M.; Hagen, J.; Argmann, C.; Wanders, R.; Houtkooper, R.H.; Hollmann, M.W.; et al. Acute detachment of hexokinase II from mitochondria modestly increases oxygen consumption of the intact mouse heart. Metabolism 2017, 72, 66–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gurel, E.; Ustunova, S.; Kapucu, A.; Yilmazer, N.; Eerbeek, O.; Nederlof, R.; Hollmann, M.W.; Demirci-Tansel, C.; Zuurbier, C.J. Hexokinase cellular trafficking in ischemia-reperfusion and ischemic preconditioning is altered in type I diabetic heart. Mol. Biol. Rep. 2013, 40, 4153–4160. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Lledo, J.I.; Lynch, M. Evolution of mutation rates: Phylogenomic analysis of the photolyase/cryptochrome family. Mol. Biol. Evol. 2009, 26, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Smeele, K.M.; Southworth, R.; Wu, R.; Xie, C.; Nederlof, R.; Warley, A.; Nelson, J.K.; Van Horssen, P.; van den Wijngaard, J.P.; Heikkinen, S.; et al. Disruption of hexokinase II-mitochondrial binding blocks ischemic preconditioning and causes rapid cardiac necrosis. Circ. Res. 2011, 108, 1165–1169. [Google Scholar] [CrossRef]
- Nederlof, R.; Xie, C.; Eerbeek, O.; Koeman, A.; Milstein, D.M.; Hollmann, M.W.; Mik, E.G.; Warley, A.; Southworth, R.; Akar, F.G.; et al. Pathophysiological consequences of TAT-HKII peptide administration are independent of impaired vascular function and ensuing ischemia. Circ. Res. 2013, 112, e8–e13. [Google Scholar] [CrossRef][Green Version]
- Correa, F.; Garcia, N.; Gallardo-Perez, J.; Carreno-Fuentes, L.; Rodriguez-Enriquez, S.; Marin-Hernandez, A.; Zazueta, C. Post-conditioning preserves glycolytic ATP during early reperfusion: A survival mechanism for the reperfused heart. Cell Physiol. Biochem. 2008, 22, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Khaliulin, I.; Ascione, R.; Maslov, L.N.; Amal, H.; Suleiman, M.S. Preconditioning or postconditioning with 8-Br-cAMP-AM protects the heart against regional ischemia and reperfusion: A role for mitochondrial permeability transition. Cells 2021, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Nederlof, R.; Weber, N.C.; Juffermans, N.P.; de Mol, B.A.; Hollmann, M.W.; Preckel, B.; Zuurbier, C.J. A randomized trial of remote ischemic preconditioning and control treatment for cardioprotection in sevoflurane-anesthetized CABG patients. BMC Anesthesiol. 2017, 17, 51. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK—Sensing energy while talking to other signaling pathways. Cell Metab. 2014, 20, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, S.C.; Parajuli, N.; Dyck, J.R. The role of AMPK in cardiomyocyte health and survival. Biochim. Biophys. Acta 2016, 1862, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Young, L.H. AMPK: Energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol. Metab. 2015, 26, 422–429. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, B.; Li, T.; Zhu, Y.; Luo, G.; Jiang, Y.; Tang, F.; Jian, Z.; Xiao, Y. AMPK activation serves a critical role in mitochondria quality control via modulating mitophagy in the heart under chronic hypoxia. Int. J. Mol. Med. 2018, 41, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zou, M.H. AMPK, mitochondrial function, and cardiovascular disease. Int. J. Mol. Sci. 2020, 21, 4987. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Hausenloy, D.J.; Andreadou, I.; Horman, S.; Bertrand, L.; Beauloye, C. AMP-activated protein kinase: A remarkable contributor to preserve a healthy heart against ROS injury. Free Radic. Biol. Med. 2021, 166, 238–254. [Google Scholar] [CrossRef]
- Paiva, M.A.; Rutter-Locher, Z.; Goncalves, L.M.; Providencia, L.A.; Davidson, S.M.; Yellon, D.M.; Mocanu, M.M. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2123–H2134. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Miller, E.J.; Wright, T.M.; Li, J.; Qi, D.; Atsina, K.; Zaha, V.; Sakamoto, K.; Young, L.H. A small molecule AMPK activator protects the heart against ischemia-reperfusion injury. J. Mol. Cell Cardiol. 2011, 51, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, J.M.; Huang, H.; Kuznicki, M.; Zheng, S.; Sun, W.; Quan, N.; Wang, L.; Yang, H.; Guo, H.M.; et al. The protective effect of trimetazidine on myocardial ischemia/reperfusion injury through activating AMPK and ERK signaling pathway. Metabolism 2016, 65, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhang, X.; Liu, W.; Huang, Q.; Yang, W.; Fu, F.; Ma, H.; Su, H.; Wang, H.; Wang, J.; et al. AMPK-regulated and Akt-dependent enhancement of glucose uptake is essential in ischemic preconditioning-alleviated reperfusion injury. PLoS ONE 2013, 8, e69910. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Cao, J.; Song, D.; Tian, L.; Chen, K.; Wang, Y.; Gao, L.; Yin, Z.; Fan, Y.; Wang, C. Autophagy is involved in the cardioprotection effect of remote limb ischemic postconditioning on myocardial ischemia/reperfusion injury in normal mice, but not diabetic mice. PLoS ONE 2014, 9, e86838. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Zhu, S.; Hu, L.; Zhu, H.; Wu, X.; Li, Q. Myocardial ischemic postconditioning promotes autophagy against ischemia reperfusion injury via the activation of the nNOS/AMPK/mTOR pathway. Int. J. Mol. Sci. 2017, 18, 614. [Google Scholar] [CrossRef]
- Nishino, Y.; Miura, T.; Miki, T.; Sakamoto, J.; Nakamura, Y.; Ikeda, Y.; Kobayashi, H.; Shimamoto, K. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc. Res. 2004, 61, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Rohailla, S.; Clarizia, N.; Sourour, M.; Sourour, W.; Gelber, N.; Wei, C.; Li, J.; Redington, A.N. Acute, delayed and chronic remote ischemic conditioning is associated with downregulation of mTOR and enhanced autophagy signaling. PLoS ONE 2014, 9, e111291. [Google Scholar] [CrossRef]
- Bouhidel, O.; Pons, S.; Souktani, R.; Zini, R.; Berdeaux, A.; Ghaleh, B. Myocardial ischemic postconditioning against ischemia-reperfusion is impaired in ob/ob mice. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1580–H1586. [Google Scholar] [CrossRef]
- Hjortbak, M.V.; Gronnebaek, T.S.; Jespersen, N.R.; Lassen, T.R.; Seefeldt, J.M.; Tonnesen, P.T.; Jensen, R.V.; Koch, L.G.; Britton, S.L.; Pedersen, M.; et al. Differences in intrinsic aerobic capacity alters sensitivity to ischemia-reperfusion injury but not cardioprotective capacity by ischemic preconditioning in rats. PLoS ONE 2020, 15, e0240866. [Google Scholar] [CrossRef]
- Hermann, R.; Marina Prendes, M.G.; Torresin, M.E.; Velez, D.; Savino, E.A.; Varela, A. Effects of the AMP-activated protein kinase inhibitor compound C on the postconditioned rat heart. J. Physiol. Sci. 2012, 62, 333–341. [Google Scholar] [CrossRef]
- Aslam, M.; Ladilov, Y. Emerging role of cAMP/AMPK signaling. Cells 2022, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Zhang, C.S.; Li, M.; Wang, W.; Wang, Z.; Hawley, S.A.; Ma, T.; Feng, J.W.; Tian, X.; Qi, Q.; et al. Hierarchical activation of compartmentalized pools of AMPK depends on severity of nutrient or energy stress. Cell Res. 2019, 29, 460–473. [Google Scholar] [CrossRef]
- Miyamoto, T.; Rho, E.; Sample, V.; Akano, H.; Magari, M.; Ueno, T.; Gorshkov, K.; Chen, M.; Tokumitsu, H.; Zhang, J.; et al. Compartmentalized AMPK signaling illuminated by genetically encoded molecular sensors and actuators. Cell Rep. 2015, 11, 657–670. [Google Scholar] [CrossRef]
- Hoffman, N.J.; Parker, B.L.; Chaudhuri, R.; Fisher-Wellman, K.H.; Kleinert, M.; Humphrey, S.J.; Yang, P.; Holliday, M.; Trefely, S.; Fazakerley, D.J.; et al. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab. 2015, 22, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lei, C.; Wang, C.; Li, N.; Srivastava, M.; Tang, M.; Zhang, H.; Choi, J.M.; Jung, S.Y.; Qin, J.; et al. Global phosphoproteomic analysis reveals ARMC10 as an AMPK substrate that regulates mitochondrial dynamics. Nat. Commun. 2019, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Li, Y.; Heusch, G. Mitogen-activated protein kinases in the heart. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2001, 363, 245–266. [Google Scholar] [CrossRef]
- Shvedova, M.; Anfinogenova, Y.; Atochina-Vasserman, E.N.; Schepetkin, I.A.; Atochin, D.N. c-Jun N-terminal kinases (JNKs) in myocardial and cerebral ischemia/reperfusion injury. Front. Pharmacol. 2018, 9, 715. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Chernysheva, G.A.; Smol’yakova, V.I.; Aliev, O.I.; Fomina, T.I.; Sandrikina, L.A.; Sukhodolo, I.V.; Ivanova, V.V.; Osipenko, A.N.; Anfinogenova, N.D.; et al. Cardioprotective effects of a selective c-Jun N-terminal kinase inhibitor in a rat model of myocardial infarction. Biomedicines 2023, 11, 714. [Google Scholar] [CrossRef]
- Chambers, J.W.; Pachori, A.; Howard, S.; Iqbal, S.; LoGrasso, P.V. Inhibition of JNK mitochondrial localization and signaling is protective against ischemia/reperfusion injury in rats. J. Biol. Chem. 2013, 288, 4000–4011. [Google Scholar] [CrossRef]
- Jang, S.; Javadov, S. Inhibition of JNK aggravates the recovery of rat hearts after global ischemia: The role of mitochondrial JNK. PLoS ONE 2014, 9, e113526. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, W.; Chopra, I.; Li, H.F.; Dougherty, C.J.; Adi, J.; Adi, N.; Wang, H.; Webster, K.A. c-Jun N-terminal kinase (JNK-1) confers protection against brief but not extended ischemia during acute myocardial infarction. J. Biol. Chem. 2011, 286, 13995–14006. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, X.; Liu, Y.; Liu, Z. Morphine postconditioning protects against reperfusion injury via inhibiting JNK/p38 MAPK and mitochondrial permeability transition pores signaling pathways. Cell Physiol. Biochem. 2016, 39, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.Y.; Huang, J.; Zhu, H.J.; Wu, H.; Xu, S.J.; Irwin, M.G.; He, S.F.; Zhang, Y. Remifentanil preconditioning confers cardioprotection via c-Jun NH(2)-terminal kinases and extracellular signal regulated kinases pathways in ex-vivo failing rat heart. Eur. J. Pharmacol. 2018, 828, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Du, L.; Liu, Q.; Zhou, L.; Hu, Z. Remote limb ischemic conditioning produces cardioprotection in rats with testicular ischemia/reperfusion injury. Exp. Physiol. 2021, 106, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Kang, P.M.; Hampe, J.; Yoshimura, K.; Noma, T.; Matsuzaki, M.; Izumo, S. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J. Biol. Chem. 2002, 277, 10244–10250. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, D.; Chen, Y.; Liu, W.; Jin, F.; Bo, L. Selective inhibition of JNK located on mitochondria protects against mitochondrial dysfunction and cell death caused by endoplasmic reticulum stress in mice with LPS-induced ALI/ARDS. Int. J. Mol. Med. 2022, 49, 85. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.W.; Lograsso, P.V. Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J. Biol. Chem. 2011, 286, 16052–16062. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Jin, C.; Liu, J.; Wang, J.; Chen, Y. Honokiol attenuates acetaminophen-induced acute liver injury by inhibiting hepatic CYP1A2 activity and improving liver mitochondrial dysfunction. Chin. Herb. Med. 2023, 15, 231–239. [Google Scholar] [CrossRef]
- Hanawa, N.; Shinohara, M.; Saberi, B.; Gaarde, W.A.; Han, D.; Kaplowitz, N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008, 283, 13565–13577. [Google Scholar] [CrossRef]
- Zhou, Q.; Lam, P.Y.; Han, D.; Cadenas, E. c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J. Neurochem. 2008, 104, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Spigolon, G.; Bonny, C.; Culman, J.; Vercelli, A.; Herdegen, T. The JNK inhibitor D-JNKI-1 blocks apoptotic JNK signaling in brain mitochondria. Mol. Cell Neurosci. 2012, 49, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Nunez, R.E.; Javadov, S.; Escobales, N. Angiotensin II-preconditioning is associated with increased PKCε/PKCδ ratio and prosurvival kinases in mitochondria. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Heslop, K.A.; Rovini, A.; Hunt, E.G.; Fang, D.; Morris, M.E.; Christie, C.F.; Gooz, M.B.; DeHart, D.N.; Dang, Y.; Lemasters, J.J.; et al. JNK activation and translocation to mitochondria mediates mitochondrial dysfunction and cell death induced by VDAC opening and sorafenib in hepatocarcinoma cells. Biochem. Pharmacol. 2020, 171, 113728. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.W.; Cherry, L.; Laughlin, J.D.; Figuera-Losada, M.; Lograsso, P.V. Selective inhibition of mitochondrial JNK signaling achieved using peptide mimicry of the Sab kinase interacting motif-1 (KIM1). ACS Chem. Biol. 2011, 6, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Win, S.; Than, T.A.; Kaplowitz, N. The regulation of JNK signaling pathways in cell death through the interplay with mitochondrial SAB and upstream post-translational effects. Int. J. Mol. Sci. 2018, 19, 3657. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, Q. Mst1 regulates post-infarction cardiac injury through the JNK-Drp1-mitochondrial fission pathway. Cell Mol. Biol. Lett. 2018, 23, 21. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Li, R.; Hu, N.; Xin, T.; Zhu, P.; Hu, S.; Ma, S.; Zhu, H.; Ren, J.; Zhou, H. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018, 14, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Ping, P.; Zhang, J.; Zheng, Y.-T.; Li, R.C.X.; Dawn, B.; Tang, X.-L.; Takano, H.; Balafanova, Z.; Bolli, R. Demonstration of selective protein kinase C-dependent activation of Src and Lck tyrosine kinases during ischemic preconditioning in conscious rabbits. Circ. Res. 1999, 85, 542–550. [Google Scholar] [CrossRef]
- Vondriska, T.M.; Zhang, J.; Song, C.; Tang, X.-L.; Cao, X.; Baines, C.P.; Pass, J.M.; Wang, S.; Bolli, R.; Ping, P. Protein kinase C e-Src modules direct signal transduction in nitric oxide-induced cardioprotection. Complex formation as a means for cardioprotective signaling. Circ. Res. 2001, 88, 1306–1313. [Google Scholar] [CrossRef]
- Lim, S.; Smith, K.R.; Lim, S.T.; Tian, R.; Lu, J.; Tan, M. Regulation of mitochondrial functions by protein phosphorylation and dephosphorylation. Cell Biosci. 2016, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Zhang, Y.; Zhong, S.; Gao, F.; Chen, Y.; Wang, B.; Zhang, Z.; Cai, W.; Li, W.; Zheng, F.; et al. N-n-butyl haloperidol iodide ameliorates oxidative stress in mitochondria induced by hypoxia/reoxygenation through the mitochondrial c-Jun N-terminal kinase/Sab/Src/reactive oxygen species pathway in H9c2 cells. Oxid. Med. Cell Longev. 2019, 2019, 7417561. [Google Scholar] [CrossRef] [PubMed]
- Arachiche, A.; Augereau, O.; Decossas, M.; Pertuiset, C.; Gontier, E.; Letellier, T.; Dachary-Prigent, J. Localization of PTP-1B, SHP-2, and Src exclusively in rat brain mitochondria and functional consequences. J. Biol. Chem. 2008, 283, 24406–24411. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Huh, J.; Wang, H.; Kang, Y.; Lou, J.; Xu, Z. Mitochondrial events responsible for morphine’s cardioprotection against ischemia/reperfusion injury. Toxicol. Appl. Pharmacol. 2016, 290, 66–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, F.; Zheng, H.; Xi, J.; Cui, X.; Xu, Z. Roles of mitochondrial Src tyrosine kinase and zinc in nitric oxide-induced cardioprotection against ischemia/reperfusion injury. Free Radic. Res. 2013, 47, 517–525. [Google Scholar] [CrossRef]
- Dawn, B.; Takano, H.; Tang, X.-L.; Kodani, E.; Banerjee, S.; Rezazadeh, S.; Qui, Y.; Bolli, R. Role of Src protein tyrosine kinases in late preconditioning against myocardial infarction. AJP Heart Circ. Physiol. 2002, 283, H549–H556. [Google Scholar] [CrossRef]
- Mocanu, M.M.; Baxter, G.F.; Yue, Y.; Yellon, D.M. The p38 MAPK inhibitor, SB203580, abrogates ischaemic preconditioning in rat heart but timing of administration is critical. Basic Res. Cardiol. 2000, 95, 472–478. [Google Scholar] [CrossRef]
- Nakano, A.; Cohen, M.V.; Critz, S.; Downey, J.M. SB203580, an inhibitor of p38 MAPK, abolishes infarct-limiting effect of ischemic preconditioning in isolated rabbit hearts. Basic Res. Cardiol. 2000, 95, 466–471. [Google Scholar] [CrossRef]
- Schulz, R.; Belosjorow, S.; Gres, P.; Jansen, J.; Michel, M.C.; Heusch, G. p38 MAP kinase is a mediator of ischemic preconditioning in pigs. Cardiovasc. Res. 2002, 55, 690–700. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heidbreder, M.; Naumann, A.; Tempel, K.; Dominiak, P.; Dendorfer, A. Remote vs. ischaemic preconditioning: The differential role of mitogen-activated protein kinase pathways. Cardiovasc. Res. 2008, 78, 108–115. [Google Scholar] [CrossRef]
- Martin, E.D.; De Nicola, G.; Marber, M.S. New therapeutic targets in cardiology: p38 Alpha mitogen-activated protein kinase for ischemic heart disease. Circulation 2012, 126, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; Clark, J.E.; Jacquet, S.; Mohammadi, S.; Arthur, J.S.; O’Keefe, S.J.; Marber, M.S. The activation of p38alpha, and not p38beta, mitogen-activated protein kinase is required for ischemic preconditioning. J. Mol.Cell Cardiol. 2010, 48, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Kumphune, S.; Bassi, R.; Jacquet, S.; Sicard, P.; Clark, J.E.; Verma, S.; Avkiran, M.; O’Keefe, S.J.; Marber, M.S. A chemical genetic approach reveals that p38alpha MAPK activation by diphosphorylation aggravates myocardial infarction and is prevented by the direct binding of SB203580. J. Biol. Chem. 2010, 285, 2968–2975. [Google Scholar] [CrossRef]
- Saurin, A.T.; Martin, J.L.; Heads, R.J.; Foley, C.; Mockridge, J.W.; Wright, M.J.; Wang, Y.; Marber, M.S. The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. Faseb J. 2000, 14, 2237–2246. [Google Scholar] [CrossRef]
- Kong, J.Y.; Klassen, S.S.; Rabkin, S.W. Ceramide activates a mitochondrial p38 mitogen-activated protein kinase: A potential mechanism for loss of mitochondrial transmembrane potential and apoptosis. Mol. Cell Biochem. 2005, 278, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Ballard-Croft, C.; Kristo, G.; Yoshimura, Y.; Reid, E.; Keith, B.J.; Mentzer, R.M.; Lasley, R.D. Acute adenosine preconditioning is mediated by p38 MAPK activation in discrete subcellular compartments. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1359–H1366. [Google Scholar] [CrossRef]
- Kumphune, S.; Surinkaew, S.; Chattipakorn, S.C.; Chattipakorn, N. Inhibition of p38 MAPK activation protects cardiac mitochondria from ischemia/reperfusion injury. Pharm. Biol. 2015, 53, 1831–1841. [Google Scholar] [CrossRef]
- Javadov, S.; Jang, S.; Agostini, B. Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: Therapeutic perspectives. Pharmacol. Ther. 2014, 144, 202–225. [Google Scholar] [CrossRef]
- Newby, L.K.; Marber, M.S.; Melloni, C.; Sarov-Blat, L.; Aberle, L.H.; Aylward, P.E.; Cai, G.; de Winter, R.J.; Hamm, C.W.; Heitner, J.F.; et al. Losmapimod, a novel p38 mitogen-activated protein kinase inhibitor, in non-ST-segment elevation myocardial infarction: A randomised phase 2 trial. Lancet 2014, 384, 1187–1195. [Google Scholar] [CrossRef]
- Turkieh, A.; El Masri, Y.; Pinet, F.; Dubois-Deruy, E. Mitophagy regulation following myocardial infarction. Cells 2022, 11, 199. [Google Scholar] [CrossRef]
- Matsuda, N.; Sato, S.; Shiba, K.; Okatsu, K.; Saisho, K.; Gautier, C.A.; Sou, Y.S.; Saiki, S.; Kawajiri, S.; Sato, F.; et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 2010, 189, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Cleland, M.M.; Xu, S.; Narendra, D.P.; Suen, D.F.; Karbowski, M.; Youle, R.J. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 2010, 191, 1367–1380. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, Z.; Li, X.; Yang, Y.; Ding, S. FUNDC1: An emerging mitochondrial and MAMs protein for mitochondrial quality control in heart diseases. Int. J. Mol. Sci. 2023, 24, 9151. [Google Scholar] [CrossRef]
- Fu, T.; Ma, Y.; Li, Y.; Wang, Y.; Wang, Q.; Tong, Y. Mitophagy as a mitochondrial quality control mechanism in myocardial ischemic stress: From bench to bedside. Cell Stress. Chaperones 2023, 28, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Siddall, H.K.; Yellon, D.M.; Ong, S.B.; Mukherjee, U.A.; Burke, N.; Hall, A.R.; Angelova, P.R.; Ludtmann, M.H.; Deas, E.; Davidson, S.M.; et al. Loss of PINK1 increases the heart’s vulnerability to ischemia-reperfusion injury. PLoS ONE 2013, 8, e62400. [Google Scholar] [CrossRef]
- Kubli, D.A.; Zhang, X.; Lee, Y.; Hanna, R.A.; Quinsay, M.N.; Nguyen, C.K.; Jimenez, R.; Petrosyan, S.; Murphy, A.N.; Gustafsson, A.B. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J. Biol. Chem. 2013, 288, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Chen, H.; Wu, Q.; Xie, K. Hydrogen-rich saline alleviates inflammation and apoptosis in myocardial I/R injury via PINK-mediated autophagy. Int. J. Mol. Med. 2019, 44, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Leng, Y.; Lei, S.; Qiu, Z.; Ming, H.; Zhang, Y.; Zhang, A.; Wu, Y.; Xia, Z. The mitochondria-targeted antioxidant MitoQ ameliorates myocardial ischemia-reperfusion injury by enhancing PINK1/Parkin-mediated mitophagy in type 2 diabetic rats. Cell Stress. Chaperones 2022, 27, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, M.; Song, Y.; Hua, Y.; Jia, H.; Liu, J.; Wang, Y. Exercise attenuates myocardial I/R injury by regulating endoplasmic reticulum stress and mitophagy through M2AChR. Antioxid. Redox Signal 2024, 40, 209–221. [Google Scholar] [CrossRef]
- Ji, W.; Wei, S.; Hao, P.; Xing, J.; Yuan, Q.; Wang, J.; Xu, F.; Chen, Y. Aldehyde dehydrogenase 2 has cardioprotective effects on myocardial ischaemia/reperfusion injury via suppressing mitophagy. Front. Pharmacol. 2016, 7, 101. [Google Scholar] [CrossRef]
- Tu, M.; Tan, V.P.; Yu, J.D.; Tripathi, R.; Bigham, Z.; Barlow, M.; Smith, J.M.; Brown, J.H.; Miyamoto, S. RhoA signaling increases mitophagy and protects cardiomyocytes against ischemia by stabilizing PINK1 protein and recruiting Parkin to mitochondria. Cell Death Differ. 2022, 29, 2472–2486. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Miyamoto, S. RHOA, a small G-protein, signals to mitophagy through regulation of PINK1 protein stability and protects cardiomyocytes against ischemia. Autophagy 2023, 19, 1865–1866. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhou, L.Y.; Wang, J.X.; Wang, Y.; Sun, T.; Zhao, B.; Yang, Y.J.; An, T.; Long, B.; Li, N.; et al. E2F1-dependent miR-421 regulates mitochondrial fragmentation and myocardial infarction by targeting Pink1. Nat. Commun. 2015, 6, 7619. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wu, Y.; Tang, J.; Zhang, C.; Cheng, H.; Jiang, Q.; Jian, C. Remifentanil protects against myocardial ischemia/reperfusion injury via miR-205-mediated regulation of PINK1. J. Toxicol. Sci. 2021, 46, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhao, M.; Yang, Y.; Xue, R.Q.; Yu, X.J.; Liu, J.K.; Zang, W.J. Acetylcholine attenuates hypoxia/reoxygenation injury by inducing mitophagy through PINK1/Parkin signal pathway in H9c2 cells. J. Cell Physiol. 2016, 231, 1171–1781. [Google Scholar] [CrossRef] [PubMed]
- Sala-Mercado, J.A.; Wider, J.; Undyala, V.V.; Jahania, S.; Yoo, W.; Mentzer, R.M., Jr.; Gottlieb, R.A.; Przyklenk, K. Profound cardioprotection with chloramphenicol succinate in the swine model of myocardial ischemia-reperfusion injury. Circulation 2010, 122, S179–S184. [Google Scholar] [CrossRef] [PubMed]
- Leonard, P.A.; Bienz, S.R.; Clegg, D.O.; Ward, J.R. Hematuria in patients with rheumatoid arthritis receiving gold and D-penicillamine. J. Rheumatol. 1987, 14, 55–59. [Google Scholar] [PubMed]
- Jiao, Y.; Wang, J.; Jia, Y.; Xue, M. Remote ischemic preconditioning protects against cerebral ischemia injury in rats by upregulating miR-204-5p and activating the PINK1/Parkin signaling pathway. Metab. Brain Dis. 2022, 37, 945–959. [Google Scholar] [CrossRef]
- Bi, W.; Jia, J.; Pang, R.; Nie, C.; Han, J.; Ding, Z.; Liu, B.; Sheng, R.; Xu, J.; Zhang, J. Thyroid hormone postconditioning protects hearts from ischemia/reperfusion through reinforcing mitophagy. Biomed. Pharmacother. 2019, 118, 109220. [Google Scholar] [CrossRef]
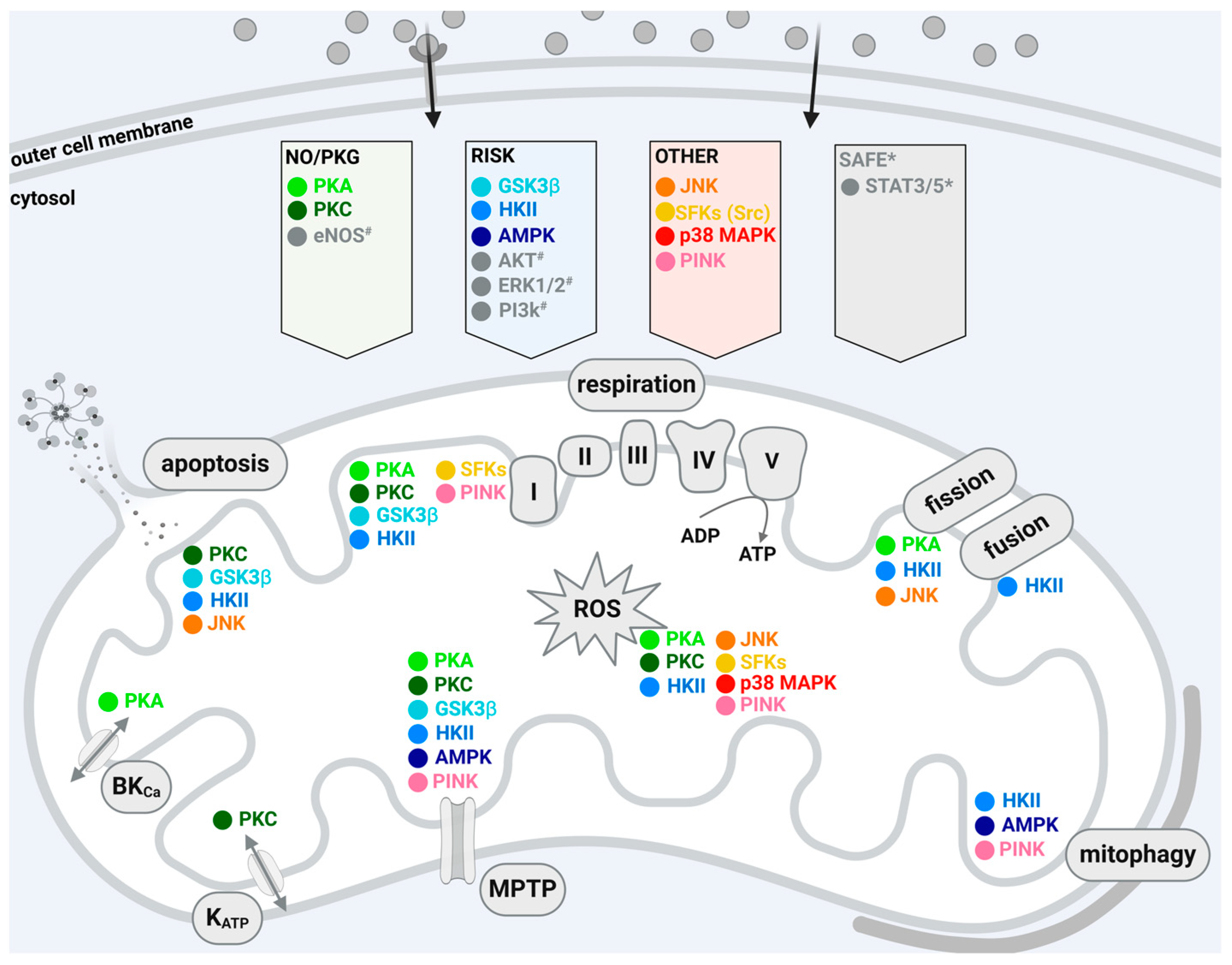

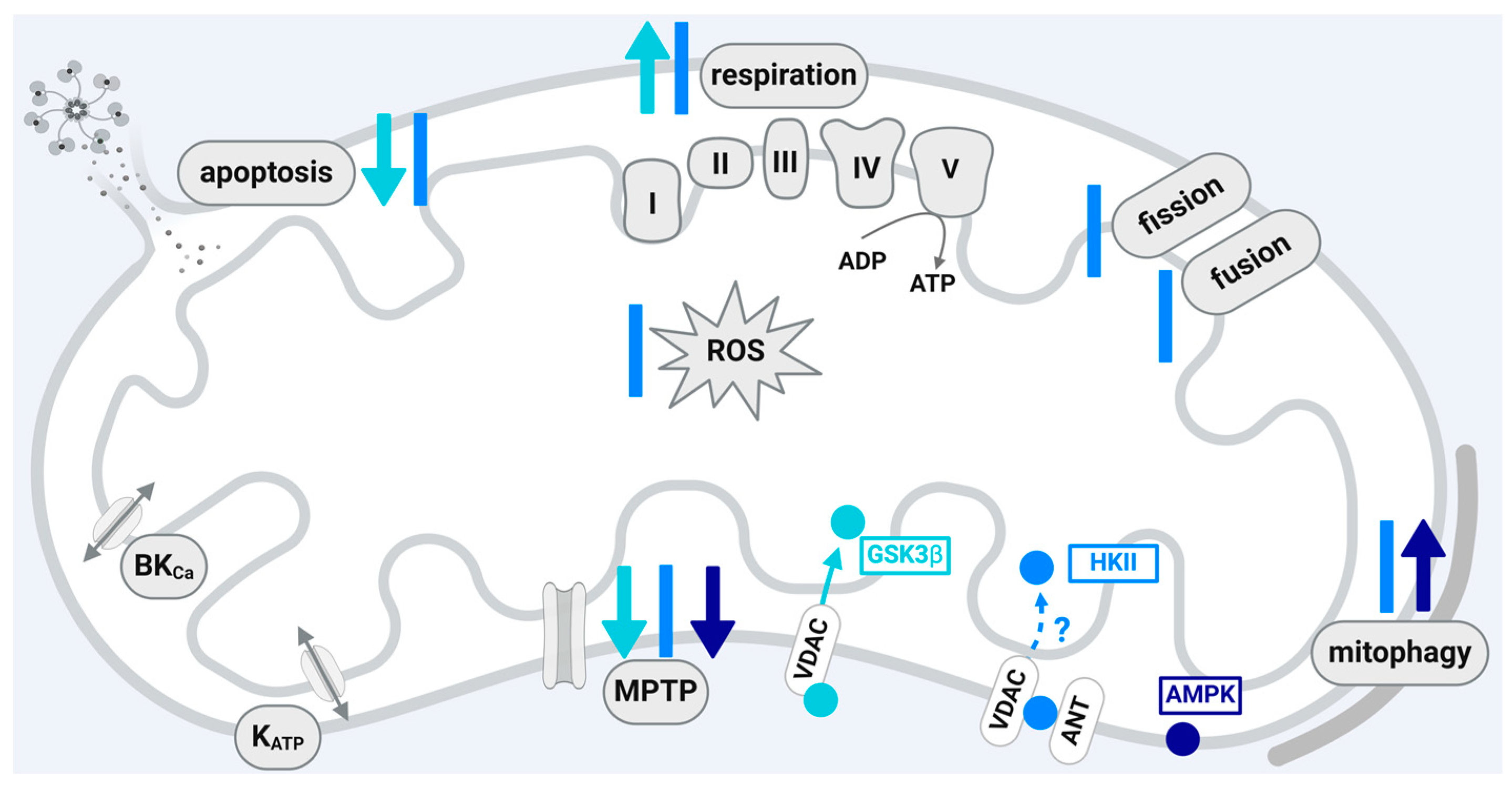
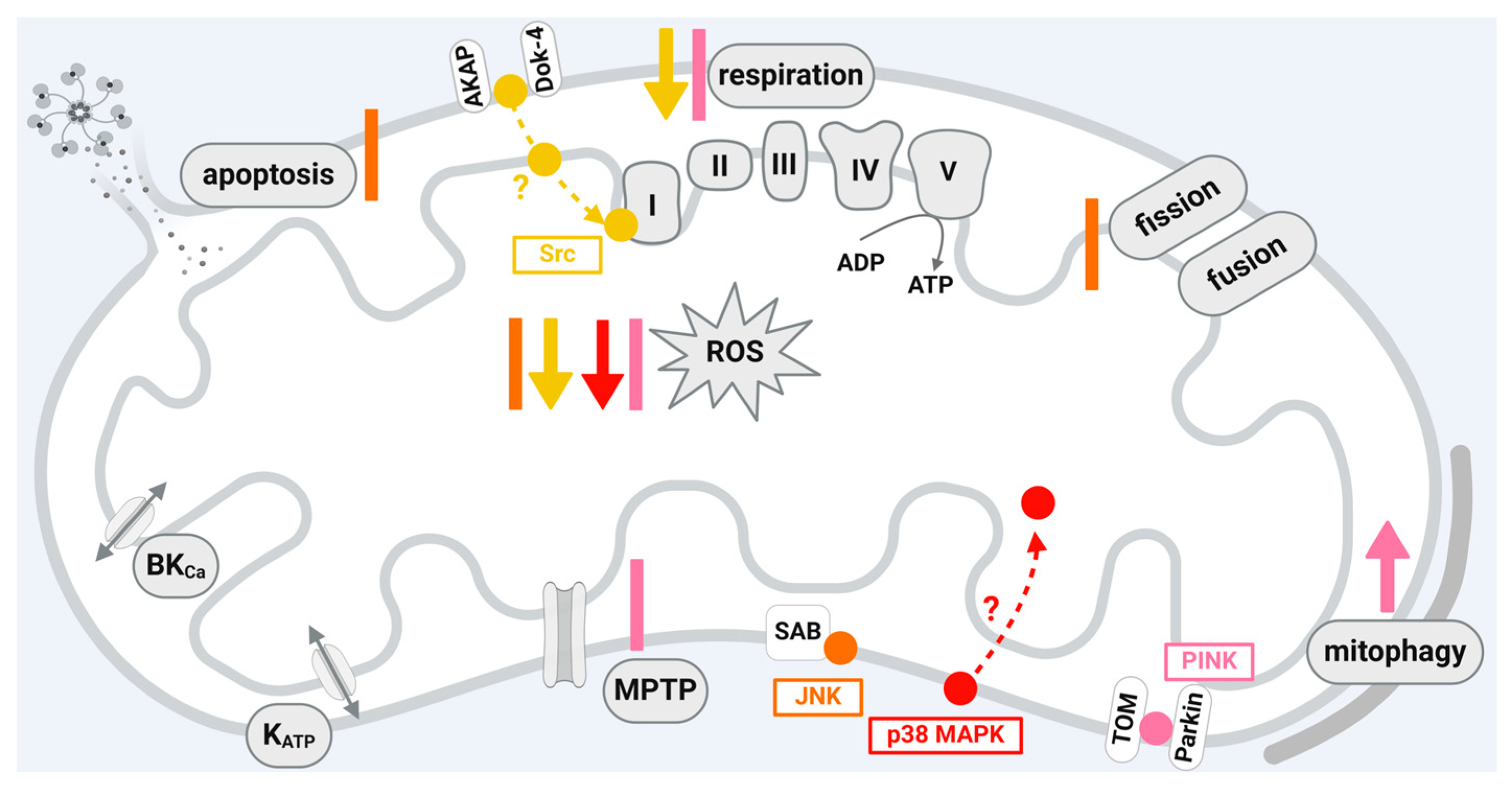
| Protein Kinase | Mitochondrial Localization | Translocation through | Interaction with |
|---|---|---|---|
| PKA | OMM: murine embryonic fibroblasts [35], mouse brain mitochondria [36]; IMM and matrix: bovine heart mitochondria [37]; rat heart mitochondria [38] | n.d. | AKAP: mouse brain mitochondria [36] ETC complex I: bovine heart mitochondria [37] Drp1: rat hearts [39] |
| PKCε | IMM: rat heart mitochondria [40] IMM and matrix: rat heart mitochondria [41] | HSP90-TOM20: rat heart mitochondria [40] HSP90-TOM70: rat cardiomyocytes [42] | JNK, p38 MAPK, ERK: mouse heart mitochondria [43] Cyt c oxidase subunit 4: neonatal rat cardiomyocytes [44] VDAC, ANT, HKII: mouse heart mitochondria [45] |
| GSK3β | possibly OMM: rat heart mitochondria [46] | VDAC2: H9C2 cells [47] | ANT: rat heart mitochondria [48] VDAC2: rat heart mitochondria [46] |
| HKII | OMM: reviewed in [49] | n.d. | AKT: neonatal rat cardiomyocytes [50]; transfected neonatal rat cardiomyocytes [51]; VDAC: HL1 cells [52], reviewed in [49] |
| AMPK | OMM: mouse gastrocnemius muscle mitochondria [53] | n.d. | MFF: transfected human embryonic kidney–293 T cells [35] |
| JNK | possibly OMM: mouse hepatocytes [54] | n.d. | SAB: mouse hepatocytes [54] |
| SFKs | IMM: rat heart mitochondria [55] Possibly matrix: HEK293 cells [56] | n.d. | AKAP121: GC2 cells [57] ANT1: rat heart mitochondria, HeLa cell mitochondria [58] ETC complex I: rat heart mitochondria [55] ETC complexes I and III: rat heart mitochondria [59] Dok-4: transfected bovine aortic endothelial cells [60] Diverse matrix proteins: HEK293 cells [56] |
| p38 MAPK | possibly matrix: mouse heart mitochondria [61] | n.d. | MnSOD: neonatal rat cardiomyocytes [62], mouse heart mitochondria [61] PKCε: mouse heart mitochondria [43] |
| PINK1 | OMM: HeLa cell mitos [63], reviewed in [64] | TOM complex: HeLa cell mitochondria [63], reviewed in [65] | E3 ubiquitin ligase Parkin complex: transduced Flp-In T-Rex HEK293 cells [66], reviewed in [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boengler, K.; Eickelmann, C.; Kleinbongard, P. Mitochondrial Kinase Signaling for Cardioprotection. Int. J. Mol. Sci. 2024, 25, 4491. https://doi.org/10.3390/ijms25084491
Boengler K, Eickelmann C, Kleinbongard P. Mitochondrial Kinase Signaling for Cardioprotection. International Journal of Molecular Sciences. 2024; 25(8):4491. https://doi.org/10.3390/ijms25084491
Chicago/Turabian StyleBoengler, Kerstin, Chantal Eickelmann, and Petra Kleinbongard. 2024. "Mitochondrial Kinase Signaling for Cardioprotection" International Journal of Molecular Sciences 25, no. 8: 4491. https://doi.org/10.3390/ijms25084491
APA StyleBoengler, K., Eickelmann, C., & Kleinbongard, P. (2024). Mitochondrial Kinase Signaling for Cardioprotection. International Journal of Molecular Sciences, 25(8), 4491. https://doi.org/10.3390/ijms25084491








