Patterns of Chromosomal Instability and Clonal Heterogeneity in Luminal B Breast Cancer: A Pilot Study
Abstract
1. Introduction
2. Results
2.1. Patients and Clinicopathological Data
2.2. CIN and CH Levels
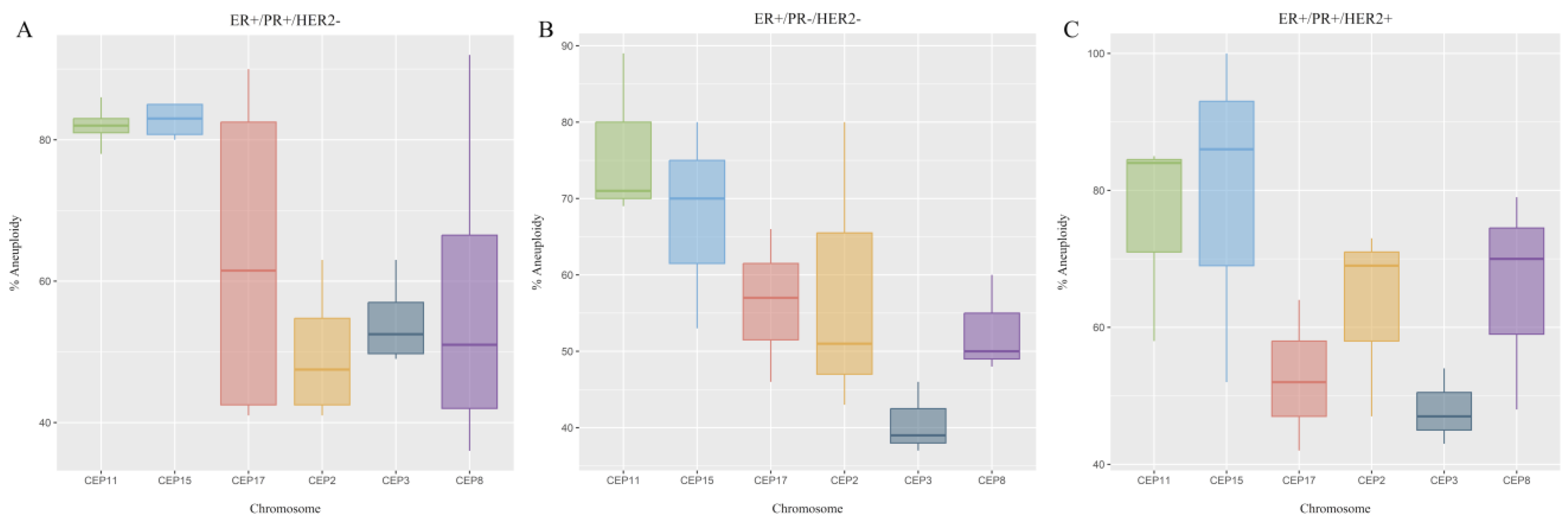
2.3. CEP Copy Number Variations (CNVs)
2.4. Determination of Stable and Unstable Aneuploidy
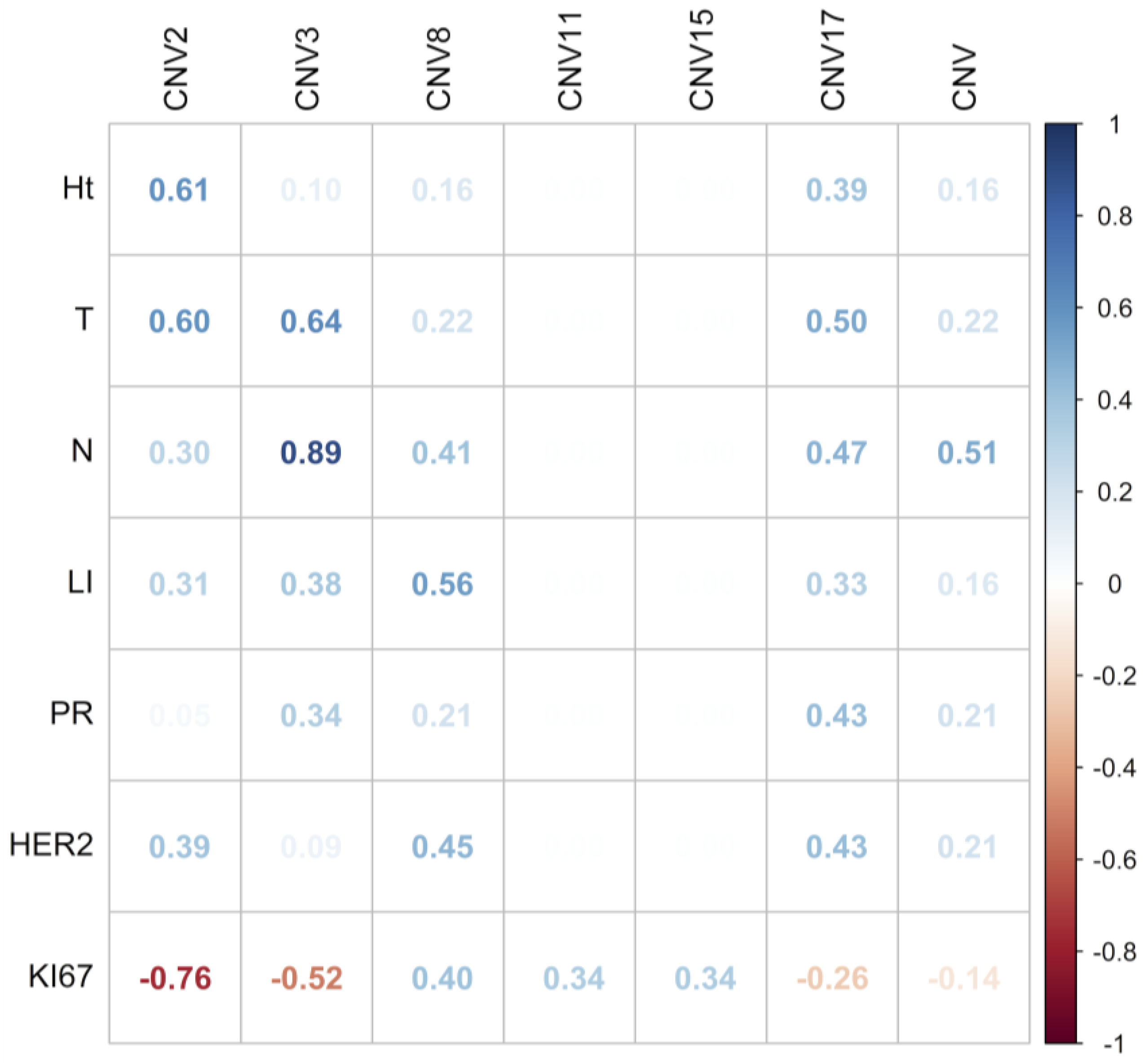
2.5. Association between CEP Copy Number Variations and CH with Clinicopathological Parameters
3. Discussion
4. Materials and Methods
4.1. Patients and Clinicopathological Data
4.2. Dual-Color Fluorescence In Situ Hybridization (FISH) Assays
4.3. Evaluation of CIN and CH
4.4. Evaluation of CEP Copy Number Variations (CNVs)
4.5. Determination of Stable and Unstable Aneuploidy
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehrgou, A.; Akouchekian, M. The Importance of BRCA1 and BRCA2 Genes Mutations in Breast Cancer Development. Med. J. Islam. Repub. Iran. 2016, 30, 369. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Haibe-Kains, B.; Desmedt, C.; Loi, S.; Culhane, A.C.; Bontempi, G.; Quackenbush, J.; Sotiriou, C. A Three-Gene Model to Robustly Identify Breast Cancer Molecular Subtypes. JNCI: J. Natl. Cancer Inst. 2012, 104, 311–325. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Sørlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated Observation of Breast Tumor Subtypes in Independent Gene Expression Data Sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Bedard, P.L. Luminal-B Breast Cancer and Novel Therapeutic Targets. Breast Cancer Res. 2011, 13, 221. [Google Scholar] [CrossRef]
- Creighton, C. The Molecular Profile of Luminal B Breast Cancer. Biologics 2012, 6, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Tao, Y.; Luo, J.; A’Hern, R.; Evans, D.B.; Bhatnagar, A.S.; Chaudri Ross, H.A.; von Kameke, A.; Miller, W.R.; Smith, I.; et al. Outcome Prediction for Estrogen Receptor-Positive Breast Cancer Based on Postneoadjuvant Endocrine Therapy Tumor Characteristics. JNCI J. Natl. Cancer Inst. 2008, 100, 1380–1388. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the Treatment of Women with Early Breast Cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Pikor, L.; Thu, K.; Vucic, E.; Lam, W. The Detection and Implication of Genome Instability in Cancer. Cancer Metastasis Rev. 2013, 32, 341–352. [Google Scholar] [CrossRef]
- Tanaka, K.; Hirota, T. Chromosomal Instability: A Common Feature and a Therapeutic Target of Cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2016, 1866, 64–75. [Google Scholar] [CrossRef]
- Geigl, J.B.; Obenauf, A.C.; Schwarzbraun, T.; Speicher, M.R. Defining ‘Chromosomal Instability’. Trends Genet. 2008, 24, 64–69. [Google Scholar] [CrossRef]
- Gagos, S.; Irminger-Finger, I. Chromosome Instability in Neoplasia: Chaotic Roots to Continuous Growth. Int. J. Biochem. Cell Biol. 2005, 37, 1014–1033. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic Instability—An Evolving Hallmark of Cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Kalimutho, M.; Nones, K.; Srihari, S.; Duijf, P.H.G.; Waddell, N.; Khanna, K.K. Patterns of Genomic Instability in Breast Cancer. Trends Pharmacol. Sci. 2019, 40, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Dayal, J.H.S.; Albergante, L.; Newman, T.J.; South, A.P. Quantitation of Multiclonality in Control and Drug-Treated Tumour Populations Using High-Throughput Analysis of Karyotypic Heterogeneity. Converg. Sci. Phys. Oncol. 2015, 1, 025001. [Google Scholar] [CrossRef]
- Fedorenko, I.V.; Wargo, J.A.; Flaherty, K.T.; Messina, J.L.; Smalley, K.S.M. BRAF Inhibition Generates a Host–Tumor Niche That Mediates Therapeutic Escape. J. Investig. Dermatol. 2015, 135, 3115–3124. [Google Scholar] [CrossRef]
- Birkbak, N.J.; Eklund, A.C.; Li, Q.; McClelland, S.E.; Endesfelder, D.; Tan, P.; Tan, I.B.; Richardson, A.L.; Szallasi, Z.; Swanton, C. Paradoxical Relationship between Chromosomal Instability and Survival Outcome in Cancer. Cancer Res. 2011, 71, 3447–3452. [Google Scholar] [CrossRef]
- Lee, K.; Jang, M.H.; Chung, Y.R.; Lee, Y.; Kang, E.; Kim, S.-W.; Kim, Y.J.; Kim, J.H.; Kim, I.A.; Park, S.Y. Prognostic Significance of Centromere 17 Copy Number Gain in Breast Cancer Depends on Breast Cancer Subtype. Hum. Pathol. 2017, 61, 111–120. [Google Scholar] [CrossRef]
- Andrade, J.R.; Gallagher, A.D.; Maharaj, J.; McClelland, S.E. Disentangling the Roles of Aneuploidy, Chromosomal Instability and Tumour Heterogeneity in Developing Resistance to Cancer Therapies. Chromosome Res. 2023, 31, 28. [Google Scholar] [CrossRef]
- Rajagopalan, H.; Lengauer, C. Aneuploidy and Cancer. Nature 2004, 432, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Ades, F.; Zardavas, D.; Bozovic-Spasojevic, I.; Pugliano, L.; Fumagalli, D.; de Azambuja, E.; Viale, G.; Sotiriou, C.; Piccart, M. Luminal B Breast Cancer: Molecular Characterization, Clinical Management, and Future Perspectives. J. Clin. Oncol. 2014, 32, 2794–2803. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Shin, H.C.; Bae, Y.K.; Kim, M.K.; Kang, S.H.; Lee, S.J.; Lee, E.H. Multiplication of Chromosome 17 Centromere Is Associated with Prognosis in Patients with Invasive Breast Cancers Exhibiting Normal HER2 and TOP2A Status. J. Breast Cancer 2012, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.V.; Ejlertsen, B.; Møller, S.; Jensen, M.-B.; Balslev, E.; Müller, S.; Knoop, A.; Mouridsen, H.T. Lack of Independent Prognostic and Predictive Value of Centromere 17 Copy Number Changes in Breast Cancer Patients with Known HER2 and TOP2A Status. Mol. Oncol. 2012, 6, 88–97. [Google Scholar] [CrossRef]
- Bartlett, J.M.; Munro, A.F.; Dunn, J.A.; McConkey, C.; Jordan, S.; Twelves, C.J.; Cameron, D.A.; Thomas, J.; Campbell, F.M.; Rea, D.W.; et al. Predictive Markers of Anthracycline Benefit: A Prospectively Planned Analysis of the UK National Epirubicin Adjuvant Trial (NEAT/BR9601). Lancet Oncol. 2010, 11, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Tibau, A.; López-Vilaró, L.; Pérez-Olabarria, M.; Vázquez, T.; Pons, C.; Gich, I.; Alonso, C.; Ojeda, B.; Ramón y Cajal, T.; Lerma, E.; et al. Chromosome 17 Centromere Duplication and Responsiveness to Anthracycline-Based Neoadjuvant Chemotherapy in Breast Cancer. Neoplasia 2014, 16, 861–867. [Google Scholar] [CrossRef]
- Lebok, P.; Mittenzwei, A.; Kluth, M.; Özden, C.; Taskin, B.; Hussein, K.; Möller, K.; Hartmann, A.; Lebeau, A.; Witzel, I.; et al. 8p Deletion Is Strongly Linked to Poor Prognosis in Breast Cancer. Cancer Biol. Ther. 2015, 16, 1080–1087. [Google Scholar] [CrossRef]
- Watkins, T.B.K.; Lim, E.L.; Petkovic, M.; Elizalde, S.; Birkbak, N.J.; Wilson, G.A.; Moore, D.A.; Grönroos, E.; Rowan, A.; Dewhurst, S.M.; et al. Pervasive Chromosomal Instability and Karyotype Order in Tumour Evolution. Nature 2020, 587, 126–132. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Ross, J.S.; Fletcher, J.A. Tumor Suppressor Genes in Breast Cancer. Pathol. Patterns Rev. 2005, 124 (Suppl S1), S16–S28. [Google Scholar] [CrossRef]
- Liu, H.; Lu, Z.-G.; Miki, Y.; Yoshida, K. Protein Kinase C δ Induces Transcription of the TP53 Tumor Suppressor Gene by Controlling Death-Promoting Factor Btf in the Apoptotic Response to DNA Damage. Mol. Cell Biol. 2007, 27, 8480–8491. [Google Scholar] [CrossRef]
- Shiovitz, S.; Korde, L.A. Genetics of Breast Cancer: A Topic in Evolution. Ann. Oncol. 2015, 26, 1291–1299. [Google Scholar] [CrossRef]
- Wang, L.; Lee, K.; Malonis, R.; Sanchez, I.; Dynlacht, B.D. Tethering of an E3 Ligase by PCM1 Regulates the Abundance of Centrosomal KIAA0586/Talpid3 and Promotes Ciliogenesis. Elife 2016, 5, e12950. [Google Scholar] [CrossRef]
- Yuan, B.-Z.; Zhou, X.; Durkin, M.E.; Zimonjic, D.B.; Gumundsdottir, K.; Eyfjord, J.E.; Thorgeirsson, S.S.; Popescu, N.C. DLC-1 Gene Inhibits Human Breast Cancer Cell Growth and in Vivo Tumorigenicity. Oncogene 2003, 22, 445–450. [Google Scholar] [CrossRef]
- Guan, C.-N.; Zhang, P.-W.; Lou, H.-Q.; Liao, X.-H.; Chen, B.-Y. DLC-1 Expression Levels in Breast Cancer Assessed by QRT-PCR Are Negatively Associated with Malignancy. Asian Pac. J. Cancer Prev. 2012, 13, 1231–1233. [Google Scholar] [CrossRef][Green Version]
- Rodrigues-Ferreira, S.; Di Tommaso, A.; Dimitrov, A.; Cazaubon, S.; Gruel, N.; Colasson, H.; Nicolas, A.; Chaverot, N.; Molinié, V.; Reyal, F.; et al. 8p22 MTUS1 Gene Product ATIP3 Is a Novel Anti-Mitotic Protein Underexpressed in Invasive Breast Carcinoma of Poor Prognosis. PLoS ONE 2009, 4, e7239. [Google Scholar] [CrossRef]
- Lovat, F.; Ishii, H.; Schiappacassi, M.; Fassan, M.; Barbareschi, M.; Galligioni, E.; Gasparini, P.; Baldassarre, G.; Croce, C.M.; Vecchione, A. LZTS1 Downregulation Confers Paclitaxel Resistance and Is Associated with Worse Prognosis in Breast Cancer. Oncotarget 2014, 5, 970–977. [Google Scholar] [CrossRef]
- Pirzio, L.M.; Pichierri, P.; Bignami, M.; Franchitto, A. Werner Syndrome Helicase Activity Is Essential in Maintaining Fragile Site Stability. J. Cell Biol. 2008, 180, 305–314. [Google Scholar] [CrossRef]
- Svoboda, M.; Sana, J.; Redova, M.; Navratil, J.; Palacova, M.; Fabian, P.; Slaby, O.; Vyzula, R. MiR-34b Is Associated with Clinical Outcome in Triple-Negative Breast Cancer Patients. Diagn. Pathol. 2012, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; Liu, Q.; Cochran, C.; Bennett, L.M.; Ding, W.; et al. A Strong Candidate for the Breast and Ovarian Cancer Susceptibility Gene BRCA1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Roylance, R.; Endesfelder, D.; Gorman, P.; Burrell, R.A.; Sander, J.; Tomlinson, I.; Hanby, A.M.; Speirs, V.; Richardson, A.L.; Birkbak, N.J.; et al. Relationship of Extreme Chromosomal Instability with Long-Term Survival in a Retrospective Analysis of Primary Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.R.; Pandis, N.; Heim, S. Tumors of the Breast. In Cancer Cytogenetics; Wiley: Hoboken, NJ, USA, 2015; pp. 426–446. [Google Scholar] [CrossRef]
- Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Genetic Instability in Colorectal Cancers. Nature 1997, 386, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.F.; Twelves, C.; Thomas, J.S.; Cameron, D.A.; Bartlett, J.M. Chromosome Instability and Benefit from Adjuvant Anthracyclines in Breast Cancer. Br. J. Cancer 2012, 107, 71–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maley, C.C.; Galipeau, P.C.; Finley, J.C.; Wongsurawat, V.J.; Li, X.; Sanchez, C.A.; Paulson, T.G.; Blount, P.L.; Risques, R.-A.; Rabinovitch, P.S.; et al. Genetic Clonal Diversity Predicts Progression to Esophageal Adenocarcinoma. Nat. Genet. 2006, 38, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, H.J.; Jang, M.H.; Lee, S.; Ahn, S.; Park, S.Y. Centromere 17 Copy Number Gain Reflects Chromosomal Instability in Breast Cancer. Sci. Rep. 2019, 9, 17968. [Google Scholar] [CrossRef]
- Lingle, W.L.; Barrett, S.L.; Negron, V.C.; D’Assoro, A.B.; Boeneman, K.; Liu, W.; Whitehead, C.M.; Reynolds, C.; Salisbury, J.L. Centrosome Amplification Drives Chromosomal Instability in Breast Tumor Development. Proc. Natl. Acad. Sci. USA 2002, 99, 1978–1983. [Google Scholar] [CrossRef]
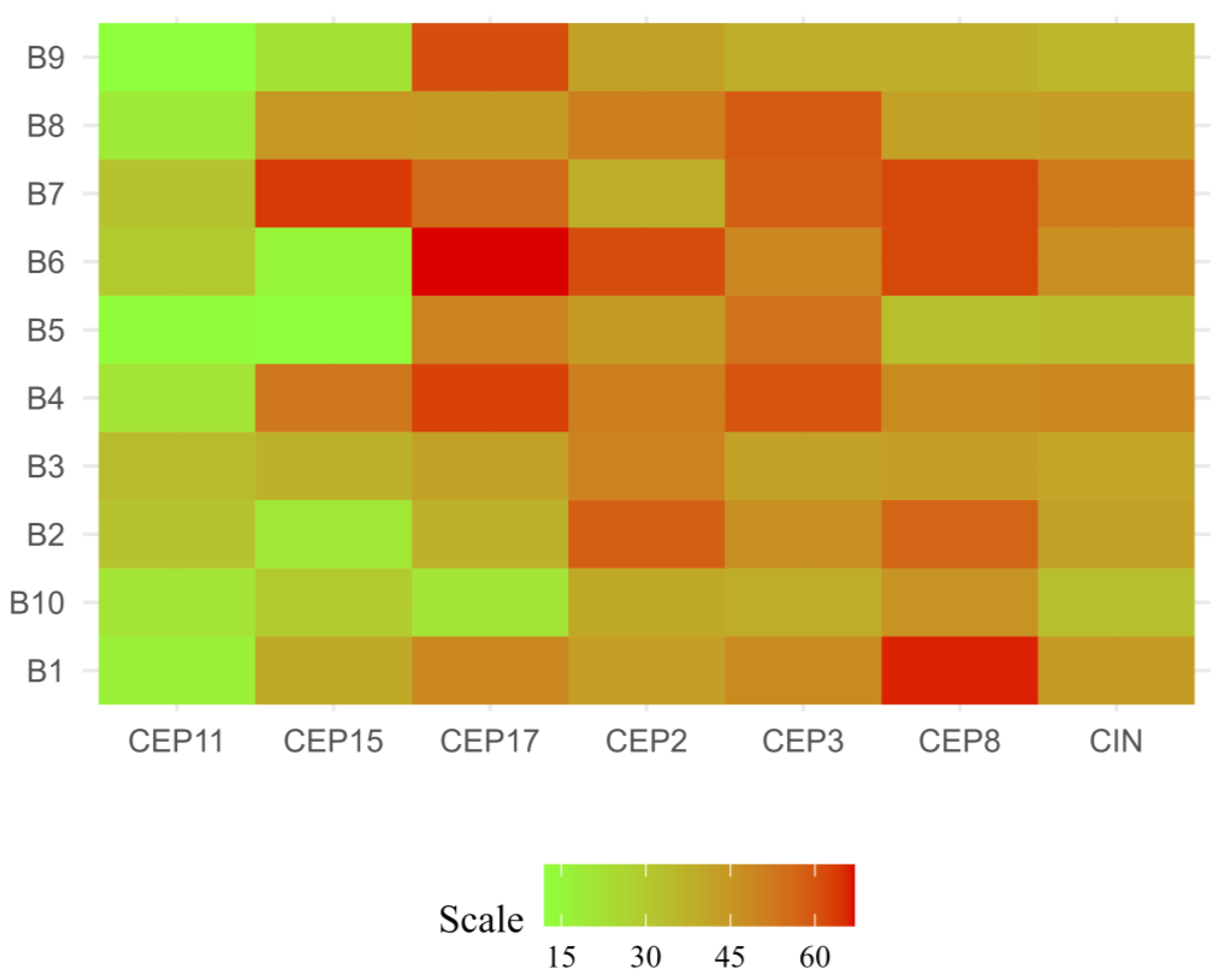
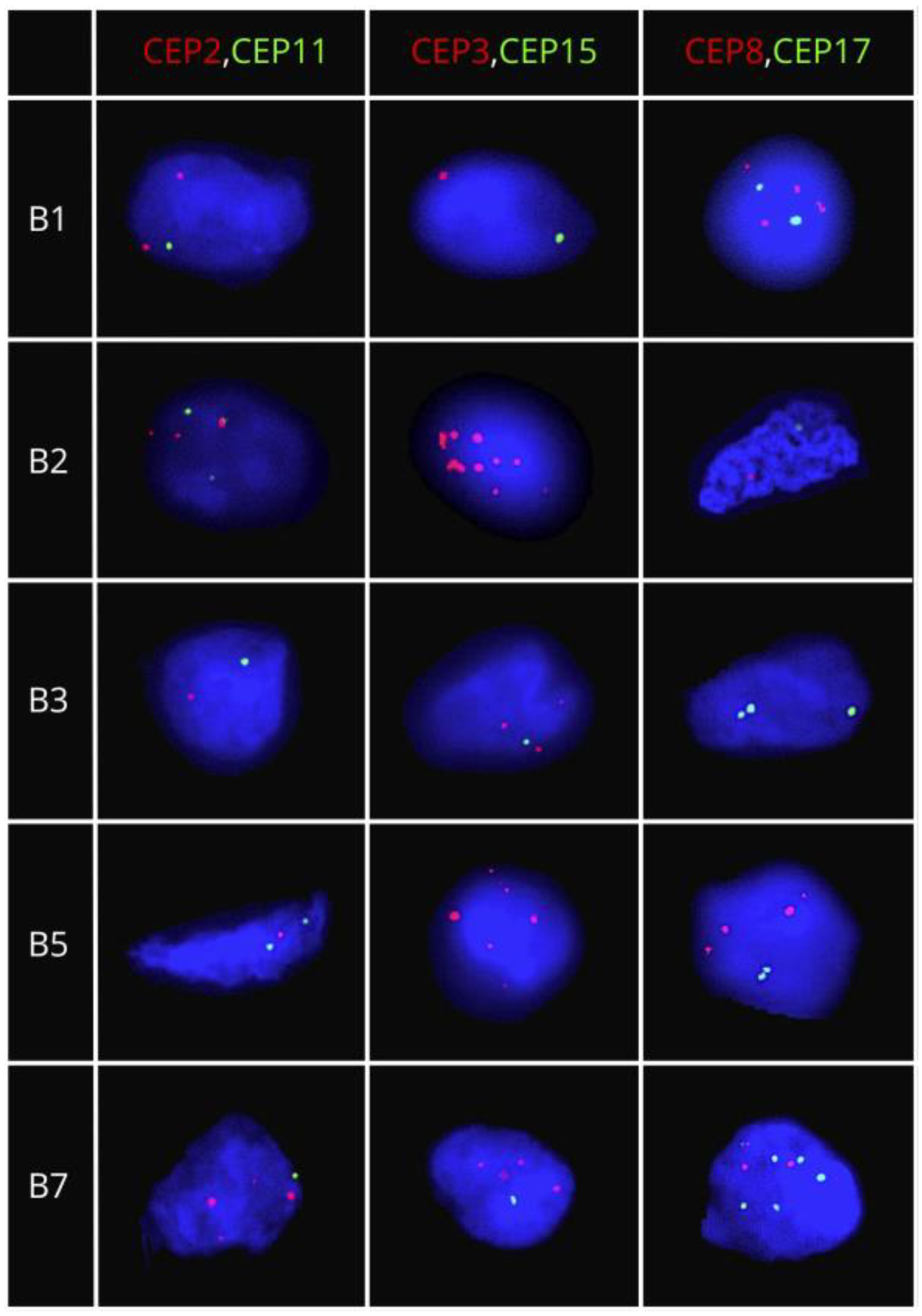

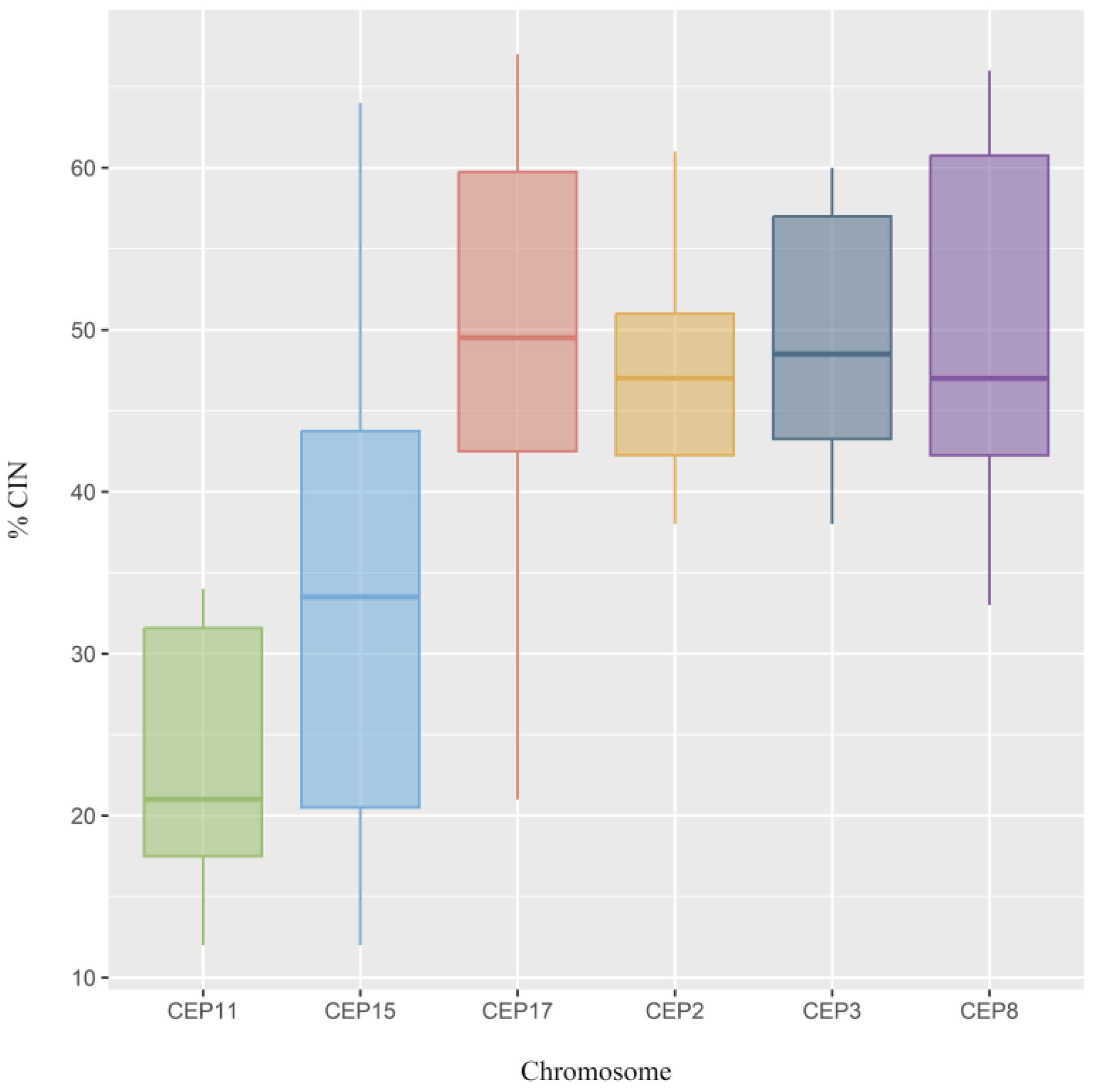
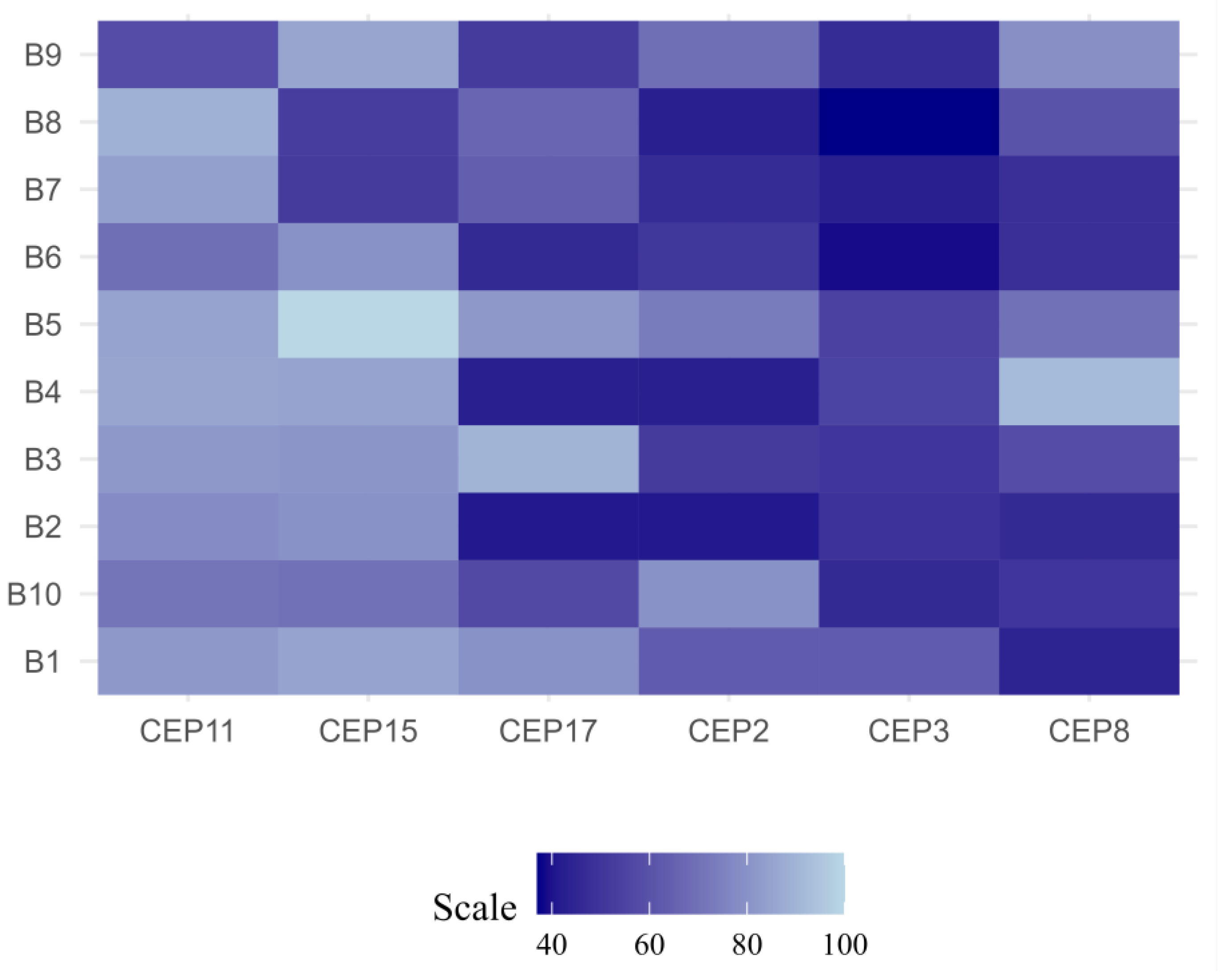
| Clinicopathological Characteristics | CIN | p-Value | CH | p-Value | ||
|---|---|---|---|---|---|---|
| Intermediate | High | High | ||||
| Total Number of patients | 9 (0.9) | 1 (0.1) | - | 10 (1) | - | |
| Age | ≥50 years | 9 (0.9) | 1 (0.1) | 0.187 | 10 (1) | 0.467 |
| Breast | Right | 6 (0.6) | 1 (0.1) | 0.788 | 7 (0.7) | 1 |
| Left | 3 (0.3) | 0 | 3 (0.3) | |||
| Histological type | Invasive ductal carcinoma | 7 (0.7) | 1 (0.1) | 0.598 | 8 (0.8) | 1 |
| Mixed carcinoma | 2 (0.2) | 0 | 2 (0.2) | |||
| Histologic grade | ll | 7 (0.7) | 1 (0.1) | 0.598 | 8 (0.8) | 1 |
| lll | 2 (0.2) | 0 | 2 (0.2) | |||
| T stage | T1 | 2 (0.2) | 0 | 0.788 | 2 (0.2) | 1 |
| T2 | 6 (0.6) | 1 (0.1) | 7 (0.7) | |||
| T3 | 1 (0.1) | 0 | 1 (0.1) | |||
| N stage | N0 | 0 | 1 (0.1) | 0.644 | 1 (0.1) | 1 |
| N1 | 3 (0.3) | 1 (0.1) | 4 (0.4) | |||
| N2 | 3 (0.3) | 0 | 3 (0.3) | |||
| N3 | 2 (0.2) | 0 | 2 (0.2) | |||
| Lymphovascular invasion | Absent | 1 (0.1) | 1 (0.1) | 0.035 * | 2 (0.2) | 1 |
| Present | 8 (0.8) | 0 | 8 (0.8) | |||
| Progesterone receptor | Positive | 6 (0.6) | 1 (0.1) | 0.49 | 7 (0.7) | 1 |
| Negative | 3 (0.3) | 0 | 3 (0.3) | |||
| HER2 status | Positive | 2 (0.2) | 1 (0.1) | 0.107 | 3 (0.3) | 1 |
| Negative | 7 (0.7) | 0 | 7 (0.7) | |||
| Ki67 index | ≥20% | 9 (0.9) | 1 (0.1) | 0.661 | 10 (1) | 0.467 |
| Receptor status | ER+/PR+/HER2− | 4 (0.4) | 0 | 0.791 | 4 (0.4) | 0.92 |
| ER+/PR+/HER2+ | 2 (0.2) | 1 (0.1) | 3 (0.3) | |||
| ER+/PR−/HER2− | 3 (0.3) | 3 (0.3) | ||||
| Gene | Location | Function | References |
|---|---|---|---|
| MSH2 | 2p22 | Mismatch repair | [29] |
| RARβ2 | 3p24 | Retinoic acid receptor | [29] |
| PRKCD | 3p21.1 | Involved in DNA damage response | [30] |
| BAP1 | 3p21.1 | Regulate ubiquitination during the DNA damage response and the cell cycle | [31] |
| MLH1 | 3p21 | Mismatch repair | [29] |
| FHIT | 3p14.2 | Plays an important role in the regulation of apoptosis | [31] |
| PMC1 | 8p22 | Encoded for a protein essential for anchoring microtubules to the centrosome | [32] |
| DLC1 | 8p22 | Promoter of apoptosis | [33,34] |
| MTUS1 | 8p22 | Slows down mitotic progression by prolonging metaphase | [35] |
| LZTS1 | 8p21 | Inhibits the Cdk1/cyclin B1 complex | [36] |
| WRN | 8p12 | Critical controller of fragile site stability, essential for preserving genome stability | [37] |
| ATM | 11q22.3 | DNA repair | [31] |
| MIR34B | 11q23.1 | Involved in DNA damage response | [38] |
| TP53 | 17p13.1 | DNA repair, cell cycle, apoptosis, and angiogenesis regulator | [31] |
| BRCA1 | 17q21.31 | DNA repair; maintains genomic stability | [39] |
| BRIP1 | 17q23.2 | Important in the normal double-strand break repair function of breast cancer | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camargo-Herrera, V.; Castellanos, G.; Rangel, N.; Jiménez-Tobón, G.A.; Martínez-Agüero, M.; Rondón-Lagos, M. Patterns of Chromosomal Instability and Clonal Heterogeneity in Luminal B Breast Cancer: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 4478. https://doi.org/10.3390/ijms25084478
Camargo-Herrera V, Castellanos G, Rangel N, Jiménez-Tobón GA, Martínez-Agüero M, Rondón-Lagos M. Patterns of Chromosomal Instability and Clonal Heterogeneity in Luminal B Breast Cancer: A Pilot Study. International Journal of Molecular Sciences. 2024; 25(8):4478. https://doi.org/10.3390/ijms25084478
Chicago/Turabian StyleCamargo-Herrera, Valentina, Giovanny Castellanos, Nelson Rangel, Guillermo Antonio Jiménez-Tobón, María Martínez-Agüero, and Milena Rondón-Lagos. 2024. "Patterns of Chromosomal Instability and Clonal Heterogeneity in Luminal B Breast Cancer: A Pilot Study" International Journal of Molecular Sciences 25, no. 8: 4478. https://doi.org/10.3390/ijms25084478
APA StyleCamargo-Herrera, V., Castellanos, G., Rangel, N., Jiménez-Tobón, G. A., Martínez-Agüero, M., & Rondón-Lagos, M. (2024). Patterns of Chromosomal Instability and Clonal Heterogeneity in Luminal B Breast Cancer: A Pilot Study. International Journal of Molecular Sciences, 25(8), 4478. https://doi.org/10.3390/ijms25084478






