The Effect of Enteric-Derived Lipopolysaccharides on Obesity
Abstract
1. Introduction
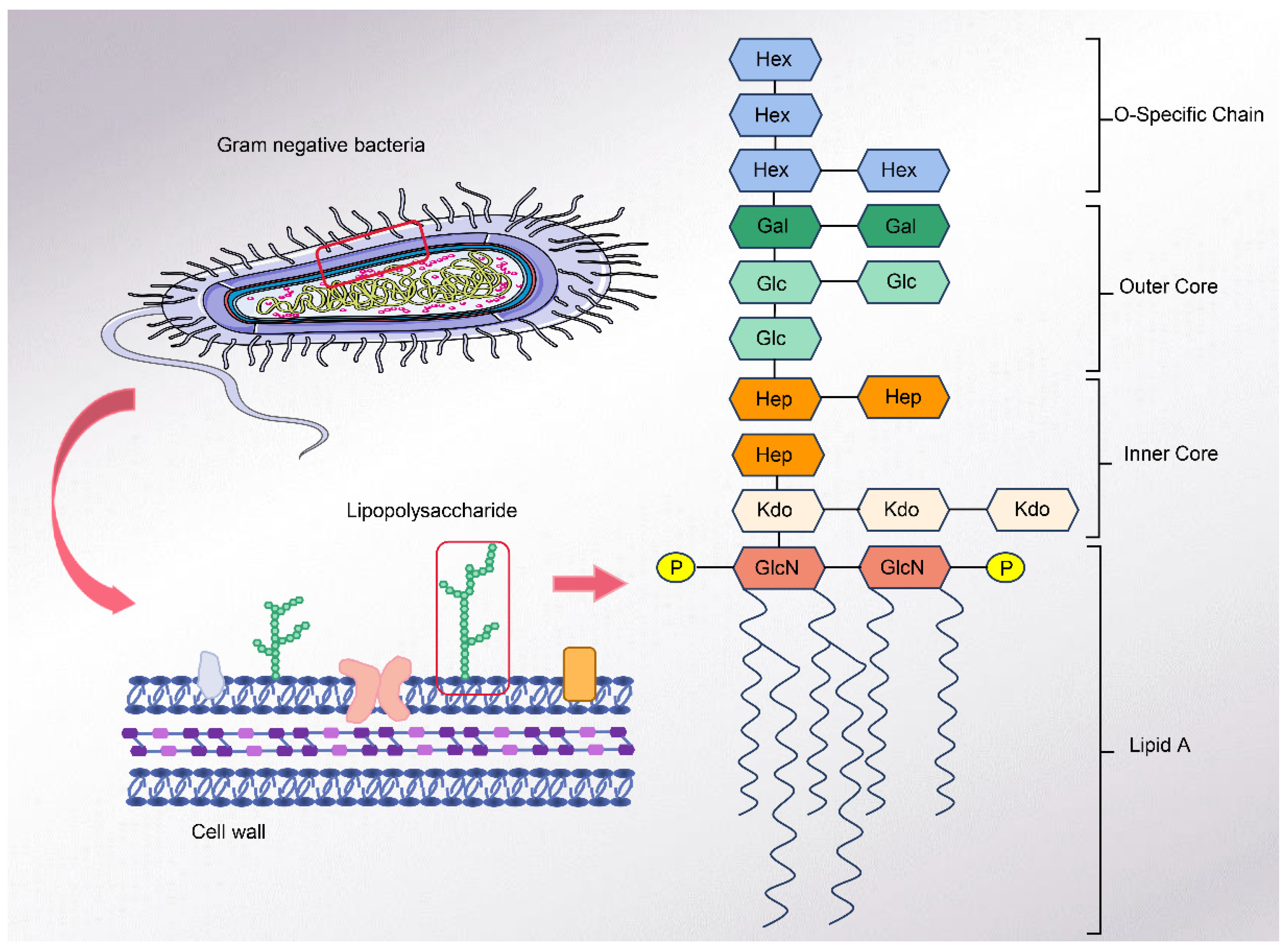
2. Lipopolysaccharide
2.1. Structure of LPS
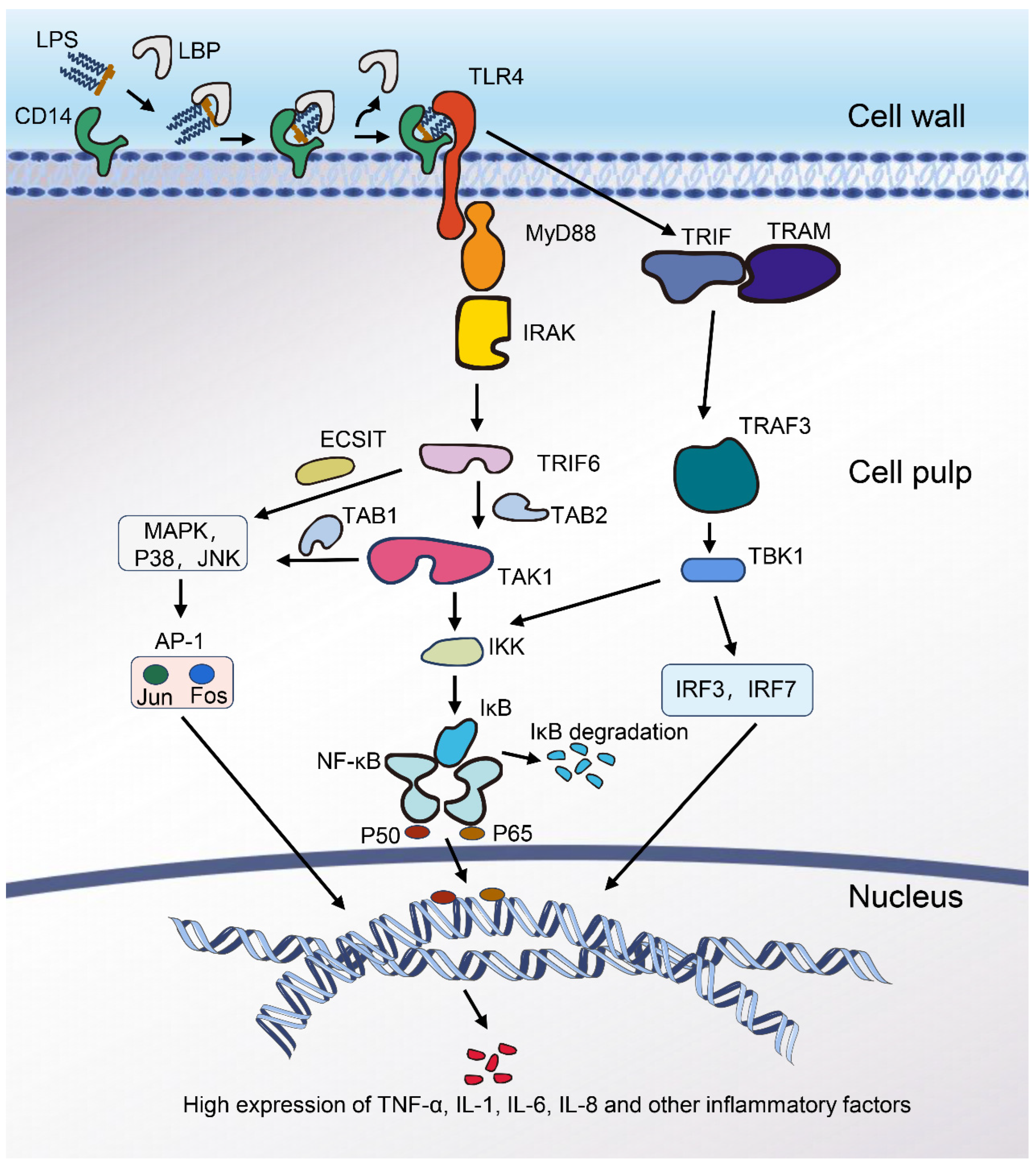
2.2. Mechanism of Inflammation Induced by LPS
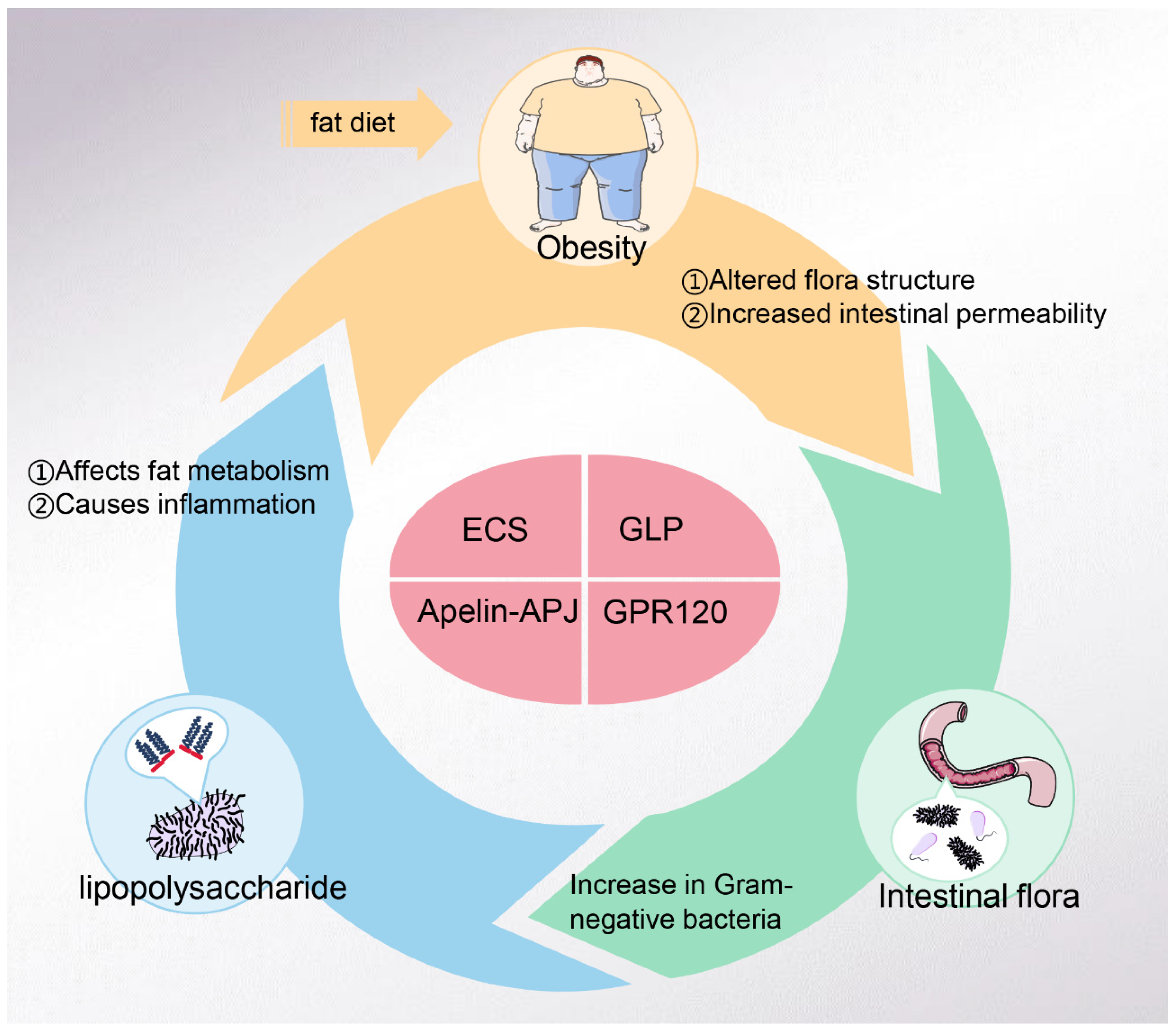
3. LPS and Obesity
4. Obesity and Intestinal Flora
5. LPS and Intestinal Flora
6. LPS-Related Targets
6.1. ECS (Endocannabinoid System)
6.2. Apelin–APJ
6.3. GLP
6.4. GPR120
7. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Pfeiffer, R. Untersuchungen über das Choleragift. Z. Hyg. Infekt. 1892, 11, 393–412. [Google Scholar] [CrossRef]
- Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. Immunol. 2003, 3, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C. Endotoxin removal: History of a mission. Blood Purif. 2014, 37 (Suppl. 1), 5–8. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Endotoxin: The uninvited guest. Biomaterials 2005, 26, 6811–6817. [Google Scholar] [CrossRef]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; de La Serre, C.B. Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet-Fed Rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Lad, N.; Murphy, A.M.; Parenti, C.; Nelson, C.P.; Williams, N.C.; Sharpe, G.R.; McTernan, P.G. Asthma and obesity: Endotoxin another insult to add to injury? Clin. Sci. 2021, 135, 2729–2748. [Google Scholar] [CrossRef]
- Moreira, A.P.B.; Texeira, T.F.S.; Ferreira, A.B.; Peluzio, M.d.C.G.; Alfenas, R.d.C.G. Influence of a high-fat diet on gut microbiota, intestinal permeabilit y and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Fender, A.C.; Dobrev, D. A Gut Feeling: Lipopolysaccharide Links Gut Dysbiosis With Inflammator y Atrial Cardiomyopathy, Obesity, and Atrial Fibrillation. Can. J. Cardiol. 2022, 38, 1976–1978. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- De La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Erridge, C.; Bennett-Guerrero, E.; Poxton, I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002, 4, 837–851. [Google Scholar] [CrossRef]
- Heine, H.; Rietschel, E.T.; Ulmer, A.J. The biology of endotoxin. Mol. Biotechnol. 2001, 19, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Duda, K.A.; Lanzetta, R.; Silipo, A.; De Castro, C.; Molinaro, A. A Journey from Structure to Function of Bacterial Lipopolysaccharides. Chem. Rev. 2022, 122, 15767–15821. [Google Scholar] [CrossRef] [PubMed]
- Schletter, J.; Heine, H.; Ulmer, A.J.; Rietschel, E.T. Molecular mechanisms of endotoxin activity. Arch. Microbiol. 1995, 164, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.P.; Lowary, T.L. New insights into lipopolysaccharide assembly and export. Curr. Opin. Chem. Biol. 2019, 53, 37–43. [Google Scholar] [CrossRef]
- Paulsson, M.; Saladin, K.; Landwehr, R. Binding of Ca2+ influences susceptibility of laminin to proteolytic digestion and interactions between domain-specific laminin fragments. Eur. J. Biochem. 1988, 177, 477–481. [Google Scholar] [CrossRef]
- Lüderitz, O.; Freudenberg, M.A.; Galanos, C.; Lehmann, V.; Rietschel, E.T.; Shaw, D.H. Lipopolysaccharides of Gram-Negative Bacteria. In Current Topics in Membranes and Transport; Bronner, F., Kleinteller, A., Eds.; Academic Press: Cambridge, MA, USA, 1982; Volume 17, pp. 79–151. [Google Scholar]
- Rietschel, E.T.; Brade, H.; Brade, L.; Brandenburg, K.; Schade, U.; Seydel, U.; Zähringer, U.; Galanos, C.; Lüderitz, O.; Westphal, O.; et al. Lipid A, the endotoxic center of bacterial lipopolysaccharides: Relati on of chemical structure to biological activity. Prog. Clin. Biol. Res. 1987, 231, 25–53. [Google Scholar]
- Garcia-Vello, P.; Di Lorenzo, F.; Zucchetta, D.; Zamyatina, A.; De Castro, C.; Molinaro, A. Lipopolysaccharide lipid A: A promising molecule for new immunity-base d therapies and antibiotics. Pharmacol. Ther. 2022, 230, 107970. [Google Scholar] [CrossRef] [PubMed]
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zähringer, U.; Seydel, U.; Di Padova, F.; et al. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994, 8, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Galanos, C.; Luderitz, O.; Rietschel, E.T.; Westphal, O.; Brade, H.; Brade, L.; Freudenberg, M.; Schade, U.; Imoto, M.; Yoshimura, H.; et al. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur. J. Biochem. 1985, 148, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Mayer, H.; Koch, M.H.; Weckesser, J.; Rietschel, E.T.; Seydel, U. Influence of the supramolecular structure of free lipid A on its biolo gical activity. Eur. J. Biochem. 1993, 218, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Schromm, A.B.; Brandenburg, K.; Loppnow, H.; Zähringer, U.; Rietschel, E.T.; Carroll, S.F.; Koch, M.H.J.; Kusumoto, S.; Seydel, U. The Charge of Endotoxin Molecules Influences Their Conformation and IL-6-Inducing Capacity. J. Immunol. 1998, 161, 5464–5471. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Heavner, G.; Nedelman, M.; Sherris, D.; Brunt, E.; Knight, D.; Ghrayeb, J. Lipopolysaccharide (LPS) neutralizing peptides reveal a lipid A bindin g site of LPS binding protein. J. Biol. Chem. 1995, 270, 17934–17938. [Google Scholar] [CrossRef] [PubMed]
- Hellman, J.; Warren, H.S. Outer membrane protein A (OmpA), peptidoglycan-associated lipoprotein (PAL), and murein lipoprotein (MLP) are released in experimental Gram- negative sepsis. J. Endotoxin Res. 2001, 7, 69–72. [Google Scholar] [CrossRef]
- Schumann, R.R.; Latz, E. Lipopolysaccharide-binding protein. Chem. Immunol. 2000, 74, 42–60. [Google Scholar] [CrossRef]
- Van Amersfoort, E.S.; Van Berkel, T.J.C.; Kuiper, J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 2003, 16, 379–414. [Google Scholar] [CrossRef]
- Matsumura, T.; Ito, A.; Takii, T.; Hayashi, H.; Onozaki, K. Endotoxin and cytokine regulation of toll-like receptor (TLR) 2 and TL R4 gene expression in murine liver and hepatocytes. J. Interferon Cytokine Res. 2000, 20, 915–921. [Google Scholar] [CrossRef]
- Yang, R.B.; Mark, M.R.; Gray, A.; Huang, A.; Xie, M.H.; Zhang, M.; Goddard, A.; Wood, W.I.; Gurney, A.L.; Godowski, P.J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular sign alling. Nature 1998, 395, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporespo nsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar] [CrossRef] [PubMed]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, M.; Zanoni, I. Toll-like receptor co-receptors as master regulators of the immune res ponse. Mol. Immunol. 2015, 63, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.L.; Alcaide, P. MyD88: At the heart of inflammatory signaling and cardiovascular disea se. J. Mol. Cell. Cardiol. 2021, 161, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Liu, Z.; Pu, Y.; Bao, Y. Immunomodulatory effects exerted by Poria Cocos polysaccharides via TL R4/TRAF6/NF-κB signaling in vitro and in vivo. Biomed. Pharmacother. 2019, 112, 108709. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya-Tsuji, J.; Kishimoto, K.; Hiyama, A.; Inoue, J.; Cao, Z.; Matsumoto, K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinas e cascade in the IL-1 signalling pathway. Nature 1999, 398, 252–256. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Régnier, C.H.; Song, H.Y.; Gao, X.; Goeddel, D.V.; Cao, Z.; Rothe, M. Identification and characterization of an IkappaB kinase. Cell 1997, 90, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Shirakabe, K.; Shibuya, H.; Irie, K.; Oishi, I.; Ueno, N.; Taniguchi, T.; Nishida, E.; Matsumoto, K. Identification of a member of the MAPKKK family as a potential mediato r of TGF-beta signal transduction. Science 1995, 270, 2008–2011. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Niu, P.; Zhao, Y.; Cheng, Y.; Chen, W.; Lin, L.; Lu, J.; Cheng, X.; Xu, Z. Impact of miR-223-3p and miR-2909 on inflammatory factors IL-6, IL-1ss, and TNF-alpha, and the TLR4/TLR2/NF-kappaB/STAT3 signaling pathway induced by lipopolysaccharide in human adipose stem cells. PLoS ONE 2019, 14, e0212063. [Google Scholar] [CrossRef]
- Jang, J.; Jung, Y.; Kim, Y.; Jho, E.H.; Yoon, Y. LPS-induced inflammatory response is suppressed by Wnt inhibitors, Dickkopf-1 and LGK974. Sci. Rep. 2017, 7, 41612. [Google Scholar] [CrossRef] [PubMed]
- Kopp, E.; Medzhitov, R.; Carothers, J.; Xiao, C.; Douglas, I.; Janeway, C.A.; Ghosh, S. ECSIT is an evolutionarily conserved intermediate in the Toll/IL-1 sig nal transduction pathway. Genes Dev. 1999, 13, 2059–2071. [Google Scholar] [CrossRef] [PubMed]
- Whitmarsh, A.J.; Davis, R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996, 74, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Chen, R.; Jiang, X.; He, J.; Li, C. TAK1 confers antibacterial protection through mediating the activation of MAPK and NF-kappaB pathways in shrimp. Fish. Shellfish. Immunol. 2022, 123, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Lopez, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Hacker, H.; Tseng, P.H.; Karin, M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat. Rev. Immunol. 2011, 11, 457–468. [Google Scholar] [CrossRef]
- Lim, K.H.; Staudt, L.M. Toll-like receptor signaling. Cold Spring Harb. Perspect. Biol. 2013, 5, a011247. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, G.; Suresh, K. [6]-Shogaol attenuates inflammation, cell proliferation via modulate NF-κB and AP-1 oncogenic signaling in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis. Biomed. Pharmacother. 2018, 98, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Putignani, L.; Bottazzo, G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010, 31, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Suriano, F.; Vieira-Silva, S.; Falony, G.; Roumain, M.; Paquot, A.; Pelicaen, R.; Regnier, M.; Delzenne, N.M.; Raes, J.; Muccioli, G.G.; et al. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: Two sides of the same coin. Microbiome 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Hersoug, L.-G.; Møller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflam mation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Yang, R.; Lyles, M.F.; Gong, D.; Nicklas, B.J. Abdominal adipose tissue cytokine gene expression: Relationship to obesity and metabolic risk factors. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E741–E747. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Gautier, T.; Deckert, V.; Nguyen, M.; Desrumaux, C.; Masson, D.; Lagrost, L. New therapeutic horizons for plasma phospholipid transfer protein (PLT P): Targeting endotoxemia, infection and sepsis. Pharmacol. Ther. 2022, 236, 108105. [Google Scholar] [CrossRef]
- Lebrun, L.J.; Pallot, G.; Nguyen, M.; Tavernier, A.; Dusuel, A.; Pilot, T.; Deckert, V.; Dugail, I.; Le Guern, N.; Pais De Barros, J.-P.; et al. Increased Weight Gain and Insulin Resistance in HF-Fed PLTP Deficient Mice Is Related to Altered Inflammatory Response and Plasma Transport of Gut-Derived LPS. Int. J. Mol. Sci. 2022, 23, 13226. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Postigo, M.; Oliva-Olivera, W.; Coin-Aragüez, L.; Ramos-Molina, B.; Giraldez-Perez, R.M.; Lhamyani, S.; Alcaide-Torres, J.; Perez-Martinez, P.; El Bekay, R.; Cardona, F.; et al. Metabolic endotoxemia promotes adipose dysfunction and inflammation in human obesity. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E319–E332. [Google Scholar] [CrossRef] [PubMed]
- Omran, F.; Murphy, A.M.; Younis, A.Z.; Kyrou, I.; Vrbikova, J.; Hainer, V.; Sramkova, P.; Fried, M.; Ball, G.; Tripathi, G.; et al. The impact of metabolic endotoxaemia on the browning process in human adipocytes. BMC Med. 2023, 21, 154. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-Cañellas, N.; Latorre, J.; Santos-González, E.; Lluch, A.; Ortega, F.; Mayneris-Perxachs, J.; Fernández-Real, J.-M.; Moreno-Navarrete, J.M. Inflammatory response to bacterial lipopolysaccharide drives iron accu mulation in human adipocytes. Biomed. Pharmacother. 2023, 166, 115428. [Google Scholar] [CrossRef] [PubMed]
- Damms-Machado, A.; Louis, S.; Schnitzer, A.; Volynets, V.; Rings, A.; Basrai, M.; Bischoff, S.C. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am. J. Clin. Nutr. 2017, 105, 127–135. [Google Scholar] [CrossRef]
- Gummesson, A.; Carlsson, L.M.; Storlien, L.H.; Backhed, F.; Lundin, P.; Lofgren, L.; Stenlof, K.; Lam, Y.Y.; Fagerberg, B.; Carlsson, B. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity 2011, 19, 2280–2282. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Castagliuolo, I.; Di Leo, V.; Buda, A.; Pinzani, M.; Palu, G.; Martines, D. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G518–G525. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Pan, L.; Liao, H.; Yao, W.; Shen, N.; Chen, C.; Liu, D.; Ge, M. High-fat diet increased NADPH-oxidase-related oxidative stress and agg ravated LPS-induced intestine injury. Life Sci. 2020, 253, 117539. [Google Scholar] [CrossRef]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulatin g the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef]
- Dornas, W.; Lagente, V. Intestinally derived bacterial products stimulate development of nonal coholic steatohepatitis. Pharmacol. Res. 2019, 141, 418–428. [Google Scholar] [CrossRef]
- Lasselin, J.; Benson, S.; Hebebrand, J.; Boy, K.; Weskamp, V.; Handke, A.; Hasenberg, T.; Remy, M.; Föcker, M.; Unteroberdörster, M.; et al. Immunological and behavioral responses to in vivo lipopolysaccharide a dministration in young and healthy obese and normal-weight humans. Brain Behav. Immun. 2020, 88, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat stora ge. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Gao, R.; Zhu, C.; Li, H.; Yin, M.; Pan, C.; Huang, L.; Kong, C.; Wang, X.; Zhang, Y.; Qu, S.; et al. Dysbiosis Signatures of Gut Microbiota Along the Sequence from Healthy, Young Patients to Those with Overweight and Obesity. Obesity 2018, 26, 351–361. [Google Scholar] [CrossRef]
- Yun, Y.; Kim, H.N.; Kim, S.E.; Heo, S.G.; Chang, Y.; Ryu, S.; Shin, H.; Kim, H.L. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol. 2017, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Kallio, K.A.; Hatonen, K.A.; Lehto, M.; Salomaa, V.; Mannisto, S.; Pussinen, P.J. Endotoxemia, nutrition, and cardiometabolic disorders. Acta Diabetol. 2015, 52, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Haghiac, M.; Surace, P.; Challier, J.C.; Guerre-Millo, M.; Singh, K.; Waters, T.; Minium, J.; Presley, L.; Catalano, P.M.; et al. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity 2011, 19, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L. The gut microbiota and obesity: From correlation to causality. Nat. Rev. Microbiol. 2013, 11, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Duburcq, T.; Tournoys, A.; Gnemmi, V.; Hubert, T.; Gmyr, V.; Pattou, F.; Jourdain, M. Impact of Obesity on Endotoxin-Induced Disseminated Intravascular Coagulation. Shock 2015, 44, 341–347. [Google Scholar] [CrossRef]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-f ree mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gerard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010, 24, 4948–4959. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.P.; Wilkens, L.R.; Shvetsov, Y.B.; Maskarinec, G.; Park, S.-Y.; Shepherd, J.A.; Boushey, C.J.; Hebert, J.R.; Wirth, M.D.; Ernst, T.; et al. Associations of the Dietary Inflammatory Index with total adiposity an d ectopic fat through the gut microbiota, LPS, and C-reactive protein in the Multiethnic Cohort-Adiposity Phenotype Study. Am. J. Clin. Nutr. 2022, 115, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Fei, N.; Zhao, L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013, 7, 880–884. [Google Scholar] [CrossRef]
- Huang, Q.; Lu, S.; Zhu, Y.; Wei, B.; Chen, Y.; Bai, F. Bacterial endotoxin-induced maternal inflammation leads to fetal intes tinal injury and affects microbial colonization in the neonatal period. J. Matern.-Fetal Neonatal Med. 2022, 35, 6917–6927. [Google Scholar] [CrossRef]
- Xue, L.; He, J.; Gao, N.; Lu, X.; Li, M.; Wu, X.; Liu, Z.; Jin, Y.; Liu, J.; Xu, J.; et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci. Rep. 2017, 7, 45176. [Google Scholar] [CrossRef] [PubMed]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Backhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef]
- Stephens, M.; von der Weid, P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432. [Google Scholar] [CrossRef]
- Anhe, F.F.; Barra, N.G.; Cavallari, J.F.; Henriksbo, B.D.; Schertzer, J.D. Metabolic endotoxemia is dictated by the type of lipopolysaccharide. Cell Rep. 2021, 36, 109691. [Google Scholar] [CrossRef]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hamalainen, A.M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef]
- Collado, M.C.; Derrien, M.; Isolauri, E.; de Vos, W.M.; Salminen, S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G.; Naslain, D.; Backhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; de Vos, W.M.; Satokari, R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Baratta, F.; Pastori, D.; Cocomello, N.; Colantoni, A.; Angelico, F.; Del Ben, M. New Insights into the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Gut-Derived Lipopolysaccharides and Oxidative Stress. Nutrients 2020, 12, 2762. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, Z.I.; Lee, S.; Kirkland, R.; Ross, M.; de La Serre, C.B. Cannabinoid receptor type-1 partially mediates metabolic endotoxemia-i nduced inflammation and insulin resistance. Physiol. Behav. 2019, 199, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.; Galic, M.A.; Wang, A.; Chambers, A.P.; McCafferty, D.M.; McKay, D.M.; Sharkey, K.A.; Pittman, Q.J. Cannabinoid 1 receptors are critical for the innate immune response to TLR4 stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R224–R231. [Google Scholar] [CrossRef] [PubMed]
- Bariani, M.V.; Rubio, A.P.D.; Cella, M.; Burdet, J.; Franchi, A.M.; Aisemberg, J. Role of the endocannabinoid system in the mechanisms involved in the LPS-induced preterm labor. Reproduction 2015, 150, 463–472. [Google Scholar]
- Cani, P.D. Crosstalk between the gut microbiota and the endocannabinoid system: Impact on the gut barrier function and the adipose tissue. Clin. Microbiol. Infect. 2012, 18, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Manca, C.; Boubertakh, B.; Leblanc, N.; Deschênes, T.; Lacroix, S.; Martin, C.; Houde, A.; Veilleux, A.; Flamand, N.; Muccioli, G.G.; et al. Germ-free mice exhibit profound gut microbiota-dependent alterations o f intestinal endocannabinoidome signaling. J. Lipid Res. 2020, 61, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, S.; Pechereau, F.; Leblanc, N.; Boubertakh, B.; Houde, A.; Martin, C.; Flamand, N.; Silvestri, C.; Raymond, F.; Di Marzo, V.; et al. Rapid and Concomitant Gut Microbiota and Endocannabinoidome Response to Diet-Induced Obesity in Mice. mSystems 2019, 4, e00407-19. [Google Scholar] [CrossRef]
- Suriano, F.; Manca, C.; Flamand, N.; Depommier, C.; Van Hul, M.; Delzenne, N.M.; Silvestri, C.; Cani, P.D.; Di Marzo, V. Exploring the endocannabinoidome in genetically obese (ob/ob) and diabetic (db/db) mice: Links with inflammation and gut microbiota. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159056. [Google Scholar] [CrossRef]
- Geurts, L.; Lazarevic, V.; Derrien, M.; Everard, A.; Van Roye, M.; Knauf, C.; Valet, P.; Girard, M.; Muccioli, G.G.; Francois, P.; et al. Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin-resistant mice: Impact on apelin regulation in adipose tissue. Front. Microbiol. 2011, 2, 149. [Google Scholar] [CrossRef]
- Nozu, T.; Miyagishi, S.; Kumei, S.; Nozu, R.; Takakusaki, K.; Okumura, T.J.J.G.H. Glucagon-like peptide-1 analog, liraglutide, improves visceral sensation and gut permeability in rats. J. Gastroenterol. Hepatol. 2018, 33, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Helmstadter, J.; Keppeler, K.; Aust, F.; Kuster, L.; Frenis, K.; Filippou, K.; Vujacic-Mirski, K.; Tsohataridis, S.; Kalinovic, S.; Kroller-Schon, S.; et al. GLP-1 Analog Liraglutide Improves Vascular Function in Polymicrobial Sepsis by Reduction of Oxidative Stress and Inflammation. Antioxidants 2021, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, L.J.; Lenaerts, K.; Kiers, D.; Pais de Barros, J.P.; Le Guern, N.; Plesnik, J.; Thomas, C.; Bourgeois, T.; Dejong, C.H.C.; Kox, M.; et al. Enteroendocrine L Cells Sense LPS after Gut Barrier Injury to Enhance GLP-1 Secretion. Cell Rep. 2017, 21, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Kahles, F.; Meyer, C.; Mollmann, J.; Diebold, S.; Findeisen, H.M.; Lebherz, C.; Trautwein, C.; Koch, A.; Tacke, F.; Marx, N.; et al. GLP-1 secretion is increased by inflammatory stimuli in an IL-6-dependent manner, leading to hyperinsulinemia and blood glucose lowering. Diabetes 2014, 63, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Maruta, K.; Takajo, T.; Akiba, Y.; Said, H.; Kaunitz, J.D. GLP-2 Acutely Prevents Endotoxin-Related Increased Intestinal Paracellular Permeability in Rats. Dig. Dis. Sci. 2020, 65, 2605–2618. [Google Scholar] [CrossRef] [PubMed]
- Akiba, Y.; Kaji, I.; Maruta, K.; Kaunitz, J.D.J.G. GLP-2 acutely diminishes LPS-related intestinal permeability in rats. Gastroenterology 2017, 152, S506. [Google Scholar] [CrossRef]
- Arana, M.R.; Tocchetti, G.N.; Zecchinati, F.; Londero, A.S.; Dominguez, C.; Perdomo, V.; Rigalli, J.P.; Villanueva, S.S.M.; Mottino, A.D. Glucagon-like peptide 2 prevents down-regulation of intestinal multidrug resistance-associated protein 2 and P-glycoprotein in endotoxemic rats. Toxicology 2017, 390, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lei, X.T.; Huang, Q.; Wang, T.; Sun, H.B.; Wang, H.Y. A novel GPR120-selective agonist promotes insulin secretion and improves chronic inflammation. Life Sci. 2021, 269, 119029. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Forte, N.; Fernandez-Rilo, A.C.; Palomba, L.; Di Marzo, V.; Cristino, L. Obesity Affects the Microbiota-Gut-Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators. Int. J. Mol. Sci. 2020, 21, 1554. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M.; Engeli, S.; Kloting, N.; Berndt, J.; Fasshauer, M.; Batkai, S.; Pacher, P.; Schon, M.R.; Jordan, J.; Stumvoll, M. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 2006, 55, 3053–3060. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Böhnke, J.; Feldpausch, M.; Gorzelniak, K.; Janke, J.; Bátkai, S.; Pacher, P.; Harvey-White, J.; Luft, F.C.; Sharma, A.M.; et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005, 54, 2838–2843. [Google Scholar] [CrossRef] [PubMed]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velazquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci. Rep. 2017, 7, 15645. [Google Scholar] [CrossRef] [PubMed]
- Suriano, F.; Manca, C.; Flamand, N.; Van Hul, M.; Delzenne, N.M.; Silvestri, C.; Cani, P.D.; Di Marzo, V. A Lipidomics- and Transcriptomics-Based Analysis of the Intestine of Genetically Obese (ob/ob) and Diabetic (db/db) Mice: Links with Inflammation and Gut Microbiota. Cells 2023, 12, 411. [Google Scholar] [CrossRef] [PubMed]
- Karwad, M.A.; Couch, D.G.; Theophilidou, E.; Sarmad, S.; Barrett, D.A.; Larvin, M.; Wright, K.L.; Lund, J.N.; O’Sullivan, S.E. The role of CB1 in intestinal permeability and inflammation. FASEB J. 2017, 31, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Cota, D. CB1 receptors: Emerging evidence for central and peripheral mechanisms that regulate energy balance, metabolism, and cardiovascular health. Diabetes Metab. Res. Rev. 2007, 23, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef]
- Li, C.; Cheng, H.; Adhikari, B.K.; Wang, S.; Yang, N.; Liu, W.; Sun, J.; Wang, Y. The Role of Apelin-APJ System in Diabetes and Obesity. Front. Endocrinol. 2022, 13, 820002. [Google Scholar] [CrossRef]
- Wang, L.; Zhan, X.; Wang, Z.; Ma, J.; Chang, X.; Zhu, X. Toll-like receptor 4 is necessary for glucose-dependent glucagon-like peptide-1 secretion in male mice. Biochem. Biophys. Res. Commun. 2019, 510, 104–109. [Google Scholar] [CrossRef]
- Lebrun, L.J.; Dusuel, A.; Xolin, M.; Le Guern, N.; Grober, J. Activation of TLRs Triggers GLP-1 Secretion in Mice. Int. J. Mol. Sci. 2023, 24, 5333. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Xie, T.; Guo, X.; Wu, D.; Li, S.; Li, X.; Lu, Y.; Wang, X. Glucagon-like peptide-1 receptor agonist exendin-4 mitigates lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. Int. Immunopharmacol. 2019, 77, 105969. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.R.; Cross, E.; Sanna, F.; Hodson, L. Dysregulation of hepatic metabolism with obesity: Factors influencing glucose and lipid metabolism. Proc. Nutr. Soc. 2022, 81, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Mandard, S.; Dray, C.; Deckert, V.; Valet, P.; Besnard, P.; Drucker, D.J.; Lagrost, L.; Grober, J. Lipopolysaccharides-mediated increase in glucose-stimulated insulin secretion: Involvement of the GLP-1 pathway. Diabetes 2014, 63, 471–482. [Google Scholar] [CrossRef]
- Hwang, I.; Park, Y.J.; Kim, Y.R.; Kim, Y.N.; Ka, S.; Lee, H.Y.; Seong, J.K.; Seok, Y.J.; Kim, J.B. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J. 2015, 29, 2397–2411. [Google Scholar] [CrossRef]
- Wichmann, A.; Allahyar, A.; Greiner, T.U.; Plovier, H.; Lunden, G.O.; Larsson, T.; Drucker, D.J.; Delzenne, N.M.; Cani, P.D.; Backhed, F. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe 2013, 14, 582–590. [Google Scholar] [CrossRef]
- Cani, P.D.; Knauf, C. A newly identified protein from Akkermansia muciniphila stimulates GLP-1 secretion. Cell Metab. 2021, 33, 1073–1075. [Google Scholar] [CrossRef]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Simon, M.C.; Strassburger, K.; Nowotny, B.; Kolb, H.; Roden, M.J.D.C. Intake of Lactobacillus reuteri Improves Incretin and Insulin Secretion in Glucose-Tolerant Humans: A Proof of Concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef]
- Qi, K.K.; Wu, J.; Xu, Z.W. Effects of PEGylated porcine glucagon-like peptide-2 therapy in weaning piglets challenged with lipopolysaccharide. Peptides 2014, 58, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Raptis, D.A.; Limani, P.; Jang, J.H.; Ungethum, U.; Tschuor, C.; Graf, R.; Humar, B.; Clavien, P.A. GPR120 on Kupffer cells mediates hepatoprotective effects of omega3-fatty acids. J. Hepatol. 2014, 60, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, C.; Hong, Y.H.; Iga, T.; Hishikawa, D.; Suzuki, Y.; Song, S.H.; Choi, K.C.; Adachi, T.; Hirasawa, A.; Tsujimoto, G.; et al. The regulation of adipogenesis through GPR120. Biochem. Biophys. Res. Commun. 2007, 354, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Yang, Y.; Zhou, Y.; Wei, H.; Peng, J. GPR120: A critical role in adipogenesis, inflammation, and energy metabolism in adipose tissue. Cell. Mol. Life Sci. 2017, 74, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Umemoto, T.; Kakei, M.; Momomura, S.I.; Kawakami, M.; Ishikawa, S.E.; Hara, K. Eicosapentaenoic acid shows anti-inflammatory effect via GPR120 in 3T3-L1 adipocytes and attenuates adipose tissue inflammation in diet-induced obese mice. Nutr. Metab. 2017, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, T.; Wang, H.Y. Ginsenoside Rb2 enhances the anti-inflammatory effect of omega-3 fatty acid in LPS-stimulated RAW264.7 macrophages by upregulating GPR120 expression. Acta Pharmacol. Sin. 2017, 38, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Li, Y.; Chen, J.; Zong, Y.; Yang, X. DHA/AA alleviates LPS-induced Kupffer cells pyroptosis via GPR120 interaction with NLRP3 to inhibit inflammasome complexes assembly. Cell Death Dis. 2021, 12, 73. [Google Scholar] [CrossRef]
- Ichimura, A.; Hirasawa, A.; Poulain-Godefroy, O.; Bonnefond, A.; Hara, T.; Yengo, L.; Kimura, I.; Leloire, A.; Liu, N.; Iida, K.; et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 2012, 483, 350–354. [Google Scholar] [CrossRef]
- Tanaka, T.; Yano, T.; Adachi, T.; Koshimizu, T.A.; Hirasawa, A.; Tsujimoto, G. Cloning and characterization of the rat free fatty acid receptor GPR120: In vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 377, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Fredborg, M.; Theil, P.K.; Jensen, B.B.; Purup, S. G protein-coupled receptor120 (GPR120) transcription in intestinal epithelial cells is significantly affected by bacteria belonging to the Bacteroides, Proteobacteria, and Firmicutes phyla. J. Anim. Sci. 2012, 90 (Suppl. 4), 10–12. [Google Scholar] [CrossRef] [PubMed]
| Table | Experimental Model | Effect | Refs. |
|---|---|---|---|
| CB1 | Male Wistar rats | Obesity, Mesenteric, Retroperitoneal fat | [104] |
| CB1 | Wild-type male C57BL/6 mice and Mice lacking CB1 receptor | Fever | [105] |
| CB1 | Virgin BALB/c female mice | Preterm labour | [106] |
| ECS | C57BL/6J, B6.V-Lepob/J mice. Germ-free Swiss Webster and Myd88—//—C57BL/6 mice | Gut permeability, Adipogenesis, Gut microbiota | [98] |
| CB1 | Male C57BL/6 J mice | Diet intake, Body weight, Body composition, Inflammation, Gut barrier, Gut microbiota | [107] |
| ECS | Conventionally Raised and Germ-free C57BL/6NTac mice | Gut microbiota, Lipid mediator levels | [108] |
| ECS | C57BL/6J male mice | Gut microbiota, Obesity | [109] |
| ECS | Genetically obese and diabetic mice | Adipose tissues, Gut microbiota, Inflammation | [110] |
| ECS, Apelin–APJ | Obesity and diabetes leptin resistance (db/db) mice and C57BL/6 mice | Gut microbiota, Inflammation, Fat tissue | [111] |
| GLP-1 | Adult male Sprague Dawley rats | Allodynia, Inflammation, Gut permeability | [112] |
| GLP-1 | C57BL/6J mice | Cardiovascular system, Oxidative stress, Inflammation, Sepsis | [113] |
| GLP-1 | Male C57BL/6J mice | Gut barrier | [114] |
| GLP-1 | C57BL/6 mice | Inflammation, Insulin secretion, Blood glucose | [115] |
| GLP-2 | Male Sprague Dawley rats | Intestinal paracellular permeability | [116] |
| GLP-2 | Rats | Gut permeability | [117] |
| GLP-2 | Adult female Wistar rats | Intestinal structure and morphology, Inflammation, Oxidative stress | [118] |
| GLP-2 | ob/ob mice | Intestinal permeability, Gut microbiota, Inflammation, Obesity | [119] |
| GPR120 | C57BL/KsJ-db/db obese diabetic mice and C57BL/KsJ-db/m mice | Inflammation, Blood glucose | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Lai, W.; Min, T.; Wei, J.; Bai, Y.; Cao, H.; Guo, J.; Su, Z. The Effect of Enteric-Derived Lipopolysaccharides on Obesity. Int. J. Mol. Sci. 2024, 25, 4305. https://doi.org/10.3390/ijms25084305
Wang K, Lai W, Min T, Wei J, Bai Y, Cao H, Guo J, Su Z. The Effect of Enteric-Derived Lipopolysaccharides on Obesity. International Journal of Molecular Sciences. 2024; 25(8):4305. https://doi.org/10.3390/ijms25084305
Chicago/Turabian StyleWang, Kai, Weiwen Lai, Tianqi Min, Jintao Wei, Yan Bai, Hua Cao, Jiao Guo, and Zhengquan Su. 2024. "The Effect of Enteric-Derived Lipopolysaccharides on Obesity" International Journal of Molecular Sciences 25, no. 8: 4305. https://doi.org/10.3390/ijms25084305
APA StyleWang, K., Lai, W., Min, T., Wei, J., Bai, Y., Cao, H., Guo, J., & Su, Z. (2024). The Effect of Enteric-Derived Lipopolysaccharides on Obesity. International Journal of Molecular Sciences, 25(8), 4305. https://doi.org/10.3390/ijms25084305






