Roles of Integrin in Cardiovascular Diseases: From Basic Research to Clinical Implications
Abstract
1. Introduction
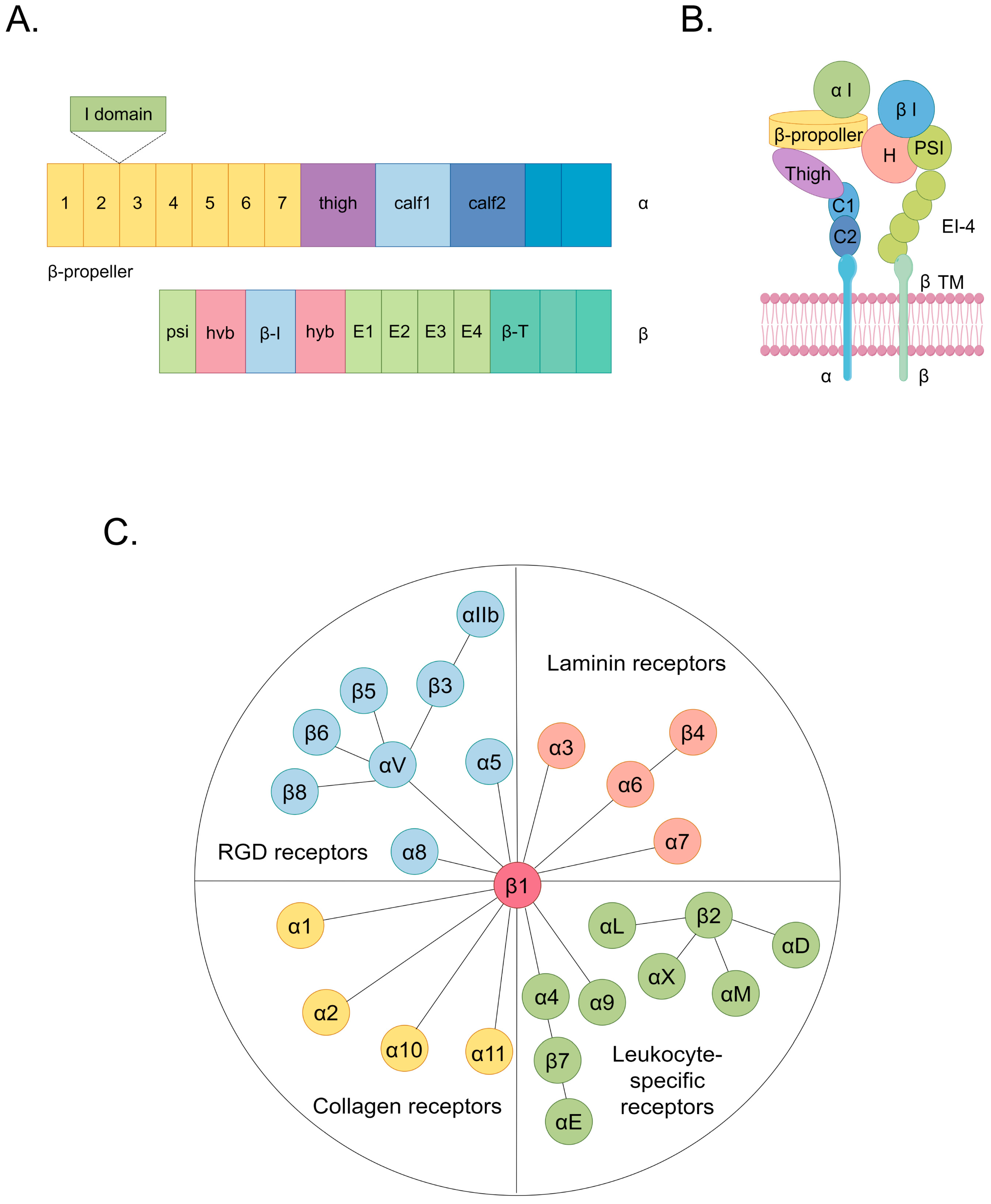
2. Potential Effects of Integrins in CVDs
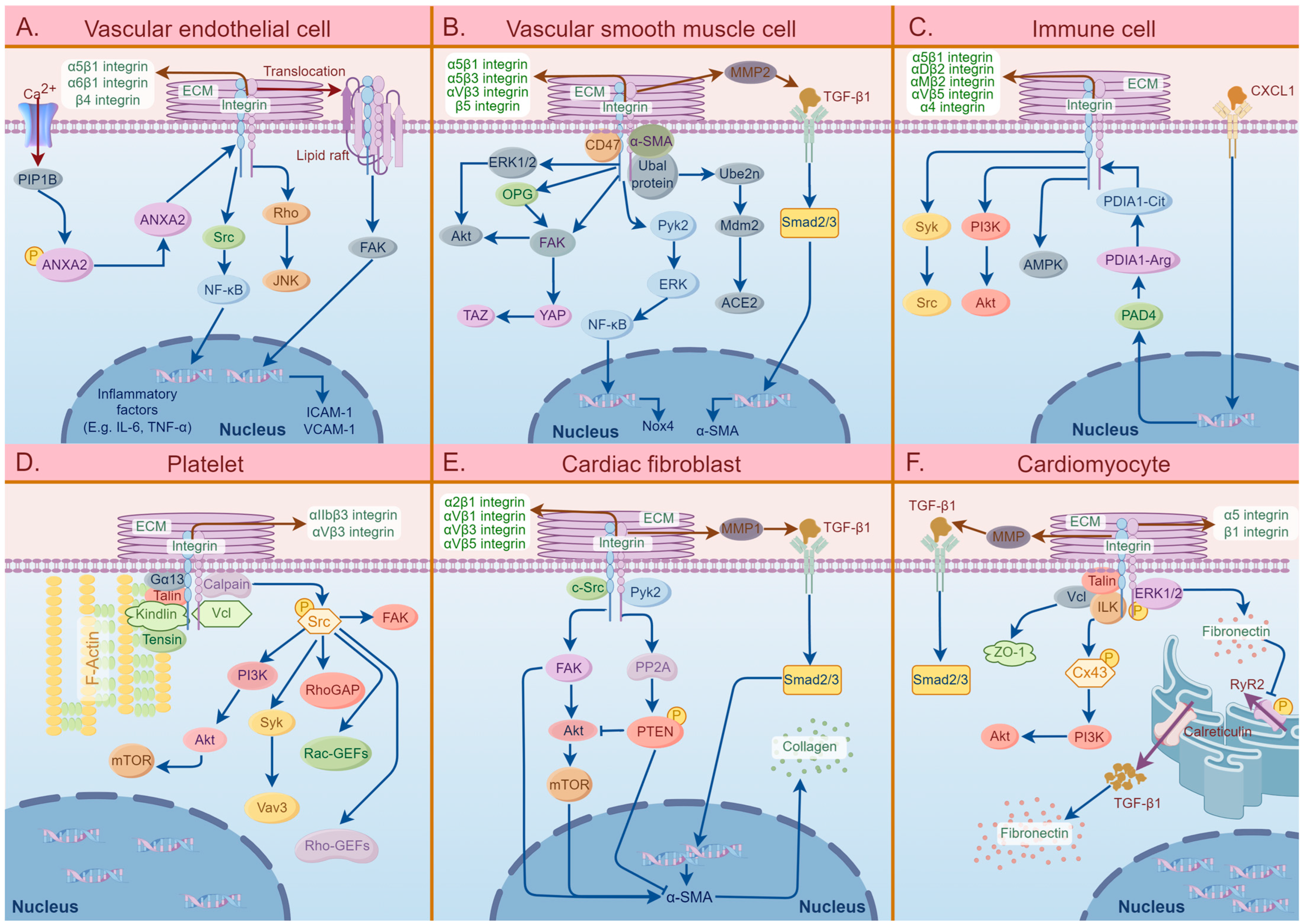
2.1. Atherosclerosis
2.1.1. Integrins That Bind to RGD Receptors
2.1.2. Integrins That Bind to Laminin Receptors
2.1.3. Integrins That Bind to Leukocyte-Specific Receptors
2.2. Cardiac Fibrosis
2.2.1. αV Integrins
2.2.2. β1 Integrins
2.2.3. Other Integrins
2.3. Arrhythmias
2.4. Hypertension
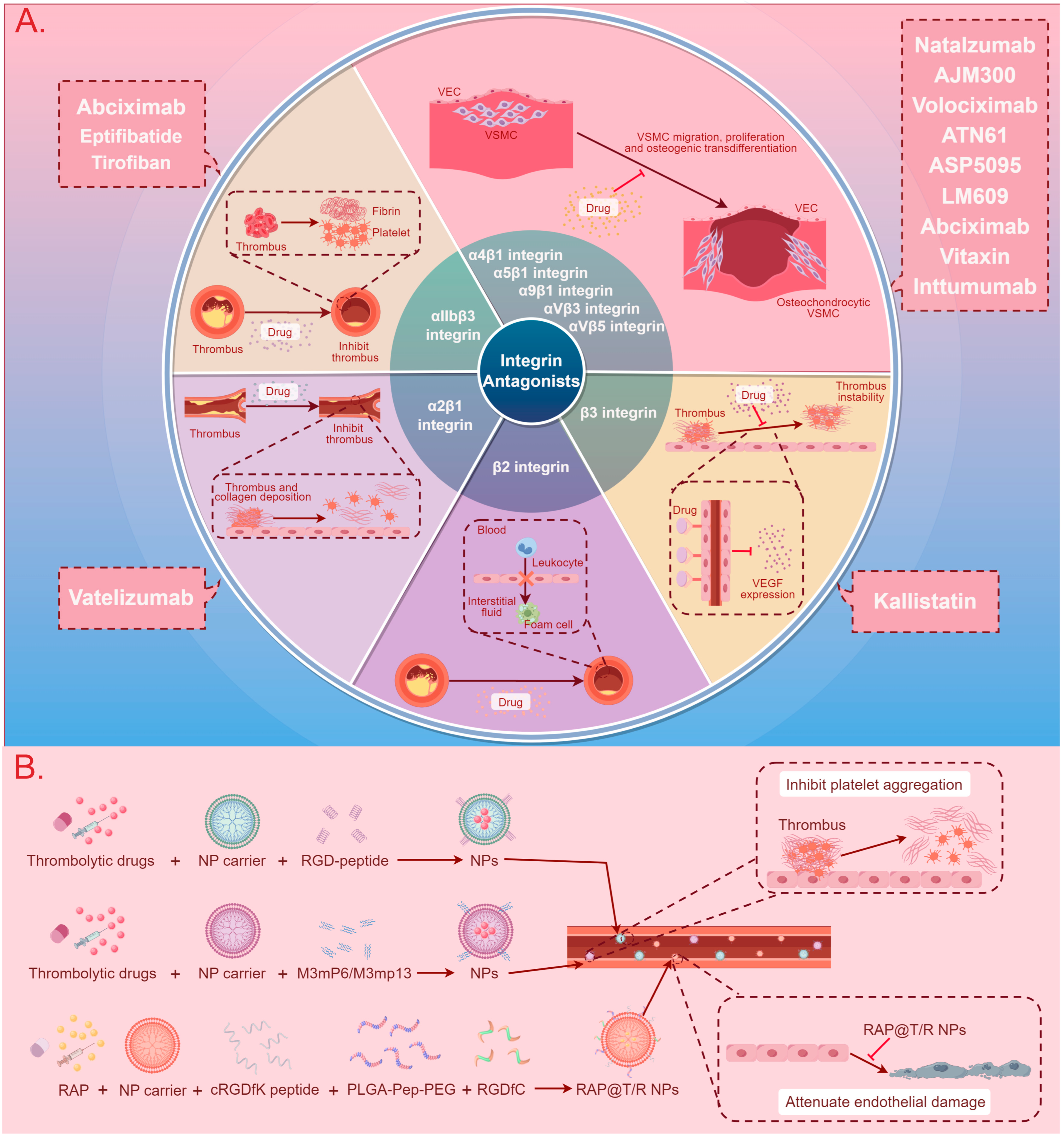
3. Integrin-Based Therapy
3.1. Integrin Antagonists, Antibodies, and Inhibitors
3.2. Nanotherapy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mensah, G.A.; Fuster, V.; Roth, G.A. A Heart-Healthy and Stroke-Free World: Using Data to Inform Global Action. J. Am. Coll. Cardiol. 2023, 82, 2343–2349. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2018, 16, 203–212. [Google Scholar] [CrossRef]
- Ferro, F.; Spelat, R.; Pandit, A.; Martin-Ventura, J.L.; Rabinovich, G.A.; Contessotto, P. Glycosylation of blood cells during the onset and progression of atherosclerosis and myocardial infarction. Trends Mol. Med. 2024, 30, 178–196. [Google Scholar] [CrossRef]
- Wulf-Johansson, H.; Lock Johansson, S.; Schlosser, A.; Trommelholt Holm, A.; Rasmussen, L.M.; Mickley, H.; Diederichsen, A.C.; Munkholm, H.; Poulsen, T.S.; Tornøe, I.; et al. Localization of microfibrillar-associated protein 4 (MFAP4) in human tissues: Clinical evaluation of serum MFAP4 and its association with various cardiovascular conditions. PLoS ONE 2013, 8, e82243. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.H.; Mouton, A.J.; DeLeon-Pennell, K.Y.; Genovese, F.; Karsdal, M.; Lindsey, M.L. Understanding cardiac extracellular matrix remodeling to develop biomarkers of myocardial infarction outcomes. Matrix Biol. 2019, 75–76, 43–57. [Google Scholar] [CrossRef]
- Toba, H.; Lindsey, M.L. Extracellular matrix roles in cardiorenal fibrosis: Potential therapeutic targets for CVD and CKD in the elderly. Pharmacol. Ther. 2019, 193, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Haverslag, R.; Pasterkamp, G.; Hoefer, I.E. Targeting adhesion molecules in cardiovascular disorders. Cardiovasc. Hematol. Disord. Drug Targets 2008, 8, 252–260. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Etienne-Manneville, S. Integrin diversity brings specificity in mechanotransduction. Biol. Cell 2018, 110, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Samarel, A.M. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2291–H2301. [Google Scholar] [CrossRef]
- Lv, H.; Wang, H.; Quan, M.; Zhang, C.; Fu, Y.; Zhang, L.; Lin, C.; Liu, X.; Yi, X.; Chen, J.; et al. Cartilage oligomeric matrix protein fine-tunes disturbed flow-induced endothelial activation and atherogenesis. Matrix Biol. J. Int. Soc. Matrix Biol. 2021, 95, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.Y.; Chu, P.H.; Chen, S.C.; Lee, T.H. MFG-E8 promotes osteogenic transdifferentiation of smooth muscle cells and vascular calcification by regulating TGF-β1 signaling. Commun. Biol. 2022, 5, 364. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Li, X.; Ramos-Echazabal, G.; Faridi, H.; Zigmond, Z.M.; Santos Falcon, N.; Hernandez, D.R.; Shehadeh, S.A.; Velazquez, O.C.; Gupta, V.; et al. A Genetic Model of Constitutively Active Integrin CD11b/CD18. J. Immunol. 2020, 205, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Zemljic-Harpf, A.E.; Godoy, J.C.; Platoshyn, O.; Asfaw, E.K.; Busija, A.R.; Domenighetti, A.A.; Ross, R.S. Vinculin directly binds zonula occludens-1 and is essential for stabilizing connexin-43-containing gap junctions in cardiac myocytes. J. Cell Sci. 2014, 127, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin αIIbβ3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Arosio, D.; Casagrande, C.; Manzoni, L. Integrin-mediated drug delivery in cancer and cardiovascular diseases with peptide-functionalized nanoparticles. Curr. Med. Chem. 2012, 19, 3128–3151. [Google Scholar] [CrossRef]
- Nishimura, M.; Kumsta, C.; Kaushik, G.; Diop, S.B.; Ding, Y.; Bisharat-Kernizan, J.; Catan, H.; Cammarato, A.; Ross, R.S.; Engler, A.J.; et al. A dual role for integrin-linked kinase and β1-integrin in modulating cardiac aging. Aging Cell 2014, 13, 431–440. [Google Scholar] [CrossRef]
- Xu, J.; Shi, G.P. Vascular wall extracellular matrix proteins and vascular diseases. Biochim. Biophys. Acta 2014, 1842, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Estevez, B.; Shen, B.; Du, X. Targeting integrin and integrin signaling in treating thrombosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.J.; Byzova, T.V.; Qin, J.; Topol, E.J.; Plow, E.F. Integrin alphaIIbbeta3 and its antagonism. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic Insights into the Oxidized Low-Density Lipoprotein-Induced Atherosclerosis. Oxid. Med. Cell Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Gisterå, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, X.; Xia, Y.; Mao, L. An update on the phenotypic switching of vascular smooth muscle cells in the pathogenesis of atherosclerosis. Cell Mol. Life Sci. 2021, 79, 6. [Google Scholar] [CrossRef] [PubMed]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef] [PubMed]
- Demos, C.; Williams, D.; Jo, H. Disturbed Flow Induces Atherosclerosis by Annexin A2-Mediated Integrin Activation. Circ. Res. 2020, 127, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, T.; Chen, Z.; Yan, M.; Li, B.; Lv, H.; Wang, C.; Xiang, S.; Shi, L.; Zhu, Y.; et al. Coupling of Integrin α5 to Annexin A2 by Flow Drives Endothelial Activation. Circ. Res. 2020, 127, 1074–1090. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, J.; Hu, T.; Ren, Z.; Li, L.; Hameed, I.; Zhang, X.; Men, C.; Guo, Y.; Xu, D.; et al. Galectin-3 exacerbates ox-LDL-mediated endothelial injury by inducing inflammation via integrin β1-RhoA-JNK signaling activation. J. Cell Physiol. 2019, 234, 10990–11000. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Chen, S.; Luo, S.; Chen, A.; Wang, L.; Tang, H.; Wang, F.; Wang, Z.; Gao, X.; Zuo, G.; et al. Shear-Induced ITGB4 Promotes Endothelial Cell Inflammation and Atherosclerosis. Oxid. Med. Cell Longev. 2022, 2022, 5842677. [Google Scholar] [CrossRef]
- Hsu, P.L.; Chen, J.S.; Wang, C.Y.; Wu, H.L.; Mo, F.E. Shear-Induced CCN1 Promotes Atheroprone Endothelial Phenotypes and Atherosclerosis. Circulation 2019, 139, 2877–2891. [Google Scholar] [CrossRef]
- Zhu, Y.; Qu, J.; He, L.; Zhang, F.; Zhou, Z.; Yang, S.; Zhou, Y. Calcium in Vascular Smooth Muscle Cell Elasticity and Adhesion: Novel Insights Into the Mechanism of Action. Front. Physiol. 2019, 10, 852. [Google Scholar] [CrossRef]
- Govatati, S.; Pichavaram, P.; Kumar, R.; Rao, G.N. Blockade of CD47 function attenuates restenosis by promoting smooth muscle cell efferocytosis and inhibiting their migration and proliferation. J. Biol. Chem. 2023, 299, 104594. [Google Scholar] [CrossRef] [PubMed]
- Krautter, F.; Hussain, M.T.; Zhi, Z.; Lezama, D.R.; Manning, J.E.; Brown, E.; Marigliano, N.; Raucci, F.; Recio, C.; Chimen, M.; et al. Galectin-9: A novel promoter of atherosclerosis progression. Atherosclerosis 2022, 363, 57–68. [Google Scholar] [CrossRef]
- Cui, K.; Podolnikova, N.P.; Bailey, W.; Szmuc, E.; Podrez, E.A.; Byzova, T.V.; Yakubenko, V.P. Inhibition of integrin α(D)β(2)-mediated macrophage adhesion to end product of docosahexaenoic acid (DHA) oxidation prevents macrophage accumulation during inflammation. J. Biol. Chem. 2019, 294, 14370–14382. [Google Scholar] [CrossRef] [PubMed]
- Favretto, G.; Cunha, R.S.D.; Dalboni, M.A.; Oliveira, R.B.; Barreto, F.C.; Massy, Z.A.; Stinghen, A.E.M. Endothelial Microparticles in Uremia: Biomarkers and Potential Therapeutic Targets. Toxins 2019, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, X.; Han, J.; Peng, W.; Fang, Z.; Zhou, Y.; Xu, X.; Lin, J.; Xiao, F.; Zhao, L.; et al. Convallatoxin Promotes M2 Macrophage Polarization to Attenuate Atherosclerosis through PPARγ-Integrin α(v)β(5) Signaling Pathway. Drug Des. Devel Ther. 2021, 15, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Sun, Y.; Yang, S.; Yu, M.; Pan, L.; Yang, J.; Yang, J.; Shao, Q.; Liu, J.; Liu, Y.; et al. Omentin-1 Modulates Macrophage Function via Integrin Receptors αvβ3 and αvβ5 and Reverses Plaque Vulnerability in Animal Models of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 757926. [Google Scholar] [CrossRef] [PubMed]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Chang, T.T.; Chang, C.C.; Chen, J.W. Fatty-Acid-Binding Protein 4 as a Novel Contributor to Mononuclear Cell Activation and Endothelial Cell Dysfunction in Atherosclerosis. Int. J. Mol. Sci. 2020, 21, 9245. [Google Scholar] [CrossRef] [PubMed]
- Marchini, T.; Mitre, L.S.; Wolf, D. Inflammatory Cell Recruitment in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 9, 635527. [Google Scholar] [CrossRef]
- Finney, A.C.; Scott, M.L.; Reeves, K.A.; Wang, D.; Alfaidi, M.; Schwartz, J.C.; Chitmon, C.M.; Acosta, C.H.; Murphy, J.M.; Alexander, J.S.; et al. EphA2 signaling within integrin adhesions regulates fibrillar adhesion elongation and fibronectin deposition. Matrix Biol. 2021, 103–104, 1–21. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, D.; Lü, S.; Zhang, Y.; Long, M. Mechanical features of endothelium regulate cell adhesive molecule-induced calcium response in neutrophils. APL Bioeng. 2019, 3, 016104. [Google Scholar] [CrossRef] [PubMed]
- Marx, C.; Novotny, J.; Salbeck, D.; Zellner, K.R.; Nicolai, L.; Pekayvaz, K.; Kilani, B.; Stockhausen, S.; Bürgener, N.; Kupka, D.; et al. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood 2019, 134, 1859–1872. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, J.; Osaka, M.; Deushi, M.; Hosoya, S.; Ishigami, A.; Maehara, T.; Yoshida, M. CXCL1-Triggered PAD4 Cytoplasmic Translocation Enhances Neutrophil Adhesion through Citrullination of PDIA1. J. Atheroscler. Thromb. 2022, 29, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Krueger, I.; Gremer, L.; Mangels, L.; Klier, M.; Jurk, K.; Willbold, D.; Bock, H.H.; Elvers, M. Reelin Amplifies Glycoprotein VI Activation and AlphaIIb Beta3 Integrin Outside-In Signaling via PLC Gamma 2 and Rho GTPases. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.S.; Kim, S.; Kwon, Y.S.; Kim, M.K.; Nam, K.S. A new function for MAP4K4 inhibitors during platelet aggregation and platelet-mediated clot retraction. Biochem. Pharmacol. 2021, 188, 114519. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, W.S.; Vesey, A.T.; Vickers, A.; Neale, A.; Moles, C.; Connell, M.; Joshi, N.V.; Lucatelli, C.; Fletcher, A.M.; Spratt, J.C.; et al. In vivo alpha-V beta-3 integrin expression in human aortic atherosclerosis. Heart 2019, 105, 1868–1875. [Google Scholar] [CrossRef]
- Sheikhvatan, M.; Boroumand, M.A.; Behmanesh, M.; Ziaee, S.; Cheraghee, S. Integrin Beta-3 Gene Polymorphism and Risk for Myocardial Infarction in Premature Coronary Disease. Iran. J. Biotechnol. 2019, 17, e1921. [Google Scholar] [CrossRef]
- Ngai, D.; Lino, M.; Bendeck, M.P. Cell-Matrix Interactions and Matricrine Signaling in the Pathogenesis of Vascular Calcification. Front. Cardiovasc. Med. 2018, 5, 174. [Google Scholar] [CrossRef]
- Al-Yafeai, Z.; Yurdagul, A., Jr.; Peretik, J.M.; Alfaidi, M.; Murphy, P.A.; Orr, A.W. Endothelial FN (Fibronectin) Deposition by α5β1 Integrins Drives Atherogenic Inflammation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2601–2614. [Google Scholar] [CrossRef]
- Budatha, M.; Zhang, J.; Schwartz, M.A. Fibronectin-Mediated Inflammatory Signaling Through Integrin α5 in Vascular Remodeling. J. Am. Heart Assoc. 2021, 10, e021160. [Google Scholar] [CrossRef]
- Murphy, J.M.; Jeong, K.; Lim, S.S. FAK Family Kinases in Vascular Diseases. Int. J. Mol. Sci. 2020, 21, 3630. [Google Scholar] [CrossRef] [PubMed]
- Dietz, M.; Kamani, C.H.; Deshayes, E.; Dunet, V.; Mitsakis, P.; Coukos, G.; Nicod Lalonde, M.; Schaefer, N.; Prior, J.O. Imaging angiogenesis in atherosclerosis in large arteries with (68)Ga-NODAGA-RGD PET/CT: Relationship with clinical atherosclerotic cardiovascular disease. EJNMMI Res. 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Park, E.J.; Kawamoto, E.; Usuda, H.; Wada, K.; Taguchi, A.; Shimaoka, M. Endothelial connexin-integrin crosstalk in vascular inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166168. [Google Scholar] [CrossRef] [PubMed]
- Aman, J.; Margadant, C. Integrin-Dependent Cell-Matrix Adhesion in Endothelial Health and Disease. Circ. Res. 2023, 132, 355–378. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, S.; Nesbitt, W.S.; Rooney, M.; Jackson, S.P. Bidirectional integrin alphaIIbbeta3 signalling regulating platelet adhesion under flow: Contribution of protein kinase C. Biochem. J. 2003, 372, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Larivière, M.; Lorenzato, C.S.; Adumeau, L.; Bonnet, S.; Hémadou, A.; Jacobin-Valat, M.J.; Noubhani, A.; Santarelli, X.; Minder, L.; Di Primo, C.; et al. Multimodal molecular imaging of atherosclerosis: Nanoparticles functionalized with scFv fragments of an anti-αIIbβ3 antibody. Nanomedicine 2019, 22, 102082. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zheng, Z.; Li, C.; Wan, J.; Wang, M. Developmental endothelial locus-1 in cardiovascular and metabolic diseases: A promising biomarker and therapeutic target. Front. Immunol. 2022, 13, 1053175. [Google Scholar] [CrossRef]
- Potashnikova, D.M.; Saidova, A.A.; Tvorogova, A.V.; Anisimova, A.S.; Botsina, A.Y.; Vasilieva, E.Y.; Margolis, L.B. CTLs From Patients With Atherosclerosis Show Elevated Adhesiveness and Distinct Integrin Expression Patterns on 2D Substrates. Front. Med. 2022, 9, 891916. [Google Scholar] [CrossRef]
- Sun, C.; Fu, Y.; Gu, X.; Xi, X.; Peng, X.; Wang, C.; Sun, Q.; Wang, X.; Qian, F.; Qin, Z.; et al. Macrophage-Enriched lncRNA RAPIA: A Novel Therapeutic Target for Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1464–1478. [Google Scholar] [CrossRef]
- Weber, K.T.; Brilla, C.G. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991, 83, 1849–1865. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Al-Hasani, J.; Sens-Albert, C.; Ghosh, S.; Trogisch, F.A.; Nahar, T.; Friede, P.A.P.; Reil, J.C.; Hecker, M. Zyxin protects from hypertension-induced cardiac dysfunction. Cell Mol. Life Sci. 2022, 79, 93. [Google Scholar] [CrossRef]
- Chen, C.; Li, R.; Ross, R.S.; Manso, A.M. Integrins and integrin-related proteins in cardiac fibrosis. J. Mol. Cell Cardiol. 2016, 93, 162–174. [Google Scholar] [CrossRef]
- Leask, A. A sticky wicket: Overexpression of integrin alpha 11 is sufficient for cardiac fibrosis. Acta Physiol. 2018, 222, e13025. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Q.; Kong, M.; Mao, L.; Yang, Y.; Xu, Y. Myocardin-related transcription factor A regulates integrin beta 2 transcription to promote macrophage infiltration and cardiac hypertrophy in mice. Cardiovasc. Res. 2022, 118, 844–858. [Google Scholar] [CrossRef]
- van Putten, S.; Shafieyan, Y.; Hinz, B. Mechanical control of cardiac myofibroblasts. J. Mol. Cell Cardiol. 2016, 93, 133–142. [Google Scholar] [CrossRef]
- Sui, S.; Hou, Y. Dual integrin αvβ3 and αvβ5 blockade attenuates cardiac dysfunction by reducing fibrosis in a rat model of doxorubicin-induced cardiomyopathy. Scand. Cardiovasc. J. 2021, 55, 287–296. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Quinones, L.; Kasiganesan, H.; Zhang, Y.; Pleasant, D.L.; Sundararaj, K.P.; Zile, M.R.; Bradshaw, A.D.; Kuppuswamy, D. β3 integrin in cardiac fibroblast is critical for extracellular matrix accumulation during pressure overload hypertrophy in mouse. PLoS ONE 2012, 7, e45076. [Google Scholar] [CrossRef]
- Sun, M.; Ishii, R.; Okumura, K.; Krauszman, A.; Breitling, S.; Gomez, O.; Hinek, A.; Boo, S.; Hinz, B.; Connelly, K.A.; et al. Experimental Right Ventricular Hypertension Induces Regional β1-Integrin-Mediated Transduction of Hypertrophic and Profibrotic Right and Left Ventricular Signaling. J. Am. Heart Assoc. 2018, 7, e13025. [Google Scholar] [CrossRef]
- Valiente-Alandi, I.; Potter, S.J.; Salvador, A.M.; Schafer, A.E.; Schips, T.; Carrillo-Salinas, F.; Gibson, A.M.; Nieman, M.L.; Perkins, C.; Sargent, M.A.; et al. Inhibiting Fibronectin Attenuates Fibrosis and Improves Cardiac Function in a Model of Heart Failure. Circulation 2018, 138, 1236–1252. [Google Scholar] [CrossRef]
- Gałdyszyńska, M.; Zwoliński, R.; Piera, L.; Szymański, J.; Jaszewski, R.; Drobnik, J. Stiff substrates inhibit collagen accumulation via integrin α2β1, FAK and Src kinases in human atrial fibroblast and myofibroblast cultures derived from patients with aortal stenosis. Biomed. Pharmacother. 2023, 159, 114289. [Google Scholar] [CrossRef]
- Hong, J.; Chu, M.; Qian, L.; Wang, J.; Guo, Y.; Xu, D. Fibrillar Type I Collagen Enhances the Differentiation and Proliferation of Myofibroblasts by Lowering α2β1 Integrin Expression in Cardiac Fibrosis. Biomed. Res. Int. 2017, 2017, 1790808. [Google Scholar] [CrossRef]
- Kanaan, R.; Medlej-Hashim, M.; Jounblat, R.; Pilecki, B.; Sorensen, G.L. Microfibrillar-associated protein 4 in health and disease. Matrix Biol. J. Int. Soc. Matrix Biol. 2022, 111, 1–25. [Google Scholar] [CrossRef]
- Sarrazy, V.; Koehler, A.; Chow, M.L.; Zimina, E.; Li, C.X.; Kato, H.; Caldarone, C.A.; Hinz, B. Integrins αvβ5 and αvβ3 promote latent TGF-β1 activation by human cardiac fibroblast contraction. Cardiovasc. Res. 2014, 102, 407–417. [Google Scholar] [CrossRef]
- Schussler, O.; Chachques, J.C.; Alifano, M.; Lecarpentier, Y. Key Roles of RGD-Recognizing Integrins During Cardiac Development, on Cardiac Cells, and After Myocardial Infarction. J. Cardiovasc. Transl. Res. 2022, 15, 179–203. [Google Scholar] [CrossRef]
- Perrucci, G.L.; Barbagallo, V.A.; Corlianò, M.; Tosi, D.; Santoro, R.; Nigro, P.; Poggio, P.; Bulfamante, G.; Lombardi, F.; Pompilio, G. Integrin ανβ5 in vitro inhibition limits pro-fibrotic response in cardiac fibroblasts of spontaneously hypertensive rats. J. Transl. Med. 2018, 16, 352. [Google Scholar] [CrossRef]
- Meagher, P.B.; Lee, X.A.; Lee, J.; Visram, A.; Friedberg, M.K.; Connelly, K.A. Cardiac Fibrosis: Key Role of Integrins in Cardiac Homeostasis and Remodeling. Cells 2021, 10, 770. [Google Scholar] [CrossRef]
- Failer, T.; Amponsah-Offeh, M.; Neuwirth, A.; Kourtzelis, I.; Subramanian, P.; Mirtschink, P.; Peitzsch, M.; Matschke, K.; Tugtekin, S.M.; Kajikawa, T.; et al. Developmental endothelial locus-1 protects from hypertension-induced cardiovascular remodeling via immunomodulation. J. Clin. Investig. 2022, 132, e126155. [Google Scholar] [CrossRef]
- Shimojo, N.; Hashizume, R.; Kanayama, K.; Hara, M.; Suzuki, Y.; Nishioka, T.; Hiroe, M.; Yoshida, T.; Imanaka-Yoshida, K. Tenascin-C may accelerate cardiac fibrosis by activating macrophages via the integrin αVβ3/nuclear factor-κB/interleukin-6 axis. Hypertension 2015, 66, 757–766. [Google Scholar] [CrossRef]
- Murray, I.R.; Gonzalez, Z.N.; Baily, J.; Dobie, R.; Wallace, R.J.; Mackinnon, A.C.; Smith, J.R.; Greenhalgh, S.N.; Thompson, A.I.; Conroy, K.P.; et al. αv integrins on mesenchymal cells regulate skeletal and cardiac muscle fibrosis. Nat. Commun. 2017, 8, 1118. [Google Scholar] [CrossRef]
- Lin, C.; Guo, Y.; Xia, Y.; Li, C.; Xu, X.; Qi, T.; Zhang, F.; Fan, M.; Hu, G.; Zhao, H.; et al. FNDC5/Irisin attenuates diabetic cardiomyopathy in a type 2 diabetes mouse model by activation of integrin αV/β5-AKT signaling and reduction of oxidative/nitrosative stress. J. Mol. Cell Cardiol. 2021, 160, 27–41. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kita, S.; Tanaka, Y.; Fukuda, S.; Obata, Y.; Okita, T.; Kawachi, Y.; Tsugawa-Shimizu, Y.; Fujishima, Y.; Nishizawa, H.; et al. A disintegrin and metalloproteinase 12 prevents heart failure by regulating cardiac hypertrophy and fibrosis. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H238–H251. [Google Scholar] [CrossRef]
- Fan, D.; Takawale, A.; Shen, M.; Samokhvalov, V.; Basu, R.; Patel, V.; Wang, X.; Fernandez-Patron, C.; Seubert, J.M.; Oudit, G.Y.; et al. A Disintegrin and Metalloprotease-17 Regulates Pressure Overload-Induced Myocardial Hypertrophy and Dysfunction Through Proteolytic Processing of Integrin β1. Hypertension 2016, 68, 937–948. [Google Scholar] [CrossRef]
- Angelini, A.; Trial, J.; Ortiz-Urbina, J.; Cieslik, K.A. Mechanosensing dysregulation in the fibroblast: A hallmark of the aging heart. Ageing Res. Rev. 2020, 63, 101150. [Google Scholar] [CrossRef]
- Niu, L.; Cheng, B.; Huang, G.; Nan, K.; Han, S.; Ren, H.; Liu, N.; Li, Y.; Genin, G.M.; Xu, F. A positive mechanobiological feedback loop controls bistable switching of cardiac fibroblast phenotype. Cell Discov. 2022, 8, 84. [Google Scholar] [CrossRef]
- Schroer, A.K.; Merryman, W.D. Mechanobiology of myofibroblast adhesion in fibrotic cardiac disease. J. Cell Sci. 2015, 128, 1865–1875. [Google Scholar] [CrossRef]
- Saraswati, S.; Lietman, C.D.; Li, B.; Mathew, S.; Zent, R.; Young, P.P. Small proline-rich repeat 3 is a novel coordinator of PDGFRβ and integrin β1 crosstalk to augment proliferation and matrix synthesis by cardiac fibroblasts. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 7885–7904. [Google Scholar] [CrossRef]
- Horii, Y.; Matsuda, S.; Toyota, C.; Morinaga, T.; Nakaya, T.; Tsuchiya, S.; Ohmuraya, M.; Hironaka, T.; Yoshiki, R.; Kasai, K.; et al. VGLL3 is a mechanosensitive protein that promotes cardiac fibrosis through liquid-liquid phase separation. Nat. Commun. 2023, 14, 550. [Google Scholar] [CrossRef]
- Takawale, A.; Zhang, P.; Patel, V.B.; Wang, X.; Oudit, G.; Kassiri, Z. Tissue Inhibitor of Matrix Metalloproteinase-1 Promotes Myocardial Fibrosis by Mediating CD63-Integrin β1 Interaction. Hypertension 2017, 69, 1092–1103. [Google Scholar] [CrossRef]
- Bouvet, M.; Claude, O.; Roux, M.; Skelly, D.; Masurkar, N.; Mougenot, N.; Nadaud, S.; Blanc, C.; Delacroix, C.; Chardonnet, S.; et al. Anti-integrin α(v) therapy improves cardiac fibrosis after myocardial infarction by blunting cardiac PW1(+) stromal cells. Sci. Rep. 2020, 10, 11404. [Google Scholar] [CrossRef]
- Gao, A.E.; Sullivan, K.E.; Black, L.D., 3rd. Lysyl oxidase expression in cardiac fibroblasts is regulated by α2β1 integrin interactions with the cellular microenvironment. Biochem. Biophys. Res. Commun. 2016, 475, 70–75. [Google Scholar] [CrossRef]
- Tannous, C.; Deloux, R.; Karoui, A.; Mougenot, N.; Burkin, D.; Blanc, J.; Coletti, D.; Lavery, G.; Li, Z.; Mericskay, M. NMRK2 Gene Is Upregulated in Dilated Cardiomyopathy and Required for Cardiac Function and NAD Levels during Aging. Int. J. Mol. Sci. 2021, 22, 3524. [Google Scholar] [CrossRef]
- Civitarese, R.A.; Talior-Volodarsky, I.; Desjardins, J.F.; Kabir, G.; Switzer, J.; Mitchell, M.; Kapus, A.; McCulloch, C.A.; Gullberg, D.; Connelly, K.A. The α11 integrin mediates fibroblast-extracellular matrix-cardiomyocyte interactions in health and disease. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H96–H106. [Google Scholar] [CrossRef]
- Hanna, A.; Humeres, C.; Frangogiannis, N.G. The role of Smad signaling cascades in cardiac fibrosis. Cell Signal 2021, 77, 109826. [Google Scholar] [CrossRef]
- Romaine, A.; Sørensen, I.W.; Zeltz, C.; Lu, N.; Erusappan, P.M.; Melleby, A.O.; Zhang, L.; Bendiksen, B.; Robinson, E.L.; Aronsen, J.M.; et al. Overexpression of integrin α11 induces cardiac fibrosis in mice. Acta Physiol. 2018, 222, e12932. [Google Scholar] [CrossRef]
- Talior-Volodarsky, I.; Arora, P.D.; Wang, Y.; Zeltz, C.; Connelly, K.A.; Gullberg, D.; McCulloch, C.A. Glycated Collagen Induces α11 Integrin Expression Through TGF-β2 and Smad3. J. Cell. Physiol. 2015, 230, 327–336. [Google Scholar] [CrossRef]
- Li, J.; Salvador, A.M.; Li, G.; Valkov, N.; Ziegler, O.; Yeri, A.; Yang Xiao, C.; Meechoovet, B.; Alsop, E.; Rodosthenous, R.S.; et al. Mir-30d Regulates Cardiac Remodeling by Intracellular and Paracrine Signaling. Circ. Res. 2021, 128, e1–e23. [Google Scholar] [CrossRef]
- Rouzaud-Laborde, C.; Delmas, C.; Pizzinat, N.; Tortosa, F.; Garcia, C.; Mialet-Perez, J.; Payrastre, B.; Sié, P.; Spreux-Varoquaux, O.; Sallerin, B.; et al. Platelet activation and arterial peripheral serotonin turnover in cardiac remodeling associated to aortic stenosis. Am. J. Hematol. 2015, 90, 15–19. [Google Scholar] [CrossRef]
- Nevers, T.; Salvador, A.M.; Velazquez, F.; Ngwenyama, N.; Carrillo-Salinas, F.J.; Aronovitz, M.; Blanton, R.M.; Alcaide, P. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J. Exp. Med. 2017, 214, 3311–3329. [Google Scholar] [CrossRef]
- Walker, J.A.; Beck, G.A.; Campbell, J.H.; Miller, A.D.; Burdo, T.H.; Williams, K.C. Anti-α4 Integrin Antibody Blocks Monocyte/Macrophage Traffic to the Heart and Decreases Cardiac Pathology in a SIV Infection Model of AIDS. J. Am. Heart Assoc. 2015, 4, e001932. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.; Yang, D.; Jiang, X.; Wang, W.E.; Yue, R.; Fang, Y. Integrin-Linked Kinase Activation Prevents Ventricular Arrhythmias Induced by Ischemia/Reperfusion Via Inhibition of Connexin 43 Remodeling. J. Cardiovasc. Transl. Res. 2021, 14, 610–618. [Google Scholar] [CrossRef]
- Quang, K.L.; Maguy, A.; Qi, X.Y.; Naud, P.; Xiong, F.; Tadevosyan, A.; Shi, Y.F.; Chartier, D.; Tardif, J.C.; Dobrev, D.; et al. Loss of cardiomyocyte integrin-linked kinase produces an arrhythmogenic cardiomyopathy in mice. Circ. Arrhythm. Electrophysiol. 2015, 8, 921–932. [Google Scholar] [CrossRef]
- Brodehl, A.; Rezazadeh, S.; Williams, T.; Munsie, N.M.; Liedtke, D.; Oh, T.; Ferrier, R.; Shen, Y.; Jones, S.J.M.; Stiegler, A.L.; et al. Mutations in ILK, encoding integrin-linked kinase, are associated with arrhythmogenic cardiomyopathy. Transl. Res. 2019, 208, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Shi, L.; Chen, X.; Cui, C.; Huang, J.; Chen, B.; Hall, D.D.; Pan, Z.; Lu, M.; et al. Integrin β1D Deficiency-Mediated RyR2 Dysfunction Contributes to Catecholamine-Sensitive Ventricular Tachycardia in Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation 2020, 141, 1477–1493. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, S.; Shao, Y.; Wu, Y.; Qin, J.; Chen, Y.; Chen, L.; Gu, H.; Wang, X.; Huang, C.; et al. Calreticulin overexpression correlates with integrin-α5 and transforming growth factor-β1 expression in the atria of patients with rheumatic valvular disease and atrial fibrillation. Int. J. Cardiol. 2013, 168, 2177–2185. [Google Scholar] [CrossRef]
- Schinner, C.; Xu, L.; Franz, H.; Zimmermann, A.; Wanuske, M.T.; Rathod, M.; Hanns, P.; Geier, F.; Pelczar, P.; Liang, Y.; et al. Defective Desmosomal Adhesion Causes Arrhythmogenic Cardiomyopathy by Involving an Integrin-αVβ6/TGF-β Signaling Cascade. Circulation 2022, 146, 1610–1626. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, D.J.; Schmeckpeper, J.; Knollmann, B.C. Animal Models to Study Cardiac Arrhythmias. Circ. Res. 2022, 130, 1926–1964. [Google Scholar] [CrossRef]
- Kamble, R.N.; Gaikwad, S.; Maske, A.; Patil, S.S. Fabrication of electrospun nanofibres of BCS II drug for enhanced dissolution and permeation across skin. J. Adv. Res. 2016, 7, 483–489. [Google Scholar] [CrossRef]
- Dieffenbach, P.B.; Mallarino Haeger, C.; Rehman, R.; Corcoran, A.M.; Coronata, A.M.F.; Vellarikkal, S.K.; Chrobak, I.; Waxman, A.B.; Vitali, S.H.; Sholl, L.M.; et al. A Novel Protective Role for Matrix Metalloproteinase-8 in the Pulmonary Vasculature. Am. J. Respir. Crit. Care Med. 2021, 204, 1433–1451. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yu, P.B. Periostin: A Novel Integrator of Hypoxic Signaling in Pulmonary Hypertension. Circ. Res. 2020, 127, 1153–1155. [Google Scholar] [CrossRef]
- Jia, D.; Zhu, Q.; Liu, H.; Zuo, C.; He, Y.; Chen, G.; Lu, A. Osteoprotegerin Disruption Attenuates HySu-Induced Pulmonary Hypertension Through Integrin αvβ3/FAK/AKT Pathway Suppression. Circ. Cardiovasc. Genet. 2017, 10, e001591. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, X.; Teng, X.; Gu, H.; Yuan, W.; Meng, J.; Li, J.; Zheng, Z.; Wei, Y.; Hu, S. Osteopontin plays important roles in pulmonary arterial hypertension induced by systemic-to-pulmonary shunt. FASEB J. 2019, 33, 7236–7251. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Martini, A.G.; Brown, R.I.; Liang, X.; Medrano, S.; Goto, S.; Narita, I.; Arend, L.J.; Sequeira-Lopez, M.L.S.; Gomez, R.A. Inhibition of the renin-angiotensin system causes concentric hypertrophy of renal arterioles in mice and humans. JCI Insight 2021, 6, e154337. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhu, H.; Wang, S.; Li, Y.; Yan, C.; Wang, J.; Ying, K. CircItgb5 promotes synthetic phenotype of pulmonary artery smooth muscle cells via interacting with miR-96-5p and Uba1 in monocrotaline-induced pulmonary arterial hypertension. Respir. Res. 2023, 24, 165. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, N.; Link, P.A.; Farkas, D.; Harmon, B.; Hudson, J.; Bogamuwa, S.; Piper, B.; Authelet, K.; Cool, C.D.; Heise, R.L.; et al. Dichotomous role of integrin-β5 in lung endothelial cells. Pulm. Circ. 2022, 12, e12156. [Google Scholar] [CrossRef] [PubMed]
- Vinaiphat, A.; Pazhanchamy, K.; JebaMercy, G.; Ngan, S.C.; Leow, M.K.; Ho, H.H.; Gao, Y.G.; Lim, K.L.; Richards, A.M.; de Kleijn, D.P.V.; et al. Endothelial Damage Arising From High Salt Hypertension Is Elucidated by Vascular Bed Systematic Profiling. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, Z.; Cui, L.; Ban, Y.; Leung, P.C.K.; Li, Y.; Ma, J. Activin A increases human trophoblast invasion by upregulating integrin β1 through ALK4. Faseb. J. 2021, 35, e21220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Che, J.; Chang, L.; Guo, M.; Bao, X.; Mu, D.; Sun, X.; Zhang, X.; Lu, W.; Xie, J. CD47- and Integrin α4/β1-Comodified-Macrophage-Membrane-Coated Nanoparticles Enable Delivery of Colchicine to Atherosclerotic Plaque. Adv. Healthc. Mater. 2022, 11, e2101788. [Google Scholar] [CrossRef]
- Pang, A.; Cheng, N.; Cui, Y.; Bai, Y.; Hong, Z.; Delaney, M.K.; Zhang, Y.; Chang, C.; Wang, C.; Liu, C.; et al. High-loading Gα(13)-binding EXE peptide nanoparticles prevent thrombosis and protect mice from cardiac ischemia/reperfusion injury. Sci. Transl. Med. 2020, 12, eaaz7287. [Google Scholar] [CrossRef]
- Peters, L.J.F.; Jans, A.; Bartneck, M.; van der Vorst, E.P.C. Immunomodulatory Nanomedicine for the Treatment of Atherosclerosis. J. Clin. Med. 2021, 10, 3185. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.X. Development of High-Drug-Loading Nanoparticles. Chempluschem 2020, 85, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Hagemeyer, C.E.; Peter, K. Targeting the platelet integrin GPIIb/IIIa. Curr. Pharm. Des. 2010, 16, 4119–4133. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Guleria, R.S.; Foster, D.M.; Lu, G.; Watson, L.E.; Sanghi, S.; Smith, M.; Dostal, D.E. Integrins: Novel therapeutic targets for cardiovascular diseases. Cardiovasc. Hematol. Agents Med. Chem. 2007, 5, 109–132. [Google Scholar] [CrossRef] [PubMed]
- van den Kerkhof, D.L.; van der Meijden, P.E.J.; Hackeng, T.M.; Dijkgraaf, I. Exogenous Integrin αIIbβ3 Inhibitors Revisited: Past, Present and Future Applications. Int. J. Mol. Sci. 2021, 22, 3366. [Google Scholar] [CrossRef]
- Vorchheimer, D.A.; Badimon, J.J.; Fuster, V. Platelet glycoprotein IIb/IIIa receptor antagonists in cardiovascular disease. JAMA 1999, 281, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.A. Controversies surrounding platelet glycoprotein IIb/IIIa inhibitors in percutaneous coronary intervention and acute coronary syndromes. Semin. Thromb. Hemost. 2004, 30, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Breuer, J.; Schneider-Hohendorf, T.; Ostkamp, P.; Herich, S.; Rakhade, S.; Antonijevic, I.; Klotz, L.; Wiendl, H.; Schwab, N. VLA-2 blockade in vivo by vatelizumab induces CD4+FoxP3+ regulatory T cells. Int. Immunol. 2019, 31, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Duplàa, C.; Couffinhal, T.; Dufourcq, P.; Llanas, B.; Moreau, C.; Bonnet, J. The integrin very late antigen-4 is expressed in human smooth muscle cell. Involvement of alpha 4 and vascular cell adhesion molecule-1 during smooth muscle cell differentiation. Circ. Res. 1997, 80, 159–169. [Google Scholar] [CrossRef]
- Lumsden, A.B.; Chen, C.; Hughes, J.D.; Kelly, A.B.; Hanson, S.R.; Harker, L.A. Anti-VLA-4 antibody reduces intimal hyperplasia in the endarterectomized carotid artery in nonhuman primates. J. Vasc. Surg. 1997, 26, 87–93. [Google Scholar] [CrossRef]
- Braun, A.; Dofiles, L.; Rousselle, S.; Guerrero, L.; Gunther, J.; Yednock, T.; Stricker-Krongrad, A.; Messersmith, E. Effects of an alpha-4 integrin inhibitor on restenosis in a new porcine model combining endothelial denudation and stent placement. PLoS ONE 2010, 5, e14314. [Google Scholar] [CrossRef] [PubMed]
- Raab-Westphal, S.; Marshall, J.F.; Goodman, S.L. Integrins as Therapeutic Targets: Successes and Cancers. Cancers 2017, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.J.; Li, M.B.; Wu, X.; Wu, S.; Zhu, W.; Chen, D.; Luo, M.; Eitenmüller, I.; Kampmann, A.; Schaper, J.; et al. Activation of the integrins alpha 5beta 1 and alpha v beta 3 and focal adhesion kinase (FAK) during arteriogenesis. Mol. Cell Biochem. 2009, 322, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qin, X.; Wang, H.; Miao, R.; Zhang, Y.; Miao, C.; Wang, Z. Effects of integrin α5β1 on the proliferation and migration of human aortic vascular smooth muscle cells. Mol. Med. Rep. 2016, 13, 1147–1155. [Google Scholar] [CrossRef]
- Yurdagul, A., Jr.; Green, J.; Albert, P.; McInnis, M.C.; Mazar, A.P.; Orr, A.W. α5β1 integrin signaling mediates oxidized low-density lipoprotein-induced inflammation and early atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Dev, R.; Doddapattar, P.; Kon, S.; Dhanesha, N.; Chauhan, A.K. Integrin α9 regulates smooth muscle cell phenotype switching and vascular remodeling. JCI Insight 2021, 6, e147134. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Tanaka, Y.; Erdman, J.; Kaneko, Y.; Saito, M.; Higashitani, C.; Smulders, R.; Lademacher, C. ASP5094, a humanized monoclonal antibody against integrin alpha-9, did not show efficacy in patients with rheumatoid arthritis refractory to methotrexate: Results from a phase 2a, randomized, double-blind, placebo-controlled trial. Arthritis Res. Ther. 2020, 22, 252. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, K.; Liu, B.; Kent, K.C. Activation of integrin receptors is required for growth factor-induced smooth muscle cell dysfunction. J. Vasc. Surg. 2000, 31, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Hoshiga, M.; Alpers, C.E.; Smith, L.L.; Giachelli, C.M.; Schwartz, S.M. Alpha-v beta-3 integrin expression in normal and atherosclerotic artery. Circ. Res. 1995, 77, 1129–1135. [Google Scholar] [CrossRef]
- van der Zee, R.; Murohara, T.; Passeri, J.; Kearney, M.; Cheresh, D.A.; Isner, J.M. Reduced intimal thickening following alpha(v)beta3 blockade is associated with smooth muscle cell apoptosis. Cell Adhes. Commun. 1998, 6, 371–379. [Google Scholar] [CrossRef]
- Liaw, L.; Skinner, M.P.; Raines, E.W.; Ross, R.; Cheresh, D.A.; Schwartz, S.M.; Giachelli, C.M. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J. Clin. Investig. 1995, 95, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Bendeck, M.P.; Irvin, C.; Reidy, M.; Smith, L.; Mulholland, D.; Horton, M.; Giachelli, C.M. Smooth muscle cell matrix metalloproteinase production is stimulated via alpha(v)beta(3) integrin. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Bae, J.S.; Park, R.W.; Kim, J.E.; Park, J.Y.; Kim, I.S. betaig-h3 triggers signaling pathways mediating adhesion and migration of vascular smooth muscle cells through alphavbeta5 integrin. Exp. Mol. Med. 2006, 38, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zou, J.; Yu, X.; Yin, S.; Tang, C. The antiatherogenic function of kallistatin and its potential mechanism. Acta Biochim. Biophys. Sin. 2020, 52, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.P.; Vaidya, B. Targeted delivery of thrombolytic agents: Role of integrin receptors. Expert. Opin. Drug Deliv. 2009, 6, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhao, X.; O’Brien, K.A.; Stojanovic-Terpo, A.; Delaney, M.K.; Kim, K.; Cho, J.; Lam, S.C.; Du, X. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature 2013, 503, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Ni, Y.; Yu, H.; Yin, H.; Yang, F.; Li, C.; Sun, D.; Pei, T.; Ma, J.; Deng, L.; et al. Inflammatory endothelium-targeted and cathepsin responsive nanoparticles are effective against atherosclerosis. Theranostics 2022, 12, 4200–4220. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Grenache, D.G.; Coleman, T.; Semenkovich, C.F.; Santoro, S.A.; Zutter, M.M. Alpha2beta1 integrin and development of atherosclerosis in a mouse model: Assessment of risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2104–2109. [Google Scholar] [CrossRef]
- Skinner, M.P.; Raines, E.W.; Ross, R. Dynamic expression of alpha 1 beta 1 and alpha 2 beta 1 integrin receptors by human vascular smooth muscle cells. Alpha 2 beta 1 integrin is required for chemotaxis across type I collagen-coated membranes. Am. J. Pathol. 1994, 145, 1070–1081. [Google Scholar]
- Akanchise, T.; Angelova, A. Ginkgo Biloba and Long COVID: In Vivo and In Vitro Models for the Evaluation of Nanotherapeutic Efficacy. Pharmaceutics 2023, 15, 1562. [Google Scholar] [CrossRef] [PubMed]
- Zerrillo, L.; Gigliobianco, M.R.; D’Atri, D.; Garcia, J.P.; Baldazzi, F.; Ridwan, Y.; Fuentes, G.; Chan, A.; Creemers, L.B.; Censi, R.; et al. PLGA Nanoparticles Grafted with Hyaluronic Acid to Improve Site-Specificity and Drug Dose Delivery in Osteoarthritis Nanotherapy. Nanomaterials 2022, 12, 2248. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Saglietto, A.; Manfredi, R.; Elia, E.; D’Ascenzo, F.; DE Ferrari, G.M.; Biondi-Zoccai, G.; Munzel, T. Cardiovascular disease burden: Italian and global perspectives. Minerva Cardiol. Angiol. 2021, 69, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Simon, Y.; Jazzar, A.; Najibeddine, H.; Normand, A.; Jacques, D. High Na(+) Salt Diet and Remodeling of Vascular Smooth Muscle and Endothelial Cells. Biomedicines 2021, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Simon, Y.; Jacques, D.; Bkaily, G. High salt-induced morphological and glycocalyx remodeling of human vascular smooth muscle cells is reversible but induces a high sodium salt-like sensitive memory. Can. J. Physiol. Pharmacol. 2023, 101, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Castro, C.; Cordasic, N.; Neureiter, D.; Amann, K.; Marek, I.; Volkert, G.; Stintzing, S.; Jahn, A.; Rascher, W.; Hilgers, K.F.; et al. Under-expression of α8 integrin aggravates experimental atherosclerosis. J. Pathol. 2015, 236, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, J.; Du, Y.; Huang, Y.; Li, J.; Liu, B.; Liu, C.J.; Zhu, Y.; Gao, Y.; Xu, Q.; et al. Cartilage oligomeric matrix protein maintains the contractile phenotype of vascular smooth muscle cells by interacting with alpha(7)beta(1) integrin. Circ. Res. 2010, 106, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.C.; Breuss, J.; Pytela, R.; Kramer, R.H. Functional expression of the alpha 7 integrin receptor in differentiated smooth muscle cells. J. Cell Sci. 1997, 110 Pt 13, 1477–1487. [Google Scholar] [CrossRef]
- Zargham, R.; Touyz, R.M.; Thibault, G. Alpha 8 Integrin overexpression in de-differentiated vascular smooth muscle cells attenuates migratory activity and restores the characteristics of the differentiated phenotype. Atherosclerosis 2007, 195, 303–312. [Google Scholar] [CrossRef]
- Hetherington, I.; Totary-Jain, H. Anti-atherosclerotic therapies: Milestones, challenges, and emerging innovations. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 3106–3117. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Bauersachs, R.M.; Anand, S.S.; Debus, E.S.; Nehler, M.R.; Patel, M.R.; Fanelli, F.; Capell, W.H.; Diao, L.; Jaeger, N.; et al. Rivaroxaban in Peripheral Artery Disease after Revascularization. N. Engl. J. Med. 2020, 382, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Howlett, J.G. Nebivolol: Vasodilator properties and evidence for relevance in treatment of cardiovascular disease. Can. J. Cardiol. 2014, 30, S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yu, L.; Zhao, Y.; Ge, J. Panvascular medicine: An emerging discipline focusing on atherosclerotic diseases. Eur. Heart J. 2022, 43, 4528–4531. [Google Scholar] [CrossRef] [PubMed]
| Disease | Cell Types | Integrin | ECM | Main Functions | Effects of Signal Modulation | Reference |
|---|---|---|---|---|---|---|
| AS | VEC | α5β1 | ANX2 Ox-LDL | Promotes the translocation of α5β1 integrins to lipid rafts and activates the endothelial inflammatory pathway | Foam cell deposition Vascular endothelial inflammation | [35,36] |
| COMP | Inhibits the activation of α5β1 integrins | Alleviates the vascular endothelial inflammation | [14] | |||
| Gal-3 | Activates the β1 integrin/RhoA/JNK signaling pathway and exacerbates ox-LDL-mediated vascular endothelial injury | Foam cell deposition Vascular endothelial inflammation | [37] | |||
| β4 | Activates the Src/NF-kB signaling pathway and promotes the expression of endothelial inflammatory factors | Vascular endothelial inflammation | [38] | |||
| α6β1 | CCN1 | Activates NF-kB and forms a positive feedback loop with CCN1 and α6β1 integrins | Oxidative stress Vascular endothelial inflammation | [39] | ||
| VSMC | α5β1 | MFG-E8 | Promotes the MMP2 expression and activates the TGF-β1/Smad2/3 signaling pathway | VSMC calcification Vascular wall calcification | [15] | |
| α-SMA | Mediates vascular tone dysregulation and VSMC migration | Vasoconstrictive dysfunction | [40] | |||
| β3 | Thrombin | Interacts with CD47 and mediates VSMC migration and proliferation | Vascular wall thickening and vascular restenosis | [41] | ||
| Immune cell | αMβ2 | ICAM-1 | Induces the adhesion and extravasation of immune cells on the vascular endothelium | Vascular endothelial inflammation | [16] | |
| Gal-9 | Activates and promotes the differentiation of monocytes to macrophages | Vascular endothelial inflammation Atherosclerotic plaque formation | [42] | |||
| αDβ2 | DHA CEP | Promotes M1 macrophage accumulation in ECM | Vascular endothelial inflammation Atherosclerotic plaque formation | [43] | ||
| αVβ5 | Cadherin ICAM-1 E-selectin | Promotes fibronectin expression and macrophage migration | Vascular endothelial inflammation Atherosclerotic plaque formation | [44] | ||
| PPARγ | Promotes M2 macrophage polarization and the expression of anti-inflammatory factors | Attenuates AS and promotes tissue repair | [45] | |||
| Omentin-1 | Induces the PI3K/Akt signaling pathway and AMPK phosphorylation | Promotes plaque stability | [46] | |||
| OPN | Attenuates vascular calcification | Promotes positive ischemic neovascularization | [47] | |||
| α4 | FABP4 | Induces macrophage adhesion | Vascular endothelial inflammation Atherosclerotic plaque formation | [48,49] | ||
| α5β1 | EphA2 | Promotes immunocyte adhesion | [50] | |||
| β2 | E-selectin ICAM-1 | Activates the Syk/Src signaling pathway and then promotes the calcium reflux of neutrophils | [51] | |||
| Promotes eosinophil adhesion | [52] | |||||
| PAD4 | Promotes neutrophil adhesion | [53] | ||||
| Platelet | αIIbβ3 | Fibrinogen | Activates Rho GTPase RAC1 and RhoA, thereby promoting cytoskeletal reorganization | Thrombus formation | [54] | |
| GNE495 PF 06260933 | Inhibits the pathogenic roles of αIIbβ3 integrins | Inhibits platelet aggregation and clot retraction | [55] | |||
| αVβ3 | Fibronectin | Promotes platelet adhesion and aggregation | Thrombus formation | [56,57] |
| Disease | Cell Types | Integrin | ECM | Main Functions | Effects of Signal Modulation | Reference |
|---|---|---|---|---|---|---|
| Cardiac fibrosis | Cardiac fibroblast | αVβ5 αVβ3 | Latent TGF-β | Activates the TGF-β1/Smad2/3/α-SMA signaling pathway and promotes collagen synthesis | Cardiac fibroblast transdifferentiation and collagen deposition | [76,77] |
| Activates the FAK/c-Src/NF-kB signaling pathway and promotes collagen synthesis | [78] | |||||
| αVβ1 | Latent TGF-β | Activates the FAK/Akt/mTOR and TGF-β/Smad2/3/α-SMA signaling pathways | [79] | |||
| Fibronectin | [80] | |||||
| CD63 | Promotes the translocation of Smad2/3 and β-catenin, thereby promoting collagen synthesis | [80] | ||||
| α2β1 | Collagen | Activates FAK and Src | Attenuates collagen deposition | [81] | ||
| Activates PP2A/PTEN signaling pathway and then inhibits Akt and α-SMA expression | [82] |
| Disease | Cell Types | Integrin | ECM | Main Functions | Effects of Signal Modulation | Reference |
|---|---|---|---|---|---|---|
| Arrhythmias | Cardiomyocyte | β1 | Activates the ILK/Akt/Cx43/PI3K/Akt signaling pathway | Diminishes cardiac remodeling and attenuates arrhythmias | [111,112,113] | |
| Activates the talin/Vcl/ZO-1/Cx43 signaling pathway, thereby promoting Cx43 stability | Stabilizes the myocardial electrical signal Attenuates arrhythmias | [17] | ||||
| Fibronectin | Promotes β1 integrin degradation and inhibits RyR2 phosphorylation | Myocardial electrical signal dysfunction | [114] | |||
| α5 | Fibronectin | Promotes ECM collagen deposition | Myocardial damage Cytoskeletal remodeling | [115] |
| Disease | Cell Types | Integrin | ECM | Main Functions | Effects of Signal Modulation | Reference |
|---|---|---|---|---|---|---|
| Hypertension | PASMC | β3 | MMP8 | Activates the FAK/YAP/TAZ signaling pathway and promotes PASMC proliferation | Vascular remodeling | [119] |
| αVβ3 | Hypoxia | Activates the Pyk2/ERK/NF-kB/H2O2 signaling pathway, thereby reducing PPARγ expression and promoting PASMC proliferation | [120] | |||
| Activates the OPG/FAK/Akt signaling pathway and promotes PASMC proliferation | [61,121] | |||||
| OPN | Activates the ERK1/2/Akt signaling pathway and promotes PASMC proliferation | [122] | ||||
| β5 | PDGF-BB | Activates the Ubal protein/Ube2n/Mdm2/ACE2 and miR-96-5p/mTOR signaling pathways, thereby promoting PASMC proliferation | [123,124] |
| Category | Related Integrin | Functions | Implication in CVD | Agents in Clinics | Reference |
|---|---|---|---|---|---|
| Integrin antagonists and antibodies | αIIbβ3 | Inhibits platelet aggregation with fibrinogen and clot retraction | Inhibits thrombosis | Abciximab, Eptifibatide, Tirofiban | [18,133,134,135,136,137] |
| α2β1 | Inhibits the adhesion of platelets to collagen Inhibits the expression of collagen and collagenase genes Inhibits the phenotypic plasticity of VSMC | Inhibits thrombosis and plaque formation | Vatelizumab | [134,138] | |
| α4β1 | Inhibits the phenotypic plasticity of VSMC | Inhibits plaque formation and platelet aggregation | Natalzumab, AJM300 | [139,140,141,142] | |
| α5β1 | Inhibits the phenotypic plasticity of VSMC | Inhibits plaque formation and platelet aggregation | Volociximab, ATN61 | [143,144,145] | |
| α9β1 | Inhibits the phenotypic plasticity of VSMC | Inhibits plaque formation and platelet aggregation | ASP5094 | [146,147] | |
| αVβ3 | Inhibits the phenotypic plasticity of VSMC | Inhibits plaque formation and platelet aggregation | LM609, Abciximab (c7E3Fab; ReoPro), Vitaxin, Inttumumab, | [143,148,149,150,151,152] | |
| αVβ5 | Inhibits the phenotypic plasticity of VSMC | Inhibits plaque formation and platelet aggregation | LM609 Inttumumab | [151,153] | |
| β2 | Inhibits leukocyte extravasation | Reduces inflammatory tissue damage | [8] | ||
| β3 | Inhibits NF-κB nuclear translocation Downregulates VEGF expression | Inhibits angiogenesis | Kallistatin | [154] | |
| Nanotherapy | αIIbβ3 | Surfaces with RGD peptide; Loading thrombolytic drugs | Thrombolysis | [19,155] | |
| αIIbβ3 | Contains M3mP6 or M3mp13 Inhibits signaling pathways of αIIbβ3 integrins | Inhibits thrombosis | [23,129,156] | ||
| ανβ3 | Contains cRGDfK peptide | [130] | |||
| αVβ3 | Releases RAP | Inhibits local inflammation | RAP@T/R NPs | [157] | |
| α4β1 | MMM NPs | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhang, Q.; Lu, Y.; Chen, J.; Liu, J.; Li, Z.; Xie, Z. Roles of Integrin in Cardiovascular Diseases: From Basic Research to Clinical Implications. Int. J. Mol. Sci. 2024, 25, 4096. https://doi.org/10.3390/ijms25074096
Zhang S, Zhang Q, Lu Y, Chen J, Liu J, Li Z, Xie Z. Roles of Integrin in Cardiovascular Diseases: From Basic Research to Clinical Implications. International Journal of Molecular Sciences. 2024; 25(7):4096. https://doi.org/10.3390/ijms25074096
Chicago/Turabian StyleZhang, Shuo, Qingfang Zhang, Yutong Lu, Jianrui Chen, Jinkai Liu, Zhuohan Li, and Zhenzhen Xie. 2024. "Roles of Integrin in Cardiovascular Diseases: From Basic Research to Clinical Implications" International Journal of Molecular Sciences 25, no. 7: 4096. https://doi.org/10.3390/ijms25074096
APA StyleZhang, S., Zhang, Q., Lu, Y., Chen, J., Liu, J., Li, Z., & Xie, Z. (2024). Roles of Integrin in Cardiovascular Diseases: From Basic Research to Clinical Implications. International Journal of Molecular Sciences, 25(7), 4096. https://doi.org/10.3390/ijms25074096






