Hybrid Materials Obtained by Immobilization of Biosynthesized Ag Nanoparticles with Antioxidant and Antimicrobial Activity
Abstract
1. Introduction
2. Results and Discussion
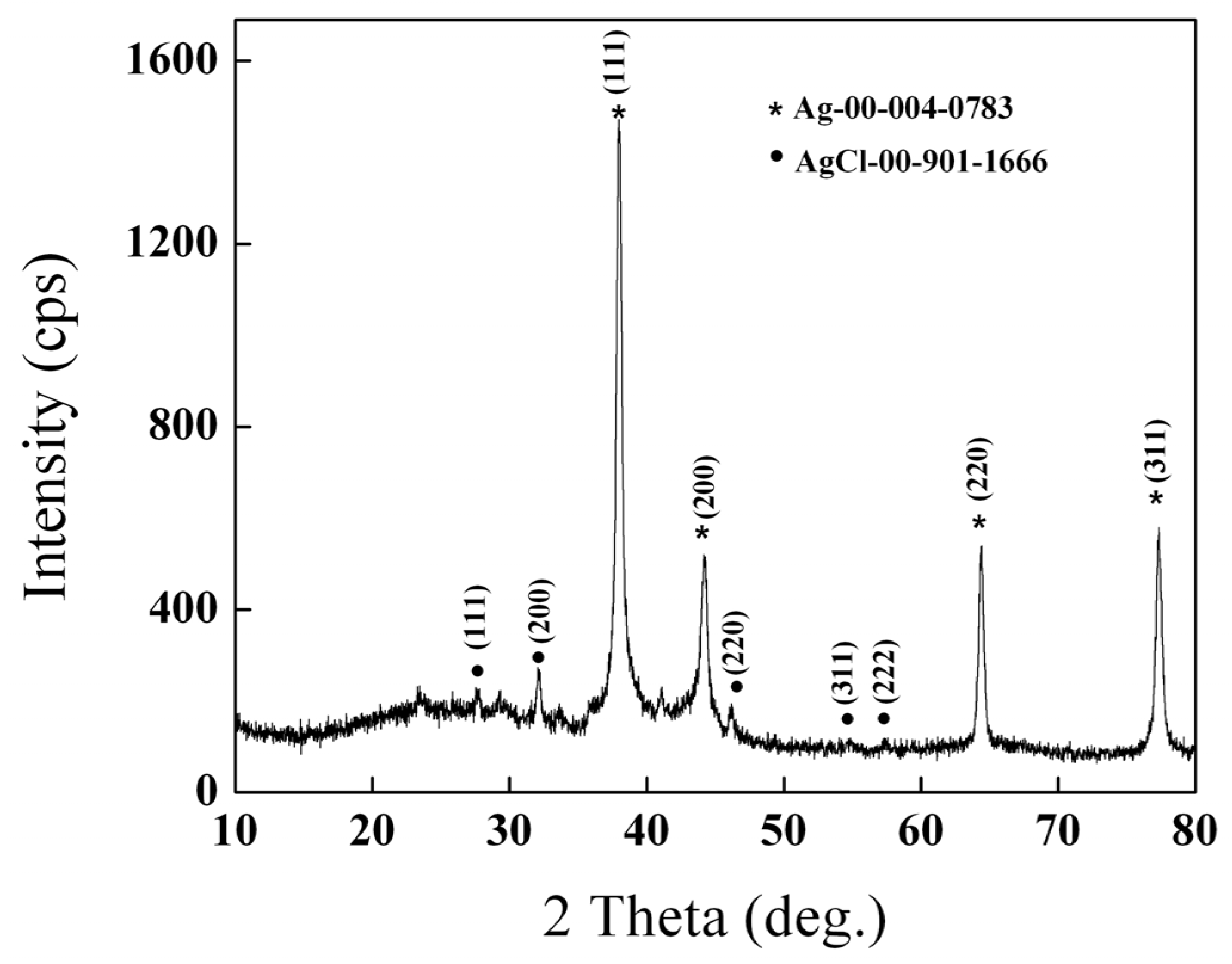
2.1. X-ray Diffraction
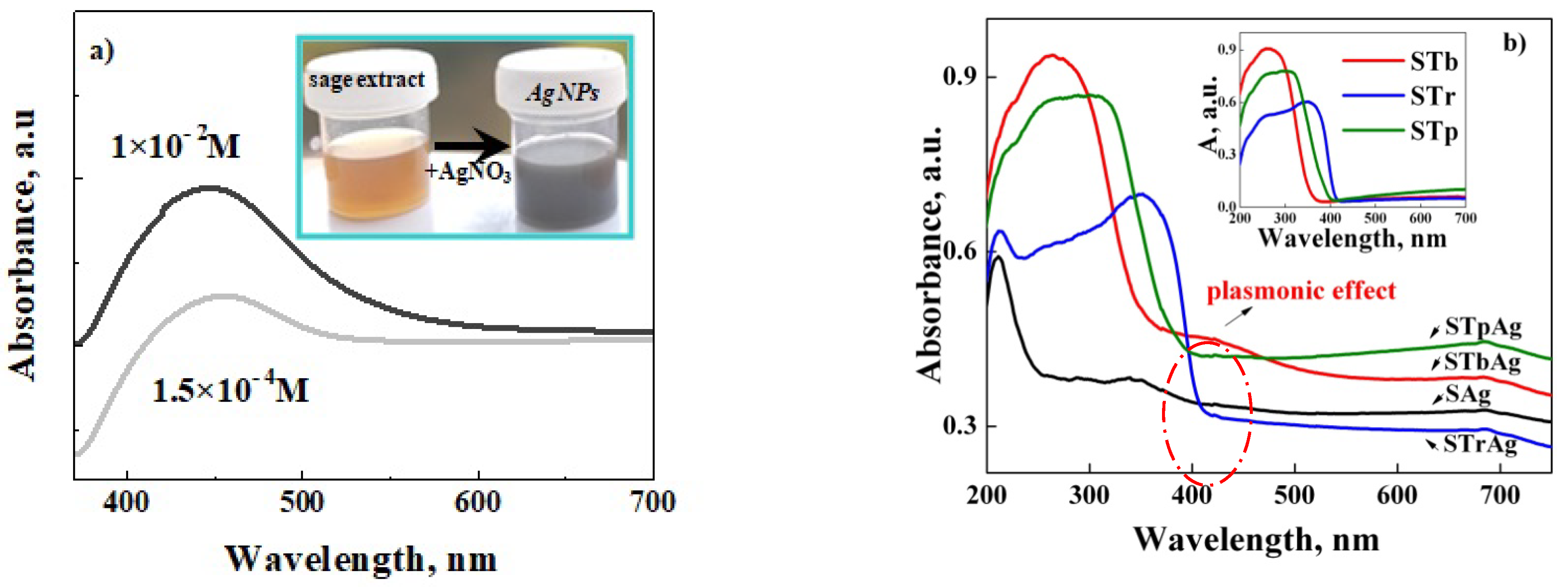
2.2. UV-Vis Absorption Analysis
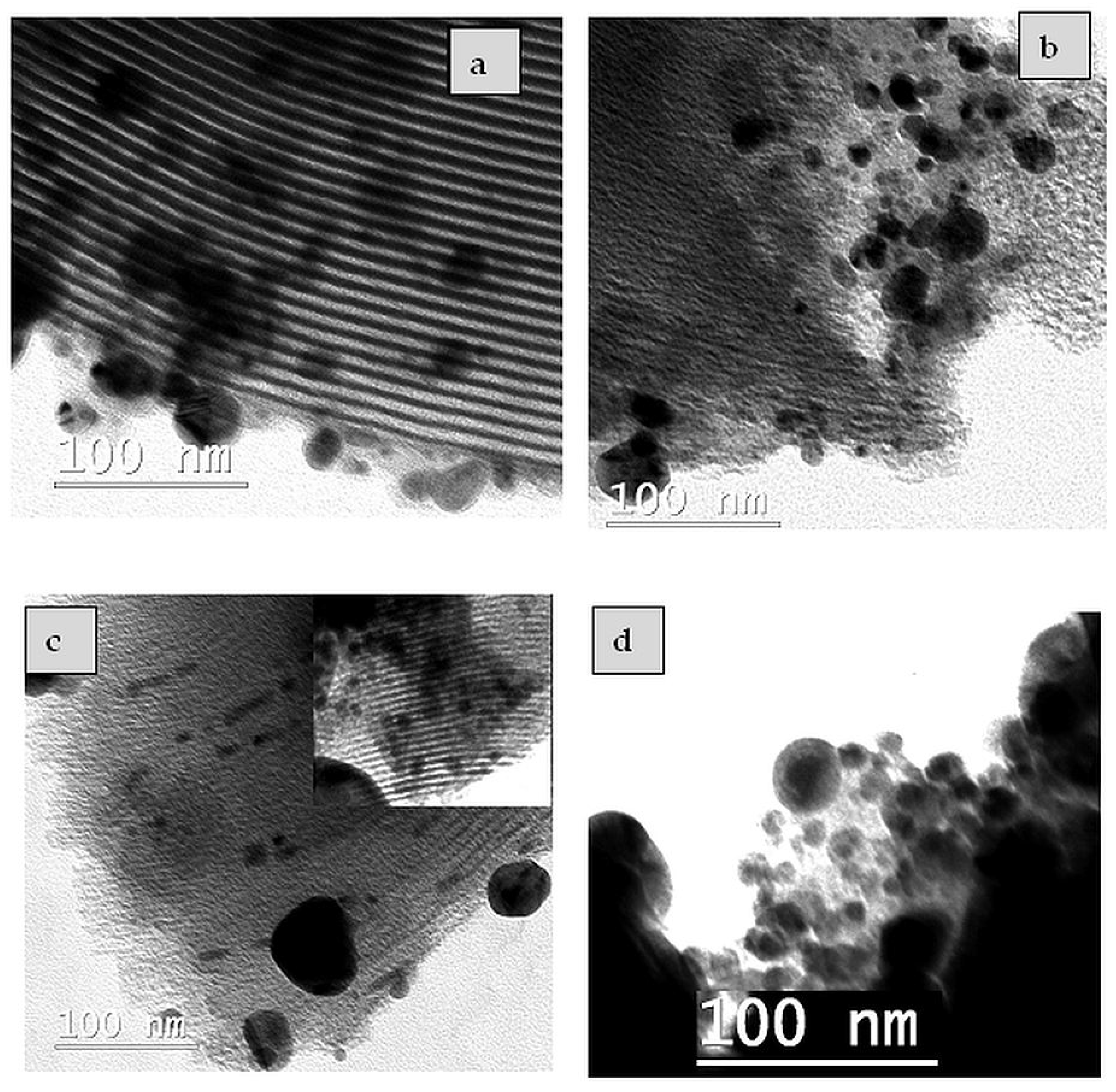
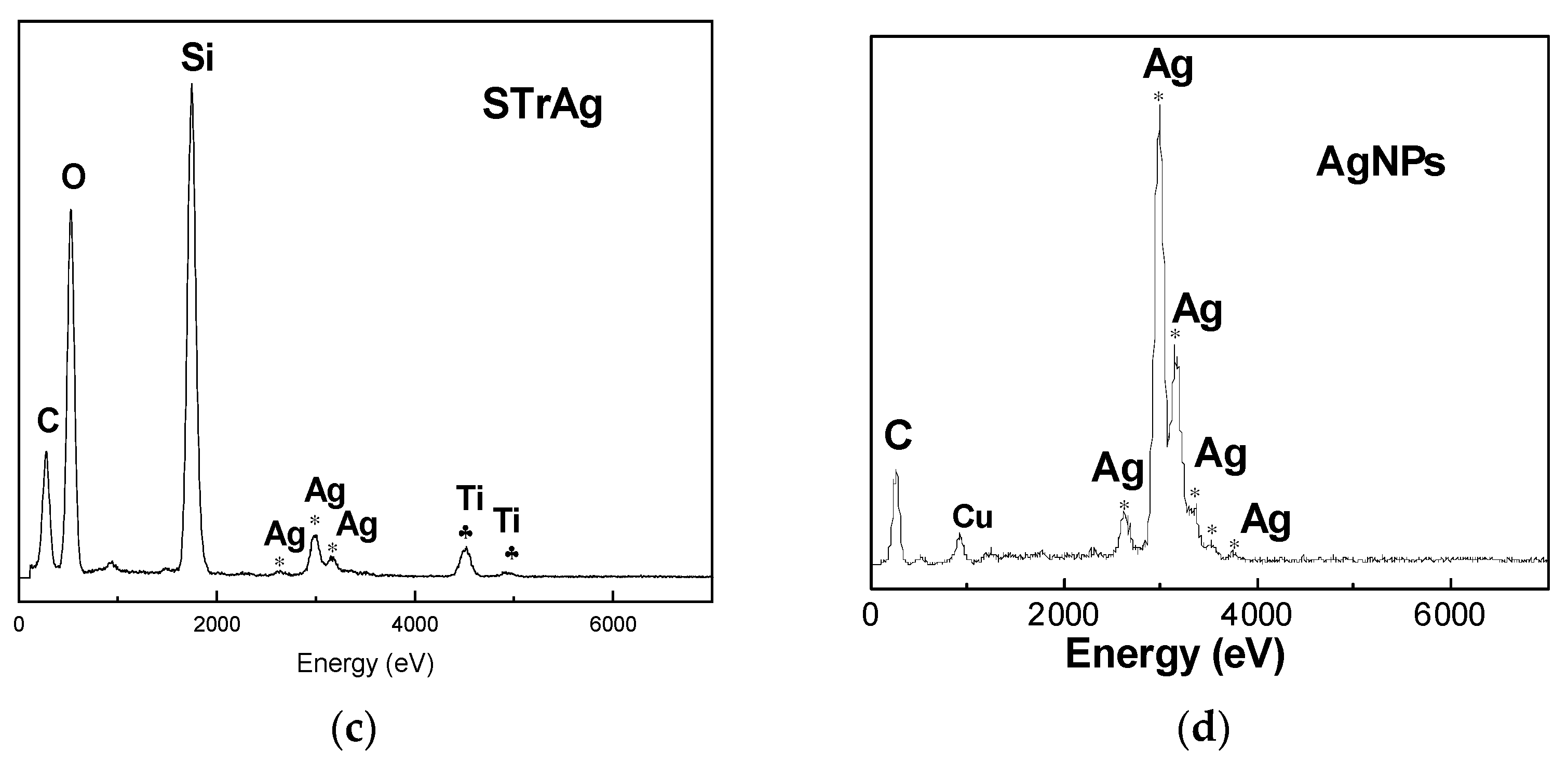
2.3. TEM/EDX Analysis
2.4. FT-IR Analysis
2.5. Photoluminescence Analysis
2.6. Dynamic Light Scattering Analysis
2.7. Antioxidant Activity
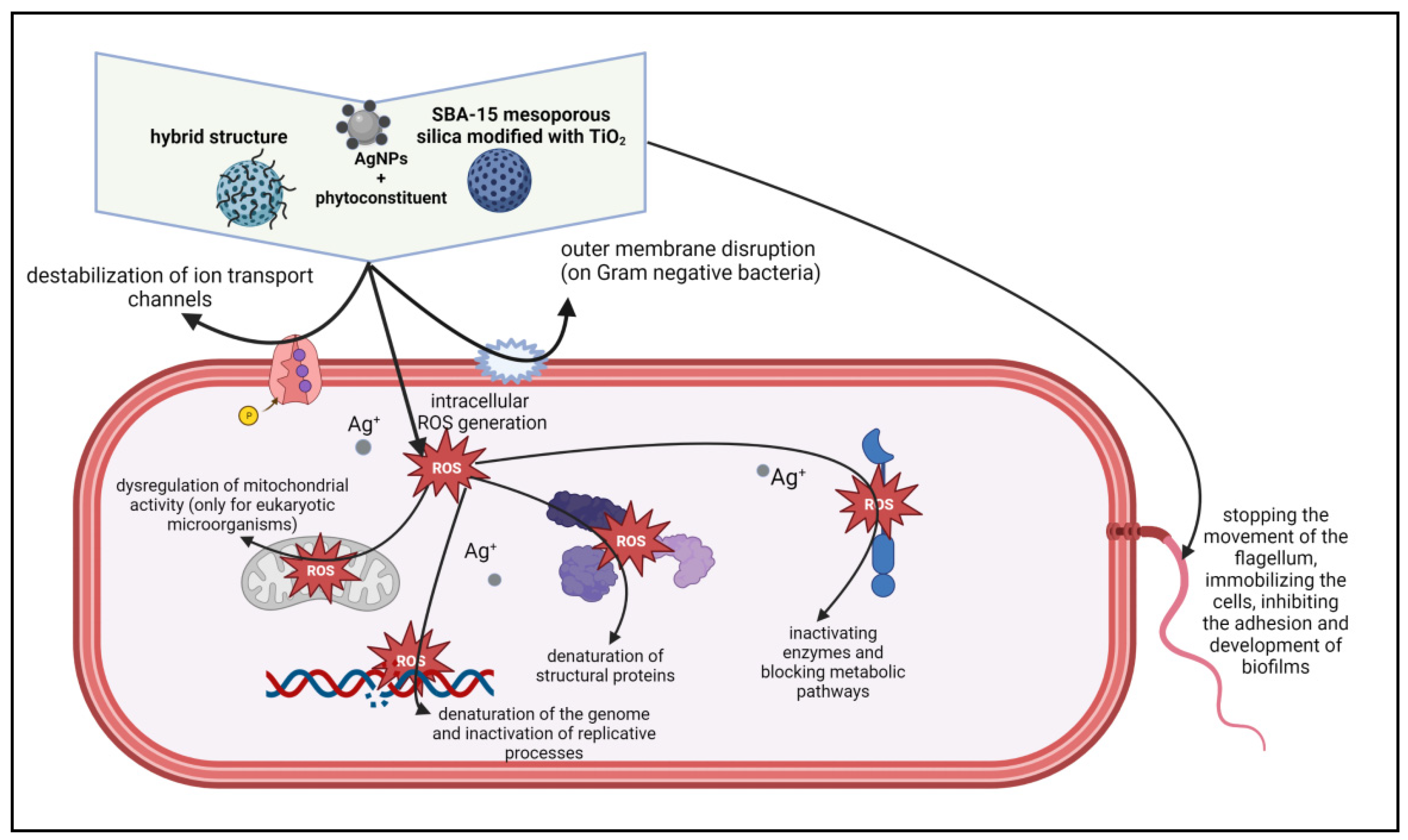
2.8. Antimicrobial Properties
3. Materials and Methods
3.1. Material Preparation
3.1.1. Chemicals
3.1.2. Synthesis of TiO2-Modified SBA-15 Supports
3.1.3. Preparation of Concentrated Extract by Nanofiltration
3.1.4. Biosynthesis and Immobilization of AgNPs
3.2. Characterization Methods
3.2.1. Characterization of Salvia officinalis Extract
3.2.2. Characterization of AgNPs before and after Immobilization
3.2.3. Characterization of Antimicrobial Test
3.2.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matatkova, O.; Michailidu, J.; Miskovska, A.; Kolouchova, I.; Masak, J.; Cejkova, A. Antimicrobial properties and applications of metal nanoparticles biosynthesized by green methods. Biotechnol. Adv. 2022, 58, 107905. [Google Scholar] [CrossRef] [PubMed]
- Santra, T.S.; Tseng, F.G.; Barik, T.K. Biosynthesis of Silver and Gold Nanoparticles for Potential Biomedical Applications—A Brief Review. J. Nanopharm. Drug Deliv. 2014, 2, 249–265. [Google Scholar] [CrossRef]
- Kieslich, C.A.; Alimirzaei, F.; Song, H.; Do, M.; Hall, P. Data-driven prediction of antiviral peptides based on periodicities of amino acid properties. In Proceedings of the 31st European Symposium on Computer Aided Process Engineering, Istanbul, Türkiye, 6–9 June 2021; Türkay, M., Gani, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 2019–2024. [Google Scholar]
- Barabadi, H.; Jounaki, K.; Pishgahzadeh, E.; Morad, H.; Bozorgchami, N.; Vahidi, H. Bioengineered metal-based antimicrobial nanomaterials for surface coatings In Antiviral and Antimicrobial Smart Coatings: Fundamentals and Applications; Kumar, A., Behera, A., Nguyen, T.A., Bilal, M., Gupta, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 489–539. [Google Scholar]
- Truong, L.B.; Medina, D.; Martínez-Sanmiguel, J.J.; Soto-Mendoza, A.; Esquivel-López, I.G.; Pérez, Y.; Muthupandian, S.; Barabadi, H.; Cholula-Díaz, J.L.; Mostafavi, E. Biogenic metal nanomaterials to combat antimicrobial resistance. In Emerging Nanomaterials and Nano-Based Drug Delivery Approaches to Combat Antimicrobial Resistance, 1st ed.; Saravanan, M., Barabadi, H., Mostafavi, E., Webster, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 261–304. [Google Scholar]
- Singla, S.; Jana, A.; Thakur, R.; Kumari, C.; Goyal, S.; Pradhan, J. Green synthesis of silver nanoparticles using Oxalis griffithii extract and assessing their antimicrobial activity. OpenNano 2022, 7, 100047. [Google Scholar] [CrossRef]
- Hashim, N.; Paramasivam, M.; Tan, J.S.; Kernain, D.; Hussina, M.H.; Brossed, N.; Gambier, F.; Raja, P.B. Green mode synthesis of silver nanoparticles using Vitis vinifera’s tannin and screening its antimicrobial activity/apoptotic potential versus cancer cells. Mater. Today Commun. 2020, 25, 101511. [Google Scholar] [CrossRef]
- Sharma, L.; Dhiman, M.; Singh, A.; Sharma, M.M. Biological synthesis of silver nanoparticles using Nyctanthes arbor-tristis L.: A green approach to evaluate antimicrobial activities. Mater. Today Proc. 2021, 43, 2915–2920. [Google Scholar] [CrossRef]
- Khanal, L.N.; Sharma, K.R.; Paudyal, H.; Parajuli, K.; Dahal, B.; Ganga, G.C.; Pokharel, Y.R.; Kalauni, S.K. Green Synthesis of Silver Nanoparticles from Root Extracts of Rubus ellipticus Sm. and Comparison of Antioxidant and Antibacterial Activity. J. Nanomater. 2022, 2022, 1832587. [Google Scholar] [CrossRef]
- Abbasi, Z.; Feizi, S.; Taghipour, E.; Ghadam, P. Green synthesis of silver nanoparticles using aqueous extract of dried Juglans regia green husk and examination of its biological properties. Green Process. Synth. 2017, 6, 477–485. [Google Scholar] [CrossRef]
- Amin, M.; Anwar, F.; Ashraf Janjua, M.R.S.; Awais Iqbal, M.; Rashid, U. Green Synthesis of Silver Nanoparticles through Reduction with Solanum xanthocarpum L. Berry Extract: Characterization, Antimicrobial and Urease Inhibitory Activities against Helicobacter pylori. Int. J. Mol. Sci. 2012, 13, 9923–9941. [Google Scholar] [CrossRef] [PubMed]
- Sellami, H.; Khan, S.A.; Ahmad, I.; Alarfaj, A.A.; Hirad, A.H.; Al-Sabri, A.E. Green Synthesis of Silver Nanoparticles Using Olea europaea Leaf Extract for Their Enhanced Antibacterial, Antioxidant, Cytotoxic and Biocompatibility Applications. Int. J. Mol. Sci. 2021, 22, 12562. [Google Scholar] [CrossRef]
- Xing, Y.; Liao, X.; Liu, X.; Li, W.; Huang, R.; Tang, J.; Xu, Q.; Li, X.; Yu, J. Characterization and Antimicrobial Activity of Silver Nanoparticles Synthesized with the Peel Extract of Mango. Materials 2021, 14, 5878. [Google Scholar] [CrossRef]
- Salari, S.; Bahabadi, S.E.; Samzadeh-Kermani, A.; Yosefzaei, F. In-Vitro Evaluation of Antioxidant and Antibacterial Potential of Green Synthesized Silver Nanoparticles Using Prosopis Farcta Fruit Extract. Iran. J. Pharm. Res. 2019, 18, 430–445. [Google Scholar] [PubMed]
- Tyavambiza, C.; Meyer, M.; Wusu, A.D.; Madiehe, A.M.; Meyer, S. The Antioxidant and In Vitro Wound Healing Activity of Cotyledon orbiculata Aqueous Extract and the Synthesized Biogenic Silver Nanoparticles. Int. J. Mol. Sci. 2022, 23, 16094. [Google Scholar] [CrossRef] [PubMed]
- Vernet-Crua, A.; Cruz, D.; Mostafavi, E.; Truong, L.; Barabadi, H.; Cholula-Díaz, J.; Guisbiers, G.; Webster, T. Green-synthesized metallic nanoparticles for antimicrobial applications. In Nanomedicine: Technologies and Applications, 2nd ed.; Webster, T.J., Ed.; Woodhead Publishing: Cambridge, UK, 2023; pp. 297–338. [Google Scholar]
- Biba, R.; Cvjetko, P.; Tkalec, M.; Košpic, K.; Štefanic, P.P.; Šikic, S.; Domijan, A.-M.; Balen, B. Effects of Silver Nanoparticles on Physiological and Proteomic Responses of Tobacco (Nicotiana tabacum) Seedlings Are Coating-Dependent. Int. J. Mol. Sci. 2022, 23, 15923. [Google Scholar] [CrossRef] [PubMed]
- Burlacu, E.; Tanase, C.; Coman, N.A.; Berta, L. A Review of Bark-Extract-Mediated Green Synthesis of Metallic Nanoparticles and Their Applications. Molecules 2019, 24, 4354. [Google Scholar] [CrossRef] [PubMed]
- Sokolik, C.G.; Lellouche, J.P. Hybrid-silica nanoparticles as a delivery system of the natural biocide carvacrol. RSC Adv. 2018, 8, 36712. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, M.; Parvu, L.; Paun, G.; Savin, S.; Albu, B.G.; Munteanu, C.; Pandele Cusu, J.; Atkinson, I.; Culita, D.C.; Petcu, G.; et al. Development of a new (bio)hybrid matrix based on Althaea officinalis and Betonica officinalis extracts loaded into mesoporous silica nanoparticles for bioactive compounds with therapeutic applications. J. Drug Deliv. Sci. Technol. 2020, 51, 605–613. [Google Scholar] [CrossRef]
- Mai, Z.; Chen, J.; Hu, Y.; Liu, F.; Fu, B.; Zhang, H.; Dong, X.; Huang, W.; Zhou, W. Novel functional mesoporous silica nanoparticles loaded with Vitamin E acetate as smart platforms for pH responsive delivery with high bioactivity. J. Coll. Interface Sci. 2017, 508, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, Z.; Subramaniam, S.; Khan, G.M.; Abeer, M.M.; Qu, Z.; Janjua, T.; Kumeria, T.; Batra, J.; Popat, A. Encapsulation and controlled release of resveratrol within functionalized mesoporous silica nanoparticles for prostate cancer therapy. Front. Bioeng. Biotechnol. 2019, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Maliki, I.; Es-Safi, I.; El Moussaoui, A.; Mechchate, H.; El Majdoub, Y.O.; Bouymajane, A.; Cacciola, F.; Mondello, L.; Elbadaoui, K. Salvia officinalis and Lippia triphylla: Chemical characterization and evaluation of antidepressant-like activity. J. Pharm. Biomed. Anal. 2021, 203, 114207. [Google Scholar] [CrossRef]
- Metwally, D.M.; Alajmi, R.A.; El-Khadragy, M.F.; Al-Quraishy, S. Silver Nanoparticles Biosynthesized with Salvia officinalis Leaf Exert Protective Effect on Hepatic Tissue Injury Induced by Plasmodium chabaudi. Front. Vet. Sci. 2021, 7, 620665. [Google Scholar] [CrossRef]
- Balčiūnaitienė, A.; Liaudanskas, M.; Puzerytė, V.; Viškelis, J.; Janulis, V.; Viškelis, P.; Jankauskaitė, V. Eucalyptus globulus and Salvia officinalis extracts mediated green synthesis of silver nanoparticles and their application as an antioxidant and antimicrobial agent. Plants 2022, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Albeladi, S.S.R.; Malik, M.A.; Al-thabaiti, S.A. Facile biofabrication of silver nanoparticles using Salvia officinalis leaf extract and its catalytic activity towards Congo red dye degradation. J. Mater. Res. Technol. 2020, 9, 10031–10044. [Google Scholar] [CrossRef]
- Huq, A. Green Synthesis of Silver Nanoparticles Using Pseudoduganella eburnea MAHUQ-39 and Their Antimicrobial Mechanisms Investigation against Drug Resistant Human Pathogens. Int. J. Mol. Sci. 2020, 21, 1510. [Google Scholar] [CrossRef] [PubMed]
- Nagasundaram, N.; Rahuman, M.A.; Raghavan, P.S. Antibacterial application studies of nanosilver incorporated products. Int. J. Pharm. Res. Bio–Sci. 2014, 3, 153–164. [Google Scholar]
- Kamli, M.R.; Alzahrani, E.A.; Albukhari, S.M.; Ahmad, A.; Sabir, J.S.M.; Malik, M.A. Combination Effect of Novel Bimetallic Ag-Ni Nanoparticles with Fluconazole against Candida albicans. J. Fungi 2022, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Ansah, I.B.; Lee, S.H.; Mun, C.; Kim, D.-H.; Park, S.-G. Interior Hotspot Engineering in Ag–Au Bimetallic Nanocomposites by In Situ Galvanic Replacement Reaction for Rapid and Sensitive Surface-Enhanced Raman Spectroscopy Detection. Int. J. Mol. Sci. 2022, 23, 11741. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.T.P.; Petri, M.V.; Valencia, E.Y.; Camargo, P.H.C.; de Torresi, S.I.C.; Spira, B. Visible light plasmon excitation of silver nanoparticles against antibiotic-resistant Pseudomonas aeruginosa. Photodiagnosis Photodyn. Ther. 2020, 31, 101908. [Google Scholar] [CrossRef] [PubMed]
- Paterno, G.M.; Ross, A.M.; Pietralunga, S.M.; Normani, S.; Vedova, N.D.; Limwongyut, J.; Bondelli, G.; Moscardi, L.; Bazan, G.C.; Scotognella, F.; et al. The impact of bacteria exposure on the plasmonic response of silver nanostructured surfaces. Chem. Phys. Rev. 2021, 2, 021401. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Dong, P.; Yang, F.; Cheng, X.; Huang, Z.; Nie, X.; Xiao, Y.; Zhang, X. Plasmon enhanced photocatalytic and antimicrobial activities of Ag-TiO2 nanocomposites under visible light irradiation prepared by DBD cold plasma treatment. Mater. Sci. Eng. C 2019, 96, 197–204. [Google Scholar] [CrossRef]
- El-Desouky, N.; Shoueir, K.R.; El-Mehasseb, I.; El-Kemary, M. Bio-inspired green manufacturing of plasmonic silver nanoparticles/Degussa using Banana Waste Peduncles: Photocatalytic, antimicrobial, and cytotoxicity evaluation. J. Mater. Res. Technol. 2021, 10, 671–686. [Google Scholar] [CrossRef]
- Pal, S.; Nisi, R.; Licciulli, A. Antibacterial Activity of In Situ Generated Silver Nanoparticles in Hybrid Silica Films. Photochem 2022, 2, 479–488. [Google Scholar] [CrossRef]
- Chand, K.; Cao, D.; Fouad, D.E.; Shah, A.H.; Lakhan, M.N.; Dayo, A.Q.; Sagar, H.J.; Zhu, K.; Mohamed, A.M.A. Photocatalytic and antimicrobial activity of biosynthesized silver and titanium dioxide nanoparticles: A comparative study. J. Mol. Liq. 2020, 316, 113821. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Roto, R.; Novarita, D.; Suwondo, K.P.; Kuswandi, B. Preparation of TiO2/AgNPs by photodeposition method using Ag(I) prezent in radiophotography wastewater and their antibacterial activity in visible light illumination. J. Environ. Chem. Eng. 2019, 7, 103178. [Google Scholar] [CrossRef]
- Yılmaz, G.E.; Göktürk, I.; Ovezova, M.; Yılmaz, F.; Kılıç, S.; Denizli, A. Antimicrobial Nanomaterials: A Review. Hygiene 2023, 3, 269–290. [Google Scholar] [CrossRef]
- Mahira, S.; Jain, A.; Khan, W.; Domb, A.J. Antimicrobial Materials—An Overview. In Antimicrobial Materials for Biomedical Applications; Domb, A.J., Kunduru, K.R., Farah, S., Eds.; The Royal Society of Chemistry: London, UK, 2019; pp. 1–37. [Google Scholar]
- Parra-Ortiz, E.; Caselli, L.; Agnoletti, M.; Skoda, M.W.A.; Li, X.; Zhao, D.; Malmsten, M. Mesoporous silica as a matrix for photocatalytic titanium dioxide nanoparticles: Lipid membrane interactions. Nanoscale 2022, 14, 12297. [Google Scholar] [CrossRef] [PubMed]
- Parra-Ortiz, E.; Malmsten, M. Photocatalytic nanoparticles from membrane interactions to antimicrobial and antiviral effects. Adv. Colloid Interface Sci. 2022, 299, 102526. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Sarkar, R.; Pakhira, B.; Ghosh, S.; Sarkar, R.; Barui, A.; Sarka, S. ROS generation by reduced graphene oxide (rGO) induced by visible light showing antibacterial activity: Comparison with graphene oxide (GO). RSC Adv. 2015, 5, 80192–80195. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef]
- Prasanna, V.L.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.-H.; Liu, Y.; Wang, H. Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- de Dicastillo, C.L.; Correa, M.G.; Martínez, F.B.; Streitt, C.; Galotto, M.J. Antimicrobial Effect of Titanium Dioxide Nanoparticles. In Antimicrobial Resistance—A One Health Perspective; Mareș, M., Lim, S.H.E., Lai, K., Cristina, R., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Preda, S.; Pandele-Cusu, J.; Petrescu, S.V.; Ciobanu, E.M.; Petcu, G.; Culita, D.C.; Apostol, N.G.; Costescu, R.M.; Raut, I.; Constantin, M.; et al. Photocatalytic and Antibacterial Properties of Doped TiO2 Nanopowders Synthesized by Sol-Gel Method. Gels 2022, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yan, X.; Shao, R.; Dai, H.; Li, S. Preparation of nano-TiO2 by liquid hydrolysis and characterization of its antibacterial activity. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 407–409. [Google Scholar] [CrossRef]
- Bucuresteanu, R.; Ionita, M.; Chihaia, V.; Ficai, A.; Trusca, R.-D.; Ilie, C.-I.; Kuncser, A.; Holban, A.-M.; Mihaescu, G.; Petcu, G.; et al. Antimicrobial Properties of TiO2 Microparticles Coated with Ca- and Cu-Based Composite Layers. Int. J. Mol. Sci. 2022, 23, 6888. [Google Scholar] [CrossRef] [PubMed]
- Kuyukina, M.S.; Makarova, M.V.; Pistsova, O.N.; Glebov, G.G.; Osipenko, M.A.; Ivshina, I.B. Exposure to metal nanoparticles changes zeta potentials of Rhodococcus cells. Heliyon 2022, 8, 11632. [Google Scholar] [CrossRef] [PubMed]
- Filip, M.; Petcu, G.; Anghel, E.M.; Petrescu, S.; Trica, B.; Osiceanu, P.; Stanica, N.; Atkinson, I.; Munteanu, C.; Mureseanu, M.; et al. FeTi-SBA-15 magnetic nanocomposites with photocatalytic properties. Catal. Today 2021, 366, 10–19. [Google Scholar] [CrossRef]
- Lv, F.; Miao, Y.; Yang, D.; Mao, B.; Bian, Z.; Zhu, F. In Situ Etching Synthesis of TiO2-SBA-15 Nanocomposite Enhancing Adsorption and Photocatalytic Degradation. Catalysts 2022, 12, 1334. [Google Scholar] [CrossRef]
- Taghdiri, M.; Doolabi, S.D. Shift the Photocatalytic Activity of P25 TiO2 Nanoparticles toward the Visible Region upon Surface Modification with Organic Hybrid of Phosphotungstate. Int. J. Photoenerg. 2020, 2020, 8870194. [Google Scholar] [CrossRef]
- Rajkumar, R.; Shivakumar, M.S.; Nathan, S.S.; Selvam, K. Pharmacological and Larvicidal Potential of Green Synthesized Silver Nanoparticles Using Carmona retusa (Vahl) Masam Leaf Extract. J. Clust. Sci. 2018, 29, 1243–1253. [Google Scholar] [CrossRef]
- Bordbar, M. Biosynthesis of Ag/almond shell nanocomposite as a cost-effective and efficient catalyst for degradation of 4-nitrophenol and organic dyes. RSC Adv. 2017, 7, 180. [Google Scholar] [CrossRef]
- Shi, J.P.; Ma, C.Y.; Xu, B.; Zhang, H.W.; Yu, C.P. Effect of light on toxicity of nanosilver to Tetrahymena pyriformis. Environ. Toxicol. Chem. 2012, 31, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, F.; Liu, P.; Gao, G.; Ding, Y.; Zhang, S.; Yang, M. Antioxidant functionalized silica-coated TiO2 nanorods to enhance the thermal and photo stability of polypropylene. Appl. Surf. Sci. 2019, 476, 682–690. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Chen, X.; Lan, W.; Xie, J. Natural phenolic compounds: Antimicrobial properties, antimicrobial mechanisms, and potential utilization in the preservation of aquatic products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Mureseanu, M.; Filip, M.; Somacescu, S.; Baran, A.; Carja, G.; Parvulescu, V. Ce, Ti modified MCM-48 mesoporous photocatalysts: Effect of the synthesis route on support and metal ion properties. Appl. Surf. Sci. 2018, 444, 235–242. [Google Scholar] [CrossRef]
- Akhavan, O. Las ing antibacterial activities of Ag–TiO2/Ag/a-TiO2 nanocomposite thin filmphotocatalysts under solar light irradiation. J. Colloid Interface Sci. 2009, 336, 117–124. [Google Scholar] [CrossRef]
- Barabadi, H.; Vahidi, H.; Mahjoub, M.A.; Kosar, Z.; Kamali, K.D.; Ponmurugan, K.; Hosseini, O.; Rashedi, M.; Saravanan, M. Emerging Antineoplastic Gold Nanomaterials for Cervical Cancer Therapeutics: A Systematic Review. J. Clust. Sci. 2020, 31, 1173–1184. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska-Büchner, E.; Flieger, W.; Pasieczna-Patkowska, S.; Franus, W.; Panek, R.; Korona-Głowniak, I.; Suśniak, K.; Rajtar, B.; Świątek, Ł.; Żuk, N.; et al. Antimicrobial and Apoptotic Efficacy of Plant-Mediated Silver Nanoparticles. Molecules 2023, 28, 5519. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.A.; Das, S.S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, M.S.; Amit Kumar Nayak, A.K. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy. Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Mohaghegh, N.; Endo-Kimura, M.; Wang, K.; Wei, Z.; Najafabadi, A.H.; Zehtabi, F.; Kouchehbaghi, N.H.; Sharma, S.; Markowska-Szczupak, A.; Kowalska, E. Apatite-coated Ag/AgBr/TiO2 nanocomposites: Insights into the antimicrobial mechanism in the dark and under visible-light irradiation. Appl. Surf. Sci. 2023, 617, 156574. [Google Scholar] [CrossRef]
- Talank, N.; Morad, H.; Barabadi, H.; Mojab, F.; Amidi, S.; Kobarfard, F.; Jounaki, M.A.M.K.; Mohammadi, N.; Salehi, G.; Ashrafizadeh, M.; et al. Bioengineering of green synthesized silver nanoparticles: In vitro physicochemical, antibacterial, biofilm inhibitory, anticoagulant, and antioxidant performance. Talanta 2022, 243, 123374–123389. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. Nanobiotechnol 2018, 16, 14. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R. Fluconazole resistance in Candida species: A current perspective. Infect. Drug Resist. 2017, 10, 137–245. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.R.; Fuchs, B.B.; Pinhati, H.M.S.; Siqueira, R.A.; Hagen, F.; Meis, J.F.; Mylonakis, E.; Colombo, A.L. Candida parapsilosis resistance to fluconazole: Molecular mechanisms and in vivo impact in infected Galleria mellonella larvae. Antimicrob. Agents Chemother. 2015, 59, 6581–6587. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Bach, H. Mechanisms of Antifungal Properties of Metal Nanoparticles. Nanomaterials 2022, 12, 4470. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Tang, C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2006, 101, 140–147. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH• Free Radical Method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]


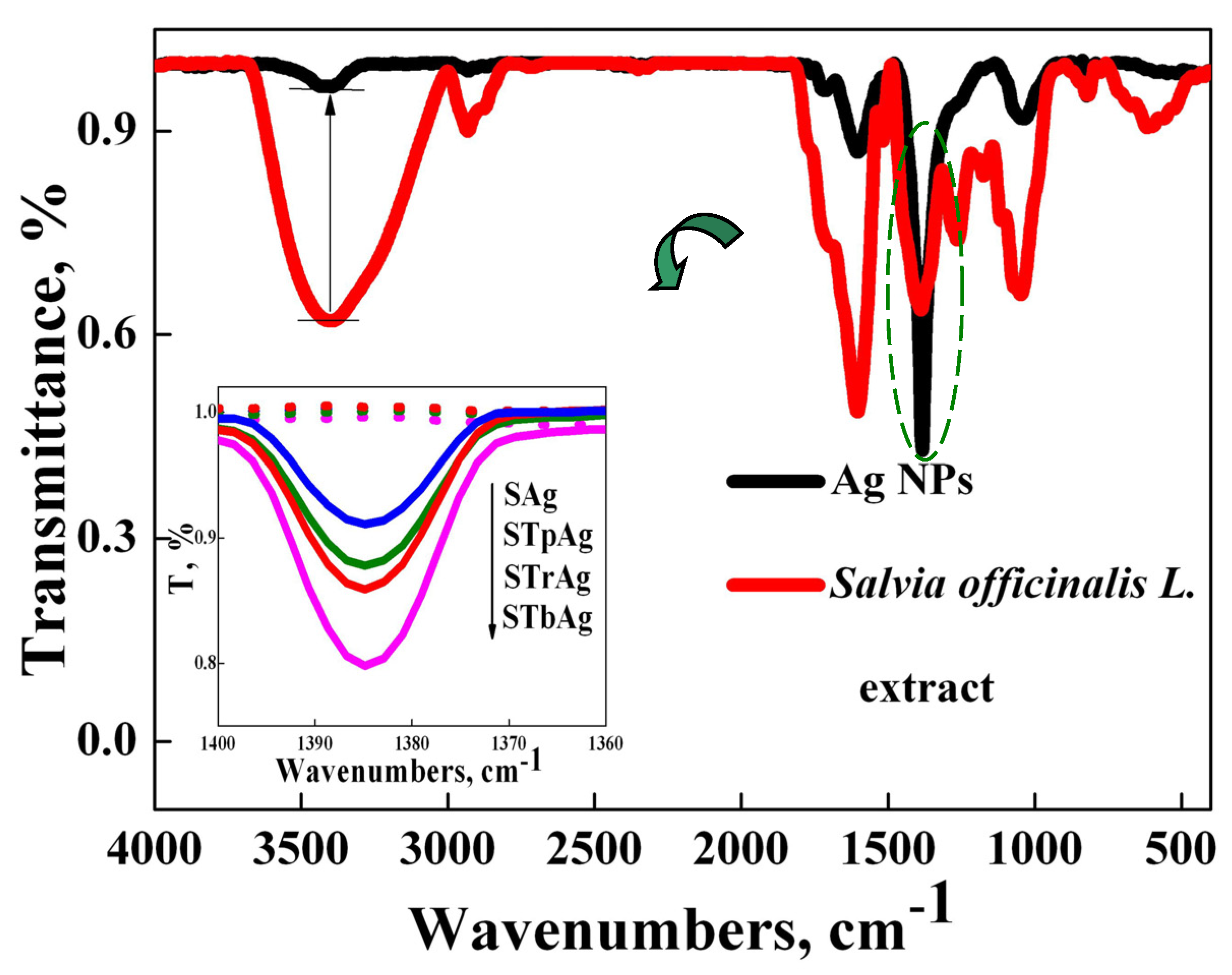
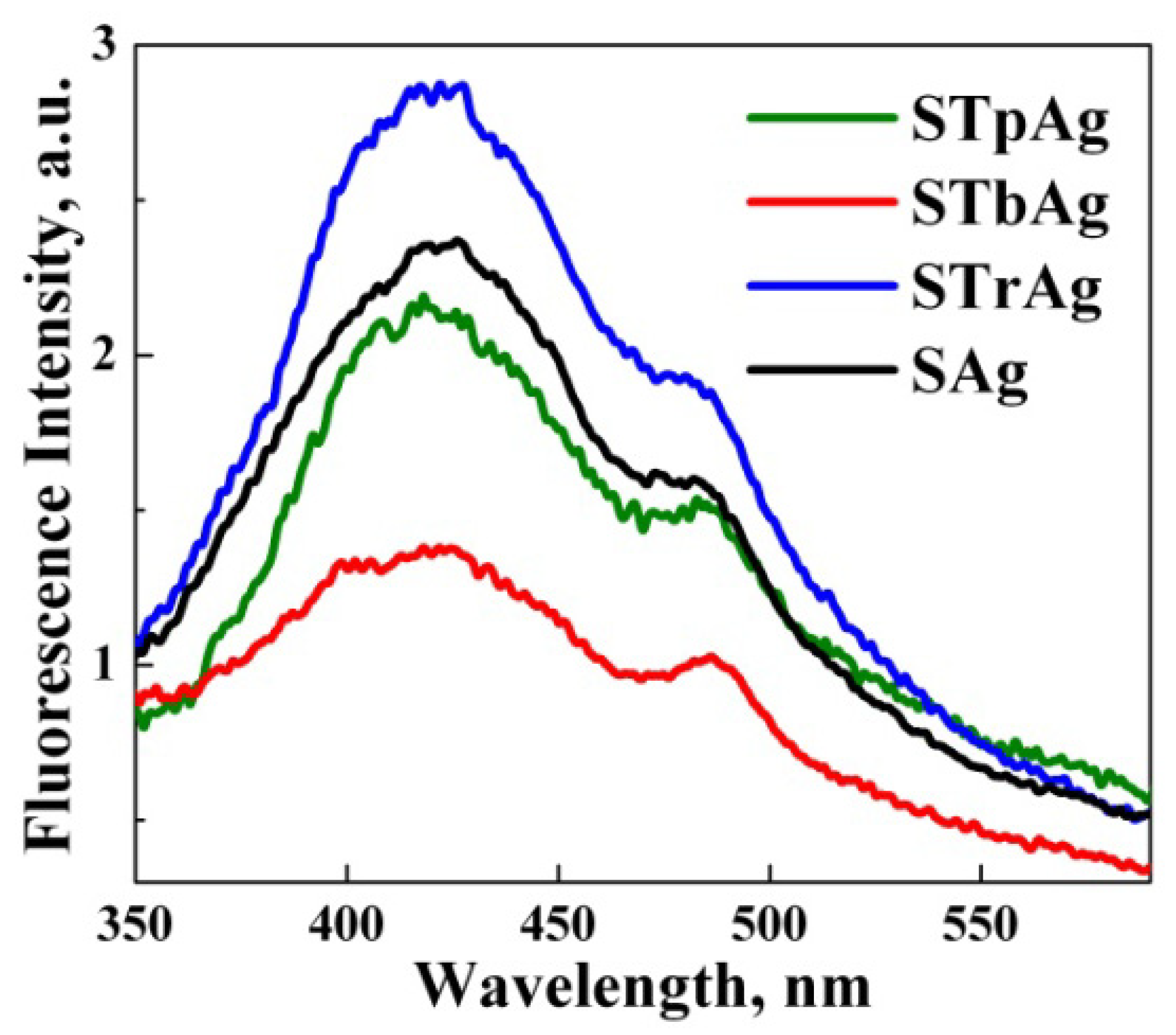
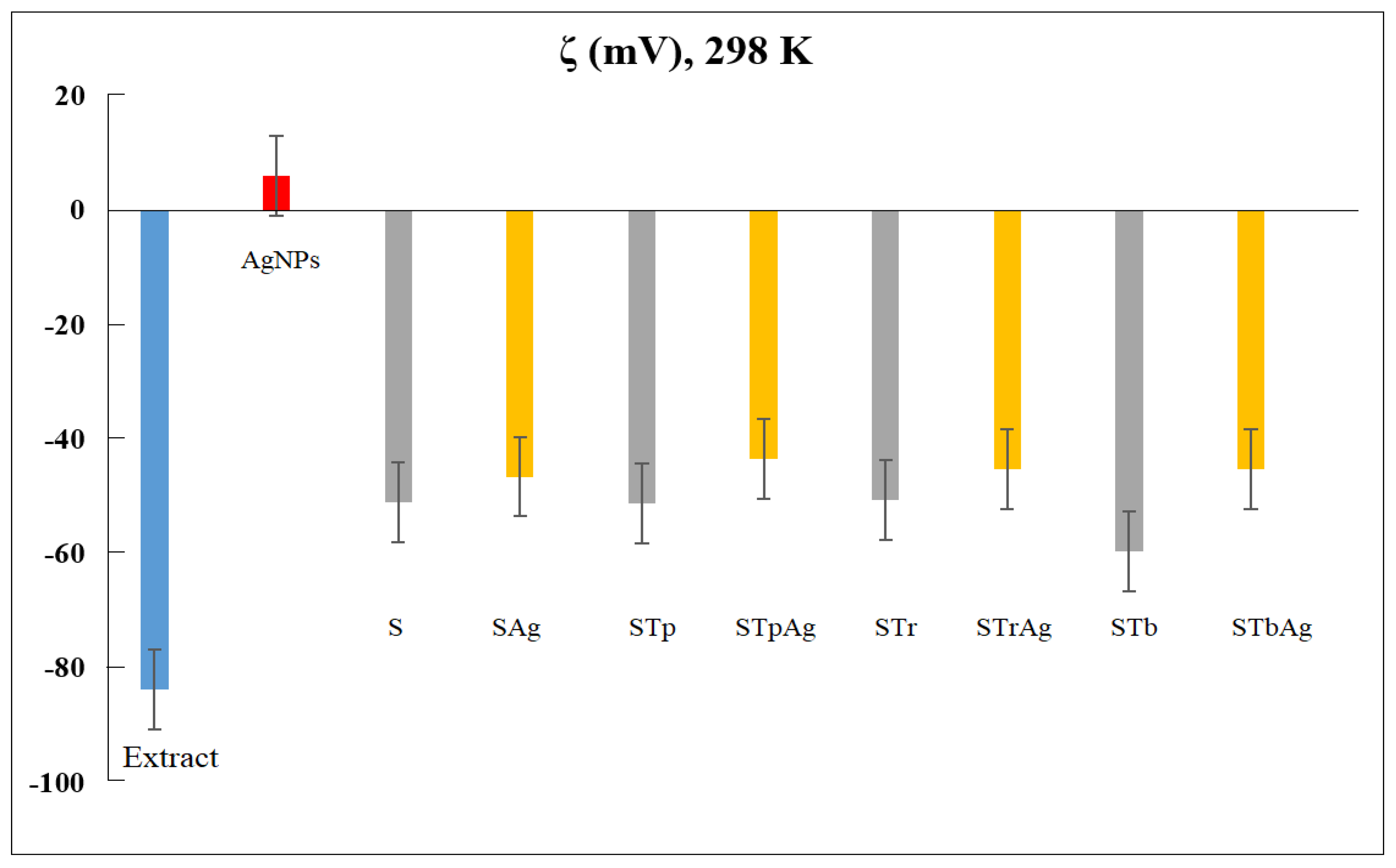



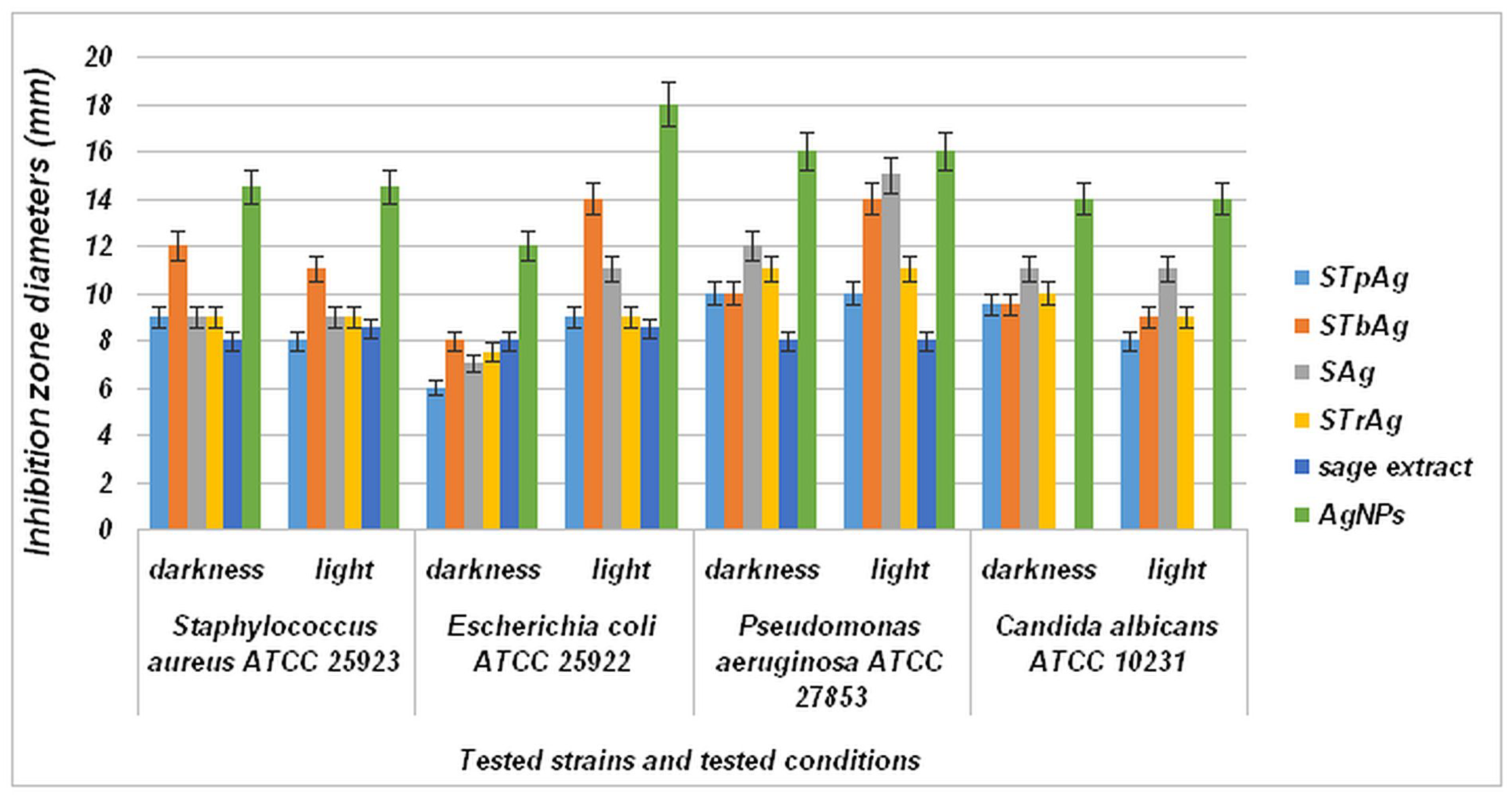
| Sample | Eg (eV) |
|---|---|
| STp | 3.11 |
| STpAg | 3.08 |
| STr | 2.95 |
| STrAg | 2.91 |
| STb | 3.45 |
| STbAg | 3.20 |
| Staphylococcus aureus ATCC 25923 | Escherichia coli ATCC 25922 | Pseudomonas aeruginosa ATCC 27853 | Candida albicans ATCC 10231 | |||||
|---|---|---|---|---|---|---|---|---|
| Darkness | Light | Darkness | Light | Darkness | Light | Darkness | Light | |
| STpAg | 54.99 | 27.49 | 27.49 | 27.49 | 13.74 | 13.74 | 3.43 | 1.71 |
| STbAg | 6.87 | 6.87 | 13.74 | 6.87 | 13.74 | 6.87 | 13.74 | 0.84 |
| SAg | 27.49 | 27.49 | 13.74 | 13.74 | 27.49 | 13.74 | 1.71 | 1.71 |
| STrAg | 13.74 | 27.49 | 13.74 | 13.74 | 13.74 | 13.74 | 1.71 | 1.71 |
| Sage extract | 139 | 139 | 2780 | 139 | 556 | 556 | 278 | 278 |
| AgNPs | 47 | 23 | 23.4 | 23.4 | 23.4 | 23.4 | 23.4 | 23.4 |
| Gentamicin | 2 | 2 | 4 | 4 | 4 | 4 | - | - |
| Fluconazole | - | - | - | - | - | - | 4 | 4 |
| Bacterial Strain | AgNPs | AgNPs i |
|---|---|---|
| E. coli ATCC 25922 | 12 | 10.6 |
| S. aureus ATCC 25923 | 14.5 | 12 |
| Sample | Support | TiO2 Phase | AgNPs |
|---|---|---|---|
| STp | SBA-15 | Anatase + Rutile 10 wt.% | - |
| STb | SBA-15 | Anatase 10 wt.% | - |
| STr | SBA-15 | Rutile 10 wt.% | - |
| SAg | SBA-15 | - | Biosynthesized AgNPs 1 wt.% |
| STpAg | SBA-15 | Anatase + Rutile 10 wt.% | Biosynthesized AgNPs 1 wt.% |
| STbAg | SBA-15 | Anatase 10 wt.% | Biosynthesized AgNPs 1 wt.% |
| STrAg | SBA-15 | Rutile 10 wt.% | Biosynthesized AgNPs 1 wt.% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petcu, G.; Ciobanu, E.M.; Paun, G.; Neagu, E.; Baran, A.; Trica, B.; Neacsu, A.; Atkinson, I.; Bucuresteanu, R.; Badaluta, A.; et al. Hybrid Materials Obtained by Immobilization of Biosynthesized Ag Nanoparticles with Antioxidant and Antimicrobial Activity. Int. J. Mol. Sci. 2024, 25, 4003. https://doi.org/10.3390/ijms25074003
Petcu G, Ciobanu EM, Paun G, Neagu E, Baran A, Trica B, Neacsu A, Atkinson I, Bucuresteanu R, Badaluta A, et al. Hybrid Materials Obtained by Immobilization of Biosynthesized Ag Nanoparticles with Antioxidant and Antimicrobial Activity. International Journal of Molecular Sciences. 2024; 25(7):4003. https://doi.org/10.3390/ijms25074003
Chicago/Turabian StylePetcu, Gabriela, Elena Madalina Ciobanu, Gabriela Paun, Elena Neagu, Adriana Baran, Bogdan Trica, Andreea Neacsu, Irina Atkinson, Razvan Bucuresteanu, Alexandra Badaluta, and et al. 2024. "Hybrid Materials Obtained by Immobilization of Biosynthesized Ag Nanoparticles with Antioxidant and Antimicrobial Activity" International Journal of Molecular Sciences 25, no. 7: 4003. https://doi.org/10.3390/ijms25074003
APA StylePetcu, G., Ciobanu, E. M., Paun, G., Neagu, E., Baran, A., Trica, B., Neacsu, A., Atkinson, I., Bucuresteanu, R., Badaluta, A., Ditu, L. M., & Parvulescu, V. (2024). Hybrid Materials Obtained by Immobilization of Biosynthesized Ag Nanoparticles with Antioxidant and Antimicrobial Activity. International Journal of Molecular Sciences, 25(7), 4003. https://doi.org/10.3390/ijms25074003











