A Clinical, Pharmacological, and Formulation Evaluation of Melatonin in the Treatment of Ocular Disorders—A Systematic Review
Abstract
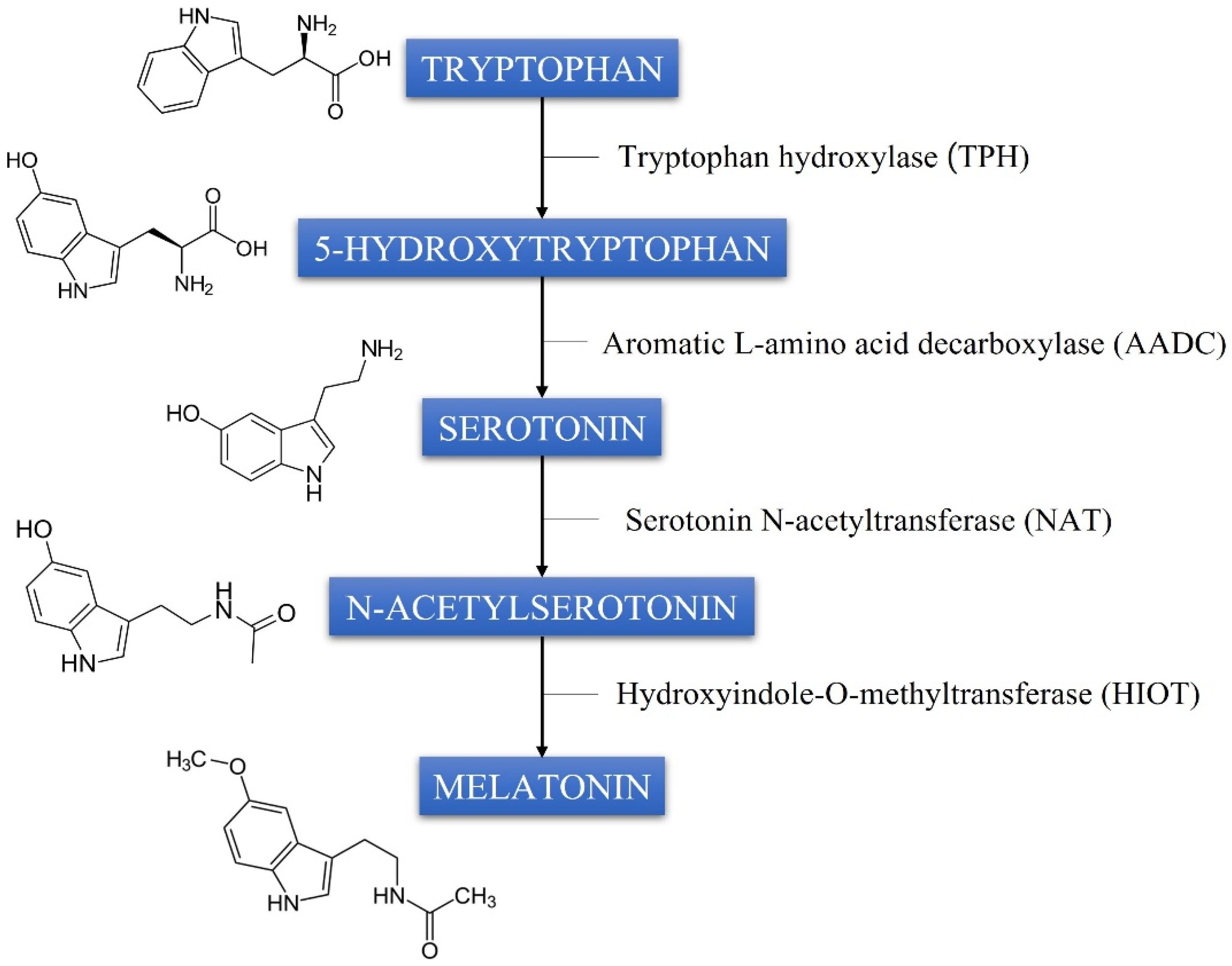
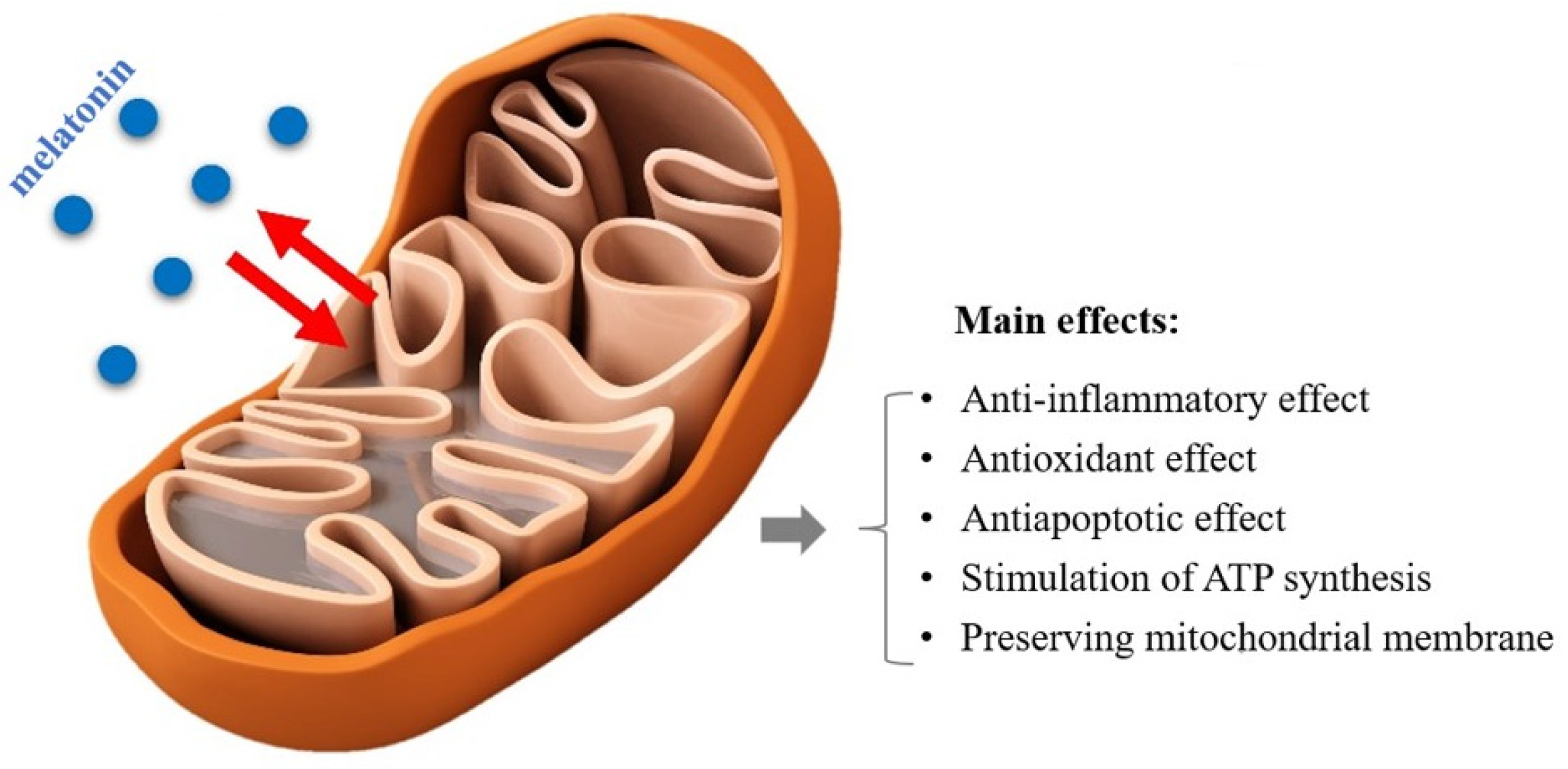
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Collection and Extraction
3. Results
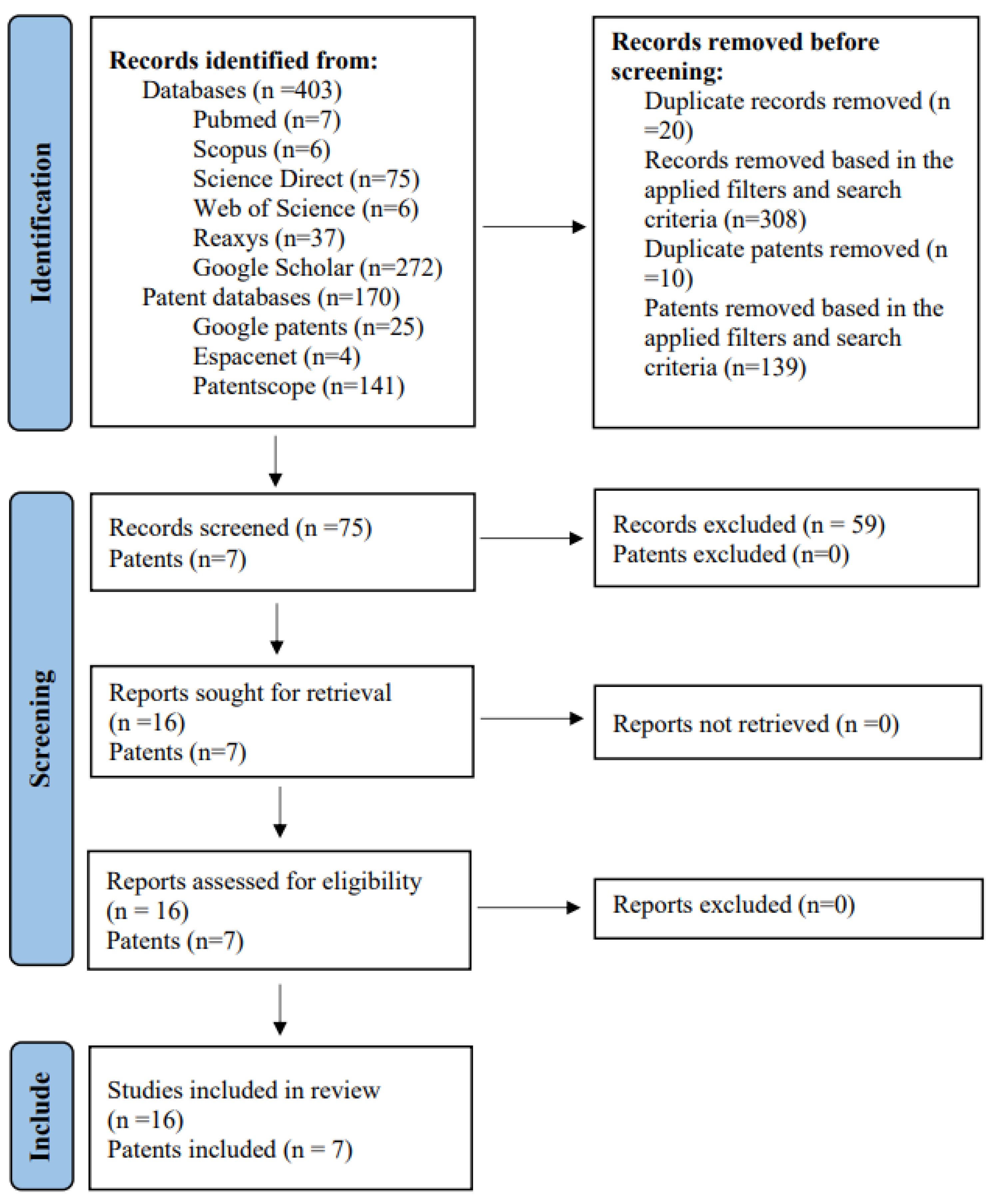
3.1. Studies Included from the Consulted Databases
3.2. The Results of the Studies
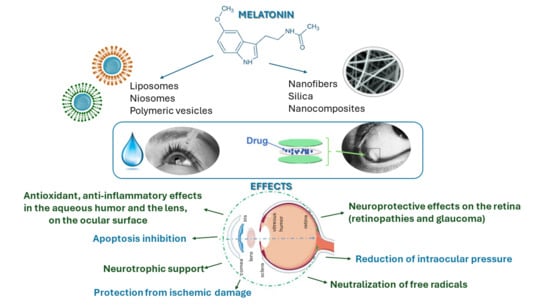
3.2.1. Melatonin Ophthalmic Indications and Drug Delivery Strategies
3.2.2. Ophthalmic Conditions Treated with Melatonin
3.2.3. Melatonin Formulations
3.3. Patent Studies
4. Discussion
Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y. Isolation of Melatonin and 5-Methoxyindole-3-acetic Acid from Bovine Pineal Glands. J. Biol. Chem. 1960, 235, 1992–1997. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Nordlund, J.J. Melatonin: Clinical pharmacology. J. Neural Transm. Suppl. 1978, 13, 339–347. [Google Scholar]
- Cavallo, A. Melatonin and human puberty: Current perspectives. J. Pineal Res. 1993, 15, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.; Gibbon, S.; Singh, P. The hypothermic effect of melatonin on core body temperature: Is more better? J. Pineal Res. 1996, 20, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Calvo, J.R.; Karbownik, M.; Qi, W.; Tan, D.X. Melatonin and Its Relation to the Immune System and Inflammation. Ann. N. Y. Acad. Sci. 2000, 917, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Karasek, M. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, B.S. The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Kimpinski, K. Role of melatonin in blood pressure regulation: An adjunct anti-hypertensive agent. Clin. Exp. Pharmacol. Physiol. 2018, 45, 755–766. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Dehpour, A.R.; Shirooie, S.; Silva, A.S.; Baldi, A.; Khan, H.; Daglia, M. Anti-inflammatory effects of Melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 2019, 59, S4-s16. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Pintor, J.; Martin, L.; Pelaez, T.; Hoyle, C.H.; Peral, A. Involvement of melatonin MT(3) receptors in the regulation of intraocular pressure in rabbits. Eur. J. Pharmacol. 2001, 416, 251–254. [Google Scholar] [CrossRef]
- Serle, J.B.; Wang, R.F.; Peterson, W.M.; Plourde, R.; Yerxa, B.R. Effect of 5-MCA-NAT, a putative melatonin MT3 receptor agonist, on intraocular pressure in glaucomatous monkey eyes. J. Glaucoma 2004, 13, 385–388. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J. The Pineal Gland: A Neurochemical Transducer. Science 1974, 184, 1341–1348. [Google Scholar] [CrossRef]
- Venegas, C.; García, J.A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; García-Corzo, L.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Karasek, M.; Stankov, B.; Lucini, V.; Scaglione, F.; Esposti, G.; Mariani, M.; Fraschini, F. Comparison of the rat pinealocyte ultrastructure with melatonin concentrations during daytime and at night. J. Pineal Res. 1990, 9, 251–257. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef]
- Wang, X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci. Ther. 2009, 15, 345–357. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. 2017, 74, 3883–3896. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Williams, D.L. Oxidative Stress and the Eye. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 179–192. [Google Scholar] [CrossRef]
- Bilbao-Malavé, V.; González-Zamora, J.; de la Puente, M.; Recalde, S.; Fernandez-Robredo, P.; Hernandez, M.; Layana, A.G.; Saenz de Viteri, M. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Age Related Macular Degeneration, Role in Pathophysiology, and Possible New Therapeutic Strategies. Antioxidants 2021, 10, 1170. [Google Scholar] [CrossRef]
- Yi, C.; Pan, X.; Yan, H.; Guo, M.; Pierpaoli, W. Effects of Melatonin in Age-Related Macular Degeneration. Ann. N. Y. Acad. Sci. 2005, 1057, 384–392. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Hemati, K.; Reiter, R.J.; Hosseinzadeh, A. Mitochondrial dysfunction in age-related macular degeneration: Melatonin as a potential treatment. Expert Opin. Ther. Targets 2020, 24, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Melo, I.M.; Martins Ferreira, C.G.; Lima da Silva Souza, E.H.; Almeida, L.L.; Bezerra de Sá, F.; Cavalcanti Lapa Neto, C.J.; Paz de Castro, M.V.; Teixeira, V.W.; Coelho Teixeira, Á.A. Melatonin regulates the expression of inflammatory cytokines, VEGF and apoptosis in diabetic retinopathy in rats. Chem.-Biol. Interact. 2020, 327, 109183. [Google Scholar] [CrossRef] [PubMed]
- Alkozi, H.A.; Navarro, G.; Franco, R.; Pintor, J. Melatonin and the control of intraocular pressure. Prog. Retin. Eye Res. 2020, 75, 100798. [Google Scholar] [CrossRef] [PubMed]
- Carracedo-Rodríguez, G.; Martínez-Águila, A.; Rodriguez-Pomar, C.; Bodas-Romero, J.; Sanchez-Naves, J.; Pintor, J. Effect of nutritional supplement based on melatonin on the intraocular pressure in normotensive subjects. Int. Ophthalmol. 2020, 40, 419–422. [Google Scholar] [CrossRef]
- Gubin, D.; Neroev, V.; Malishevskaya, T.; Cornelissen, G.; Astakhov, S.Y.; Kolomeichuk, S.; Yuzhakova, N.; Kabitskaya, Y.; Weinert, D. Melatonin mitigates disrupted circadian rhythms, lowers intraocular pressure, and improves retinal ganglion cells function in glaucoma. J. Pineal Res. 2021, 70, e12730. [Google Scholar] [CrossRef]
- Musumeci, T.; Bucolo, C.; Carbone, C.; Pignatello, R.; Drago, F.; Puglisi, G. Polymeric nanoparticles augment the ocular hypotensive effect of melatonin in rabbits. Int. J. Pharm. 2013, 440, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Bucolo, C.; Drago, F.; Salomone, S.; Pignatello, R. Cationic solid lipid nanoparticles enhance ocular hypotensive effect of melatonin in rabbit. Int. J. Pharm. 2015, 478, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Arranz-Romera, A.; Davis, B.M.; Bravo-Osuna, I.; Esteban-Pérez, S.; Molina-Martínez, I.T.; Shamsher, E.; Ravindran, N.; Guo, L.; Cordeiro, M.F.; Herrero-Vanrell, R. Simultaneous co-delivery of neuroprotective drugs from multi-loaded PLGA microspheres for the treatment of glaucoma. J. Control. Release 2019, 297, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Cammalleri, M.; Pezzino, S.; Corsaro, R.; Pescosolido, N.; Bagnoli, P.; Rusciano, D. Hypotensive Effect of Nanomicellar Formulation of Melatonin and Agomelatine in a Rat Model: Significance for Glaucoma Therapy. Diagnostics 2020, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, X.; Zhuang, X.; Liu, Y.; Li, S. Co-delivery of dexamethasone and melatonin by drugs laden PLGA nanoparticles for the treatment of glaucoma. J. Drug Deliv. Sci. Technol. 2020, 60, 102086. [Google Scholar] [CrossRef]
- Dal Monte, M.; Cammalleri, M.; Amato, R.; Pezzino, S.; Corsaro, R.; Bagnoli, P.; Rusciano, D. A Topical Formulation of Melatoninergic Compounds Exerts Strong Hypotensive and Neuroprotective Effects in a Rat Model of Hypertensive Glaucoma. Int. J. Mol. Sci. 2020, 21, 9267. [Google Scholar] [CrossRef] [PubMed]
- Del Valle Bessone, C.; Fajreldines, H.D.; de Barboza, G.E.D.; Tolosa de Talamoni, N.G.; Allemandi, D.A.; Carpentieri, A.R.; Quinteros, D.A. Protective role of melatonin on retinal ganglionar cell: In vitro an in vivo evidences. Life Sci. 2019, 218, 233–240. [Google Scholar] [CrossRef]
- García-Caballero, C.; Lieppman, B.; Arranz-Romera, A.; Molina-Martínez, I.T.; Bravo-Osuna, I.; Young, M.; Baranov, P.; Herrero-Vanrell, R. Photoreceptor preservation induced by intravitreal controlled delivery of GDNF and GDNF/melatonin in rhodopsin knockout mice. Mol. Vis. 2018, 24, 733–745. [Google Scholar]
- Del Valle Bessone, C.; Martinez, S.M.; Luna, J.D.; Marquez, M.A.; Ramírez, M.L.; Allemandi, D.A.; Carpentieri, Á.R.; Quinteros, D.A. Neuroprotective effect of melatonin loaded in ethylcellulose nanoparticles applied topically in a retinal degeneration model in rabbits. Exp. Eye Res. 2020, 200, 108222. [Google Scholar] [CrossRef]
- Romeo, A.; Bonaccorso, A.; Carbone, C.; Lupo, G.; Daniela Anfuso, C.; Giurdanella, G.; Caggia, C.; Randazzo, C.; Russo, N.; Romano, G.L.; et al. Melatonin loaded hybrid nanomedicine: DoE approach, optimization and in vitro study on diabetic retinopathy model. Int. J. Pharm. 2022, 627, 122195. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Manno, D.; Serra, A.; Musumeci, T.; Pepe, V.; Tisserand, C.; Puglisi, G. Innovative hybrid vs polymeric nanocapsules: The influence of the cationic lipid coating on the “4S”. Colloids Surf. B Biointerfaces 2016, 141, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Mickaela Martinez, S.; Inda, A.; Marina Garcia, A.; María Bermúdez, J.; Emilio Gonzo, E.; Herrero-Vanrell, R.; Domingo Luna, J.; Alberto Allemandi, D.; Alejandra Quinteros, D. Development of melatonin-loaded, human-serum-albumin nanoparticles formulations using different methods of preparation for ophthalmic administration. Int. J. Pharm. 2022, 628, 122308. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, H.D.; Abuzar, S.M.; Lee, J.Y.; Jin, S.E.; Kim, E.K.; Hwang, S.J. Intracorneal melatonin delivery using 2-hydroxypropyl-β-cyclodextrin ophthalmic solution for granular corneal dystrophy type 2. Int. J. Pharm. 2017, 529, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Doğanlar, Z.B.; Doğanlar, O.; Kurtdere, K.; Güçlü, H.; Chasan, T.; Turgut, E. Melatonin prevents blood-retinal barrier breakdown and mitochondrial dysfunction in high glucose and hypoxia-induced in vitro diabetic macular edema model. Toxicol In Vitro 2021, 75, 105191. [Google Scholar] [CrossRef] [PubMed]
- Aranda, M.L.; Narvaez, O.; Altschuler, F.; Calanni, J.S.; González Fleitas, M.F.; Sande, P.H.; Dorfman, D.; Concha, L.; Rosenstein, R.E. Chronobiotic effect of melatonin in experimental optic neuritis. Neuropharmacology 2021, 182, 108401. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Ge, Y.; Ye, Z.; Pan, X.; Yan, Y.; Mao, Z.; Ye, J. Anti-oxidative and mucin-compensating dual-functional nano eye drops for synergistic treatment of dry eye disease. Appl. Mater. Today 2022, 27, 101411. [Google Scholar] [CrossRef]
- Ozsolak, F.B.; Bhat, B. Nanoparticle Formulations for Delivery of Nucleic Acid Complexes. U.S. Patent Application No. US 20180311176 A1, 1 November 2018. [Google Scholar]
- Rolland, A.B.; Blanco, E.M. Formulations of Cannabidiol Derivatives and Their Use as Modulators of Cannabinoid Receptor Type 2 (CB2). Patent Application No. WO Patent 2,020,163,612 A1, 13 August 2020. [Google Scholar]
- Inman, D.M.; Lambert, W.S.; Calkins, D.J.; Horner, P.J. α-Lipoic Acid Antioxidant Treatment Limits Glaucoma-Related Retinal Ganglion Cell Death and Dysfunction. PLoS ONE 2013, 8, e65389. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Osuna, I.; Andrés-Guerrero, V.; Pastoriza Abal, P.; Molina-Martínez, I.T.; Herrero-Vanrell, R. Pharmaceutical microscale and nanoscale approaches for efficient treatment of ocular diseases. Drug Deliv. Transl. Res. 2016, 6, 686–707. [Google Scholar] [CrossRef]
- Zhao, M.; Rodríguez-Villagra, E.; Kowalczuk, L.; Le Normand, M.; Berdugo, M.; Levy-Boukris, R.; El Zaoui, I.; Kaufmann, B.; Gurny, R.; Bravo-Osuna, I.; et al. Tolerance of high and low amounts of PLGA microspheres loaded with mineralocorticoid receptor antagonist in retinal target site. J. Control. Release 2017, 266, 187–197. [Google Scholar] [CrossRef]
- Begovac, P.C.C.; Robert, L.; Li, M. Injectable and Biodegradable Polymer Formulations for Controlled Release of Bioactive Agents. AU Patent 2,021,277,631 A1, 23 December 2021. [Google Scholar]
- Carmona, G.; Gonzalez, F.C.; Heidebrecht, R.; Miller, R.J.; Oberli, M.A.; Peritt, D.; Sewell, J.A.; Smith, D.M.; Veiseh, O.; Wotton, P.K. Methods, Compositions, and Implantable Elements Comprising Active Cells. US Patent 20,200,263,196 A1, 20 August 2020. [Google Scholar]
- Smith, D.M.; Peritt, D.; Veiseh, O.; Heidebrecht, R.; Miller, R.J. Methods, Compositions, and Implantable Elements Comprising Stem Cells. WO Patent 2,019,195,056 A1, 10 October 2019. [Google Scholar]
- Roizman, K.; Requard, J.J., III; de Cogan, F.J. Topical Delivery of Therapeutic Agents Using Cell-Penetrating Peptides for the Treatment of Age-Related Macular Degeneration and Other Eye Diseases. US Patent 20,210,085,797 A1, 25 March 2021. [Google Scholar]
- Wu, K.; Shalaev, E.; Mohanty, P.S.; Wan, J. Solid Complex Comprising (Z)-7-((1R,2R,3R,5S)-2-((S,E)-5-(2,5-Dichlorothiophen-3-Yl)-3-Hydroxypent-1-En-1-Yl)-3,5-Dihydroxycyclopentyl)Hept-5-Enamide, Preparation and Uses Thereof. WO Patent 2,019,023,211 A1, 31 January 2019. [Google Scholar]
- Lanier, O.L.; Manfre, M.G.; Bailey, C.; Liu, Z.; Sparks, Z.; Kulkarni, S.; Chauhan, A. Review of Approaches for Increasing Ophthalmic Bioavailability for Eye Drop Formulations. AAPS Pharmscitech 2021, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Alany, R.G. Controlled and Continuous Release Ocular Drug Delivery Systems: Pros and Cons. Curr. Drug Deliv. 2012, 9, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jones, L.; Gu, F.X. Nanomaterials for Ocular Drug Delivery. Macromol. Biosci. 2012, 12, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Pal Kaur, I.; Kanwar, M. Ocular Preparations: The Formulation Approach. Drug Dev. Ind. Pharm. 2002, 28, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.S.; Ebrahimnejad, P.; Fatahi, Y.; D’Emanuele, A.; Dinarvand, R. Recent Developments of Nanostructures for the Ocular Delivery of Natural Compounds. Front. Chem. 2022, 10, 850757. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Popescu-Pelin, G.; Socol, G. Degradation Behavior of Polymers Used as Coating Materials for Drug Delivery—A Basic Review. Polymers 2021, 13, 1272. [Google Scholar] [CrossRef] [PubMed]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Bíró, T.; Aigner, Z. Current Approaches to Use Cyclodextrins and Mucoadhesive Polymers in Ocular Drug Delivery—A Mini-Review. Sci. Pharm. 2019, 87, 15. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration Enhancers in Ocular Drug Delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef]
- Chetoni, P.; Burgalassi, S.; Monti, D.; Tampucci, S.; Tullio, V.; Cuffini, A.M.; Muntoni, E.; Spagnolo, R.; Zara, G.P.; Cavalli, R. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: Pharmacokinetic studies on rabbits. Eur. J. Pharm. Biopharm. 2016, 109, 214–223. [Google Scholar] [CrossRef]
- Ahmad, I.; Pandit, J.; Sultana, Y.; Mishra, A.K.; Hazari, P.P.; Aqil, M. Optimization by design of etoposide loaded solid lipid nanoparticles for ocular delivery: Characterization, pharmacokinetic and deposition study. Mater. Sci. Eng. C 2019, 100, 959–970. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, J.; Zhang, X.; Pan, W. Nanostructured lipid carrier (NLC) coated with Chitosan Oligosaccharides and its potential use in ocular drug delivery system. Int. J. Pharm. 2011, 403, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Andreani, T.; Egea, M.A.; Garcia, M.L.; Souto, S.B.; Silva, A.M.; Souto, E.B. Design of cationic lipid nanoparticles for ocular delivery: Development, characterization and cytotoxicity. Int. J. Pharm. 2014, 461, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Calpena, A.C.; Clares, B.; Andreani, T.; Egea, M.A.; Veiga, F.J.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Biopharmaceutical evaluation of epigallocatechin gallate-loaded cationic lipid nanoparticles (EGCG-LNs): In vivo, in vitro and ex vivo studies. Int. J. Pharm. 2016, 502, 161–169. [Google Scholar] [CrossRef]
- Lakhani, P.; Patil, A.; Wu, K.-W.; Sweeney, C.; Tripathi, S.; Avula, B.; Taskar, P.; Khan, S.; Majumdar, S. Optimization, stabilization, and characterization of amphotericin B loaded nanostructured lipid carriers for ocular drug delivery. Int. J. Pharm. 2019, 572, 118771. [Google Scholar] [CrossRef]
- Salmaso, S.; Caliceti, P. Stealth Properties to Improve Therapeutic Efficacy of Drug Nanocarriers. J. Drug Deliv. 2013, 2013, 374252. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Gu, Y.; Cheng, Y.; Cao, F. Recent advance of nanoparticle-based topical drug delivery to the posterior segment of the eye. Expert Opin. Drug Deliv. 2018, 15, 687–701. [Google Scholar] [CrossRef]
- Swenor, B.K.; Ehrlich, J.R. Ageing and vision loss: Looking to the future. Lancet Glob. Health 2021, 9, e385–e386. [Google Scholar] [CrossRef]
- Shah, M.; Cabrera-Ghayouri, S.; Christie, L.-A.; Held, K.S.; Viswanath, V. Translational Preclinical Pharmacologic Disease Models for Ophthalmic Drug Development. Pharm. Res. 2019, 36, 58. [Google Scholar] [CrossRef]
- Henrich, P.B.; Monnier, C.A.; Halfter, W.; Haritoglou, C.; Strauss, R.W.; Lim, R.Y.H.; Loparic, M. Nanoscale Topographic and Biomechanical Studies of the Human Internal Limiting Membrane. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2561–2570. [Google Scholar] [CrossRef]
- Rowe-Rendleman, C.L.; Durazo, S.A.; Kompella, U.B.; Rittenhouse, K.D.; Di Polo, A.; Weiner, A.L.; Grossniklaus, H.E.; Naash, M.I.; Lewin, A.S.; Horsager, A.; et al. Drug and Gene Delivery to the Back of the Eye: From Bench to Bedside. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2714–2730. [Google Scholar] [CrossRef]
- Halfter, W.; Oertle, P.; Monnier, C.A.; Camenzind, L.; Reyes-Lua, M.; Hu, H.; Candiello, J.; Labilloy, A.; Balasubramani, M.; Henrich, P.B.; et al. New concepts in basement membrane biology. FEBS J. 2015, 282, 4466–4479. [Google Scholar] [CrossRef]
- Peynshaert, K.; Devoldere, J.; Forster, V.; Picaud, S.; Vanhove, C.; De Smedt, S.C.; Remaut, K. Toward smart design of retinal drug carriers: A novel bovine retinal explant model to study the barrier role of the vitreoretinal interface. Drug Deliv. 2017, 24, 1384–1394. [Google Scholar] [CrossRef]
- Viceconti, M.; Pappalardo, F.; Rodriguez, B.; Horner, M.; Bischoff, J.; Musuamba Tshinanu, F. In silico trials: Verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods 2021, 185, 120–127. [Google Scholar] [CrossRef]
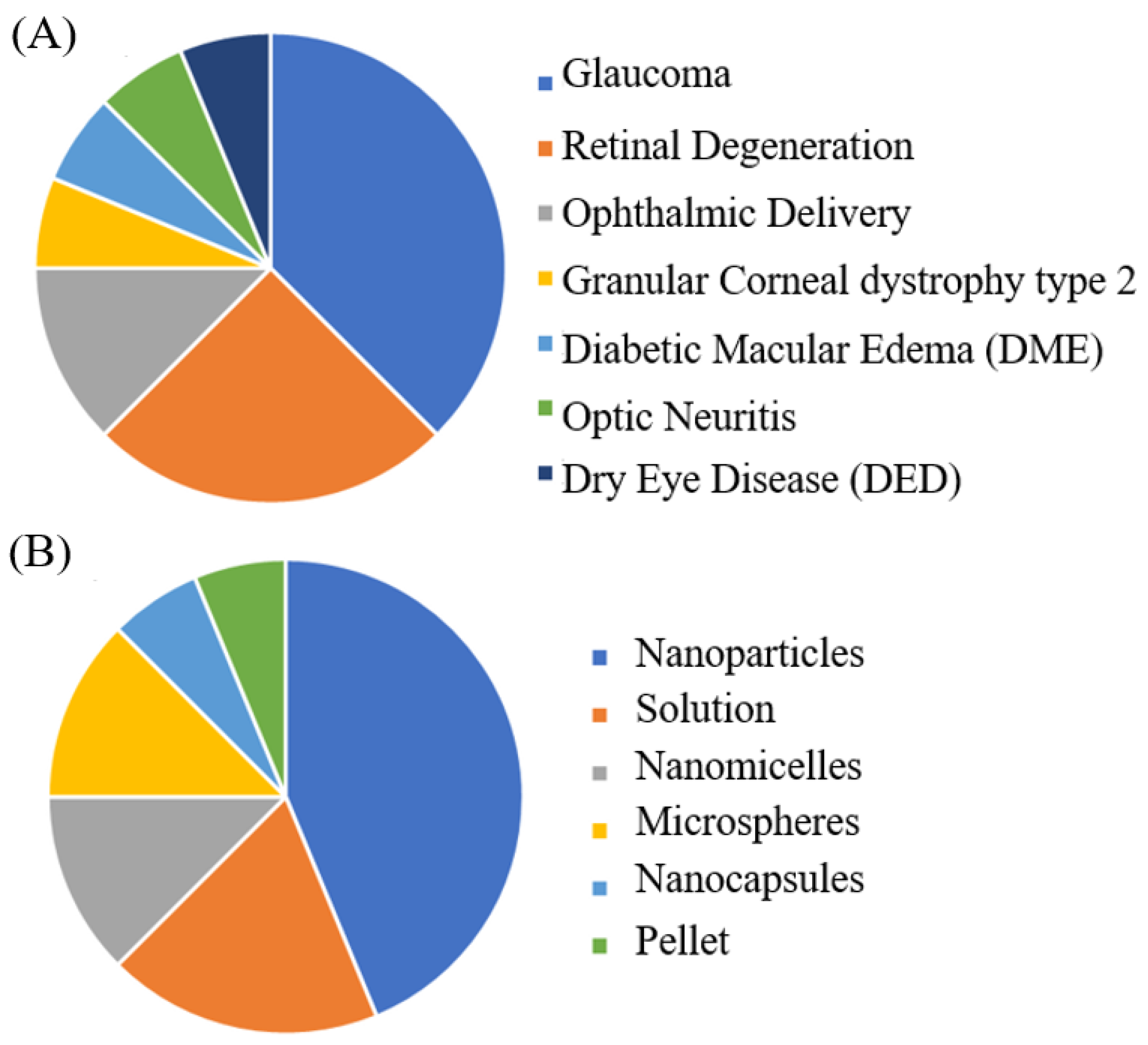
| Therapeutical Indication | Type of Formulation | Route of Administration | Materials | Loaded Drug/Concentration | Size, PDI, ZP, EE% | In Vivo/Animal Model | Effects/Properties | References |
|---|---|---|---|---|---|---|---|---|
| Glaucoma | Nanoparticles (NPs) | Topical | PLGA, PLGA-PEG, Boeringher Ingelheim KG (Ingelheim am Rhein, Germany) | 1, 3 and 5% (w/w) to polymer | Size < 300 nm PDI < 0.25 ZP from −8.2 to −32.2 mV EE% from 44.15 to 78.20% | Yes, Male albino rabbits of New Zealand |
| [32] |
| Cationic solid lipid nanoparticles (cSLNs) | Topical | Softisan® 100, by Sasol GmbH (Hamburg, Germany) DDAB, SA or PA, Sigma–Aldrich (Milan, Italy) | 0.05% (w/v) | Size = 150–300 nm PDI < 0.3 ZP from −3.99 to +61 mV EE% = 83–96% | Yes, Male albino rabbits of New Zealand |
| [33] | |
| Microspheres (MSs) | Intravitreal (ITV) injection | PLGA, Evonik España (Granollers, Spain) | MEL = 45.80 µg/mg MSs Coenzyme Q10, Sigma-Aldrich (St. Louis, MO., USA) (CoQ10) = 35.71 µg/mg MSs Dexamethasone (DX) = 115.86 µg/mg MSs | Size: 29.04 μm EE%: CoQ10 = 96.42%; DX = 78.20%; MEL = 61.83% | Yes, Adult male Dark Agouti rats |
| [34] | |
| Nanomicelles | Topical | Soluplus®, BASF, Ludwigshafen, Germany | MEL and agomelatine, Molekula, Darlington, UK 0.4–0.8% (w/v) | Size = 61 nm PDI = 0.068 | Yes, Rats Sprague Dawley |
| [35] | |
| NPs | Topical | PLGA | MEL 5% (w/v) Dexamethasone, Sigma Aldrich (Shanghai, China) (Dex) 5% (w/v) | Size = 468 nm PDI = 0.283 ZP = −21.7 mV EE% MEL = 87.25% EE% Dex = 89.41% | Yes, 5-week-old rabbits |
| [36] | |
| Nanomicelles | Topical | Soluplus® | MEL and agomelatine 0.4% (w/v) | × | Yes, Rats Sprague Dawley |
| [37] | |
| Retinal degeneration | Injectable solution | ITV injection | × | 1 mg/mL | × | Yes, New Zealand albino rabbits |
| [38] |
| MSs | ITV injection | PLGA, Resomer®503, Boehringer Ingelheim Pharma GmbH & Co, Ingelheim, Germany vitamin E, Sigma-Aldrich, Schnelldorf, Germany | MEL, Sigma-Aldrich, Schnelldorf, Germany 10% (w/w) to polymer Glial cell line-derived neurotrophic factor (GDNF, R&D, Systems, Minneapolis, MN, USA) 0.01% (w/w)to polymer | Size = 21.6–24.6 µm EE% GDNF = 26.4–39.5% EE% MEL = 39.2% | Yes, Rhodopsin null mice |
| [39] | |
| NPs | Topical | Ethylcellulose, Colorcon® (Buenos Aires, Argentina) | 1–2 mg/mL | Size = 147–179 nm; PDI = 0.092–0.2; ZP = −30 mV; EE% = 67–73% | Yes, New Zealand white female rabbits |
| [40] | |
| NPs | Topical | PLGA-PEG, DDAB, CTAB, Merck Life Science S.r.L. (Milan, Italy) | 1% (w/w) to polymer | Size = 190 nm PDI = 0.2 ZP = 39.8 mV EE% = 79.85% | Yes, Male New Zealand albino rabbits |
| [41] | |
| Ophthalmic delivery | Polymeric and hybrid aqueous-core nanocapsules (NCs) | × | PLA, Sigma (Milan, Italy) TCT, Farmalabor (Bari, Italy) DDAB, Sigma (Milan, Italy) CTAB, Sigma (Milan, Italy) | 0.03% (w/w) | Size < 250 nm PDI < 0.2 ZP from −36 to +30 mV EE% = 87–90% | No in vivo studies have been conducted |
| [42] |
| NPs | Topical and subconjunctival | HAS, Laboratorio de Hemoderivados UNC (Cordoba, Argentina) | 1 mg/mL | Size = 160–180 nm PDI = 0.06 ZP = from −30 to −40 mV EE% = 18–21% | Yes, New Zealand rabbits |
| [43] | |
| Granular corneal dystrophy type 2 (GCD2) | Eye drop | Topical | HPβCD, Alfa Aesar (Haverhill, MA, USA) | 2.75 mg/mL | × | Yes, New Zealand albino rabbits |
| [44] |
| Diabetic macular edema (DME) | Solution | × | × | 0.1, 0.5, and 1 mM | × | No in vivo studies have been conducted |
| [45] |
| Optic neuritis | Pellet | Subcutaneous | Vegetable oils, Sigma Chemical Co. (St Louis, MO, USA) | 20 mg | 2.5 mm × 1 mm (diameter × length) | Yes, Male Wistar rats |
| [46] |
| Dry eye disease (DED) | NPs | Topical | Polydopamine | MEL 20% tavilermide (Tav) 2% | Size < 250 nm ZP from −20 to +5 | Yes, Female BALB/c mice |
| [47] |
| Background | Targeted Diseases | Using MEL | Applicant/Manufacturer/Assignee | Patent Number | Publication Date | Reference |
|---|---|---|---|---|---|---|
| Biodegradable injectable polymer formulations that permit an extended or sustained release of a bioactive agent. | Chronic ocular disorder such as age-related macular degeneration (AMD) and others. | Bioactive agents include, but are not limited to, bevacizumab, ranibizumab, aflibercept, and lampalizumab. Additional bioactive agents are hormones like MEL. | W. L. Gore & Associates, Inc., Newark, DE, USA | AU 2,021,277,631 A1 | 23 December 2021 | [53] |
| Methods, compositions, and implantable elements comprising active cells (e.g., an engineered RPE cell) which may express a therapeutic agent useful for the treatment of a disease. | Neurodegenerative disorders and others. | The therapeutic agent may be any biological substance, such as nucleic acid, a polypeptide (like MEL), a lipid, a sugar, or a small molecule. | SIGILON THERAPEUTICS, Inc., Cambridge, MA, USA | US 20,200,263,196 A1 | 20 August 2020 | [54] |
| Methods, compositions, and implantable elements comprising stem cells (e.g., a mesenchymal stem function cell (MSFC)) which produce a therapeutic agent. | Neurodegenerative disorders and others. | The therapeutic agent may be any biological substance, such as a nucleic acid, a polypeptide (like MEL), a lipid, a sugar, or a small molecule. | SIGILON THERAPEUTICS, Inc., Cambridge, MA, USA | WO 2,019,195,056 A1 | 10 October 2019 | [55] |
| Nanoparticle formulations for nucleic acid complexed delivery for increasing gene expression in a targeted manner. | Diseases treatable with oligonucleotides targeting associated mRNA (intraocular melanoma, ocular cicatricial pemphigoid, optic neuritis, neuromyelitis optica). | Ligands (such as MEL or others) can be conjugated to the particle to target the nucleic acid complex to a specific cell type or tissue of interest. | Translate Bio MA, Inc., Lexington, MA, USA | US 20,180,311,176 A1 | 1 November 2018 | [48] |
| The topical delivery of therapeutic agents using cell-penetrating peptides. | AMD and other eye diseases. | The therapeutic agent is or includes an anti-dyslipidemic agent, an antioxidant (such as MEL), an anti-inflammatory agent, a complement inhibitor, a neuroprotector or an anti-angiogenic agent, or any combination thereof. | MacRegen, Inc., Birmingham, AL, USA | US 20,210,085,797 A1 | 25 March 2021 | [56] |
| Formulations of cannabidiol derivatives and their use as modulators of cannabinoid receptor type 2 in the treatment of various diseases. | Demyelinating diseases (such as neuromyelitis optica) and others. | Pharmaceutical vehicle optionally comprises a stabilizer, emulsifier, polymer, antioxidant (such as MEL), or any combination thereof. | EMERALD HEALTH PHARMACEUTICALS Inc., San Diego, CA, USA | WO 2,020,163,612 A1 | 13 August 2020 | [49] |
| Implants of solid complexes for the treatment of ophthalmic diseases. | Glaucoma and others. | To improve the stability of the therapeutic agent, the intraocular implant may comprise excipients like preservatives, an antioxidant (such as MEL), buffering agents, chelating agents, electrolytes, and others. | ALLERGAN, Inc., Irvine, CA, USA | WO 2,019,023,211 A1 | 31 January 2019 | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, A.; Kazsoki, A.; Musumeci, T.; Zelkó, R. A Clinical, Pharmacological, and Formulation Evaluation of Melatonin in the Treatment of Ocular Disorders—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3999. https://doi.org/10.3390/ijms25073999
Romeo A, Kazsoki A, Musumeci T, Zelkó R. A Clinical, Pharmacological, and Formulation Evaluation of Melatonin in the Treatment of Ocular Disorders—A Systematic Review. International Journal of Molecular Sciences. 2024; 25(7):3999. https://doi.org/10.3390/ijms25073999
Chicago/Turabian StyleRomeo, Alessia, Adrienn Kazsoki, Teresa Musumeci, and Romána Zelkó. 2024. "A Clinical, Pharmacological, and Formulation Evaluation of Melatonin in the Treatment of Ocular Disorders—A Systematic Review" International Journal of Molecular Sciences 25, no. 7: 3999. https://doi.org/10.3390/ijms25073999
APA StyleRomeo, A., Kazsoki, A., Musumeci, T., & Zelkó, R. (2024). A Clinical, Pharmacological, and Formulation Evaluation of Melatonin in the Treatment of Ocular Disorders—A Systematic Review. International Journal of Molecular Sciences, 25(7), 3999. https://doi.org/10.3390/ijms25073999