Pharmacological Activation of Piezo1 Channels Enhances Astrocyte–Neuron Communication via NMDA Receptors in the Murine Neocortex
Abstract
1. Introduction
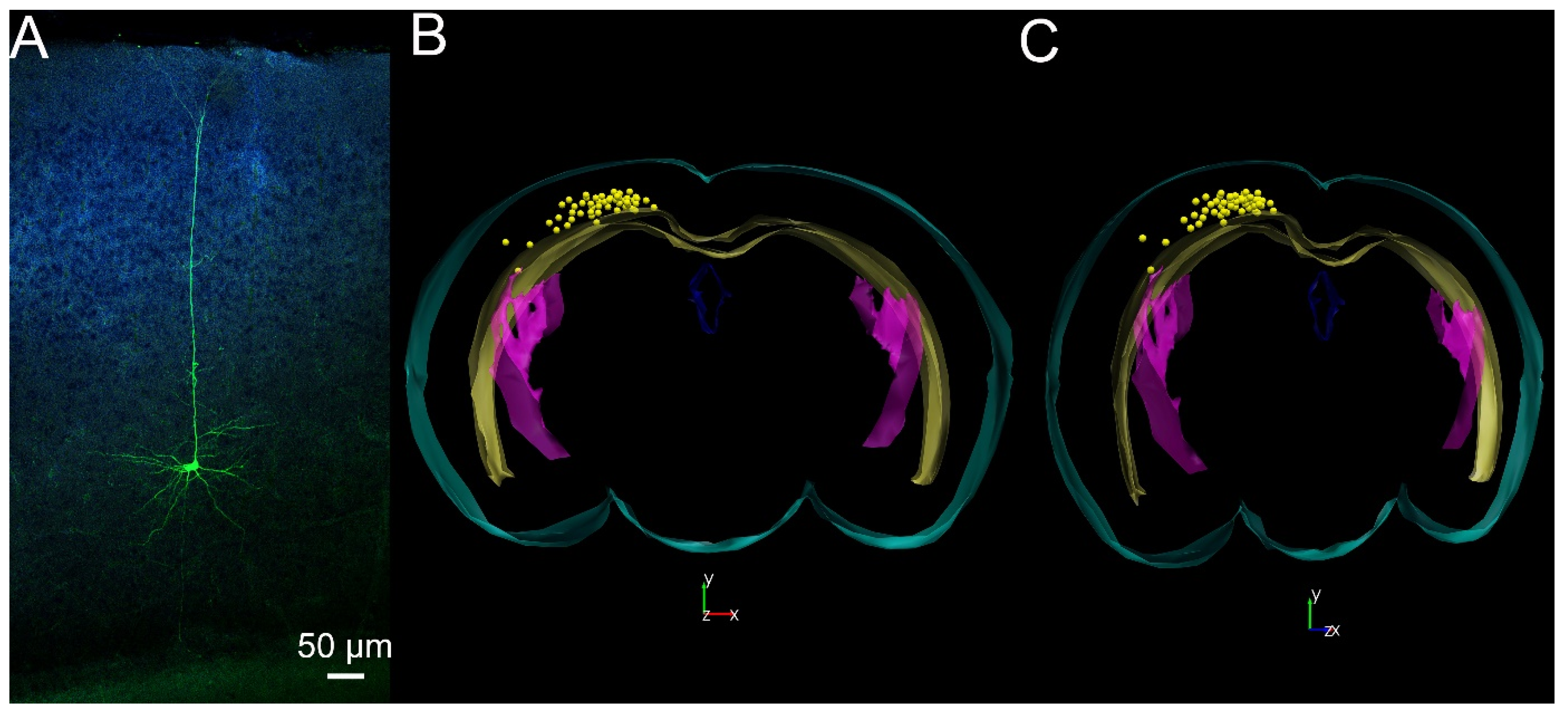
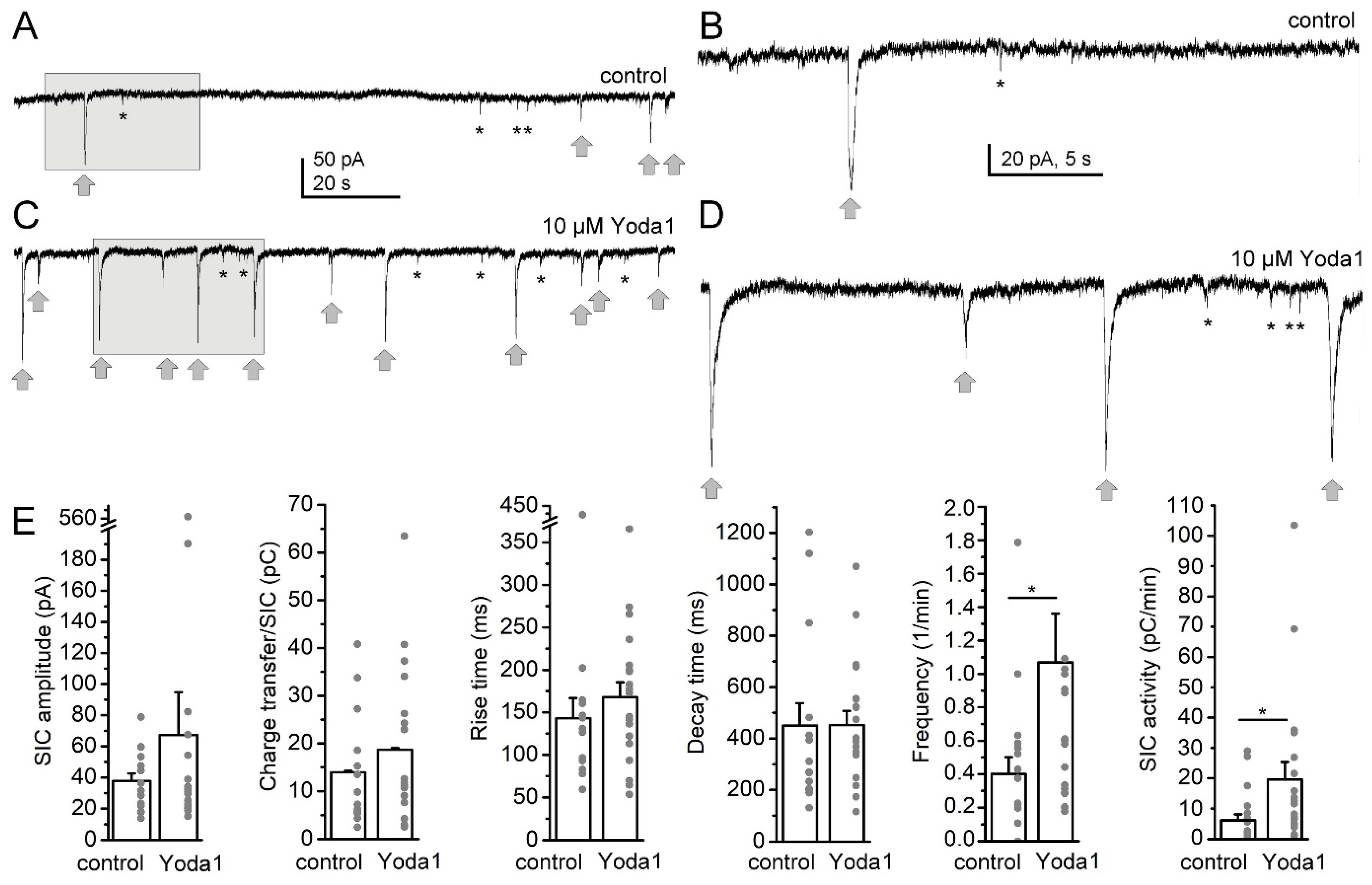
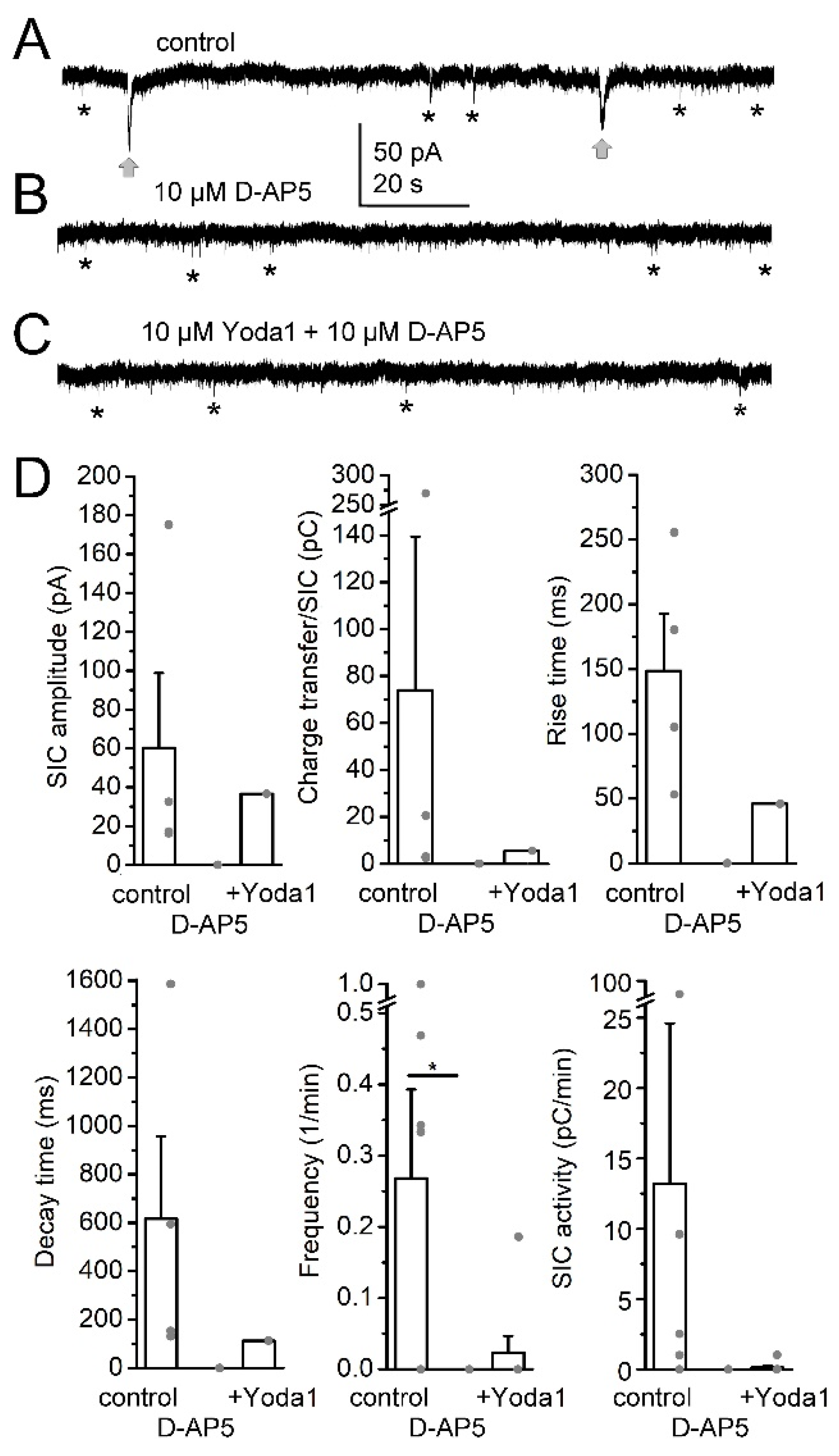
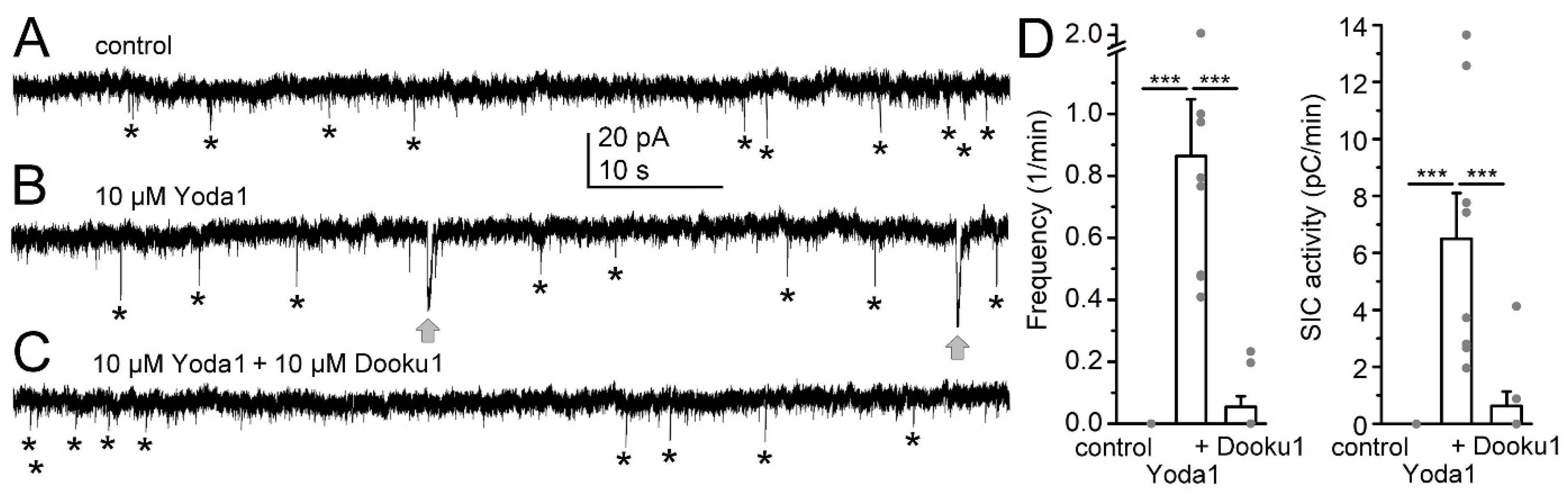
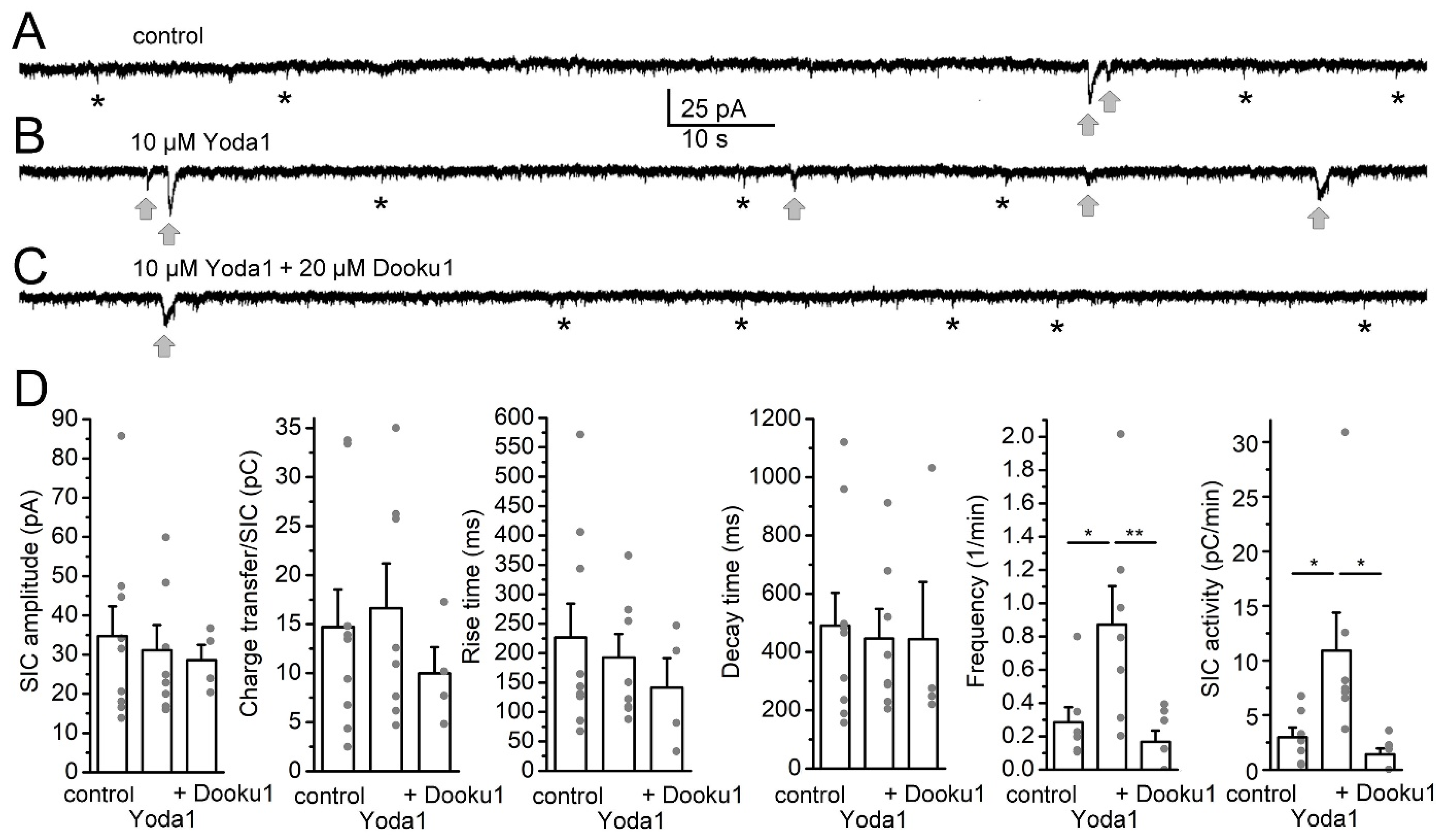
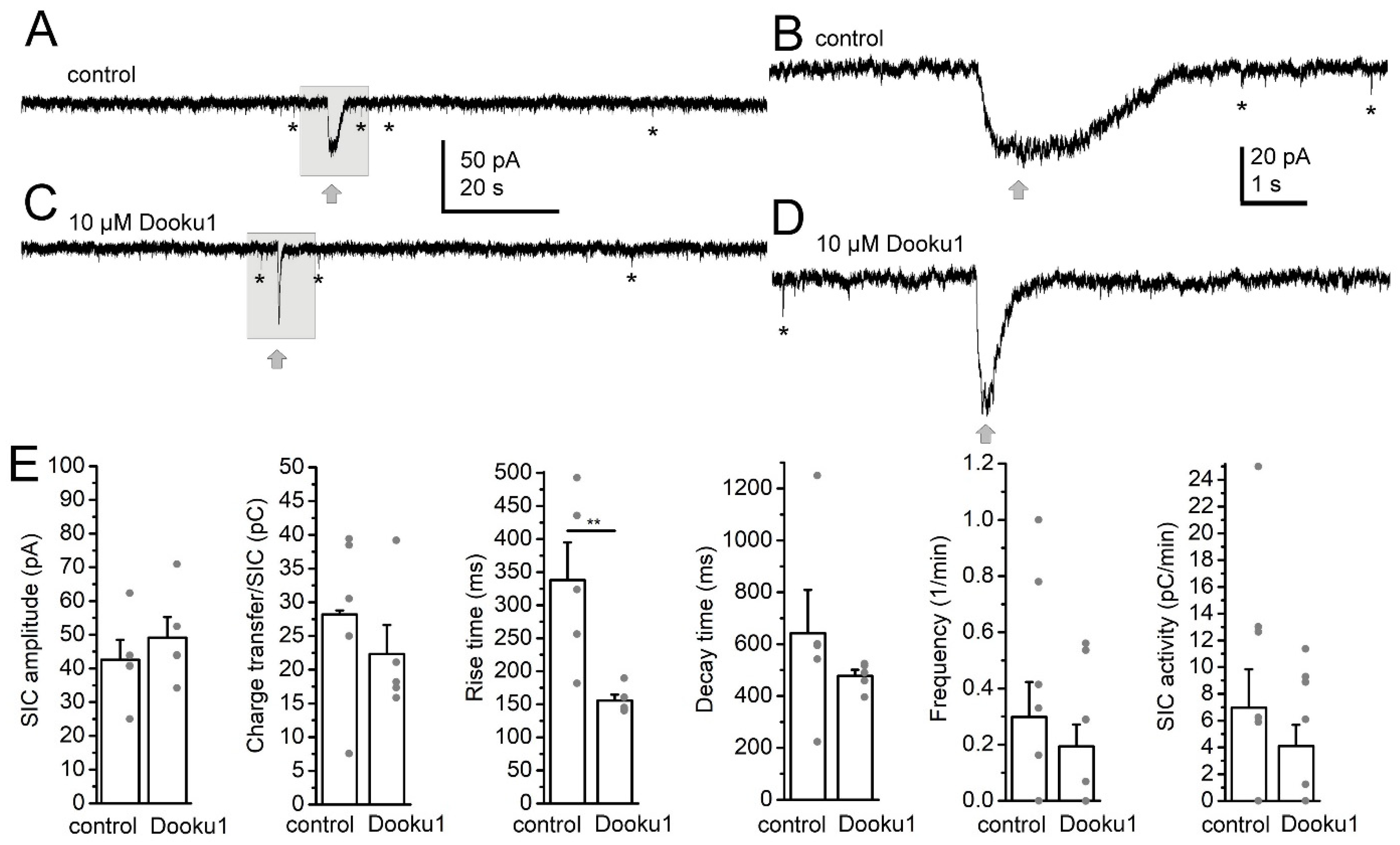
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals, Solutions
4.2. Animals, Preparation
4.3. Ex Vivo Electrophysiology
4.4. Morphological Analysis
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.S.; Woo, S.H.; Dubin, A.E.; Moshourab, R.A.; Wetzel, C.; Petrus, M.; Mathur, J.; Bégay, V.; Coste, B.; Mainquist, J.; et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014, 516, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.; Chesler, A.T. PIEZO2 ion channels in proprioception. Curr. Opin. Neurobiol. 2022, 75, 102572. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.; Yu, F.; Zhang, X.; Pang, Y.; Zhao, W.; Sun, P.; Li, L. Mechanosensitive Piezo1 channel in physiology and pathophysiology of the central nervous system. Ageing Res. Rev. 2023, 90, 102026. [Google Scholar] [CrossRef] [PubMed]
- Dienes, B.; Bazsó, T.; Szabó, L.; Csernoch, L. The role of the Piezo1 mechanosensitive channel in the musculoskeletal system. Int. J. Mol. Sci. 2023, 24, 6513. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife 2019, 8, e49631. [Google Scholar] [CrossRef] [PubMed]
- Savadipour, A.; Nims, R.J.; Rashidi, N.; Garcia-Castorena, J.M.; Tang, R.; Marushack, G.K.; Oswald, S.J.; Liedtke, W.B.; Guilak, F. Membrane stretch as the mechanism of activation of PIEZO1 ion channels in chondrocytes. Proc. Natl. Acad. Sci. USA 2023, 120, e2221958120. [Google Scholar] [CrossRef]
- Ortuste Quiroga, H.P.; Ganassi, M.; Yokoyama, S.; Nakamura, K.; Yamashita, T.; Raimbach, D.; Hagiwara, A.; Harrington, O.; Breach-Teji, J.; Asakura, A.; et al. Fine-tuning of Piezo1 expression and activity ensures efficient myoblast fusion during skeletal myogenesis. Cells 2022, 11, 393. [Google Scholar] [CrossRef]
- Beech, D.J.; Kalli, A.C. Force sensing by Piezo channels in cardiovascular health and disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2228–2239. [Google Scholar] [CrossRef]
- Friedrich, E.E.; Hong, Z.; Xiong, S.; Zhong, M.; Di, A.; Rehman, J.; Komarova, Y.A.; Malik, A.B. Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc. Natl. Acad. Sci. USA 2019, 116, 12980–12985. [Google Scholar] [CrossRef]
- Dalghi, M.G.; Ruiz, W.G.; Clayton, D.R.; Montalbetti, N.; Daugherty, S.L.; Beckel, J.M.; Carattino, M.D.; Apodaca, G. Functional roles for PIEZO1 and PIEZO2 in urothelial mechanotransduction and lower urinary tract interoception. JCI Insight 2021, 6, e152984. [Google Scholar] [CrossRef]
- Aresta Branco, M.S.L.; Gutierrez Cruz, A.; Borhani Peikani, M.; Mutafova-Yambolieva, V.N. Sensory neurons, PIEZO channels and PAC1 receptors regulate the mechanosensitive release of soluble ectonucleotidases in the murine urinary bladder lamina propria. Int. J. Mol. Sci. 2023, 24, 7322. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, L.; Paff, M.; Tang, W.C. Initial studies of mechanical compression on neurogenesis with neonatal neural stem cells. Nanomedicine 2012, 8, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, T.; Uesugi, Y.; Kaneko, N.; Yoshida, W.; Sawamoto, K.; Inagaki, N. Shootin1b mediates a mechanical clutch to produce force for neuronal migration. Cell Rep. 2018, 25, 624–639.e6. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Innes-Gold, S.; Cohen, A.E. Membrane tension propagation couples axon growth and collateral branching. Sci. Adv. 2022, 8, eabo1297. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Tan, R.; Wang, X.; Zhou, X.; Ma, R.; Ma, X.; Li, Y.; Liu, R.; Zhang, C.; Liu, X.; et al. Transcranial magneto-acoustic stimulation attenuates synaptic plasticity impairment through the activation of Piezo1 in Alzheimer’s disease mouse model. Research 2023, 6, 0130. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Cui, Y.; Wang, H.; Jiang, J.; Zhang, T.; Sun, S.; Zhou, Z.; Zhong, Y.; Xiao, B. Astrocytic Piezo1-mediated mechanotransduction determines adult neurogenesis and cognitive functions. Neuron 2022, 110, 2984–2999.e8. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Estevez, M.; Rolle, S.O.; Mampay, M.; Dev, K.K.; Sheridan, G.K. Piezo1 regulates calcium oscillations and cytokine release from astrocytes. Glia 2020, 68, 145–160. [Google Scholar] [CrossRef]
- Parpura, V.; Basarsky, T.A.; Liu, F.; Jeftinija, K.; Jeftinija, S.; Haydon, P.G. Glutamate-mediated astrocyte-neuron signalling. Nature 1994, 369, 744–747. [Google Scholar] [CrossRef]
- Malarkey, E.B.; Parpura, V. Mechanisms of glutamate release from astrocytes. Neurochem. Int. 2008, 52, 142–154. [Google Scholar] [CrossRef]
- Panatier, A.; Robitaille, R. Astrocytic mGluR5 and the tripartite synapse. Neuroscience 2016, 323, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Papouin, T.; Oliet, S.H. Organization, control and function of extrasynaptic NMDA receptors. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130601. [Google Scholar] [CrossRef] [PubMed]
- Dore, K.; Stein, I.S.; Brock, J.A.; Castillo, P.E.; Zito, K.; Sjöström, P.J. Unconventional NMDA receptor signaling. J. Neurosci. 2017, 37, 10800–10807. [Google Scholar] [CrossRef] [PubMed]
- Höft, S.; Griemsmann, S.; Seifert, G.; Steinhäuser, C. Heterogeneity in expression of functional ionotropic glutamate and GABA receptors in astrocytes across brain regions: Insights from the thalamus. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130602. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, F.; Shigemoto, R. Metabotropic glutamate receptors. Cell Tissue Res. 2006, 326, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Fellin, T.; Gomez-Gonzalo, M.; Gobbo, S.; Carmignoto, G.; Haydon, P.G. Astrocytic glutamate is not necessary for the generation of epileptiform neuronal activity in hippocampal slices. J. Neurosci. 2006, 26, 9312–9322. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.R.; Bollands, M.; Morris, P.G.; Jones, S. Mechanisms regulating spill-over of synaptic glutamate to extrasynaptic NMDA receptors in mouse substantia nigra dopaminergic neurons. Eur. J. Neurosci. 2015, 42, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Csemer, A.; Kovács, A.; Maamrah, B.; Pocsai, K.; Korpás, K.; Klekner, Á.; Szücs, P.; Nánási, P.P.; Pál, B. Astrocyte- and NMDA receptor-dependent slow inward currents differently contribute to synaptic plasticity in an age-dependent manner in mouse and human neocortex. Aging Cell 2023, 22, e13939. [Google Scholar] [CrossRef]
- Hertelendy, P.; Varga, D.P.; Menyhárt, Á.; Bari, F.; Farkas, E. Susceptibility of the cerebral cortex to spreading depolarization in neurological disease states: The impact of aging. Neurochem. Int. 2019, 127, 125–136. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B. The Mouse Brain in Stereotaxic Coordinates, 4th ed.; Elsevier: San Diego, CA, USA, 2013. [Google Scholar]
- Pál, B. On the functions of astrocyte-mediated neuronal slow inward currents. Neural Regen. Res. 2024. [Google Scholar]
- Fellin, T.; Pascual, O.; Gobbo, S.; Pozzan, T.; Haydon, P.G.; Carmignoto, G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 2004, 43, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Lauderdale, K.; Murphy, T.; Tung, T.; Davila, D.; Binder, D.K.; Fiacco, T.A. Osmotic edema rapidly increases neuronal excitability through activation of NMDA receptor-dependent slow inward currents in juvenile and adult hippocampus. ASN Neuro 2015, 7, 1759091415605115. [Google Scholar] [CrossRef] [PubMed]
- Syková, E.; Vargová, L. Extrasynaptic transmission and the diffusion parameters of the extracellular space. Neurochem. Int. 2008, 52, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Pál, B. Astrocyte-dependent slow inward currents (SICs) participate in neuromodulatory mechanisms in the pedunculopontine nucleus (PPN). Front. Cell Neurosci. 2017, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Maneshi, M.M.; Maki, B.; Gnanasambandam, R.; Belin, S.; Popescu, G.K.; Sachs, F.; Hua, S.Z. Mechanical stress activates NMDA receptors in the absence of agonists. Sci. Rep. 2017, 7, 39610. [Google Scholar] [CrossRef] [PubMed]
- Belin, S.; Maki, B.A.; Catlin, J.; Rein, B.A.; Popescu, G.K. Membrane stretch gates NMDA receptors. J. Neurosci. 2022, 42, 5672–5680. [Google Scholar] [CrossRef] [PubMed]
- Breau, M.A.; Bonnet, I.; Stoufflet, J.; Xie, J.; De Castro, S.; Schneider-Maunoury, S. Extrinsic mechanical forces mediate retrograde axon extension in a developing neuronal circuit. Nat. Commun. 2017, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Coakley, S.; Shi, R.; Shen, K. Dendrites use mechanosensitive channels to proofread ligand-mediated neurite extension during morphogenesis. Dev. Cell 2022, 57, 1615–1629.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xian, Q.; Hou, X.; Wong, K.F.; Zhu, T.; Chen, Z.; He, D.; Kala, S.; Murugappan, S.; Jing, J.; et al. The mechanosensitive ion channel Piezo1 contributes to ultrasound neuromodulation. Proc. Natl. Acad. Sci. USA 2023, 120, e2300291120. [Google Scholar] [CrossRef]
- Bonansco, C.; Couve, A.; Perea, G.; Ferradas, C.Á.; Roncagliolo, M.; Fuenzalida, M. Glutamate released spontaneously from astrocytes sets the threshold for synaptic plasticity. Eur. J. Neurosci. 2011, 33, 1483–1492. [Google Scholar] [CrossRef]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 2018, 174, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gallego, I.; Pérez-Rodríguez, M.; Coatl-Cuaya, H.; Flores, G.; Rodríguez-Moreno, A. Adenosine and astrocytes determine the developmental dynamics of spike timing-dependent plasticity in the somatosensory cortex. J. Neurosci. 2022, 42, 6038–6052. [Google Scholar] [CrossRef]
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Le, D.T.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 16148–16153. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, L.P.; Qin, X.H.; Li, S.J.; Zhang, M.; Wang, Q.; Hu, H.H.; Fang, Y.Y.; Gao, Y.B.; Li, X.W.; et al. Astrocytic adenosine 5′-triphosphate release regulates the proliferation of neural stem cells in the adult hippocampus. Stem Cells 2013, 31, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Ahmed, A.; Jayasi, J.; Womac, A.; Sally, O.; Bae, C. Inflammation condition sensitizes Piezo1 mechanosensitive channel in mouse cerebellum astrocyte. Front. Cell Neurosci. 2023, 17, 1200946. [Google Scholar] [CrossRef] [PubMed]
- Szabó, L.; Balogh, N.; Tóth, A.; Angyal, Á.; Gönczi, M.; Csiki, D.M.; Tóth, C.; Balatoni, I.; Jeney, V.; Csernoch, L.; et al. The mechanosensitive Piezo1 channels contribute to the arterial medial calcification. Front. Physiol. 2022, 13, 1037230. [Google Scholar] [CrossRef]
- Qu, J.; Zong, H.F.; Shan, Y.; Zhang, S.C.; Guan, W.P.; Yang, Y.; Zhao, H.L. Piezo1 suppression reduces demyelination after intracerebral hemorrhage. Neural Regen. Res. 2023, 18, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Baksa, B.; Bayasgalan, T.; Szentesi, P.; Csemer, A.; Pál, B. Orexinergic actions modify occurrence of slow inward currents on neurons in the pedunculopontine nucleus. Neuroreport 2019, 30, 933–938. [Google Scholar] [CrossRef]
- Botello-Smith, W.M.; Jiang, W.; Zhang, H.; Ozkan, A.D.; Lin, Y.C.; Pham, C.N.; Lacroix, J.J.; Luo, Y. A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1. Nat. Commun. 2019, 10, 4503. [Google Scholar] [CrossRef]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical activation of the mechanotransduction channel Piezo1. Elife 2015, 4, e07369. [Google Scholar] [CrossRef]
- Davies, J.; Watkins, J.C. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982, 235, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.L.; Cuthbertson, K.; Endesh, N.; Rode, B.; Blythe, N.M.; Hyman, A.J.; Hall, S.J.; Gaunt, H.J.; Ludlow, M.J.; Foster, R.; et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br. J. Pharmacol. 2018, 175, 1744–1759. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Blaquiere, M.; Janvier, A.; Cresto, N.; Lana, C.; Genin, A.; Hirbec, H.; Audinat, E.; Faucherre, A.; Barbier, E.L.; et al. PIEZO1 expression at the glio-vascular unit adjusts to neuroinflammation in seizure conditions. Neurobiol. Dis. 2023, 187, 106297. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wanggou, S.; Bodalia, A.; Zhu, M.; Dong, W.; Fan, J.J.; Yin, W.C.; Min, H.K.; Hu, M.; Draghici, D.; et al. A feedforward mechanism mediated by mechanosensitive ion channel PIEZO1 and tissue mechanics promotes glioma aggression. Neuron 2018, 100, 799–815.e7. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Hu, T.; Qiu, O.; Su, Y.; Gu, J.; Xia, Z. Effect of Piezo1 overexpression on peritumoral brain edema in glioblastomas. AJNR Am. J. Neuroradiol. 2020, 41, 1423–1429. [Google Scholar] [CrossRef]
- Hong, R.; Yang, D.; Jing, Y.; Chen, S.; Tian, H.; Yang, Y. PIEZO1-related physiological and pathological processes in CNS: Focus on the gliomas. Cancers 2023, 15, 883. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csemer, A.; Sokvári, C.; Maamrah, B.; Szabó, L.; Korpás, K.; Pocsai, K.; Pál, B. Pharmacological Activation of Piezo1 Channels Enhances Astrocyte–Neuron Communication via NMDA Receptors in the Murine Neocortex. Int. J. Mol. Sci. 2024, 25, 3994. https://doi.org/10.3390/ijms25073994
Csemer A, Sokvári C, Maamrah B, Szabó L, Korpás K, Pocsai K, Pál B. Pharmacological Activation of Piezo1 Channels Enhances Astrocyte–Neuron Communication via NMDA Receptors in the Murine Neocortex. International Journal of Molecular Sciences. 2024; 25(7):3994. https://doi.org/10.3390/ijms25073994
Chicago/Turabian StyleCsemer, Andrea, Cintia Sokvári, Baneen Maamrah, László Szabó, Kristóf Korpás, Krisztina Pocsai, and Balázs Pál. 2024. "Pharmacological Activation of Piezo1 Channels Enhances Astrocyte–Neuron Communication via NMDA Receptors in the Murine Neocortex" International Journal of Molecular Sciences 25, no. 7: 3994. https://doi.org/10.3390/ijms25073994
APA StyleCsemer, A., Sokvári, C., Maamrah, B., Szabó, L., Korpás, K., Pocsai, K., & Pál, B. (2024). Pharmacological Activation of Piezo1 Channels Enhances Astrocyte–Neuron Communication via NMDA Receptors in the Murine Neocortex. International Journal of Molecular Sciences, 25(7), 3994. https://doi.org/10.3390/ijms25073994





