Meeting the Challenge of Controlling Viral Immunopathology
Abstract
1. Introduction
| Disease | Immunopathogenesis | Key Immune Cells/Cytokines | Refs. |
|---|---|---|---|
| Dengue virus | Formation of immune complexes (virus-antibody) depositing in blood vessels, triggering inflammation and vascular leakage. Cytokine storm resulting from infection of inflammatory cells | B cells, defective CD4+ and CD8+ T cells, macrophages; Dengue-specific antibodies, TNF-α, IL-2, IL-6 | [4,5,6] |
| EBV | Potential molecular mimicry triggering autoimmune reactions against self-tissues | CD4+ and CD8+ T cells, B cells; EBV-specific antibodies | [7,8] |
| HBV | Chronic infection triggers CD8-mediated inflammation, leading to liver damage. | B cells, CD4+ and CD8+ T cells, macrophages; HBV-specific antibodies, IFN-γ, TNF-α, IL-1, IL-6 | [9] |
| HCV | Immune complex deposition leads to chronic inflammation and liver damage. | B cells, macrophages; HCV-specific antibodies | [10] |
| HSV | T cell-mediated chronic inflammatory response in eye and brain | CD4+ and CD8+ T cells, NK cells, IFN-γ, TNF-α, IL-1, IL-6, IL-17 | [11,12,13] |
| LCMV | T cell-mediated inflammation and Immune complexes in kidney and skin | CD8+ T cells, macrophages; IFN-γ, TGF-beta, IL-10, IL-7. May also involve CD4+ T cells and B cells in specific contexts. | [14] |
| RSV | Th2-biased immune response with release of proinflammatory cytokines and eosinophil recruitment | Neutrophils, ROS production, Netosis, NLRP3, CD4+ T cells, eosinophils; IL-3, IL-4, IL-5, IL-10, IL-13, IL-17 | [15,16] |
| SARS-CoV-1 and 2 | Combined inflammatory response (cytokine storm) and direct viral damage to endothelial cells, and T cell-mediated damage to endothelial cells | CD4+ and CD8+ T cells, macrophages, NK cells; IL-1, IL-6, TNF-α, IFN-γ. | [17,18,19] |
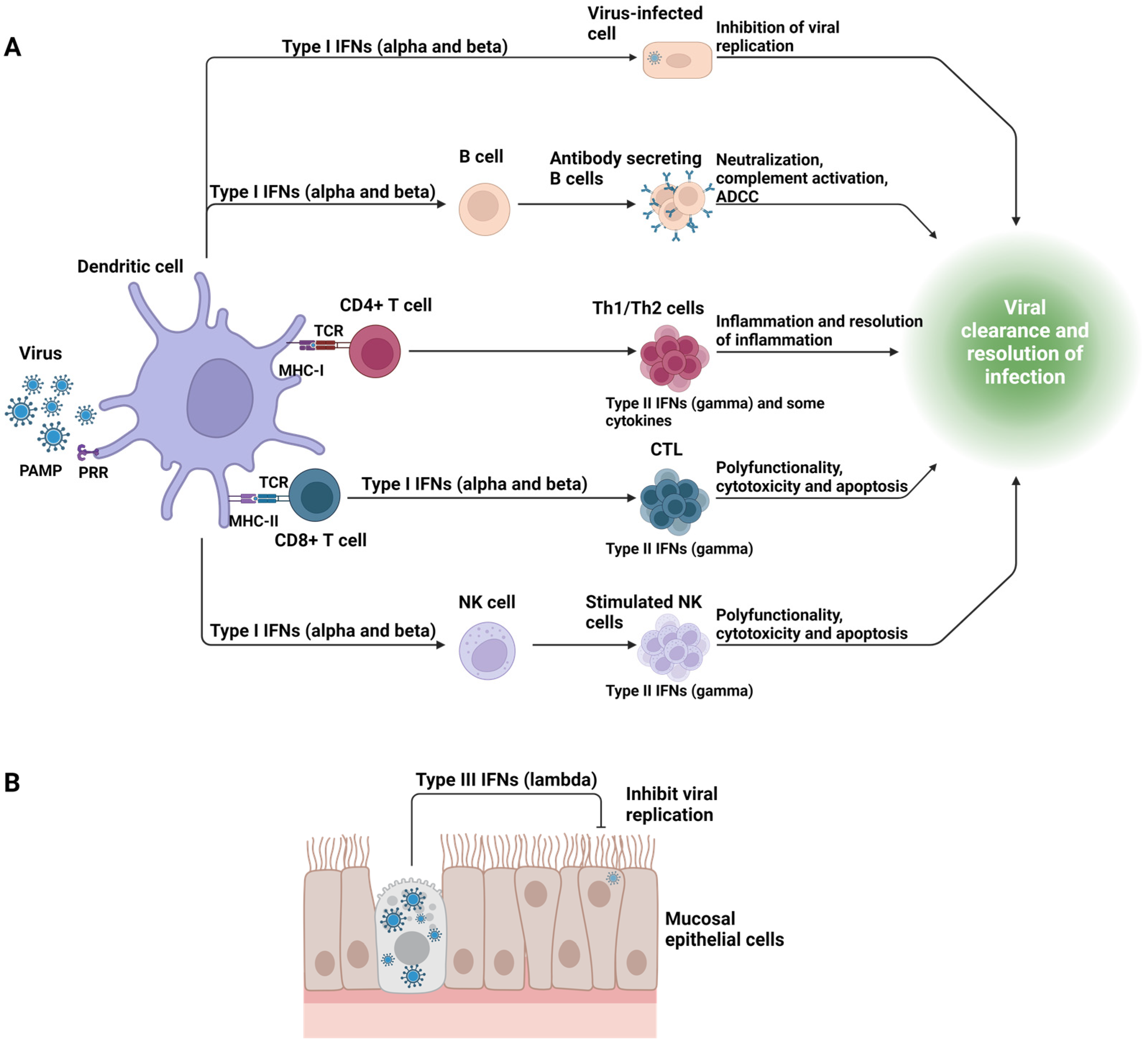
2. Overview of the First Responders to Viral Infection and Their Impact on the Outcome
3. The Principal Components of Innate Immunity That Affect the Outcome of Viral Infections
4. Targeting Innate Immune Components to Minimize Pathology Associated with Viral Infections
5. Overview of the Principal Adaptive Immune Components That Participate in Viral Immunopathology
6. Some Approaches Available to Diminish the Impact Lesions Caused by Adaptive Immune Responses to Viruses
Author Contributions
Funding
Conflicts of Interest
References
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.-C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Yasir, M.; Goyal, A.; Sonthalia, S. Corticosteroid Adverse Effects. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kuczera, D.; Assolini, J.P.; Tomiotto-Pellissier, F.; Pavanelli, W.R.; Silveira, G.F. Highlights for Dengue Immunopathogenesis: Antibody-Dependent Enhancement, Cytokine Storm, and Beyond. J. Interferon Cytokine Res. 2018, 38, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Mady, B.J.; Erbe, D.V.; Kurane, I.; Fanger, M.W.; Ennis, F.A. Antibody-dependent enhancement of dengue virus infection mediated by bispecific antibodies against cell surface molecules other than Fc gamma receptors. J. Immunol. 1991, 147, 3139–3144. [Google Scholar] [CrossRef]
- Khanam, A.; Gutiérrez-Barbosa, H.; Lyke, K.E.; Chua, J.V. Immune-Mediated Pathogenesis in Dengue Virus Infection. Viruses 2022, 14, 2575. [Google Scholar] [CrossRef]
- Fujinami, R.S.; Oldstone, M.B.A. Molecular mimicry as a mechanism for virus-induced autoimmunity. Immunol. Res. 1989, 8, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Thorley-Lawson, D.A.; Hawkins, J.B.; Tracy, S.I.; Shapiro, M. The pathogenesis of Epstein-Barr virus persistent infection. Curr. Opin. Virol. 2013, 3, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Chisari, F.V. Hepatitis B virus transgenic mice: Insights into the virus and the disease. Hepatology 1995, 22 Pt 1, 1316–1325. [Google Scholar] [CrossRef]
- Irshad, M.; Gupta, P.; Irshad, K. Immunopathogenesis of Liver Injury During Hepatitis C Virus Infection. Viral Immunol. 2019, 32, 112–120. [Google Scholar] [CrossRef]
- Doymaz, M.Z.; Rouse, B.T. Herpetic stromal keratitis: An immunopathologic disease mediated by CD4+ T lymphocytes. Invest. Ophthalmol. Vis. Sci. 1992, 33, 2165–2173. [Google Scholar]
- Rajasagi, N.K.; Rouse, B.T. The Role of T Cells in Herpes Stromal Keratitis. Front. Immunol. 2019, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Antony, F.; Rouse, B.T.; Suryawanshi, A. Role of Innate Interferon Responses at the Ocular Surface in Herpes Simplex Virus-1-Induced Herpetic Stromal Keratitis. Pathogens 2023, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ramachandran, S.; Mann, M.; Popkin, D.L. Role of lymphocytic choriomeningitis virus (LCMV) in understanding viral immunology: Past, present and future. Viruses 2012, 4, 2650–2669. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Unger, S.A.; Walton, M.; Schwarze, J. The Human Immune Response to Respiratory Syncytial Virus Infection. Clin. Microbiol. Rev. 2017, 30, 481–502. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, J.J.; Avellaneda, A.M.; Salazar-Ardiles, C.; Maya, J.E.; Kalergis, A.M.; Lay, M.K. Host Components Contributing to Respiratory Syncytial Virus Pathogenesis. Front. Immunol. 2019, 10, 2152. [Google Scholar] [CrossRef] [PubMed]
- Bordallo, B.; Bellas, M.; Cortez, A.F.; Vieira, M.; Pinheiro, M. Severe COVID-19: What have we learned with the immunopathogenesis? Adv. Rheumatol. 2020, 60, 50. [Google Scholar] [CrossRef]
- Wong, L.R.; Perlman, S. Immune dysregulation and immunopathology induced by SARS-CoV-2 and related coronaviruses—Are we our own worst enemy? Nat. Rev. Immunol. 2022, 22, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Theron, M.; Huang, K.J.; Chen, Y.W.; Liu, C.C.; Lei, H.Y. A probable role for IFN-gamma in the development of a lung immunopathology in SARS. Cytokine 2005, 32, 30–38. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Chan, M.; Zhou, S.; Wang, J.; Reed, G.; Bronson, R.; Arnold, M.M.; Knipe, D.M.; Finberg, R.W. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 2004, 101, 1315–1320. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Jouanguy, E.; Ugolini, S.; Smahi, A.; Elain, G.; Romero, P.; Segal, D.; Sancho-Shimizu, V.; Lorenzo, L.; Puel, A.; et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science 2007, 317, 1522–1527. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Zhao, Y.; Ma, X.; Yi, H. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J. Zhejiang Univ. Sci. B 2021, 22, 609–632. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Jones, E.A.; Popova, L.; Kwinn, L.; Haynes, L.M.; Jones, L.P.; Tripp, R.A.; Walsh, E.E.; Freeman, M.W.; Golenbock, D.T.; Anderson, L.J.; et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000, 1, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.; Sato, A.; Akira, S.; Medzhitov, R.; Iwasaki, A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 2003, 198, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Reinert, L.S.; Lopušná, K.; Winther, H.; Sun, C.; Thomsen, M.K.; Nandakumar, R.; Mogensen, T.H.; Meyer, M.; Vægter, C.; Nyengaard, J.R.; et al. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat. Commun. 2016, 7, 13348. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Wu, J.; Wu, Y.T.; Du, F.; Aroh, C.; Yan, N.; Sun, L.; Chen, Z.J. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 2013, 341, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.M.; Ma, X.; Abdullah, S.W.; Zheng, H. Activation and Inhibition of the NLRP3 Inflammasome by RNA Viruses. J. Inflamm. Res. 2021, 14, 1145–1163. [Google Scholar] [CrossRef]
- Gimenez, F.; Bhela, S.; Dogra, P.; Harvey, L.; Varanasi, S.K.; Jaggi, U.; Rouse, B.T. The inflammasome NLRP3 plays a protective role against a viral immunopathological lesion. J. Leukoc. Biol. 2016, 99, 647–657. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Jiang, Z.; Waggoner, S.N.; Sharma, S.; Cole, L.E.; Waggoner, L.; Vanaja, S.K.; Monks, B.G.; Ganesan, S.; Latz, E.; et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 2010, 11, 395–402. [Google Scholar] [CrossRef]
- Pulendran, B.; Tang, H.; Denning, T.L. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr. Opin. Immunol. 2008, 20, 61–67. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I.; Ravindran, R.; Stewart, S.; Alam, M.; Kwissa, M.; et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, J.F.; Woda, B.A.; Habu, S.; Okumura, K.; Welsh, R.M. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J. Immunol. 1983, 131, 1531–1538. [Google Scholar] [CrossRef]
- Biron, C.A.; Byron, K.S.; Sullivan, J.L. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 1989, 320, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Etzioni, A.; Eidenschenk, C.; Katz, R.; Beck, R.; Casanova, J.L.; Pollack, S. Fatal varicella associated with selective natural killer cell deficiency. J. Pediatr. 2005, 146, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, U.; Matundan, H.H.; Yu, J.; Hirose, S.; Mueller, M.; Wormley, F.L., Jr.; Ghiasi, H. Essential role of M1 macrophages in blocking cytokine storm and pathology associated with murine HSV-1 infection. PLoS Pathog. 2021, 17, e1009999. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.; Joseph, J.; Shirey, K.A.; Keegan, A.D.; Boukhvalova, M.S.; Vogel, S.N.; Blanco, J.C.G. Protection against influenza-induced Acute Lung Injury (ALI) by enhanced induction of M2a macrophages: Possible role of PPARγ/RXR ligands in IL-4-induced M2a macrophage differentiation. Front. Immunol. 2022, 13, 968336. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, A.; Marois, I.; Cloutier, D.; Verreault, C.; Cantin, A.M.; Richter, M.V. The prostanoid 15-deoxy-Δ12,14-prostaglandin-j2 reduces lung inflammation and protects mice against lethal influenza infection. J. Infect. Dis. 2012, 205, 621–630. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, T.; Wei, X.; Yang, S.; Tian, Z. Type 1 innate lymphoid cells contribute to the pathogenesis of chronic hepatitis B. Innate Immun. 2015, 21, 665–673. [Google Scholar] [CrossRef]
- Monticelli, L.A.; Sonnenberg, G.F.; Abt, M.C.; Alenghat, T.; Ziegler, C.G.; Doering, T.A.; Angelosanto, J.M.; Laidlaw, B.J.; Yang, C.Y.; Sathaliyawala, T.; et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011, 12, 1045–1054. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, L.; Xiao, Z.; Li, M.; Wu, X.; Li, W.; Li, X.; Zhao, Q.; Wu, Y.; Zhang, H.; et al. Protective Role of γδ T Cells in Different Pathogen Infections and Its Potential Clinical Application. J. Immunol. Res. 2018, 2018, 5081634. [Google Scholar] [CrossRef]
- Sabbaghi, A.; Miri, S.M.; Keshavarz, M.; Mahooti, M.; Zebardast, A.; Ghaemi, A. Role of γδ T cells in controlling viral infections with a focus on influenza virus: Implications for designing novel therapeutic approaches. Virol. J. 2020, 17, 174. [Google Scholar] [CrossRef]
- Jameson, J.M.; Cruz, J.; Costanzo, A.; Terajima, M.; Ennis, F.A. A role for the mevalonate pathway in the induction of subtype cross-reactive immunity to influenza A virus by human gammadelta T lymphocytes. Cell Immunol. 2010, 264, 71–77. [Google Scholar] [CrossRef]
- Andreakos, E. Type I and type III interferons: From basic biology and genetics to clinical development for COVID-19 and beyond. Semin. Immunol. 2024, 72, 101863. [Google Scholar] [CrossRef]
- Bencze, D.; Fekete, T.; Pázmándi, K. Type I Interferon Production of Plasmacytoid Dendritic Cells under Control. Int. J. Mol. Sci. 2021, 22, 4190. [Google Scholar] [CrossRef]
- Dalskov, L.; Gad, H.H.; Hartmann, R. Viral recognition and the antiviral interferon response. EMBO J. 2023, 42, e112907. [Google Scholar] [CrossRef]
- Murphy, K.T.P.; Walport, M.; Janeway, C. Janeway’s Immunobiology; Garland Science, Taylor & Francis Group, LLC: New York, NY, USA, 2008. [Google Scholar]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Sommereyns, C.; Paul, S.; Staeheli, P.; Michiels, T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008, 4, e1000017. [Google Scholar] [CrossRef]
- Okabayashi, T.; Kojima, T.; Masaki, T.; Yokota, S.; Imaizumi, T.; Tsutsumi, H.; Himi, T.; Fujii, N.; Sawada, N. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res. 2011, 160, 360–366. [Google Scholar] [CrossRef]
- Mordstein, M.; Neugebauer, E.; Ditt, V.; Jessen, B.; Rieger, T.; Falcone, V.; Sorgeloos, F.; Ehl, S.; Mayer, D.; Kochs, G.; et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J. Virol. 2010, 84, 5670–5677. [Google Scholar] [CrossRef]
- Mordstein, M.; Kochs, G.; Dumoutier, L.; Renauld, J.C.; Paludan, S.R.; Klucher, K.; Staeheli, P. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008, 4, e1000151. [Google Scholar] [CrossRef]
- Doldan, P.; Dai, J.; Metz-Zumaran, C.; Patton, J.T.; Stanifer, M.L.; Boulant, S. Type III and Not Type I Interferons Efficiently Prevent the Spread of Rotavirus in Human Intestinal Epithelial Cells. J. Virol. 2022, 96, e0070622. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Park, S.H. An Impaired Inflammatory and Innate Immune Response in COVID-19. Mol. Cells 2021, 44, 384–391. [Google Scholar] [CrossRef]
- Wilson, E.B.; Yamada, D.H.; Elsaesser, H.; Herskovitz, J.; Deng, J.; Cheng, G.; Aronow, B.J.; Karp, C.L.; Brooks, D.G. Blockade of Chronic Type I Interferon Signaling to Control Persistent LCMV Infection. Science 2013, 340, 202–207. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Ng, C.; Lee, A.M.; Sullivan, B.M.; Sheehan, K.C.F.; Welch, M.; Schreiber, R.D.; Carlos de la Torre, J.; Oldstone, M.B.A. Persistent LCMV Infection Is Controlled by Blockade of Type I Interferon Signaling. Science 2013, 340, 207–211. [Google Scholar] [CrossRef]
- Ng, C.T.; Sullivan, B.M.; Teijaro, J.R.; Lee, A.M.; Welch, M.; Rice, S.; Sheehan, K.C.; Schreiber, R.D.; Oldstone, M.B. Blockade of interferon Beta, but not interferon alpha, signaling controls persistent viral infection. Cell Host Microbe 2015, 17, 653–661. [Google Scholar] [CrossRef]
- Davidson, S.; Crotta, S.; McCabe, T.M.; Wack, A. Pathogenic potential of interferon αβ in acute influenza infection. Nat. Commun. 2014, 5, 3864. [Google Scholar] [CrossRef]
- Rigaux, P.; Killoran, K.E.; Qiu, Z.; Rosenberg, H.F. Depletion of alveolar macrophages prolongs survival in response to acute pneumovirus infection. Virology 2012, 422, 338–345. [Google Scholar] [CrossRef]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Parry, B.; Ravid, S.; Clark, S.; Schrimpf, M.R.; et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 2022, 606, 576–584. [Google Scholar] [CrossRef]
- Sefik, E.; Qu, R.; Junqueira, C.; Kaffe, E.; Mirza, H.; Zhao, J.; Brewer, J.R.; Han, A.; Steach, H.R.; Israelow, B.; et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature 2022, 606, 585–593. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Mulik, S.; Sharma, S.; Reddy, P.B.; Sehrawat, S.; Rouse, B.T. Ocular neovascularization caused by herpes simplex virus type 1 infection results from breakdown of binding between vascular endothelial growth factor A and its soluble receptor. J. Immunol. 2011, 186, 3653–3665. [Google Scholar] [CrossRef]
- Thomas, J.; Gangappa, S.; Kanangat, S.; Rouse, B.T. On the essential involvement of neutrophils in the immunopathologic disease: Herpetic stromal keratitis. J. Immunol. 1997, 158, 1383–1391. [Google Scholar] [CrossRef]
- Veras, F.P.; Gomes, G.F.; Silva, B.M.S.; Caetité, D.B.; Almeida, C.; Silva, C.M.S.; Schneider, A.H.; Corneo, E.S.; Bonilha, C.S.; Batah, S.S.; et al. Targeting neutrophils extracellular traps (NETs) reduces multiple organ injury in a COVID-19 mouse model. Respir. Res. 2023, 24, 66. [Google Scholar] [CrossRef]
- Biswas, P.S.; Banerjee, K.; Kim, B.; Rouse, B.T. Mice Transgenic for IL-1 Receptor Antagonist Protein Are Resistant to Herpetic Stromal Keratitis: Possible Role for IL-1 in Herpetic Stromal Keratitis Pathogenesis1. J. Immunol. 2004, 172, 3736–3744. [Google Scholar] [CrossRef]
- Banerjee, K.; Biswas, P.S.; Kim, B.; Lee, S.; Rouse, B.T. CXCR2-/- mice show enhanced susceptibility to herpetic stromal keratitis: A role for IL-6-induced neovascularization. J. Immunol. 2004, 172, 1237–1245. [Google Scholar] [CrossRef]
- Mulik, S.; Berber, E.; Sehrawat, S.; Rouse, B.T. Controlling viral inflammatory lesions by rebalancing immune response patterns. Front. Immunol. 2023, 14, 1257192. [Google Scholar] [CrossRef]
- Jhan, M.K.; HuangFu, W.C.; Chen, Y.F.; Kao, J.C.; Tsai, T.T.; Ho, M.R.; Shen, T.J.; Tseng, P.C.; Wang, Y.T.; Lin, C.F. Anti-TNF-α restricts dengue virus-induced neuropathy. J. Leukoc. Biol. 2018, 104, 961–968. [Google Scholar] [CrossRef]
- Kim, K.S.; Jung, H.; Shin, I.K.; Choi, B.R.; Kim, D.H. Induction of interleukin-1 beta (IL-1β) is a critical component of lung inflammation during influenza A (H1N1) virus infection. J. Med. Virol. 2015, 87, 1104–1112. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, W.; Huang, H.; Zhu, H.; Zhou, P.; Zhu, H.; Ju, D. Inhibition of the inflammatory cytokine tumor necrosis factor-alpha with etanercept provides protection against lethal H1N1 influenza infection in mice. Crit. Care 2013, 17, R301. [Google Scholar] [CrossRef]
- Caine, E.A.; Scheaffer, S.M.; Arora, N.; Zaitsev, K.; Artyomov, M.N.; Coyne, C.B.; Moley, K.H.; Diamond, M.S. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat. Commun. 2019, 10, 280. [Google Scholar] [CrossRef]
- Elneil, S.; Lalezari, J.P.; Pourhassan, N.Z. Case study of a critically ill person with COVID-19 on ECMO successfully treated with leronlimab. J. Transl. Autoimmun. 2021, 4, 100097. [Google Scholar] [CrossRef]
- Latinovic, O.S.; Reitz, M.; Heredia, A. CCR5 Inhibitors and HIV-1 Infection. J. AIDS HIV Treat. 2019, 1, 1–5. [Google Scholar] [CrossRef]
- Marques, R.E.; Guabiraba, R.; Del Sarto, J.L.; Rocha, R.F.; Queiroz, A.L.; Cisalpino, D.; Marques, P.E.; Pacca, C.C.; Fagundes, C.T.; Menezes, G.B.; et al. Dengue virus requires the CC-chemokine receptor CCR5 for replication and infection development. Immunology 2015, 145, 583–596. [Google Scholar] [CrossRef]
- Tan, A.C.; Mifsud, E.J.; Zeng, W.; Edenborough, K.; McVernon, J.; Brown, L.E.; Jackson, D.C. Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol. Pharm. 2012, 9, 2710–2718. [Google Scholar] [CrossRef]
- Lau, Y.F.; Tang, L.H.; Ooi, E.E.; Subbarao, K. Activation of the innate immune system provides broad-spectrum protection against influenza A viruses with pandemic potential in mice. Virology 2010, 406, 80–87. [Google Scholar] [CrossRef]
- Abdul-Careem, M.F.; Firoz Mian, M.; Gillgrass, A.E.; Chenoweth, M.J.; Barra, N.G.; Chan, T.; Al-Garawi, A.A.; Chew, M.V.; Yue, G.; van Roojen, N.; et al. FimH, a TLR4 ligand, induces innate antiviral responses in the lung leading to protection against lethal influenza infection in mice. Antivir. Res. 2011, 92, 346–355. [Google Scholar] [CrossRef]
- Zhang, B.; Chassaing, B.; Shi, Z.; Uchiyama, R.; Zhang, Z.; Denning, T.L.; Crawford, S.E.; Pruijssers, A.J.; Iskarpatyoti, J.A.; Estes, M.K.; et al. Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science 2014, 346, 861–865. [Google Scholar] [CrossRef]
- Edwards, L.; Ferenczy, A.; Eron, L.; Baker, D.; Owens, M.L.; Fox, T.L.; Hougham, A.J.; Schmitt, K.A. Self-administered topical 5% imiquimod cream for external anogenital warts. HPV Study Group. Human PapillomaVirus. Arch. Dermatol. 1998, 134, 25–30. [Google Scholar] [CrossRef] [PubMed]
- McGowan, D.; Herschke, F.; Pauwels, F.; Stoops, B.; Last, S.; Pieters, S.; Scholliers, A.; Thoné, T.; Van Schoubroeck, B.; De Pooter, D.; et al. Novel Pyrimidine Toll-like Receptor 7 and 8 Dual Agonists to Treat Hepatitis B Virus. J. Med. Chem. 2016, 59, 7936–7949. [Google Scholar] [CrossRef]
- Lanford, R.E.; Guerra, B.; Chavez, D.; Giavedoni, L.; Hodara, V.L.; Brasky, K.M.; Fosdick, A.; Frey, C.R.; Zheng, J.; Wolfgang, G.; et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 2013, 144, 1508–1517.e10. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Pryde, D.C.; Jones, P.; Adam, F.M.; Benson, N.; Bish, G.; Calo, F.; Ciaramella, G.; Dixon, R.; Duckworth, J.; et al. Design and optimisation of orally active TLR7 agonists for the treatment of hepatitis C virus infection. Bioorg. Med. Chem. Lett. 2011, 21, 2389–2393. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xie, X.; Feng, X.L.; Xu, L.; Han, J.B.; Yu, D.; Zou, Q.C.; Liu, Q.; Li, X.; Ma, G.; et al. Specific inhibition of the NLRP3 inflammasome suppresses immune overactivation and alleviates COVID-19 like pathology in mice. EBioMedicine 2022, 75, 103803. [Google Scholar] [CrossRef]
- Van Rooijen, N.; Sanders, A.; van den Berg, T.K. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J. Immunol. Methods 1996, 193, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Rowe, W.P. Protective effect of pre-irradiation on lymphocytic choriomeningitis infection in mice. Proc. Soc. Exp. Biol. Med. 1956, 92, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Levey, R.H.; Trainin, N.; Law, L.W.; Black, P.H.; Rowe, W.P. Lymphocytic Choriomeningitis Infection in Neonataily Thymectomized Mice Bearing Diffusion Chambers Containing Thymus. Science 1963, 142, 481–485. [Google Scholar] [CrossRef]
- Thomsen, A.R.; Bro-Jørgensen, K.; Jensen, B.L. Lymphocytic choriomeningitis virus-induced immunosuppression: Evidence for viral interference with T-cell maturation. Infect. Immun. 1982, 37, 981–986. [Google Scholar] [CrossRef]
- Borrow, P. Mechanisms of viral clearance and persistence. J. Viral. Hepat. 1997, 4 (Suppl. S2), 16–24. [Google Scholar] [CrossRef] [PubMed]
- Oldstone, M.B.A. Immunopathology of Persistent Viral Infections. Hosp. Pract. 1982, 17, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.S.; Ahmed, R. Immune conflicts in lymphocytic choriomeningitis virus. Springer Semin. Immunopathol. 1995, 17, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Rouse, B.T.; Sehrawat, S. Immunity and immunopathology to viruses: What decides the outcome? Nat. Rev. Immunol. 2010, 10, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Borrow, P.; Oldstone, M. Lymphocytic choriomeningitis virus. In Viral Pathogenesis; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1997; pp. 593–627. [Google Scholar]
- Thimme, R.; Wieland, S.; Steiger, C.; Ghrayeb, J.; Reimann, K.A.; Purcell, R.H.; Chisari, F.V. CD8+ T Cells Mediate Viral Clearance and Disease Pathogenesis during Acute Hepatitis B Virus Infection. J. Virol. 2003, 77, 68–76. [Google Scholar] [CrossRef]
- Maini, M.K.; Boni, C.; Lee, C.K.; Larrubia, J.R.; Reignat, S.; Ogg, G.S.; King, A.S.; Herberg, J.; Gilson, R.; Alisa, A.; et al. The Role of Virus-Specific Cd8+ Cells in Liver Damage and Viral Control during Persistent Hepatitis B Virus Infection. J. Exp. Med. 2000, 191, 1269–1280. [Google Scholar] [CrossRef]
- Oldstone, M.B.; Sinha, Y.N.; Blount, P.; Tishon, A.; Rodriguez, M.; von Wedel, R.; Lampert, P.W. Virus-induced alterations in homeostasis: Alteration in differentiated functions of infected cells in vivo. Science 1982, 218, 1125–1127. [Google Scholar] [CrossRef]
- Moskophidis, D.; Lechner, F.; Pircher, H.; Zinkernagel, R.M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 1993, 362, 758–761. [Google Scholar] [CrossRef]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef]
- Yu, S.L.; Kuan, W.P.; Wong, C.K.; Li, E.K.; Tam, L.S. Immunopathological roles of cytokines, chemokines, signaling molecules, and pattern-recognition receptors in systemic lupus erythematosus. Clin. Dev. Immunol. 2012, 2012, 715190. [Google Scholar] [CrossRef] [PubMed]
- Christiaansen, A.F.; Knudson, C.J.; Weiss, K.A.; Varga, S.M. The CD4 T cell response to respiratory syncytial virus infection. Immunol. Res. 2014, 59, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chentoufi, A.A.; BenMohamed, L. Mucosal Herpes Immunity and Immunopathology to Ocular and Genital Herpes Simplex Virus Infections. Clin. Dev. Immunol. 2012, 2012, 149135. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; Diefenbach, R.J.; Miranda-Saksena, M.; Bosnjak, L.; Kim, M.; Jones, C.; Douglas, M.W. The cycle of human herpes simplex virus infection: Virus transport and immune control. J. Infect. Dis. 2006, 194 (Suppl. S1), S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Knickelbein, J.E.; Hendricks, R.L.; Charukamnoetkanok, P. Management of herpes simplex virus stromal keratitis: An evidence-based review. Surv. Ophthalmol. 2009, 54, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.S.; Rouse, B.T. Early events in HSV keratitis--setting the stage for a blinding disease. Microbes Infect. 2005, 7, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Rouse, B.T. Immunopathogenesis of herpetic ocular disease. Immunol. Res. 1997, 16, 375–386. [Google Scholar] [CrossRef]
- Chucair-Elliott, A.J.; Jinkins, J.; Carr, M.M.; Carr, D.J. IL-6 Contributes to Corneal Nerve Degeneration after Herpes Simplex Virus Type I Infection. Am. J. Pathol. 2016, 186, 2665–2678. [Google Scholar] [CrossRef]
- Sehrawat, S.; Suvas, S.; Sarangi, P.P.; Suryawanshi, A.; Rouse, B.T. In vitro-generated antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions. J. Virol. 2008, 82, 6838–6851. [Google Scholar] [CrossRef]
- Suvas, S.; Kumaraguru, U.; Pack, C.D.; Lee, S.; Rouse, B.T. CD4+CD25+ T Cells Regulate Virus-specific Primary and Memory CD8+ T Cell Responses. JEM 2003, 198, 889–901. [Google Scholar] [CrossRef]
- Lee, S.; Zheng, M.; Kim, B.; Rouse, B.T. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J. Clin. Investig. 2002, 110, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Mulik, S.; Xu, J.; Reddy, P.B.; Rajasagi, N.K.; Gimenez, F.; Sharma, S.; Lu, P.Y.; Rouse, B.T. Role of miR-132 in angiogenesis after ocular infection with herpes simplex virus. Am. J. Pathol. 2012, 181, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, S.K.; Reddy, P.B.J.; Bhela, S.; Jaggi, U.; Gimenez, F.; Rouse, B.T. Azacytidine Treatment Inhibits the Progression of Herpes Stromal Keratitis by Enhancing Regulatory T Cell Function. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, S.K.; Donohoe, D.; Jaggi, U.; Rouse, B.T. Manipulating Glucose Metabolism during Different Stages of Viral Pathogenesis Can Have either Detrimental or Beneficial Effects. J. Immunol. 2017, 199, 1748–1761. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef]
- Cleary, S.J.; Pitchford, S.C.; Amison, R.T.; Carrington, R.; Robaina Cabrera, C.L.; Magnen, M.; Looney, M.R.; Gray, E.; Page, C.P. Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology. Br. J. Pharmacol. 2020, 177, 4851–4865. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C. Impaired interferon signature in severe COVID-19. Nat. Rev. Immunol. 2020, 20, 353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef] [PubMed]
- Choto, T.A.; Makupe, I.; Cakana, A.Z.; Sibanda, E.N.; Mduluza, T. Excessive neutrophil recruitment promotes typical T-helper 17 responses in Coronavirus disease 2019 patients. PLoS ONE 2022, 17, e0273186. [Google Scholar] [CrossRef]
- Tang, L.; Yin, Z.; Hu, Y.; Mei, H. Controlling Cytokine Storm Is Vital in COVID-19. Front. Immunol. 2020, 11, 570993. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Rutigliano, J.A.; Johnson, T.R. Respiratory Syncytial Virus Immunobiology and Pathogenesis. Virology 2002, 297, 1–7. [Google Scholar] [CrossRef]
- Kosanovich, J.L.; Eichinger, K.M.; Lipp, M.A.; Gidwani, S.V.; Brahmbhatt, D.; Yondola, M.A.; Perkins, T.N.; Empey, K.M. Exacerbated lung inflammation following secondary RSV exposure is CD4+ T cell-dependent and is not mitigated in infant BALB/c mice born to PreF-vaccinated dams. Front. Immunol. 2023, 14, 1206026. [Google Scholar] [CrossRef] [PubMed]
- Sigurs, N.; Aljassim, F.; Kjellman, B.; Robinson, P.D.; Sigurbergsson, F.; Bjarnason, R.; Gustafsson, P.M. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010, 65, 1045–1052. [Google Scholar] [CrossRef]
- Bueno, S.M.; González, P.A.; Pacheco, R.; Leiva, E.D.; Cautivo, K.M.; Tobar, H.E.; Mora, J.E.; Prado, C.E.; Zúñiga, J.P.; Jiménez, J.; et al. Host immunity during RSV pathogenesis. Int. Immunopharmacol. 2008, 8, 1320–1329. [Google Scholar] [CrossRef]
- You, D.; Marr, N.; Saravia, J.; Shrestha, B.; Lee, G.I.; Turvey, S.E.; Brombacher, F.; Herbert, D.R.; Cormier, S.A. IL-4Rα on CD4+ T cells plays a pathogenic role in respiratory syncytial virus reinfection in mice infected initially as neonates. J. Leukoc. Biol. 2013, 93, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Pérez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- Alansari, K.; Toaimah, F.H.; Almatar, D.H.; El Tatawy, L.A.; Davidson, B.L.; Qusad, M.I.M. Monoclonal Antibody Treatment of RSV Bronchiolitis in Young Infants: A Randomized Trial. Pediatrics 2019, 143. [Google Scholar] [CrossRef]
- Belkaid, Y.; Rouse, B.T. Natural regulatory T cells in infectious disease. Nat. Immunol. 2005, 6, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, B.J.; Cui, W.; Amezquita, R.A.; Gray, S.M.; Guan, T.; Lu, Y.; Kobayashi, Y.; Flavell, R.A.; Kleinstein, S.H.; Craft, J.; et al. Production of IL-10 by CD4+ regulatory T cells during the resolution of infection promotes the maturation of memory CD8+ T cells. Nat. Immunol. 2015, 16, 871–879. [Google Scholar] [CrossRef]
- Suvas, S.; Azkur, A.K.; Kim, B.S.; Kumaraguru, U.; Rouse, B.T. CD4+CD25+ Regulatory T Cells Control the Severity of Viral Immunoinflammatory Lesions. J. Immunol. 2004, 172, 4123–4132. [Google Scholar] [CrossRef] [PubMed]
- Karkhah, A.; Javanian, M.; Ebrahimpour, S. The role of regulatory T cells in immunopathogenesis and immunotherapy of viral infections. Infect. Genet. Evol. 2018, 59, 32–37. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Veiga-Parga, T.; Rajasagi, N.K.; Reddy, P.B.; Sehrawat, S.; Sharma, S.; Rouse, B.T. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J. Immunol. 2011, 187, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.L.; Wang, C.T.; Yang, S.J.; Leu, C.H.; Chen, S.H.; Wu, C.L.; Shiau, A.L. IL-6 ameliorates acute lung injury in influenza virus infection. Sci. Rep. 2017, 7, 43829. [Google Scholar] [CrossRef] [PubMed]
- Loebbermann, J.; Schnoeller, C.; Thornton, H.; Durant, L.; Sweeney, N.P.; Schuijs, M.; O’Garra, A.; Johansson, C.; Openshaw, P.J. IL-10 Regulates Viral Lung Immunopathology during Acute Respiratory Syncytial Virus Infection in Mice. PLoS ONE 2012, 7, e32371. [Google Scholar] [CrossRef] [PubMed]
- Im, S.J.; Hashimoto, M.; Gerner, M.Y.; Lee, J.; Kissick, H.T.; Burger, M.C.; Shan, Q.; Hale, J.S.; Lee, J.; Nasti, T.H.; et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 2016, 537, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.T.; Tsai, H.F.; Liao, H.J.; Lin, Y.J.; Chen, L.; Chen, P.J.; Hsu, P.N. PD-1 blockage reverses immune dysfunction and hepatitis B viral persistence in a mouse animal model. PLoS ONE 2012, 7, e39179. [Google Scholar] [CrossRef]
- Araki, K.; Turner, A.P.; Shaffer, V.O.; Gangappa, S.; Keller, S.A.; Bachmann, M.F.; Larsen, C.P.; Ahmed, R. mTOR regulates memory CD8 T-cell differentiation. Nature 2009, 460, 108–112. [Google Scholar] [CrossRef]
- Berber, E.; Rouse, B.T. Controlling viral inflammatory lesions by inhibiting fatty acid metabolism. Microbes Infect. 2023, 25, 105141. [Google Scholar] [CrossRef] [PubMed]
- Budd, A.; Alleva, L.; Alsharifi, M.; Koskinen, A.; Smythe, V.; Müllbacher, A.; Wood, J.; Clark, I. Increased survival after gemfibrozil treatment of severe mouse influenza. Antimicrob. Agents Chemother. 2007, 51, 2965–2968. [Google Scholar] [CrossRef] [PubMed]
- Sumbria, D.; Berber, E.; Rouse, B.T. Supplementing the Diet with Sodium Propionate Suppresses the Severity of Viral Immuno-inflammatory Lesions. J. Virol. 2021, 95, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Olendzki, B.; Bucci, V.; Cawley, C.; Maserati, R.; McManus, M.; Olednzki, E.; Madziar, C.; Chiang, D.; Ward, D.V.; Pellish, R.; et al. Dietary manipulation of the gut microbiome in inflammatory bowel disease patients: Pilot study. Gut Microbes 2022, 14, 2046244. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c(-) Patrolling Monocyte Hematopoiesis and CD8(+) T Cell Metabolism. Immunity 2018, 48, 992–1005.e8. [Google Scholar] [CrossRef] [PubMed]
- Lanford, R.E.; Hildebrandt-Eriksen, E.S.; Petri, A.; Persson, R.; Lindow, M.; Munk, M.E.; Kauppinen, S.; Ørum, H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 2010, 327, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Bhela, S.; Mulik, S.; Gimenez, F.; Reddy, P.B.J.; Richardson, R.L.; Varanasi, S.K.; Jaggi, U.; Xu, J.; Lu, P.Y.; Rouse, B.T. Role of miR-155 in the Pathogenesis of Herpetic Stromal Keratitis. Am. J. Pathol. 2015, 185, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.R.; Dangi, T.; Palacio, N.; Sanchez, S.; Penaloza-MacMaster, P. Adoptive B cell therapy for chronic viral infection. Front. Immunol. 2022, 13, 908707. [Google Scholar] [CrossRef]
- Somers, E.C.; Eschenauer, G.A.; Troost, J.P.; Golob, J.L.; Gandhi, T.N.; Wang, L.; Zhou, N.; Petty, L.A.; Baang, J.H.; Dillman, N.O.; et al. Tocilizumab for Treatment of Mechanically Ventilated Patients With COVID-19. Clin. Infect. Dis. 2020, 73, e445–e454. [Google Scholar] [CrossRef]
- Della-Torre, E.; Campochiaro, C.; Cavalli, G.; Luca, G.D.; Napolitano, A.; Marca, S.L.; Boffini, N.; Prat, V.D.; Terlizzi, G.D.; Lanzillotta, M.; et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: An open-label cohort study. Ann. Rheum. Dis. 2020, 79, 1277–1285. [Google Scholar] [CrossRef]
- Huang, Z.; Chavda, V.P.; Vora, L.K.; Gajjar, N.; Apostolopoulos, V.; Shah, N.; Chen, Z.-S. 2-Deoxy-D-Glucose and its Derivatives for the COVID-19 Treatment: An Update. Front. Pharmacol. 2022, 13, 899633. [Google Scholar] [CrossRef]
- Bramante, C.T.; Beckman, K.B.; Mehta, T.; Karger, A.B.; Odde, D.J.; Tignanelli, C.J.; Buse, J.B.; Johnson, D.M.; Watson, R.H.B.; Daniel, J.J.; et al. Metformin reduces SARS-CoV-2 in a Phase 3 Randomized Placebo Controlled Clinical Trial. medRxiv 2023. preprint. [Google Scholar] [CrossRef]
- Daniels, L.B.; Ren, J.; Kumar, K.; Bui, Q.M.; Zhang, J.; Zhang, X.; Sawan, M.A.; Eisen, H.; Longhurst, C.A.; Messer, K. Relation of prior statin and anti-hypertensive use to severity of disease among patients hospitalized with COVID-19: Findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. PLoS ONE 2021, 16, e0254635. [Google Scholar] [CrossRef]
- Singla, A.; Harun, N.; Dilling, D.F.; Merchant, K.; McMahan, S.; Ingledue, R.; French, A.; Corral, J.A.; Korbee, L.; Kopras, E.J.; et al. Safety and efficacy of sirolimus in hospitalised patients with COVID-19 pneumonia. Respir. Investig. 2024, 62, 216–222. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef]
- Sumbria, D.; Berber, E.; Miller, L.; Rouse, B.T. Modulating glutamine metabolism to control viral immuno-inflammatory lesions. Cell. Immunol. 2021, 370, 104450. [Google Scholar] [CrossRef]
- Dupraz, L.; Magniez, A.; Rolhion, N.; Richard, M.L.; Da Costa, G.; Touch, S.; Mayeur, C.; Planchais, J.; Agus, A.; Danne, C.; et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 2021, 36, 109332. [Google Scholar] [CrossRef]
- Brown, E.M.; Kenny, D.J.; Xavier, R.J. Gut Microbiota Regulation of T Cells During Inflammation and Autoimmunity. Annu. Rev. Immunol. 2019, 37, 599–624. [Google Scholar] [CrossRef]
- Gambichler, T.; Reuther, J.; Scheel, C.H.; Becker, J.C. On the use of immune checkpoint inhibitors in patients with viral infections including COVID-19. J. ImmunoTherapy Cancer 2020, 8, e001145. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Intan, D.; Hananto, J.E.; Putri, C.; Kurniawan, A. Pre-admission glucagon-like peptide-1 receptor agonist (GLP-1RA) and mortality from coronavirus disease 2019 (Covid-19): A systematic review, meta-analysis, and meta-regression. Diabetes Res. Clin. Pract. 2021, 179, 109031. [Google Scholar] [CrossRef]
- Alharbi, S.H. Anti-inflammatory role of glucagon-like peptide 1 receptor agonists and its clinical implications. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222367. [Google Scholar] [CrossRef]
- Pang, J.; Feng, J.N.; Ling, W.; Jin, T. The anti-inflammatory feature of glucagon-like peptide-1 and its based diabetes drugs-Therapeutic potential exploration in lung injury. Acta Pharm. Sin. B 2022, 12, 4040–4055. [Google Scholar] [CrossRef]
| PRR | Viral PAMPs | Viruses | Refs. |
|---|---|---|---|
| TLR2 | Envelope proteins | HSV | [20] |
| TLR3 | dsRNA | HSV, MCMV, Rotavirus, Poliovirus | [21,22] |
| TLR4 | Fusion protein | RSV | [23] |
| TLR7/8 | ssRNA | RNA viruses | [24] |
| TLR9 | dsDNA | DNA viruses | [25] |
| MDA5/RIGI | RNA | RNA viruses | [26] |
| cGAS | cytosolic DNA | HSV, HIV-1 | [27,28] |
| NALP3 inflammasome | RNA, ion channels | RNA viruses HSV | [29,30] |
| AIM2 inflammasome | cytosolic DNA | MCMV | [31] |
| Strategies | In Vivo phenotype | Refs. |
|---|---|---|
| Macrophage directed | (i) Depletion of macrophages using clodronate liposomes affected viral disease outcome | [63] |
| (ii) Targeting proinflammatory macrophages and pyroptosis affected COVID-19 outcome in murine models | [64,65] | |
| (iii) Administration of drugs or select cytokines-induced anti-inflammatory M2 macrophages leading to attenuation of viral pathology | [39] | |
| Neutrophil directed approaches | (i) Neutrophil depletion using moAb attenuated HSV-1 induced ocular lesions | [66,67] |
| (ii) Disrupting neutrophil extracellular traps mitigated multiple organ injury in COVID-19 mouse model | [68] | |
| Cytokine directed approaches | Blockade of IL-6, IL-1b mitigated HSK lesion severity | [69,70] |
| Inhibition of IL-1, IL-6, IL-17 impacted COVID-19 disease | [71] | |
| Targeting of TNF-α attenuated dengue lesions | [72] | |
| Inhibition of IL-1β and TNF-α reduces influenza severity in mice | [73,74] | |
| Blockade of interferon beta-controlled chronic LCMV infection | [59] | |
| Interferon lambda administration controlled Zika virus in the female reproductive tract | [75] | |
| Chemokine blockade | Blockade of CCR2 CXCR3 was effective to mitigate influenza lesions | [71] |
| CCR5 inhibition conferred benefits in COVID-19 disease | [76] | |
| CCR5 blockade impacted CCR5 trophic HIV-1 levels in affected patients | [77] | |
| CCR5 blockade impacted dengue disease development | [78] | |
| Targeting Toll-like receptors/cytosolic viral sensors | Provision of TLR-2, TLR-3, TLR-4 agonists affected influenza disease in mice | [79,80,81] |
| TLR-5 agonist flagellin cured rotavirus infection in mice | [82] | |
| TLR-7 agonist for human warts induced by papillomavirus | [83] | |
| TLR-7 agonist mitigated HBV and HCV disease | [84,85,86] | |
| NLRP3 inhibition reduced COVID-19 disease severity in mice | [87] |
| In Vivo Model Systems | Example Approach | Refs. |
|---|---|---|
| Removing or blocking the products of proinflammatory T cells | IL-17R KO mice in HSV infection | [138] |
| IL-6 deficient mice infected with influenza | [139] | |
| Expanding the numbers and functions of regulatory cells and cytokines | Adoptive transfer of Treg cells in HSV-infected SCID mice | [136] |
| Immune suppressive function of IL-10 in RSV-infected mice | [140] | |
| Restoring lost protective cell function | Targeting exhausted T cells in LCMV | [141] |
| Blockade of PD-1 and PD-L1 interaction with moAb in mice with HBV persistence | [142] | |
| Exploiting differences in metabolic requirements of inflammatory and immunoprotective responses | Targeting mTOR in LCMV | [143] |
| Targeting glucose and fatty acid metabolism in HSV infection | [144] | |
| Activating PPAR-α with an agonist molecule in influenza-infected mice | [145] | |
| Changing nutritional environment during infection | Supplementing diet with short-chain fatty acid in HSV | [146] |
| Consumption of prebiotics in inflammatory bowel disease | [147] | |
| High fiber diet supplemented mice infected with influenza | [148] | |
| Supplementing diet with short-chain fatty acid in HSV | [146] | |
| Changing the expression of host molecules that impact on adaptive cell activities such as micro RNAs | Blocking miR122 with antagomir in HCV | [149] |
| Using miR-155 antagomirs in ocular HSV infection | [150] | |
| Adoptive transfer of cells that counter inflammatory reactants | Adoptive transfer of virus-specific B cells in LCMV infected model | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berber, E.; Mulik, S.; Rouse, B.T. Meeting the Challenge of Controlling Viral Immunopathology. Int. J. Mol. Sci. 2024, 25, 3935. https://doi.org/10.3390/ijms25073935
Berber E, Mulik S, Rouse BT. Meeting the Challenge of Controlling Viral Immunopathology. International Journal of Molecular Sciences. 2024; 25(7):3935. https://doi.org/10.3390/ijms25073935
Chicago/Turabian StyleBerber, Engin, Sachin Mulik, and Barry T. Rouse. 2024. "Meeting the Challenge of Controlling Viral Immunopathology" International Journal of Molecular Sciences 25, no. 7: 3935. https://doi.org/10.3390/ijms25073935
APA StyleBerber, E., Mulik, S., & Rouse, B. T. (2024). Meeting the Challenge of Controlling Viral Immunopathology. International Journal of Molecular Sciences, 25(7), 3935. https://doi.org/10.3390/ijms25073935








