Abstract
The variability in mortality in sepsis could be a consequence of genetic variability. The glucocorticoid system and the intermediate TSC22D3 gene product—glucocorticoid-induced leucine zipper—are clinically relevant in sepsis, which is why this study aimed to clarify whether TSC22D3 gene polymorphisms contribute to the variance in sepsis mortality. Blood samples for DNA extraction were obtained from 455 patients with a sepsis diagnosis according to the Sepsis-III criteria and from 73 control subjects. A SNP TaqMan assay was used to detect single-nucleotide polymorphisms (SNPs) in the TSC22D3 gene. Statistical and graphical analyses were performed using the SPSS Statistics and GraphPad Prism software. C-allele carriers of rs3747406 have a 2.07-fold higher mortality rate when the sequential organ failure assessment (SOFA) score is higher than eight. In a multivariate COX regression model, the SNP rs3747406 with a SOFA score ≥ 8 was found to be an independent risk factor for 30-day survival in sepsis. The HR was calculated to be 2.12, with a p-value of 0.011. The wild-type allele was present in four out of six SNPs in our cohort. The promoter of TSC22D3 was found to be highly conserved. However, we discovered that the C-allele of rs3747406 poses a risk for sepsis mortality for SOFA Scores higher than 6.
1. Introduction
Sepsis is a life-threatening syndrome associated with organ dysfunction caused by a misled immunologic response to an infectious agent [1]. In a clinical setting, organ dysfunction is indicated by an increase in the Sequential Organ Failure Assessment (SOFA) score of 2 points or more [1]. The first indication is often a significantly reduced general condition characterized by non-specific symptoms such as chills, hyperventilation, decreased vigilance, tachycardia, and hypotension [2]. Sepsis is, therefore, a serious illness and usually requires treatment in the intensive care unit [3]. In most cases, sepsis is triggered by a bacterial infection. Fungi, viruses, or parasites are less frequently responsible. The most common pathogens include E. coli, streptococci, staphylococci, pseudomonads, bacilli, enterococci, Enterobacter spp., Candida spp., and Klebsiella [4]. It is accompanied by an incidence of 189 hospital-treated adult cases per 100,000 person years and a mortality rate of 26.7% worldwide [5]. The mortality rate varies considerably between different patient groups due to a large number of known and unknown factors. These factors include health status, concomitant medical treatment, and the constitution of the human genome, which characterizes various physiological and biochemical systems [6,7]. In this sense, there is an urgent need to clarify genetic factors that may influence the clinical outcome of sepsis. Furthermore, evidence of the unexplored causalities for variation in sepsis mortality targets patient enrichment so that only a certain group of patients may benefit from a distinct therapy [8,9,10]. Patient enrichment can be generally defined as a design feature or strategy by which patients who meet eligibility criteria are allocated into different treatment arms or study cohorts. Genetic variants may be an important factor in narrowing down these study cohorts. An interesting candidate gene could be TSC22D3, which codes for GILZ—the glucocorticoid-induced leucine zipper. GILZ is an important mediator of the glucocorticoid system, which is physiologically and biochemically important in sepsis. Respectively, both GILZ and the glucocorticoid system are clinically relevant in sepsis and regulate the adaptive and innate axis of the immune system [11,12]. The glucocorticoid system can be therapeutically influenced by glucocorticoids, which are frequently prescribed in the treatment of sepsis with variable success [13,14,15]. Current guidelines recommend glucocorticoid treatment for patients with persistent shock who require vasopressors, as there is evidence of faster shock reversal and reduced vasopressor dependency. A recent meta-analysis suggests a potential benefit of glucocorticoid treatment in a subset of patients with septic shock who are severely ill and have a pulmonary infection, but further research is needed to validate these findings and better understand the differences within sepsis patients [16]. The GILZ, as a crucial mediator of the glucocorticoid system, promotes the inhibitory effect on the pro-inflammation and stimulates the anti-inflammation as a blocker of pro-inflammatory cascades, such as the NF-kB and MAPK pathways [17,18,19]. Through these pathways, the GILZ affects cells of adaptive and innate immunity [20,21,22,23], which, in turn, influence the pathogenesis and progression of sepsis [24,25]. Hence, there is a clear indication that the GILZ might have a pivotal role in the development and clinical outcome of sepsis. The aim of this study was, therefore, to investigate the following hypotheses: (1) genetic variations in the TSC22D3 gene are detectable in a cohort of sepsis and control subjects, and (2) the genetic variants detected have an impact on the outcome of sepsis.
2. Results
2.1. Patients’ Characteristics
Samples from 455 patients diagnosed with sepsis and 73 control patients with abdominal surgery but without sepsis were analyzed. A total of 455 patients were genotyped for the assessed SNPs, and 40 patients were excluded from survival analysis due to missing values for 30-day mortality. The majority of septic patients were male (64.9%), the median SOFA Score was 8.5, the 30-day mortality was 30.1%, and the focus of infection was on the lower respiratory tract (Table 1).

Table 1.
Baseline characteristics of sepsis patients.
The majority of control subjects were male (52.1%), the median age was 64 years, and the median SOFA score was 3 (Table 2).

Table 2.
Baseline characteristics of control patients.
2.2. Polymorphism Analysis in the Cohort
We tested our first hypothesis and searched for six single-nucleotide polymorphisms (SNPs) in the GILZ gene sequence in the genome of septic patients and in the genome of our control subjects. Although they are the most frequent and have a reasonable global allele frequency, four of the six SNPs (rs3924026, rs4300127, rs73525022, and rs4342758) had only the wild-type allele present in the cohort. Only two SNPs (rs3747406 and rs17254207) had both alleles present in the analyzed individuals (Table 3). There was no difference between sepsis and control patients regarding the allele frequencies of both alleles of the tested polymorphisms (p = n.s.).

Table 3.
Single-nucleotide polymorphisms (SNPs) were examined, including SNP type, global allele frequency, and the total number of subjects with the corresponding genotype.
2.3. Impact of Polymorphisms on Sepsis Survival
To assess the effect of the genotype on survival, we focused only on the SNPs where the minor allele was present in the patient cohort studied: rs17254207 and rs3747406. We investigated whether the polymorphisms correlated with sepsis survival. The rs17254207 polymorphism showed no correlation with sepsis survival (r = 0.013; p = 0.838). In contrast, the rs3747406 polymorphism showed a correlation with the 30-day sepsis mortality in critically ill sepsis patients. The Kaplan–Meyer analysis showed that the negative impact of the C-allele on survival increases with a rising SOFA score (Figure 1a–c). C-allele carriers with a SOFA score ≥ 6 have a 1.90-fold higher mortality rate (Figure 1b), whereas a SOFA score ≥ 8 leads to a 2.03-fold higher mortality rate within the C-allele carriers (Figure 1c).
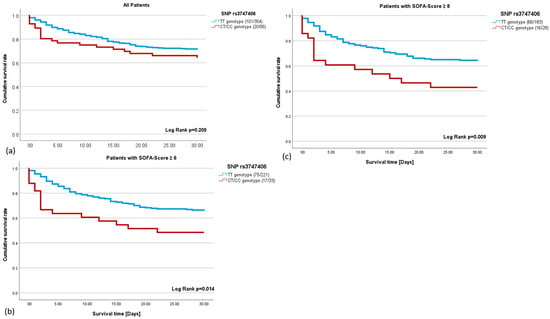
Figure 1.
(a–c): The 30-day survival rate in sepsis using the Kaplan–Meier analysis. (a). The survival curve of the T-allele carriers (blue) and C-allele carriers (red) for the single nucleotide polymorphism (SNP) rs3747406 (HR = 1.36; p = 0.215). (b): Septic patients with a sequential organ failure assessment (SOFA) score ≥ 6 and T-allele (blue), and septic patients with a SOFA score ≥ 6 and C-allele (red) for the SNP rs3747406 (HR = 1.90; p = 0.017). (c): Septic patients with a SOFA score ≥ 8 and T-allele (blue), and septic patients with a SOFA score ≥ 8 and C-allele (red) for the SNP rs3747406 (HR = 2.027; p = 0.011).
Univariate and multivariate COX regression was performed to analyze the impact of various factors on survival. Interestingly, the rs3747406 polymorphism was the strongest prognostic factor in a multivariate model, with a hazard ratio of 2.1 and independent of age and gender.
The rs3747406SNP with a SOFA score ≥ 8 was found to be an independent risk factor for 30-day mortality in sepsis in a multivariate Cox regression model (HR = 2.12, p = 0.0011, CI (1.193; 3.796)); (Table 4). The C-allele was an independent risk factor for 30-day mortality in sepsis in critically ill patients. C-allele carriers with a SOFA score ≥ 8 had a 2.12-fold higher mortality rate in sepsis.

Table 4.
Both univariate and multivariate Cox regression were conducted to analyze the rs3747406 SNP in a sample of 211 patients with a SOFA score ≥ 8.
2.4. The GILZ mRNA Expression Analysis in Septic Patients
To elucidate the possible underlying mechanism for the presence of the SNP rs3747406 C-allele, GILZ mRNA expression was analyzed depending on genotype. Interestingly, GILZ mRNA expression was decreased in TT genotypes (p = 0.0059; Figure 2) but increased in C-allele carriers over the time course of sepsis from day 1 to day 8 (p = 0.0093; Figure 2).
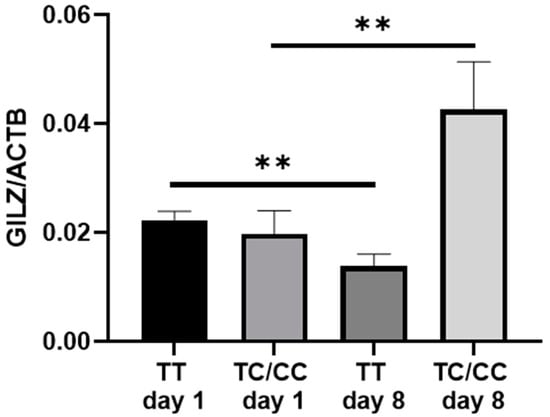
Figure 2.
Quantitative glucocorticoid-induced leucine zip (GILZ) mRNA expression in all rs3747406 SNP genotypes on day 1 and day 8 of sepsis diagnosis. The quantitative gene expression was normalized to the housekeeping gene ß-actin, computed using the 2−∆CT method. TT day 1 to day 8 n = 87; ** p = 0.0059; TC/CC day 1 to day 8 n = 13, ** p = 0.0093.
3. Discussion
This is the first study examining polymorphism in the TSC22D3 gene in septic patients. After database analysis, we investigated six polymorphisms within the TSC22D3 gene (NCBI.snp. and thermofisher.org, both accessed on 26 January 2024) [26,27]. The TSC22D3 gene sequence is highly conserved in a European cohort. The cohort analyzed here had only the wild-type allele in four out of six polymorphisms, and there were no differences in the allele distribution between the sepsis and the control group. Only rs3747406 and rs17254207 showed both alleles with low frequencies of the minor alleles.
In a further analysis, we examined whether these minor alleles might have an impact on sepsis survival. In fact, a correlation between rs3747406 and sepsis survival was demonstrated in sepsis patients with a SOFA score ≥ 6.
To date, little has been published about polymorphisms in the TSC22D3 gene, and our data suggest that the TSC22D3 gene is highly conserved in European populations. TSC22D3 belongs to the evolutionary conserved TSC-22 domain family, which is associated with the transforming growth factor-β 1 (TGF-β1)-stimulated clone 22 (TSC22) family, which compromises proteins with a leucine zipper domain and a TSC-box [28,29,30]. A limited amount of data is currently available on the function of this protein family, especially regarding the TSC-22 homologous gene-1 (THG-1)/TSC22 domain family member 4 (TSC22D4) [31]. The TSC-22 domain family includes four members: TSC22D1/TSC-22, TSC22D2/KIAA0669, TSC22D3/GILZ, and TSC22D4/THG-1. The best-characterized member of this protein family is TSC22D1, which possesses a tumor-suppressor function, and TGF-β1-stimulated clone 22 (TSC-22/TSC22D1) was first identified as a target gene of TGF-β1 in mouse osteosarcoma cells [32,33]. In addition, our studies indicated a low frequency of TSC-22 SNP alleles in the population [34]. Hence, the low frequency of mutant alleles in our analysis is consistent with published data.
We are able to show that the rs3747406 polymorphism influences sepsis survival in critically ill patients and GILZ expression over the time course of sepsis. Thus, the presence of this SNP rs3747406 in the genome can be considered as a possible explanation for the variance in sepsis survival. Although the exact mechanism of C-allele activity remains elusive, we have shown that C-allele carriers have a 2-fold higher GILZ mRNA expression on day 8 than on day 1 of sepsis diagnosis and a 3-fold higher expression than T-allele carriers on day 8. Hence, polymorphism appears to affect GILZ expression over the course of sepsis. In this context, it should be discussed that the effect of the polymorphism rs3747406 in a multivariate analysis depends on the SOFA score and, to a lesser extent, on age. The SNP rs3747406 is located near the 5′-untranslated region of the GILZ-encoding mRNA [35]. Previous studies have shown that different 5′-untranslated regions can lead to the expression of altered isoforms of the same mRNA [36,37,38]. Remarkably, it has been reported that both the leucine zipper and the C-terminus rather than the N-terminus of the GILZ amino acid sequence are essential for homodimerization and NF-kB arrest in the cytosol, respectively [39]. Therefore, it can be hypothesized that the C-allele of the rs3747406 may contribute to an impaired structure of the GILZ protein, which in turn may lead to inadequate regulation of the immune system via the GILZ, aggravating the immunopathology of sepsis [40]. Another explanation for the effect of the SNP could be that the polymorphism alters the binding of transcription factors [41,42]. Since patients with higher SOFA Score have an altered concentration of transcription factors (e.g., EZH2), an altered binding of transcription factors could explain the SOFA Score-dependent effect of the SNP [43].
A further explanation for both observations that C-allele carriers have a higher mortality rate depending on the SOFA Score and a higher GILZ expression is the finding that the GILZ is responsible for reduced responsiveness of monocytes to lipopolysaccharides [44]. A crucial influence of the GILZ is the observed inhibition of IFN-y production, which in turn is essential for the initiation of pro-inflammatory M1 macrophages [45,46]. Excessively reduced reactivity of monocytes and reduced differentiation to pro-inflammatory M1 macrophages may lead to reduced bacterial clearance in the initial phase of sepsis disease [47,48]. This may also explain why the effects of the C-allele correlate with the SOFA Score—the higher the risk of death, the higher the SOFA Score and the higher the need for a well-proportioned and synchronized host immune response to the infectious agent [49,50].
Our data address an important clinical question about the outcome of sepsis. One question that has not yet been adequately addressed is the reason for the variation in mortality rates in sepsis, which emphasizes the importance of further research [51,52]. Therefore, there is an urgent need to identify the factors responsible for the different mortality rates in sepsis. In order to find candidate genes that can shed light on the variability in sepsis mortality, this study focused on the TSC22D3 gene and its product GILZ as one of the key glucocorticoid mediators, which has been considered a promising protein for sepsis survival in mouse models [53,54]. This study focused on the investigation of polymorphisms in the GILZ gene, their presence in septic patients and control subjects, and their impact on the sepsis mortality rate. We chose surgical patients as a control group, as we aimed to include patients with comparable risk for sepsis development. If we had seen differences in the occurrence of one variant, either the sepsis or the control group, this variant could possibly make one group more prone to sepsis development. However, we did not see any differences in genotype distribution between the control group and septic patients. Hence, the anti-inflammatory reaction in sepsis patients could be altered, which impacts pathogenesis. The effect of the rs3747406 SNP was dependent on the SOFA Score but independent of age and gender [35,55]. The data presented show that different polymorphisms in the human genome are an essential component for understanding the different mortality rates in sepsis. For example, the deletion allele of the NFκB1 insertion-deletion (-94ins/delATTG) polymorphism has been associated with an increased 30-day mortality rate in septic patients [56]. As described above, the GILZ is a crucial mediator of the glucocorticoid system, which, in turn, is of clinical importance in sepsis [12,57]. Thus, the hypothesis that the rs3747406 polymorphism is a possible marker to discriminate sepsis patients who benefit from corticosteroid therapy needs to be tested in further studies. It can be speculated that the polymorphism can alter GILZ expression after glucocorticoid treatment. GILZ expression is susceptible to induction by glucocorticoids. Dexamethasone can increase GILZ transcripts more than tenfold in human RA synovial fibroblasts at concentrations as low as 1 nM. Furthermore, GILZ expression decreases when circulating cortisol is reduced in humans [58]. Hence, as the polymorphism is in a regulatory region of the TSC22D3 gene, it might alter responsiveness to glucocorticoids, e.g., via glucocorticoid receptor binding in septic patients. This question is further emphasized by the observations of Schäfer and colleagues that the presence of a specific NF-kB1 promoter polymorphism would lead to an association of hydrocortisone with a higher mortality rate in sepsis, prompting clinicians to consider patient groups with different mortality rates [59]. Our data and the specific factors previously identified could empower clinicians to differentiate patient groups with distinct mortality rates, essentially allowing precision medicine [8,60]. Individualized treatment will play a central role in medical guidelines, with increasing evidence that hydrocortisone is not suitable for every patient with sepsis. For example, Antcliffe and colleagues showed that two transcriptomic configurations can be distinguished in septic patients, one of which has an increased mortality rate when receiving corticosteroids [9]. In addition, König and colleagues showed that the serum IFNγ/IL10 ratio can be used to predict survival in patients receiving hydrocortisone [10]. There are also several biomarkers that may serve as potential parameters for predicting the efficacy of corticosteroids in sepsis. For example, Bentzer and colleagues demonstrated that corticosteroid treatment increases 28-day survival when plasma cytokine levels of CCL4, IL3, and IL6A are elevated and decreases when the levels of these biomarkers are low [61].
A limiting aspect for the interpretation of our results is the number of severely ill patients whose genome is analyzed for the rs3747406 polymorphism. Samples from patients of other ethnicities need to be analyzed for this genotype to determine decisively whether the C-allele is a marker for patient enrichment. We calculated the expected allele distribution for rs3747406 with the European allele frequency C = 0.17, which differs slightly from the global distribution of C = 0.22. Of note, the frequency of rs3747406 polymorphisms in the African population is C = 0.47; thus, further research is required to assess the impact of the polymorphism on sepsis mortality in ethnicities other than Europeans. In addition, further research should be conducted to determine how the mechanism of action affects the 30-day survival rate in sepsis when C-allele carriers of SNP rs3747406 have increased quantitative GILZ mRNA. For this reason, further studies are essential. Another limitation that has to be mentioned is the relatively high number of missing values in the baseline characteristics, which is due to the multicentric approach of our study and has to be optimized in future research.
4. Materials and Methods
4.1. Patient Recruitment
Enlistment of sepsis patients to the SepsisDataNet.NRW project and controls were approved by the Ethics Committee of the medical faculty of the Ruhr University of Bochum (No. 18-6606—BR) and by the Ethics Committees of the University of Münster (No. 2017-513-b-S) and the University of Bonn and accomplished if the criteria of the Sepsis-III definition were fulfilled by the sepsis patients [1]. Patients were recruited at the Knappschaftskrankenhaus Bochum GmbH (University of Bochum), St. Elisabeth Gruppe GmbH, and Herford (University of Bochum), as well as at the Clinics of Anesthesiology (University of Münster and University of Bonn). In the SepsisDataNet.NRW, peripheral blood was collected within the first 36 h after diagnosis of sepsis (day one), day four, and day eight after study inclusion. Informed consent was given by all patients or their representatives. Control subjects were defined as patients who had undergone surgery in the abdominal cavity and did not have sepsis according to the Sepsis-III definition [1].
4.2. Blood Sample Collection, Preparation, and Storage
Whole blood was collected from control subjects and patients who fulfilled the criteria of sepsis according to the Sepsis III definition [1]. The DNA-Exact tube (Sarstedt, Nümbrecht, Germany) and RNA Tube (Applied Biosystems, Life Technologies, Darmstadt, Germany) were used for DNA and RNA blood extraction, respectively. Total DNA and RNA were extracted from whole blood samples using the QIAamp and RNeasy kits, respectively, according to the manufacturer’s instructions (QIAGEN, Hilden, Germany). DNA and RNA sample aliquots were stored at −80 °C until further analysis.
4.3. SNP TaqMan Assay
In order to examine the SNPs rs3747406, rs3924026, rs4300127, rs73525022, rs4342758 and rs17254207 in samples from 455 sepsis patients and 73 control probands, SNP TaqMan assay (Thermo Fisher Scientific, Darmstadt, Germany) was utilized for standard qPCR (CFX Connect Real-Time System, Bio-Rad Labs, Hercules, CA, USA) using the TaqMan Genotyping Master Mix (Thermo Fisher Scientific, Darmstadt, Germany). The selection of the SNPs was based on a review of the NCBI database. A total of 24 µL of the final SNP reaction mix (consisting of 12.5 µL Master Mix, 1.25 µL SNP TaqMan Assay Primer, 10.25 µL nuclease-free water) and 1 µL of DNA (10 ng/µL) were dispensed in an optical reaction plate. RT-PCR amplification was performed under the following conditions: one denaturation step at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and at 60 °C for 1 min. Each genotype was assigned after the fluorescence emission of every sample was recorded at the VIC and FAM dye wavelengths.
4.4. GILZ mRNA Quantification
The RT-qPCR was performed in duplicate using the GoTaq qPCR Master Mix (Promega, Madison, WI, USA) and the subsequent primers on a CFX Connect Real-Time System (Bio-Rad Labs, Hercules, CA, USA). A total 25 µL reaction mix (consisting of 12.5 µL GoTaq qPCR Master Mix, 2.5 µL cDNA (10 ng/µL), 1 µL sense primer, 1 µL anti-sense primer, 8 µL nuclease-free water) was distributed in an optical reaction plate. RT-qPCR amplification was carried out under the following conditions: a primary denaturation step at 95 °C for 2 min, followed by 40 cycles at 95 °C for 30 s and at 60 °C for 30 s. The following primers were utilized: TSC22D3_mRNA—forward primer sequence 5′->3′ GTTAAGCTGGACAACAGTGCCT, reverse primer sequence 5′->3′ TTCTCCACCAGCTCTCGGAT (Eurofins Scientific SE, Hamburg, Germany). The relative GILZ mRNA expression was standardized to the housekeeping gene ß-actin, forward primer sequence 5′->3′ CCTTCCTGGGCATGGAGT, reverse primer sequence 5′->3′ CAGGGCAGTGATCTCCTTCT (Eurofins Scientific SE, Hamburg, Germany) and calculated using the 2−∆∆CT method.
4.5. Statistical Analyses
Characteristics of the individuals involved are described as numbers and percentages for categorical variables, means and standard deviations (±SD), or medians with assigned interquartile ranges for continuous variables. Kaplan–Meier survival curves were generated to evaluate the survival rate of the septic groups divided by the presence of the SNP rs3747406 genotype. The Log Rank test was used to determine the statistical significance of the survival effect observed. The hazard ratio was determined using univariate Cox Regression analysis. We evaluated the independence of the SNP rs3747406 on survival from the SOFA score, age, gender, and hydrocortisone administration using a multivariate Cox Regression analysis. Univariate and multivariate analyses were used to calculate hazard ratios with corresponding 95% confidence intervals to approximate the extent of associations between covariates and the time of death. The Hardy–Weinberg equilibrium was assessed for the polymorphism, and Chi quadrat values were calculated for the variation in allele distribution of each polymorphism between the sepsis and control groups. p values of less than 0.05 were considered statistically significant. All statistical analyses were performed using SPSS Statistics (Version 28.0.0.0, IBM, Armonk, NY, USA).
5. Conclusions
This study has first shown that the GILZ gene is highly conserved. However, this study identified the SNP rs3747406 in the TSC22D3 gene encoding the GILZ as a potential marker for predicting 30-day mortality in sepsis, depending on the SOFA Score. This SNP is, therefore, a conceivable factor in explaining the different mortality rates. Further studies are needed to verify whether the SNP rs3747406 can be used as a potential clinical indicator for the prediction of 30-day survival in sepsis and whether there is a survival effect of increased GILZ expression in terms of personalized medicine.
Author Contributions
Conceptualization: K.R. and M.A.; methodology: S.R. and P.T.; software: B.K.; validation: T.R., D.Z. and B.M.; formal analysis: S.R., K.R. and M.A.; investigation: B.K., M.U., K.R. and SepsisDataNet.NRW Research Group; resources: M.A. and SepsisDataNet.NRW Research Group data curation: H.N., B.E., U.L., E.S., D.H., S.F.E., L.B. and SepsisDataNet.NRW Research Group; writing—original draft preparation: S.R.; writing—review and editing: D.Z., L.B. and K.R.; visualization: K.R. and S.R.; supervision: K.R. and M.A.; project administration: K.R., B.K., M.A. and SepsisDataNet.NRW Research Group; funding acquisition: M.A., K.R. and B.K. and SepsisDataNet.NRW Research Group. All authors have read and agreed to the published version of the manuscript.
Funding
The SepsisDataNet.NRW (Grant EFRE-0800984) research group was funded by the European Regional Development Fund of the European Union.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study. This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Ruhr-Universität Bochum (No. 18-6606—BR) and by the Ethics Committees of the University of Münster (No. 2017-513-b-S) and the University of Bonn.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Karin Schork and Martin Eisenacher from the Core Unit Bioinformatics of the Medical Faculty of the Ruhr-University Bochum (CUBiMed.RUB) for their consulting regarding the statistical analysis. We acknowledge support by the Open Access Publication Funds of the Ruhr-Universität Bochum.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- He, Y.; Xu, J.; Shang, X.; Fang, X.; Gao, C.; Sun, D.; Yao, L.; Zhou, T.; Pan, S.; Zou, X.; et al. Clinical characteristics and risk factors associated with ICU-acquired infections in sepsis: A retrospective cohort study. Front. Cell Infect. Microbiol. 2022, 12, 962470. [Google Scholar] [CrossRef]
- Kuye, I.; Anand, V.; Klompas, M.; Chan, C.; Kadri, S.S.; Rhee, C. Prevalence and Clinical Characteristics of Patients With Sepsis Discharge Diagnosis Codes and Short Lengths of Stay in U.S. Hospitals. Crit. Care Explor. 2021, 3, e0373. [Google Scholar] [CrossRef]
- Fleischmann, C.; Thomas-Rueddel, D.O.; Hartmann, M.; Hartog, C.S.; Welte, T.; Heublein, S.; Dennler, U.; Reinhart, K. Hospital Incidence and Mortality Rates of Sepsis. Dtsch. Arztebl. Int. 2016, 113, 159–166. [Google Scholar]
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef]
- Fathi, M.; Markazi-Moghaddam, N.; Ramezankhani, A. A systematic review on risk factors associated with sepsis in patients admitted to intensive care units. Aust. Crit. Care 2019, 32, 155–164. [Google Scholar] [CrossRef]
- Konishi, M.; Mori, K.; Majima, T.; Ueda, K.; Teramoto, S.; Sakamoto, M.; Tsujimoto, M.; Maeda, K.; Mikasa, K.; Sawaki, M.; et al. Clinical analysis of patients with sepsis--comparison between underlying diseases. Kansenshogaku Zasshi 1998, 72, 681–687. [Google Scholar] [CrossRef][Green Version]
- Cohen, J.; Blumenthal, A.; Cuellar-Partida, G.; Evans, D.M.; Finfer, S.; Li, Q.; Ljungberg, J.; Myburgh, J.; Peach, E.; Powell, J.; et al. The relationship between adrenocortical candidate gene expression and clinical response to hydrocortisone in patients with septic shock. Intensive Care Med. 2021, 47, 974–983. [Google Scholar] [CrossRef]
- Antcliffe, D.B.; Burnham, K.L.; Al-Beidh, F.; Santhakumaran, S.; Brett, S.J.; Hinds, C.J.; Ashby, D.; Knight, J.C.; Gordon, A.C. Transcriptomic Signatures in Sepsis and a Differential Response to Steroids. From the VANISH Randomized Trial. Am. J. Respir. Crit. Care Med. 2018, 199, 980–986. [Google Scholar] [CrossRef]
- König, R.; Kolte, A.; Ahlers, O.; Oswald, M.; Krauss, V.; Roell, D.; Sommerfeld, O.; Dimopoulos, G.; Tsangaris, I.; Antoniadou, E.; et al. Use of IFNγ/IL10 Ratio for Stratification of Hydrocortisone Therapy in Patients With Septic Shock. Front. Immunol. 2021, 12, 607217. [Google Scholar] [CrossRef]
- Strehl, C.; Ehlers, L.; Gaber, T.; Buttgereit, F. Glucocorticoids-All-Rounders Tackling the Versatile Players of the Immune System. Front. Immunol. 2019, 10, 1744. [Google Scholar] [CrossRef] [PubMed]
- Shimba, A.; Ikuta, K. Control of immunity by glucocorticoids in health and disease. Semin. Immunopathol. 2020, 42, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Zen, M.; Canova, M.; Campana, C.; Bettio, S.; Nalotto, L.; Rampudda, M.; Ramonda, R.; Iaccarino, L.; Doria, A. The kaleidoscope of glucorticoid effects on immune system. Autoimmun. Rev. 2011, 10, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Bellissant, E.; Bollaert, P.E.; Briegel, J.; Keh, D.; Kupfer, Y. Corticosteroids for treating sepsis. Cochrane Database Syst. Rev. 2015, 2015, CD002243. [Google Scholar] [PubMed]
- Hibbert Kathryn, A. The Evolving Understanding of Glucocorticoid Treatment in Septic Shock. NEJM Evid. 2023, 2, EVIDe2300105. [Google Scholar] [PubMed]
- Bruscoli, S.; Riccardi, C.; Ronchetti, S. GILZ as a Regulator of Cell Fate and Inflammation. Cells 2021, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Berrebi, D.; Bruscoli, S.; Cohen, N.; Foussat, A.; Migliorati, G.; Bouchet-Delbos, L.; Maillot, M.-C.; Portier, A.; Couderc, J.; Galanaud, P.; et al. Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: An anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood 2003, 101, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Ayroldi, E.; Migliorati, G.; Bruscoli, S.; Marchetti, C.; Zollo, O.; Cannarile, L.; D’Adamio, F.; Riccardi, C. Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor kappaB. Blood 2001, 98, 743–753. [Google Scholar] [CrossRef]
- Bereshchenko, O.; Migliorati, G.; Bruscoli, S.; Riccardi, C. Glucocorticoid-Induced Leucine Zipper: A Novel Anti-inflammatory Molecule. Front. Pharmacol. 2019, 10, 308. [Google Scholar] [CrossRef]
- Cannarile, L.; Delfino, D.V.; Adorisio, S.; Riccardi, C.; Ayroldi, E. Implicating the Role of GILZ in Glucocorticoid Modulation of T-Cell Activation. Front. Immunol. 2019, 10, 1823. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Mouly, E.; Hamdi, H.; Maillot, M.-C.; Pallardy, M.; Godot, V.; Capel, F.; Balian, A.; Naveau, S.; Galanaud, P.; et al. GILZ expression in human dendritic cells redirects their maturation and prevents antigen-specific T lymphocyte response. Blood 2006, 107, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Hoppstädter, J.; Diesel, B.; Linnenberger, R.; Hachenthal, N.; Flamini, S.; Minet, M.; Leidinger, P.; Backes, C.; Grässer, F.; Meese, E.; et al. Amplified Host Defense by Toll-Like Receptor-Mediated Downregulation of the Glucocorticoid-Induced Leucine Zipper (GILZ) in Macrophages. Front. Immunol. 2018, 9, 3111. [Google Scholar] [CrossRef] [PubMed]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Home—SNP—NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/ (accessed on 26 January 2024).
- Thermo Fisher Scientific—DE. Available online: https://www.thermofisher.com/de/de/home.html (accessed on 26 January 2024).
- Goto, N.; Suzuki, H.; Zheng, L.; Okano, Y.; Okita, Y.; Watanabe, Y.; Kato, Y.; Kato, M. Promotion of squamous cell carcinoma tumorigenesis by oncogene-mediated THG-1/TSC22D4 phosphorylation. Cancer Sci. 2023, 114, 3972–3983. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Suzuki, H.; Nakajo, Y.; Nakano, A.; Kato, M. Regulation of c-MYC transcriptional activity by transforming growth factor-beta 1-stimulated clone 22. Cancer Sci. 2018, 109, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Pépin, A.; Espinasse, M.-A.; Latré de Laté, P.; Szely, N.; Pallardy, M.; Biola-Vidamment, A. TSC-22 Promotes Interleukin-2-Deprivation Induced Apoptosis in T-Lymphocytes. J. Cell Biochem. 2016, 117, 1855–1868. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.T.P.; Hipolito, C.J.; Suzuki, H.; Xie, R.; Kim Tuyen, H.D.; ten Terasaka, N.D.P.; Goto, Y.; Suga, H.; Kato, M. Generation of non-standard macrocyclic peptides specifically binding TSC-22 homologous gene-1. Biochem. Biophys. Res. Commun. 2019, 516, 445–450. [Google Scholar] [CrossRef]
- Kamimura, R.; Uchida, D.; Kanno, S.; Shiraishi, R.; Hyodo, T.; Sawatani, Y.; Shimura, M.; Hasegawa, T.; Tsubura-Okubo, M.; Yaguchi, E.; et al. Identification of Binding Proteins for TSC22D1 Family Proteins Using Mass Spectrometry. Int. J. Mol. Sci. 2021, 22, 10913. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, H.; Zheng, J.; Yu, P.; Xu, L.; Jiang, P.; Gao, J.; Wang, H.; Zhang, Y. Transforming Growth Factor β1 Signal is Crucial for Dedifferentiation of Cancer Cells to Cancer Stem Cells in Osteosarcoma. Stem Cells 2013, 31, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, F.; Yamada, Y.; Kuroe, A.; Someya, Y.; Kubota, A.; Ihara, Y.; Takahashi, K.; Seino, Y. Human TSC-22 Gene: No Association with Type 2 Diabetes. Intern. Med. 2001, 40, 993–997. [Google Scholar] [CrossRef] [PubMed][Green Version]
- NCBI.snp. Available online: https://www.ncbi.nlm.nih.gov/snp/rs3747406 (accessed on 11 September 2023).
- Hinnebusch, A.G.; Ivanov, I.P.; Sonenberg, N. Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 2016, 352, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Sedman, S.A.; Gelembiuk, G.W.; Mertz, J.E. Translation initiation at a downstream AUG occurs with increased efficiency when the upstream AUG is located very close to the 5’ cap. J. Virol. 1990, 64, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Ryczek, N.; Łyś, A.; Makałowska, I. The Functional Meaning of 5’UTR in Protein-Coding Genes. Int. J. Mol. Sci. 2023, 24, 2976. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, B.; Massetti, M.; Bruscoli, S.; Macchiarulo, A.; Di Virgilio, R.; Velardi, E.; Donato, V.; Migliorati, G.; Riccardi, C. Glucocorticoid-induced leucine zipper (GILZ)/NF-kappaB interaction: Role of GILZ homo-dimerization and C-terminal domain. Nucleic Acids Res. 2007, 35, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Ballegeer, M.; Vandewalle, J.; Eggermont, M.; van Isterdael, G.; Dejager, L.; de Decruyenaere, J.B.L.; Vandenbroucke, R.E.; Libert, C. Overexpression of Gilz Protects Mice Against Lethal Septic Peritonitis. Shock 2019, 52, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Rump, K.; Siffert, W.; Peters, J.; Adamzik, M. The Transcription Factor NMP4 Binds to the AQP5 Promoter and Is a Novel Transcriptional Regulator of the AQP5 Gene. DNA Cell Biol. 2016, 35, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Klenke, S.; Rump, K.; Buschkamp, K.; Engler, A.; Peters, J.; Siffert, W.; Frey, U.H. Characterization of the PLCB1 promoter and regulation by early growth response transcription factor EGR-1. Eur. J. Pharmacol. 2014, 742, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, Z.; Liu, X.; Liu, N.; Bao, X.; Sun, H.; Meng, Q.; Ren, H.; Bai, J.; Zhou, X.; et al. Lymphocyte expression of EZH2 is associated with mortality and secondary infectious complications in sepsis. Int. Immunopharmacol. 2020, 89, 107042. [Google Scholar] [CrossRef]
- Hamdi, H.; Bigorgne, A.; Naveau, S.; Balian, A.; Bouchet-Delbos, L.; Cassard-Doulcier, A.-M.; Maillot, M.-C.; Durand-Gasselin, I.; Prévot, S.; Delaveaucoupet, J.; et al. Glucocorticoid-induced leucine zipper: A key protein in the sensitization of monocytes to lipopolysaccharide in alcoholic hepatitis. Hepatology 2007, 46, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Bruscoli, S.; Sorcini, D.; Flamini, S.; Gagliardi, A.; Adamo, F.; Ronchetti, S.; Migliorati, G.; Bereshchenko, O.; Riccardi, C. Glucocorticoid-Induced Leucine Zipper Inhibits Interferon-Gamma Production in B Cells and Suppresses Colitis in Mice. Front. Immunol. 2018, 9, 1720. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Löms Ziegler-Heitbrock, H.W.; Frankenberger, M.; Wedel, A. Tolerance to Lipopolysaccharide in Human Blood Monocytes. Immunobiology 1995, 193, 217–223. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Fu, M.; Xin, H.-B. Polarizing Macrophages In Vitro. Methods Mol. Biol. 2018, 1784, 119–126. [Google Scholar] [PubMed]
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Mélot, C.; Vincent, J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001, 286, 1754–1758. [Google Scholar] [CrossRef] [PubMed]
- Berkestedt, I.; Herwald, H.; Ljunggren, L.; Nelson, A.; Bodelsson, M. Elevated plasma levels of antimicrobial polypeptides in patients with severe sepsis. J. Innate Immun. 2010, 2, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef]
- Ellouze, M.; Vigouroux, L.; Tcherakian, C.; Woerther, P.-L.; Guguin, A.; Robert, O.; Surenaud, M.; Tran, T.; Calmette, J.; Barbin, T.; et al. Overexpression of GILZ in macrophages limits systemic inflammation while increasing bacterial clearance in sepsis in mice. Eur. J. Immunol. 2020, 50, 589–602. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, W.; Shi, X.-M. Glucocorticoid-induced leucine zipper (GILZ) mediates glucocorticoid action and inhibits inflammatory cytokine-induced COX-2 expression. J. Cell Biochem. 2008, 103, 1760–1771. [Google Scholar] [CrossRef]
- Asselin-Labat, M.-L.; David, M.; Biola-Vidamment, A.; Lecoeuche, D.; Zennaro, M.-C.; Bertoglio, J.; Pallardy, M. GILZ, a new target for the transcription factor FoxO3, protects T lymphocytes from interleukin-2 withdrawal–induced apoptosis. Blood 2004, 104, 215–223. [Google Scholar] [CrossRef]
- Adamzik, M.; Schäfer, S.; Frey, U.H.; Becker, A.; Kreuzer, M.; Winning, S.; Frede, S.; Steinmann, J.; Fandrey, J.; Zacharowski, K.; et al. The NFKB1 Promoter Polymorphism (−94ins/delATTG) Alters Nuclear Translocation of NF-κB1 in Monocytes after Lipopolysaccharide Stimulation and Is Associated with Increased Mortality in Sepsis. Anesthesiology 2013, 118, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, J.; Libert, C. GILZ in sepsis: “Poor is the pupil who does not surpass his master”. Eur. J. Immunol. 2020, 50, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Aliska, G.; Nafrialdi, N.; Lie, K.C.; Setiabudy, R.; Putra, A.E.; Widyahening, I.S.; Harahap, A.R. The role of the glucocorticoid receptor and its impact on steroid response in moderate-severe COVID-19 patients. Eur. J. Pharmacol. 2023, 943, 175555. [Google Scholar] [CrossRef]
- Schäfer, S.T.; Gessner, S.; Scherag, A.; Rump, K.; Frey, U.H.; Siffert, W.; Westendorf, A.M.; Steinmann, J.; Peters, J.; Adamzik, M. Hydrocortisone fails to abolish NF-κB1 protein nuclear translocation in deletion allele carriers of the NFKB1 promoter polymorphism (-94ins/delATTG) and is associated with increased 30-day mortality in septic shock. PLoS ONE 2014, 9, e104953. [Google Scholar] [CrossRef]
- Wong, H.R.; Cvijanovich, N.Z.; Anas, N.; Allen, G.L.; Thomas, N.J.; Bigham, M.T.; Weiss, S.L.; Fitzgerald, J.; Checchia, P.A.; Meyer, K.; et al. Developing a Clinically Feasible Personalized Medicine Approach to Pediatric Septic Shock. Am. J. Respir. Crit. Care Med. 2014, 191, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Bentzer, P.; Fjell, C.; Walley, K.R.; Boyd, J.; Russell, J.A. Plasma cytokine levels predict response to corticosteroids in septic shock. Intensive Care Med. 2016, 42, 1970–1979. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).