Oxidative Stress in Sepsis: A Focus on Cardiac Pathology
Abstract
1. Introduction
2. Results
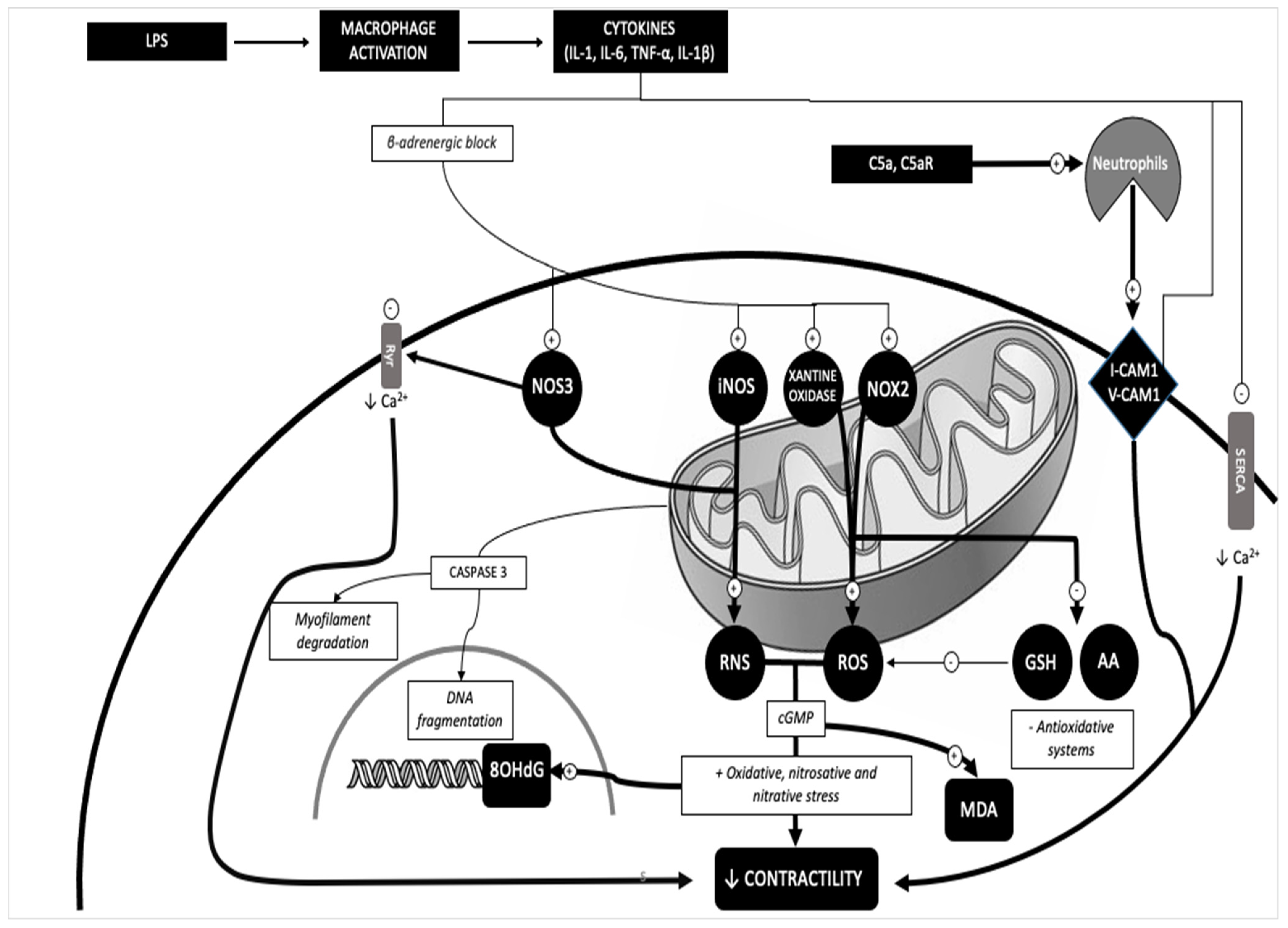
3. Discussion
4. Materials and Methods
4.1. Case Selection
4.2. IHC
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef]
- WHO. Current evidence, identifying gaps and future directions. In Global Report on the Epidemiology and Burden of Sepsis; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Guarino, M.; Perna, B.; Cesaro, A.E.; Maritati, M.; Spampinato, M.D.; Contini, C.; De Giorgio, R. 2023 Update on Sepsis and Septic Shock in Adult Patients: Management in the Emergency Department. J. Clin. Med. 2023, 12, 3188. [Google Scholar] [CrossRef]
- Chiu, C.; Legrand, M. Epidemiology of sepsis and septic shock. Curr. Opin. Anaesthesiol. 2021, 34, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Bosmann, M.; Ward, P.A. The Inflammatory Response in Sepsis. Trends Immunol. 2013, 34, 129. [Google Scholar] [CrossRef] [PubMed]
- Sprung, C.L.; Annane, D.; Keh, D.; Moreno, R.; Singer, M.; Freivogel, K.; Weiss, Y.G.; Benbenishty, J.; Kalenka, A.; Forst, H.; et al. Hydrocortisone Therapy for Patients with Septic Shock. N. Engl. J. Med. 2008, 358, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F. Oxidative stress and mitochondrial dysfunction in sepsis. Br. J. Anaesth. 2011, 107, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Andrades, M.T.; Morina, A.; Spasić, S.; Spasojević, I. Bench-to-bedside review: Sepsis—From the redox point of view. Crit. Care 2011, 15, 230. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Solé, C.; Pinilla-González, V.; Lillo-Moya, J.; González-Fernández, T.; Saso, L.; Rodrigo, R. Integrated approach to reducing polypharmacy in older people: Exploring the role of oxidative stress and antioxidant potential therapy. Redox Rep. 2024, 29, 2289740. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Lee, Y.; Kellum, J.A. A new perspective on NO pathway in sepsis and ADMA lowering as a potential ther-apeutic approach. Crit. Care. 2022, 26, 246. [Google Scholar] [CrossRef] [PubMed]
- Cerretani, D.; Bello, S.; Cantatore, S.; Fiaschi, A.I.; Montefrancesco, G.; Neri, M.; Pomara, C.; Riezzo, I.; Fiore, C.; Bonsignore, A.; et al. Acute administration of 3,4-methylenedioxymethamphetamine (MDMA) induces oxidative stress, lipoperoxidation and TNFα-mediated apoptosis in rat liver. Pharmacol. Res. 2011, 64, 517–527. [Google Scholar] [CrossRef]
- Sikora, J.P.; Karawani, J.; Sobczak, J. Neutrophils and the Systemic Inflammatory Response Syndrome (SIRS). Int. J. Mol. Sci. 2023, 24, 13469. [Google Scholar] [CrossRef]
- Karapetsa, M.; Pitsika, M.; Goutzourelas, N.; Stagos, D.; Tousia Becker, A.; Zakynthinos, E. Oxidative status in ICU patients with septic shock. Food Chem. Toxicol. 2013, 61, 106–111. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, P.; Gao, J.; Lin, Y.; Chen, L.; Shang, X. Sepsis-associated encephalopathy: From pathophysiology to clinical management. Int. Immunopharmacol. 2023, 124 Pt A, 110800. [Google Scholar] [CrossRef]
- Abelli, J.; Méndez-Valdés, G.; Gómez-Hevia, F.; Bragato, M.C.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Potential Antiox-idant Multitherapy against Complications Occurring in Sepsis. Biomedicines 2022, 10, 3088. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as bio-markers of tissue damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Nedeva, C. Inflammation and cell death of the innate and adaptive immune system during sepsis. Biomolecules 2021, 11, 1011. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Coelho Junior, H.J.; Marzetti, E. Cell death and inflammation: The role of mitochondria in health and disease. Cells 2021, 10, 537. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef]
- Aki, T.; Unuma, K.; Uemura, K. The Role of Peroxiredoxins in the Regulation of Sepsis. Antioxidants 2022, 11, 126. [Google Scholar] [CrossRef]
- Pravda, J. Hydrogen peroxide and disease: Towards a unified system of pathogenesis and therapeutics. Mol. Med. 2020, 26, 41. [Google Scholar] [CrossRef]
- Robinson, N.; Ganesan, R.; Hegedűs, C.; Kovács, K.; Kufer, T.A.; Virág, L. Programmed necrotic cell death of macro-phages: Focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019, 26, 101239. [Google Scholar] [CrossRef]
- Turillazzi, E.; Cerretani, D.; Cantatore, S.; Fiaschi, A.I.; Frati, P.; Micheli, L.; Neri, M.; Cipolloni, L.; Di Paolo, M.; Pinchi, E.; et al. Myocardial oxidative damage is induced by cardiac Fas-dependent and mitochondria-dependent apoptotic pathways in human cocaine-related overdose. Sci. Rep. 2017, 7, 44262. [Google Scholar] [CrossRef]
- Brown, G.C.; Borutaite, V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim. Biophys. Acta 2004, 1658, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Cassina, A.; Radi, R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electrontransport. Arch. Biochem. Biophys. 1996, 328, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Standiford, T.J.; Rahman, A.; Newstead, M.; Holland, S.M.; Dinauer, M.C.; Liu, Q.; Malik, A.B. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: Studies in p47phox-/- and gp91phox-/- mice. J. Immunol. 2002, 168, 3974–3982. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Barale, C.; Melchionda, E.; Penna, C.; Pagliaro, P. Platelets and Cardioprotection: The Role of Nitric Oxide and Carbon Oxide. Int. J. Mol. Sci. 2023, 24, 6107. [Google Scholar] [CrossRef]
- Pall, M.L. The NO/ONOO-cycle as the central cause of heart failure. Int. J. Mol. Sci. 2013, 14, 22274–22330. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, E.; Fiaccadori, E.; Donadello, K.; Taccone, F.S.; Franchi, F.; Scolletta, S. Myocardial depression in sepsis: From pathogenesis to clinical manifestations and treatment. J. Crit. Care. 2014, 29, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Landesberg, G.; Gilon, D.; Meroz, Y.; Georgieva, M.; Levin, P.D.; Goodman, S.; Avidan, A.; Beeri, R.; Weissman, C.; Jaffe, A.S.; et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur. Heart J. 2012, 33, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Zanotti-Cavazzoni, S.L.; Hollenberg, S.M. Cardiac dysfunction in severe sepsis and septic shock. Curr. Opin. Crit. Care 2009, 15, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Ozer, E.K.; Goktas, M.T.; Kilinc, I.; Toker, A.; Bariskaner, H.; Ugurluoglu, C.; Iskit, A.B. Infliximab alleviates the mortality, mesenteric hypoperfusion, aortic dysfunction, and multiple organ damage in septic rats. Can. J. Physiol. Pharmacol. 2017, 95, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Xianchu, L.; Lan, P.Z.; Qiufang, L.; Yi, L.; Xiangcheng, R.; Wenqi, H.; Yang, D. Naringin protects against lipopolysaccha-ride-induced cardiac injury in mice. Environ. Toxicol. Pharmacol. 2016, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Wang, B.; He, B.; Feng, M.; Yu, Y. LPS induces cardiomyocyte necroptosis through the Ripk3/Pgam5 signaling pathway. J. Recept. Signal Transduct. Res. 2021, 41, 32–37. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Misra, M.K.; Sarwat, M.; Bhakuni, P.; Tuteja, R.; Tuteja, N. Oxidative stress and ischemic myocardial syndromes. Med. Sci. Monit. 2009, 15, RA209–RA219. [Google Scholar]
- Gauthier, L.D.; Greenstin, J.L.; Cortassa, S.; O’Rourke, B.; Winslow, R.L. A computational model of reactive oxygen species and redox balance in cardiac mitochondria. Biophys. J. 2013, 105, 1045–1056. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagar, H.; Piao, S.; Kim, C.S. Role of mitochondrial oxidative stress in sepsis. Acute Crit. Care 2018, 33, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Huet, O.; Dupic, L.; Harrois, A.; Duranteau, J. Oxidative stress and endothelial dysfunction during sepsis. Front. Biosci. 2011, 16, 1986–1995. [Google Scholar] [CrossRef]
- Kayar, S.R.; Banchero, N. Volume density and distribution of mitochondria in myocardial growth and hypertrophy. Respir. Physiol. 1987, 70, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Beretta, M.; Santos, C.X.; Molenaar, C.; Hafstad, A.D.; Miller, C.C.; Revazian, A.; Betteridge, K.; Schröder, K.; Streckfuß Bömeke, K.; Doroshow, J.H. Nox4 regulates InsP3 receptor dependent Ca2+ release into mitochondria to promote cell survival. EMBO J. 2020, 39, e103530. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, J.; Kim, M.; Do, J.; Lee, D.; Han, H. Low dielectric constant polyimide aerogel composite films with low water uptake. Polym. J. 2016, 48, 829–834. [Google Scholar] [CrossRef]
- Weiss, J.N.; Korge, P.; Honda, H.M.; Ping, P. Role of the mitochondrial permeability transition in myocardial disease. Circ. Res. 2003, 93, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Larche, J.; Lancel, S.; Hassoun, S.M.; Favory, R.; Decoster, B.; Marchetti, P.; Chopin, C.; Neviere, R. Inhibition of mi-tochondrial permeability transition prevents sepsis induced myocardial dysfunction and mortality. J. Am. Coll. Cardiol. 2006, 48, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Tao, J.; Qiu, J.; Lu, H.; Wu, J.; Zhu, H.; Li, R.; Mui, D.; Toan, S.; Chang, X.; et al. DNA-PKcs promotes sepsis-induced multiple organ failure by triggering mitochondrial dysfunction. J. Adv. Res. 2022, 41, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ravingerová, T.; Kindernay, L.; Barteková, M.; Ferko, M.; Adameová, A.; Zohdi, V.; Bernátová, I.; Ferenczyová, K.; Lazou, A. The molecular mechanisms of iron metabolism and its role in cardiac dysfunction and cardioprotection. Int. J. Mol. Sci. 2020, 21, 7889. [Google Scholar] [CrossRef]
- Li, N.; Wang, W.; Zhou, H.; Wu, Q.; Duan, M.; Liu, C.; Wu, H.; Deng, W.; Shen, D.; Tang, Q. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic. Biol. Med. 2020, 160, 303–318. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2020, 31, 107–125. [Google Scholar] [CrossRef]
- Han, C.; Liu, Y.; Dai, R.; Ismail, N.; Su, W.; Li, B. Ferroptosis and its potential role in human diseases. Front. Pharmacol. 2020, 11, 239. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, B.; Fang, J.; Qin, T.; Dai, C.; Shuai, W.; Huang, H. Ferrostatin-1 alleviates lipopolysaccharide-induced cardiac dysfunction. Bioengineered 2021, 12, 9367–9376. [Google Scholar] [CrossRef]
- Liu, V.W.; Zhang, C.; Nagley, P. Mutations in mitochondrial DNA accumulate differentially in three different human tissues during ageing. Nucleic Acids Res. 1998, 26, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Hanke, T.; Erasmi, A.W.; Bechtel, M.J.; Scharfschwerdt, M.; Meissner, C.; Sievers, H.H.; Gosslau, A. Mitochondrial DNA deletions and the aging heart. Exp. Gerontol. 2006, 41, 508–517. [Google Scholar] [CrossRef]
- Barja, G.; Herrero, A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000, 14, 312–318. [Google Scholar] [CrossRef]
- Riezzo, I.; Cerretani, D.; Fiore, C.; Bello, S.; Centini, F.; D’Errico, S.; Fiaschi, A.I.; Giorgi, G.; Neri, M.; Pomara, C.; et al. Enzymatic-nonenzymatic cellular antioxidant defense systems response and immunohisto-chemical detection of MDMA, VMAT2, HSP70, and apoptosis as biomarkers for MDMA (Ecstasy) neurotoxicity. J Neurosci. Res. 2010, 88, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, V.; Makris, D.; Mantzarlis, K.; Zakynthinos, E. Sepsis-Induced Cardiomyopathy: Oxidative Implications in the Initiation and Resolution of the Damage. Oxid. Med. Cell. Longev. 2017, 2017, 7393525. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M.; Lambeth, J.D.; Nauseef, W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002, 397, 342–344. [Google Scholar] [CrossRef]
- Lambeth, J.D.; Cheng, D.; Arnold, R.S.; Edens, W.A. Novel homologs of gp91phox. Trends Biochem. Sci. 2000, 25, 459–461. [Google Scholar] [CrossRef]
- Hernansanz-Agustín, P.; Enríquez, J.A. Generation of Reactive Oxygen Species by Mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Lu, X.; Feng, Q. Pivotal Role of gp91phox-Containing NADH Oxidase in Lipopolysaccharide-Induced Tumor Necrosis Factor-α Expression and Myocardial Depression. Circulation 2005, 111, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Rongjin, H.; Feng, C.; Jun, K.; Shirong, L. Oxidative Stress-Induced Protein of SESTRIN2 in Cardioprotection Effect. Dis. Markers 2022, 2022, 7439878. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, T.; Kaiser, W.J.; Bertrand, M.J.; Vandenabeele, P. Molecular crosstalk between apoptosis, necroptosis, and survival signaling. Mol. Cell Oncol. 2015, 2, e975093. [Google Scholar] [CrossRef] [PubMed]
- Lafont, E.; Hartwig, T.; Walczak, H. Paving trail’s path with ubiquitin. Trends Biochem. Sci. 2018, 43, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Vucur, M.; Roderburg, C.; Kaiser, L.; Schneider, A.T.; Roy, S.; Loosen, S.H.; Luedde, M.; Trautwein, C.; Koch, A.; Tacke, F.; et al. Elevated serum levels of mixed lineage kinase domain like protein predict survival of patients during intensive care unit treatment. Dis. Markers 2018, 2018, 1983421. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Xu, J.; Ruan, W.; Li, S.; Xiao, F. PPAR γ activation prevents septic cardiac dysfunction via inhibition of apoptosis and necroptosis. Oxid. Med. Cell. Longev. 2017, 2017, 8326749. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, V.V.S.; Reis, J.F.; de Souza Gomes, R.; Navegantes, K.C.; Monteiro, M.C. Dual Behavior of Exosomes in Septic Cardiomyopathy. Adv. Exp. Med. Biol. 2017, 998, 101–112. [Google Scholar] [CrossRef]
- Maiese, A.; Frati, P.; Del Duca, F.; Santoro, P.; Manetti, A.C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. Myocardial Pathology in COVID-19-Associated Cardiac Injury: A Systematic Review. Diagnostics 2021, 11, 1647. [Google Scholar] [CrossRef]
- Maccio, U.; Zinkernagel, A.S.; Shambat, S.M.; Zeng, X.; Cathomas, G.; Ruschitzka, F.; Schuepbach, R.A.; Moch, H.; Varga, Z. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine 2021, 63, 103182. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Wendel Garcia, P.D.; Fumeaux, T.; Guerci, P.; Heuberger, D.M.; Montomoli, J.; Roche-Campo, F.; Schuepbach, R.A.; Hilty, M.P.; RISC-19-ICU Investigators. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine 2020, 25, 100449. [Google Scholar] [CrossRef]
- Wenzel, P.; Kopp, S.; Göbel, S.; Jansen, T.; Geyer, M.; Hahn, F.; Kreitner, K.F.; Escher, F.; Schultheiss, H.P.; Münzel, T. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc. Res. 2020, 116, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; O’brien, J.; Tollefsen, K.E.; Kim, Y.; Chauhan, V.; Yauk, C.; Huliganga, E.; Rudel, R.A.; Kay, J.E.; Helm, J.S.; et al. Reactive Oxygen Species in the Adverse Outcome Pathway Framework: Toward Creation of Harmonized Consensus Key Events. Front. Toxicol. 2022, 4, 887135. [Google Scholar] [CrossRef]
- Piccioni, A.; Franza, L.; Rosa, F.; Candelli, M.; Covino, M.; Ferrara, M.; Volonnino, G.; Bertozzi, G.; Zamponi, M.V.; Maiese, A.; et al. The role of SARS-CoV-2 infection in promoting abnormal immune response and sepsis: A comparison between SARS-CoV-2-related sepsis and sepsis from other causes. Infect. Med. 2023, 2, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, Y.; Fang, Y.; Wang, C.; Xue, Y.; Wang, F.; Cheng, J.; Ren, H.; Wang, J.; Guo, W.; et al. Pathogenetic mechanisms of septic cardiomyopathy. J. Cell. Physiol. 2022, 237, 49–58. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, Y.; Liu, W.; Liu, W.; Dong, J.; Liu, Q.; Hao, H.; Ren, H. Research progress on the activation mechanism of NLRP3 inflammasome in septic car-diomyopathy. Immun. Inflamm. Dis. 2023, 11, e1039. [Google Scholar] [CrossRef]
- Hollenberg, S.M.; Singer, M. Pathophysiology of sepsis-induced cardiomyopathy. Nat. Rev. Cardiol. 2021, 18, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Maiese, A.; Scatena, A.; Costantino, A.; Chiti, E.; Occhipinti, C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Frati, P.; Fineschi, V. Expression of MicroRNAs in Sepsis-Related Organ Dysfunction: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9354. [Google Scholar] [CrossRef] [PubMed]
- La Russa, R.; Maiese, A.; Viola, R.V.; De Matteis, A.; Pinchi, E.; Frati, P.; Fineschi, V. Searching for highly sensitive and specific biomarkers for sepsis: State-of-the-art in post-mortem diagnosis of sepsis through immunohistochemical analysis. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419855226. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.; Turatello, L.; De Salvia, A.; Neri, M.; Turillazzi, E.; La Russa, R.; Viola, R.V.; Frati, P.; Fineschi, V. Septic cardiomyopathy: The value of lactoferrin and CD15 as specific markers to corroborate a definitive diagnosis. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418776526. [Google Scholar] [CrossRef] [PubMed]
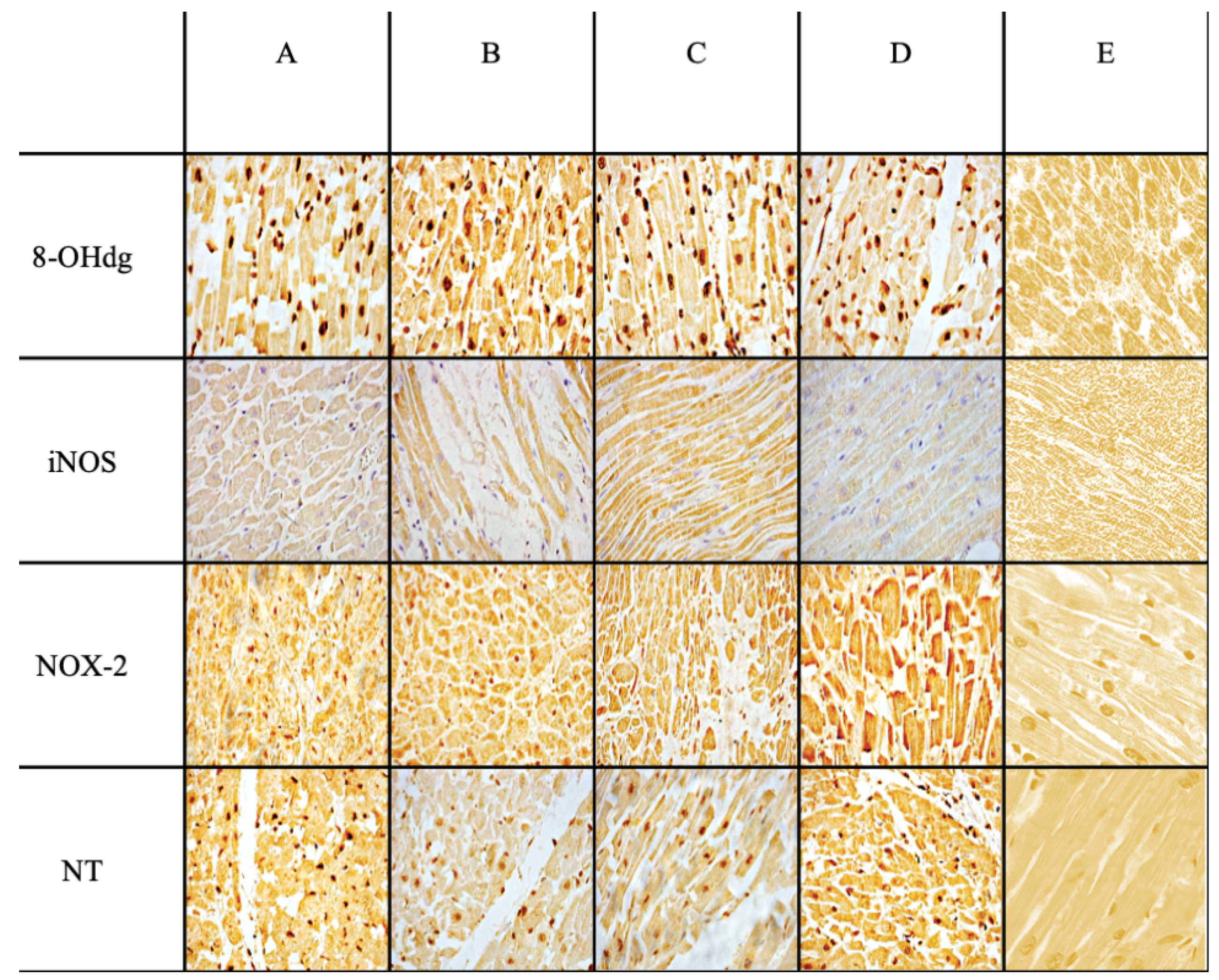
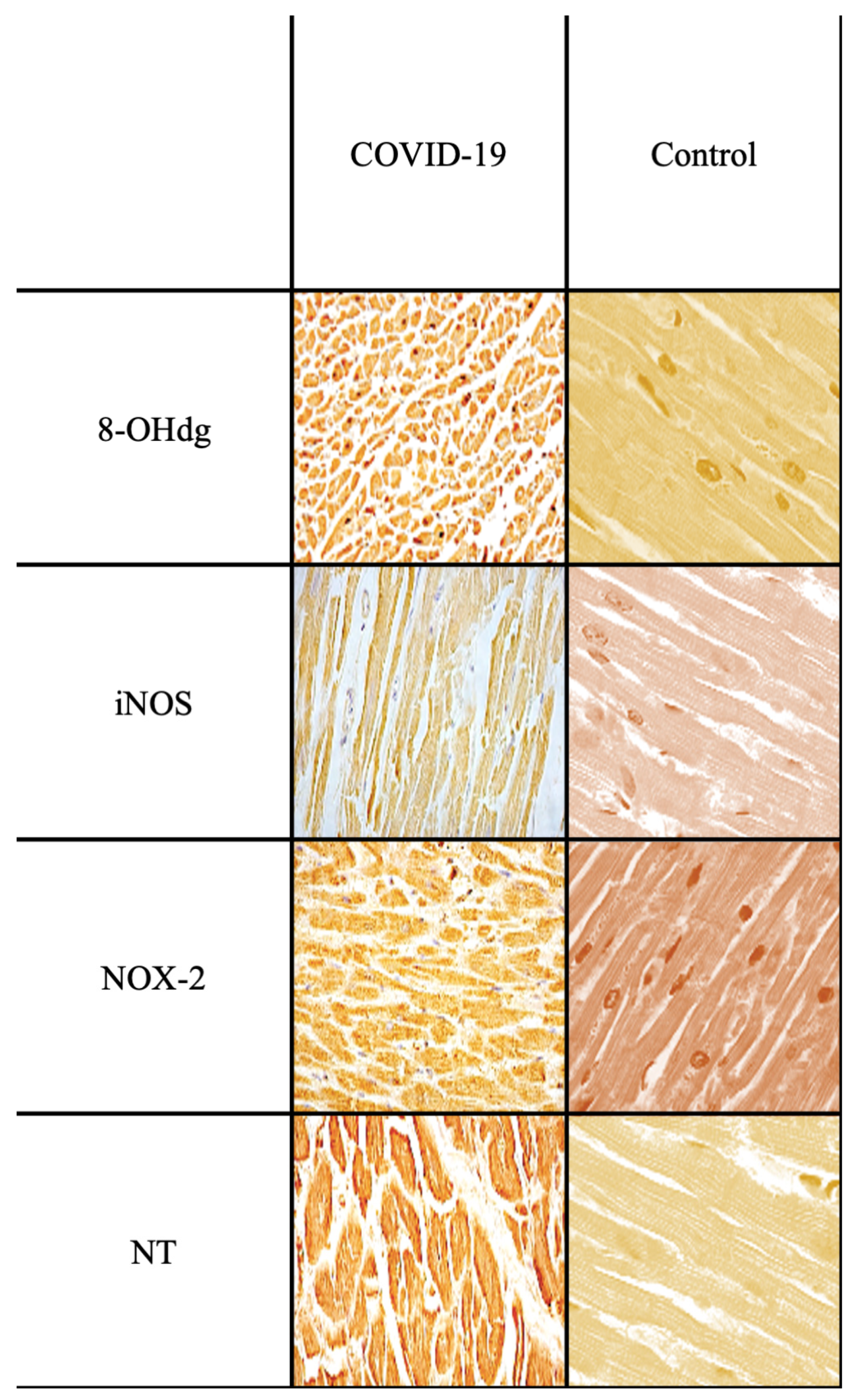
| Ab | Control Group | Sepsis Group | Statistical Value | Sepsis vs. Control |
|---|---|---|---|---|
| Anti-iNOS | +/− | +++ | *** | *** |
| Anti-NOX-2 | +/− | +++ | *** | *** |
| Anti-Nitrotyrosine | + | +++ | *** | *** |
| Anti-8-OHdG | +/− | +++ | *** | *** |
| Case | Sex, Age | Cause of Death Attributed |
|---|---|---|
| CASE 1 | F, 57 y.o. | Septic shock secondary to pneumonia |
| CASE 2 | M, 62 y.o. | Septic shock secondary to Fournier’s gangrene |
| CASE 3 | M, 68 y.o. | Septic shock secondary to peritonitis due to bowel perforation |
| CASE 4 | F, 75 y.o. | Septic shock secondary to peritonitis due to VAP |
| CASE 5 | M, 27 y.o. | Septic shock secondary to pneumonia |
| CASE 6 | M, 45 y.o. | Septic shock secondary to COVID-19 pneumonia |
| CASE 7 | M, 68 y.o. | Septic shock secondary to cholecystitis |
| CASE 8 | F, 85 y.o. | Septic shock secondary to pneumonia |
| CASE 9 | M, 62 y.o. | Septic shock secondary to bowel perforation |
| CASE 10 | F, 56 y.o. | Septic shock secondary to peritonitis due to enterocolitis |
| NC | M, 22 y.o | Head trauma from a shotgun |
| NC | F, 30 y.o. | Traffic accident resulting in the immediate death |
| NC | M, 44 y.o. | Traffic accident resulting in the immediate death |
| NOX-2 | gp91-phox, goat polyclonal antibody, sc-5826 | Santa Cruz Biotechnology, Inc., Dallas, TX, USA |
| NT | nitrotyrosine, mouse monoclonal antibody, sc-32757 | Santa Cruz Biotechnology, Inc., Dallas, TX, USA |
| iNOS | nitric oxide sinthases-2, mouse monoclonal antibody, sc-7271 | Santa Cruz Biotechnology, Inc., Dallas, TX, USA |
| 8-OHdG | 8-hidroxy-2′-deoxyguanosine, mouse monoclonal antibody, N45.1 | JaICA, Nikken SEIL Co., Fukuroi, Shizuoka, Japan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertozzi, G.; Ferrara, M.; Di Fazio, A.; Maiese, A.; Delogu, G.; Di Fazio, N.; Tortorella, V.; La Russa, R.; Fineschi, V. Oxidative Stress in Sepsis: A Focus on Cardiac Pathology. Int. J. Mol. Sci. 2024, 25, 2912. https://doi.org/10.3390/ijms25052912
Bertozzi G, Ferrara M, Di Fazio A, Maiese A, Delogu G, Di Fazio N, Tortorella V, La Russa R, Fineschi V. Oxidative Stress in Sepsis: A Focus on Cardiac Pathology. International Journal of Molecular Sciences. 2024; 25(5):2912. https://doi.org/10.3390/ijms25052912
Chicago/Turabian StyleBertozzi, Giuseppe, Michela Ferrara, Aldo Di Fazio, Aniello Maiese, Giuseppe Delogu, Nicola Di Fazio, Vittoria Tortorella, Raffaele La Russa, and Vittorio Fineschi. 2024. "Oxidative Stress in Sepsis: A Focus on Cardiac Pathology" International Journal of Molecular Sciences 25, no. 5: 2912. https://doi.org/10.3390/ijms25052912
APA StyleBertozzi, G., Ferrara, M., Di Fazio, A., Maiese, A., Delogu, G., Di Fazio, N., Tortorella, V., La Russa, R., & Fineschi, V. (2024). Oxidative Stress in Sepsis: A Focus on Cardiac Pathology. International Journal of Molecular Sciences, 25(5), 2912. https://doi.org/10.3390/ijms25052912







