The Heterogeneity of Post-Menopausal Disease Risk: Could the Basis for Why Only Subsets of Females Are Affected Be Due to a Reversible Epigenetic Modification System Associated with Puberty, Menstrual Cycles, Pregnancy and Lactation, and, Ultimately, Menopause?
Abstract
1. Purpose: Discuss the Potential Role of Variables Leading to Disease Risk in the Post-Menopausal State Having Important Roles during the Reproductive Stage of the Life Cycle, and Then Focus on Potential Mechanisms That Are Involved
2. Background/Introduction
3. Variables and Factors Influencing Post-Menopause Diseases and Conditions
4. Summary of Points Potentially Relevant to Post-Menopausal Conditions
- Not all women experience these conditions (i.e., osteoporosis, osteoarthritis, dementia, obesity, cardiovascular disease) after menopause, and those that do usually only experience a subset of them.
- For some of the conditions, such as OP and others, there appears to be a genetic component to disease risk, as it “runs in families”.
- Most if not all of the post-menopausal conditions can be linked directly or indirectly to reproductive activities such as pregnancy and lactation.
- Early menopause and elevated parity contribute to OP risk and low bone density, respectively. Early menopause is also linked to other post-menopausal conditions [77].
- Inflammation appears to be a “catalyst” for post-menopausal disease progression but possibly not an inducer of the conditions.
- AA.
- Epigenetic modifications of the genome via direct methylation or indirectly via modification of histones, as well as modification of RNA can occur under a variety of circumstances (discussed in [78,79,80,81,82]). Many of these alterations are reversible modifications that allow for modulation and regulation of gene expression and function. Several of such alterations have been implicated in diseases or conditions relevant to menopause, such as osteoporosis and calcium signaling [83,84,85], cardiovascular disease [86,87], osteoarthritis [88,89,90], the nervous system and the brain [91,92,93,94], obesity [95,96], and vascular inflammation [97].
- BB.
- CC.
- Epigenetic modifications occur with the onset of menopause [1,100] and in those with post-menopausal conditions such as OP (discussed in [1,11]), cardiovascular conditions [101], and others [98,102]. However, in many of these conditions, their relationship to disease induction versus arising as a consequence of the diseases/conditions is not known.
- DD.
- Treatment of individuals undergoing gender transitioning with hormones such as estrogen leads to epigenetic modification of the genome of blood cells [103].
- EE.
- FF.
- GG.
- HH.
- Pregnancy is associated with a downregulation of inflammation in a majority (~70%) of those with inflammatory autoimmune diseases such as rheumatoid arthritis, and disease activity is influenced by menstrual cycle variations in hormone levels [74].
- II.
- Pregnancy is associated with cognitive changes [104].
- JJ.
5. Involvement of Epigenetic Modification across the Lifespan of Females
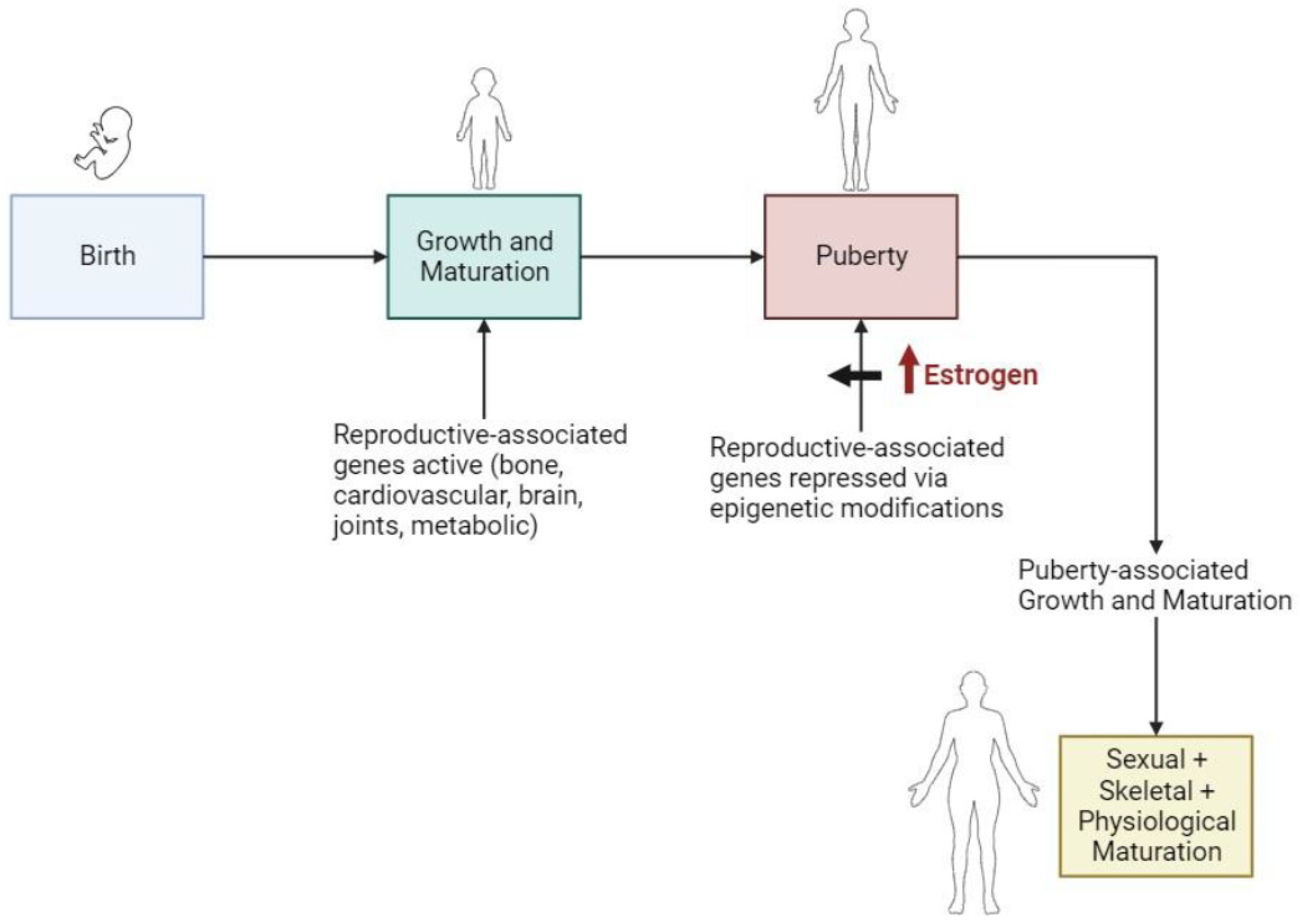
5.1. During Puberty and Gender Transitioning
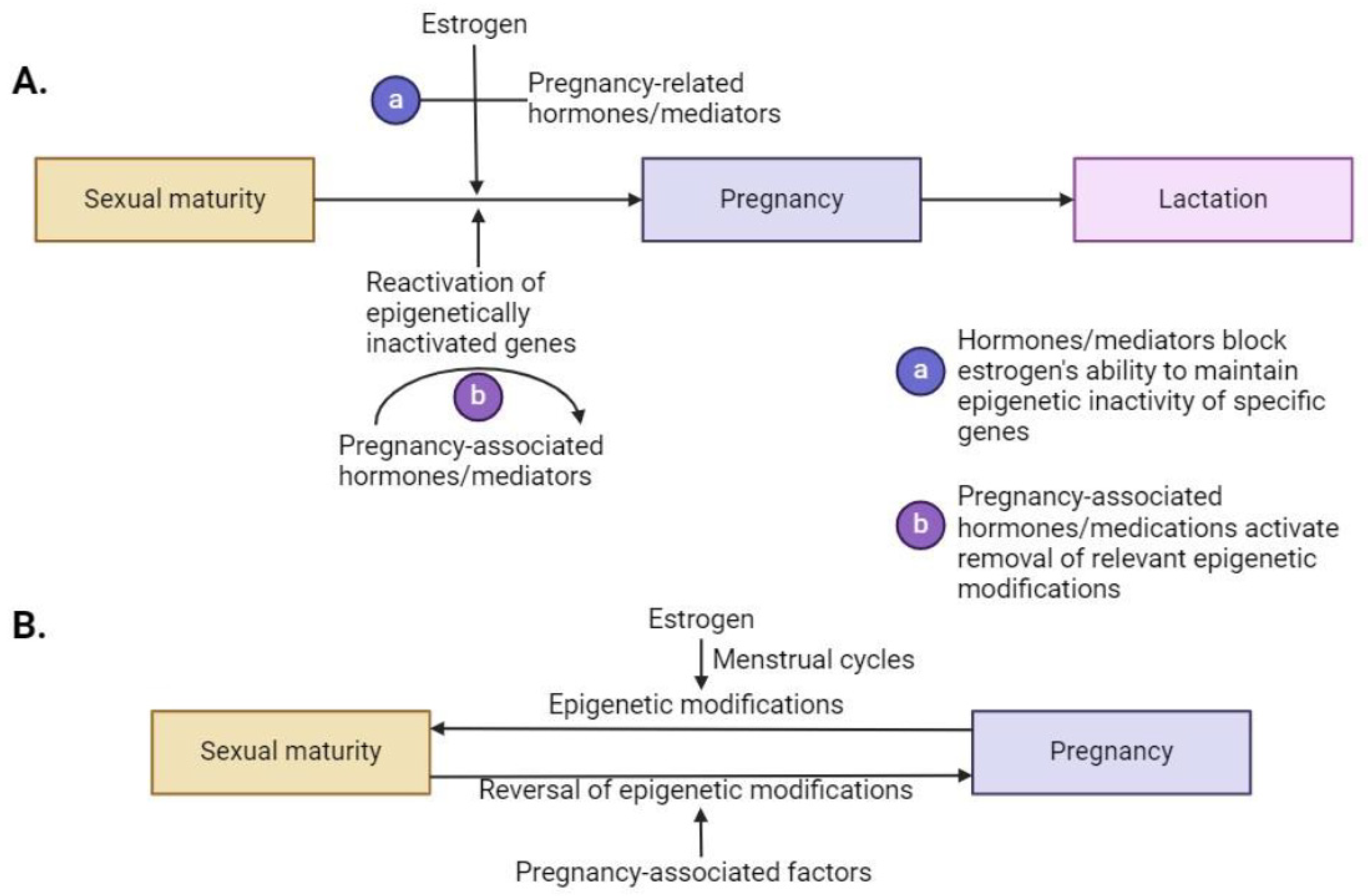
5.2. During Pregnancy and Lactation
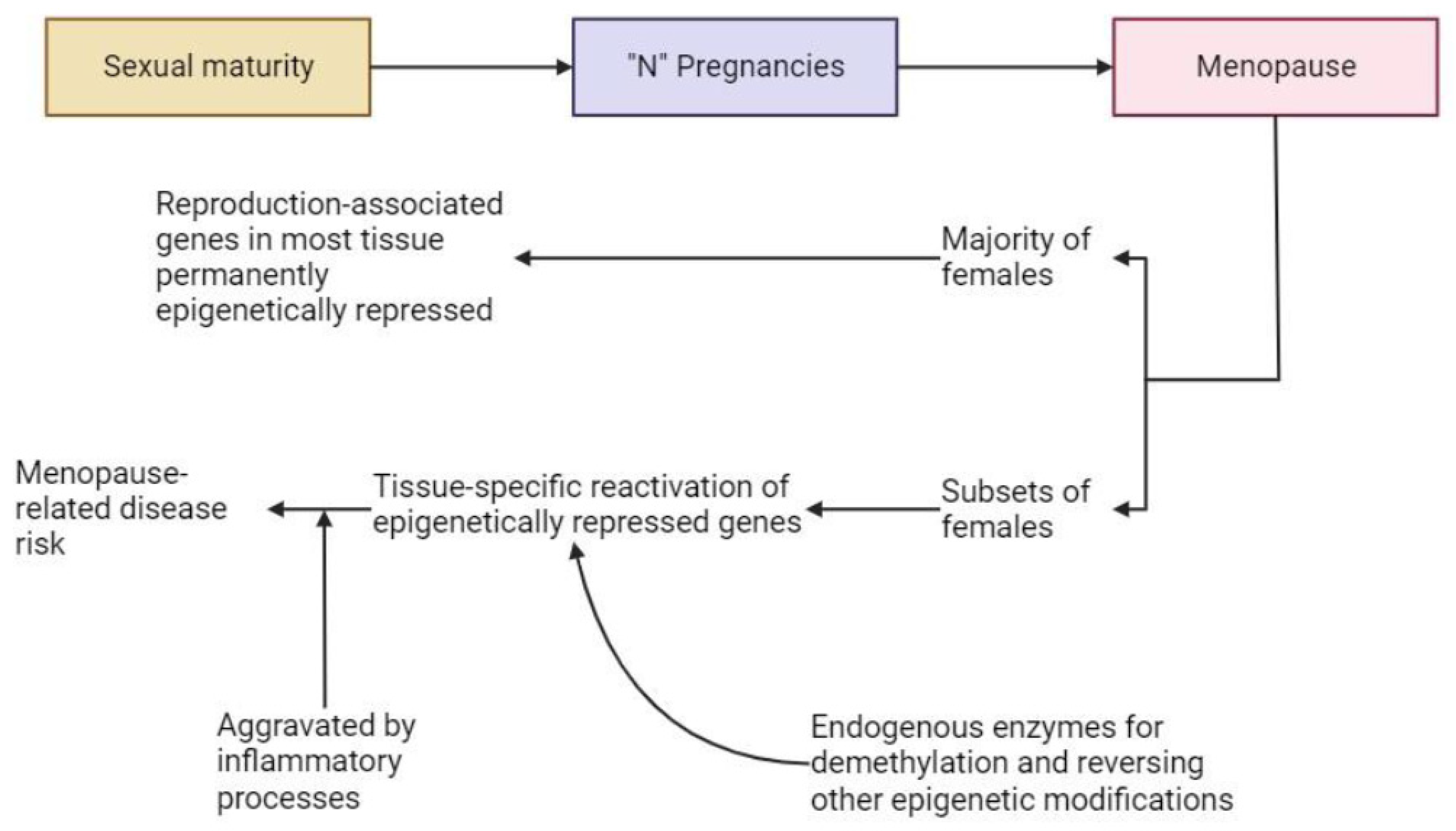
5.3. During Menopause and during Post-Menopausal Interventions
6. The Epigenetic Modification/Demodification/Remodification Hypothesis to Address the Development of Post-Menopausal Diseases and Disease Risk
7. What Cells May Be Involved in the Relevant Epigenetic Modifications Outlined in Section 6?
8. Conclusions and the Way Forward
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hart, D.A. Sex Differences in Biological Systems and the Conundrum of Menopause: Potential Commonalities in Post-Menopausal Disease Mechanisms. Int. J. Mol. Sci. 2022, 23, 4119. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A. Sex differences in musculoskeletal injury and disease risks across the lifespan: Are there unique subsets of females at higher risk than males for these conditions at distinct stages of the life cycle? Front. Physiol. 2023, 14, 1127689. [Google Scholar] [CrossRef] [PubMed]
- Harrod, C.G.; Bendok, B.R.; Batjer, H.H. Interactions between melatonin and estrogen may regulate cerebrovascular function in women: Clinical implications for the effective use of HRT during menopause and aging. Med. Hypotheses 2005, 64, 725–735. [Google Scholar] [CrossRef]
- Gannon, O.; Robison, L.; Custozzo, A.; Zuloaga, K. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem. Int. 2018, 127, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.U.; Masson, E.A. Neuroendocrinology of female aging. Gend. Med. 2005, 2, 41–56. [Google Scholar] [CrossRef]
- Yoo, J.E.; Shin, D.W.; Han, K.; Kim, D.; Won, H.; Lee, J.; Kim, S.; Nam, G.E.; Park, H.S. Female reproductive factors and the risk of dementia: A nationwide cohort study. Eur. J. Neurol. 2020, 27, 1448–1458. [Google Scholar] [CrossRef]
- Gannon, O.J.; Naik, J.S.; Riccio, D.; Mansour, F.M.; Abi-Ghanem, C.; Salinero, A.E.; Kelly, R.D.; Brooks, H.L.; Zuloaga, K.L. Menopause causes metabolic and cognitive impairments in a chronic cerebral hypoperfusion model of vascular contributions to cognitive impairment and dementia. Biol. Sex Differ. 2023, 14, 34. [Google Scholar] [CrossRef]
- Hogervorst, E.; Temple, S.; O’Donnell, E. Sex differences in dementia. Curr. Top. Behav. Neurosci. 2023, 62, 309–331. [Google Scholar] [CrossRef]
- Szoeke, C.; Downie, S.; Parker, A.; Phillips, S. Sex hormones, vascular factors and cognition. Front. Neuroendocr. 2021, 62, 100927. [Google Scholar] [CrossRef]
- Hart, D.A. Osteoarthritis as an Umbrella Term for Different Subsets of Humans Undergoing Joint Degeneration: The Need to Address the Differences to Develop Effective Conservative Treatments and Prevention Strategies. Int. J. Mol. Sci. 2022, 23, 15365. [Google Scholar] [CrossRef]
- Hart, D.A. Regulation of Bone by Mechanical Loading, Sex Hormones, and Nerves: Integration of Such Regulatory Complexity and Implications for Bone Loss during Space Flight and Post-Menopausal Osteoporosis. Biomolecules 2023, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.; Smart, C.; Goldschmidt, L.; Price, V.; Scott, J. Fat mass, weight and body shape changes at menopause—Causes and consequences: A narrative review. Climacteric 2023, 26, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Speth, R.C.; D’ambra, M.; Ji, H.; Sandberg, K. A heartfelt message, estrogen replacement therapy: Use it or lose it. Am. J. Physiol. Circ. Physiol. 2018, 315, H1765–H1778. [Google Scholar] [CrossRef]
- Kondapalli, A.V.; Kamanda-Kosseh, M.; Williams, J.M.; Shiau, S.; Bucovsky, M.; Colon, I.; Shane, E.; Cohen, A. Clinical characteristics of pregnancy and lactation associated osteoporosis: An online survey study. Osteoporos. Int. 2023, 34, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Samelson, E.J.; Hannan, M.T. Epidemiology of osteoporosis. Curr. Rheumatol. Rep. 2006, 8, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A. Homo sapiens—A species not designed for space flight: Health risks in low Earth orbit and beyond, including potential risks when traveling beyond the geomagnetic field of Earth. Life 2023, 13, 757. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.E.; Troisi, R.; Wise, L.A.; Palmer, J.R.; Titus-Ernstoff, L.; Strohsnitter, W.C.; Hatch, E.E. Menarche, Menopause, Years of Menstruation, and the Incidence of Osteoporosis: The Influence of Prenatal Exposure to Diethylstilbestrol. J. Clin. Endocrinol. Metab. 2014, 99, 594–601. [Google Scholar] [CrossRef]
- Rai, S.K.; Shaki, O.; Gupta, T.P.; Upreti, V.; Patil, D. Does parity and duration of lactation have any effect on the bone mineral density of the femur and lumbar spine in Indian women? A cross-sectional study from the Northeast region of India. J. Fam. Med. Prim. Care 2021, 10, 2886–2892. [Google Scholar] [CrossRef]
- Seo, E.; Lee, Y.; Kim, H.C. Association between Parity and Low Bone Density among Postmenopausal Korean Women. J. Prev. Med. Public Health 2021, 54, 284–292. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Cong, H. Association between parity and bone mineral density in postmenopausal women. BMC Women’s Health 2022, 22, 87. [Google Scholar] [CrossRef]
- de Bakker, C.M.J.; Burt, L.A.; Gabel, L.; Hanley, D.A.; Boyd, S.K. Associations between Breastfeeding History and Early Postmenopausal Bone Loss. Calcif. Tissue Int. 2019, 106, 264–273. [Google Scholar] [CrossRef]
- Sochocka, M.; Karska, J.; Pszczołowska, M.; Ochnik, M.; Fułek, M.; Fułek, K.; Kurpas, D.; Chojdak-Łukasiewicz, J.; Rosner-Tenerowicz, A.; Leszek, J. Cognitive Decline in Early and Premature Menopause. Int. J. Mol. Sci. 2023, 24, 6566. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, G.T.; Coughlan, G.T.; Betthauser, T.J.; Betthauser, T.J.; Boyle, R.; Boyle, R.; Koscik, R.L.; Koscik, R.L.; Klinger, H.M.; Klinger, H.M.; et al. Association of Age at Menopause and Hormone Therapy Use with Tau and β-Amyloid Positron Emission Tomography. JAMA Neurol. 2023, 80, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Karamitrou, E.K.; Anagnostis, P.; Vaitsi, K.; Athanasiadis, L.; Goulis, D.G. Early menopause and premature ovarian insufficiency are associated with increased risk of dementia: A systematic review and meta-analysis of observational studies. Maturitas 2023, 176, 107792. [Google Scholar] [CrossRef]
- Cezarino, P.Y.A.; Simoes, R.D.S.; Baracat, E.C.; Junior, J.M.S. Are women living with HIV prone to osteoporosis in post-menopause? A systematic review. Rev. Assoc. Med. Bra. (1992) 2018, 64, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Vaquero, C.; Hernández, J.L.; Olmos, J.M.; Cerdà, D.; Calleja, C.H.; López, J.A.M.; Arboleya, L.; del Rey, F.J.A.; Pardo, S.M.; Vilamajó, I.R.; et al. High incidence of clinical fragility fractures in postmenopausal women with rheumatoid arthritis. A case-control study. Bone 2023, 168, 116654. [Google Scholar] [CrossRef]
- Shen, L.; Lv, J.; Li, J.; Zhou, J.; Wang, X. Managing osteoporosis in COPD. Endocr. Metab. Immune Disord.-Drug Targets 2023, 23, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, X.; Di, J.; Zhou, H.; Niu, H.; Chen, L.; Sha, Q.; Yang, M. The relationship between dietary inflammatory index and bone mineral density in CKD patients. Ther. Apher. Dial. 2023, 28, 69–79. [Google Scholar] [CrossRef]
- Gil-Montoya, J.A.; Garrido-Martínez, M.; Barrios-Rodríguez, R.; Ramos-García, P.; Lenouvel, D.; Montes-Castillo, C.; Martínez-Ramírez, M.J. Association between low bone mineral density and periodontitis in generally healthy perimenopausal women. J. Periodontol. 2020, 92, 95–103. [Google Scholar] [CrossRef]
- Torres, H.M.; Arnold, K.M.; Oviedo, M.; Westendorf, J.J.; Weaver, S.R. Inflammatory Processes Affecting Bone Health and Repair. Curr. Osteoporos. Rep. 2023, 21, 842–853. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Ser. Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Yigitol, I.; Gulumsek, E.; Ozturk, H.A.; Arici, F.N.; Akbal, K.; Pirinci, O.; Karacay, M.; Cihan, T.N.; Totik, Z.G.; Akyildiz, M.A.; et al. Serum periostin levels are significantly higher in patients with primary hyperthyroidism and closely related to os-teoporosis. Exp. Clin. Endocrinol. Diabetes 2023, 131, 449–455. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, M.; Liang, J.; Yang, K.; Li, F.; Zhou, Z.; Qiu, M.; Chen, H.; Cai, Z.; Cui, W.; et al. Neuropeptide Y-Mediated Gut Microbiota Alterations Aggravate Postmenopausal Osteoporosis. Adv. Sci. 2023, 10, e2303015. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Kumar, V.; Rohilla, A. Understanding Osteoporosis: Human Bone Density, Genetic Mechanisms, Gut Microbiota, and Future Prospects. Probiotics Antimicrob. Proteins 2023, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, W.; Wang, J.; Huang, J.; Lv, J.; Zhao, H.; Guo, L. Serum anti-Müllerian hormone levels are associated with low bone mineral density in premenopausal women. Biomarkers 2020, 25, 693–700. [Google Scholar] [CrossRef]
- Karlamangla, A.S.; Shieh, A.; A Greendale, G.; Yu, E.W.; Burnett-Bowie, S.-A.M.; Sluss, P.M.; Martin, D.; Morrison, A.; Finkelstein, J.S. Anti-Mullerian Hormone as Predictor of Future and Ongoing Bone Loss During the Menopause Transition. J. Bone Miner. Res. 2020, 37, 1224–1232. [Google Scholar] [CrossRef]
- Abrahamsen, B.; Madsen, J.S.; Tofteng, C.L.; Stilgren, L.; Bladbjerg, E.M.; Kristensen, S.R.; Brixen, K.; Mosekilde, L. A Common Methylenetetrahydrofolate Reductase (C677T) Polymorphism Is Associated with Low Bone Mineral Density and Increased Fracture Incidence after Menopause: Longitudinal Data from the Danish Osteoporosis Prevention Study. J. Bone Miner. Res. 2003, 18, 723–729. [Google Scholar] [CrossRef]
- Catheline, S.E.; Kaiser, E.; Eliseev, R.A. Mitochondrial Genetics and Function as Determinants of Bone Phenotype and Aging. Curr. Osteoporos. Rep. 2023, 21, 540–551. [Google Scholar] [CrossRef]
- Himič, V.; Syrmos, N.; Ligarotti, G.K.I.; Kato, S.; Fehlings, M.G.; Ganau, M. The role of genetic and epigenetic factors in determining the risk of spinal fragility fractures: New insights in the management of spinal osteoporosis. Quant. Imaging Med. Surg. 2023, 13, 7632–7645. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Y.; Wang, D.; Zhang, C.; Song, H.; Ni, G. Role of irisin in bone diseases. Front. Endocrinol. 2023, 14, 1212892. [Google Scholar] [CrossRef]
- Cheng, C.-Y. Risk of osteoporosis among individuals with varicose veins: A multi-institution cohort study. Arch. Osteoporos. 2023, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Davies, D.A.; Albensi, B.C. The Interaction between NF-κB and Estrogen in Alzheimer’s Disease. Mol. Neurobiol. 2022, 60, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Au, A.; Feher, A.; McPhee, L.; Jessa, A.; Oh, S.; Einstein, G. Estrogens, inflammation and cognition. Front. Neuroendocr. 2016, 40, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Skoczek-Rubińska, A.; Muzsik-Kazimierska, A.; Chmurzynska, A.; Jamka, M.; Walkowiak, J.; Bajerska, J. Inflammatory Potential of Diet Is Associated with Biomarkers Levels of Inflammation and Cognitive Function among Postmenopausal Women. Nutrients 2021, 13, 2323. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.S.; Gannon, O.J.; Salinero, A.E.; Zuloaga, K.L. Contributions of sex to cerebrovascular function and pathology. Brain Res. 2018, 1710, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.R.; Fasano, R.; Paolisso, G. Adiponectin and Cognitive Decline. Int. J. Mol. Sci. 2020, 21, 2010. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-G.; Pang, Z.-Q.; Wu, T.-Y.; Zhang, Y.-H.; Xuan, W.-Q.; Wang, Z.; Yu, X.; Li, Y.-C.; Guo, C.; Wang, Z.-Y. Vitamin D deficiency exacerbates Alzheimer-like pathologies by reducing antioxidant capacity. Free Radic. Biol. Med. 2020, 161, 139–149. [Google Scholar] [CrossRef] [PubMed]
- McCollum, L.E.; Das, S.R.; Xie, L.; de Flores, R.; Wang, J.; Xie, S.X.; Wisse, L.E.; Yushkevich, P.A.; Wolk, D.A. Oh brother, where art tau? Amyloid, neurodegeneration, and cognitive decline without elevated tau. NeuroImage Clin. 2021, 31, 102717. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Hershey, L. Implications of the Approval of Lecanemab for Alzheimer Disease Patient Care. Neurology 2023, 101, 610–620. [Google Scholar] [CrossRef]
- Hussain, S.M.; Wang, Y.; Giles, G.G.; Graves, S.; Wluka, A.E.; Cicuttini, F.M. Female Reproductive and Hormonal Factors and Incidence of Primary Total Knee Arthroplasty Due to Osteoarthritis. Arthritis Rheumatol. 2018, 70, 1022–1029. [Google Scholar] [CrossRef]
- De Roover, A.; Escribano-Núñez, A.; Monteagudo, S.; Lories, R. Fundamentals of osteoarthritis: Inflammatory mediators in osteoarthritis. Osteoarthr. Cartil. 2023, 31, 1303–1311. [Google Scholar] [CrossRef]
- Horváth, E.; Sólyom, Á.; Székely, J.; Nagy, E.E.; Popoviciu, H. Inflammatory and Metabolic Signaling Interfaces of the Hypertrophic and Senescent Chondrocyte Phenotypes Associated with Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 16468. [Google Scholar] [CrossRef]
- Prather, H.; Cheng, J. Relationship of Chronic Systemic Inflammation to Both Chronic Lifestyle-Related Diseases and Osteoarthritis: The Case for Lifestyle Medicine for Osteoarthritis. HSS J. 2023, 19, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Qadri, M.M. Targeting CD44 Receptor Pathways in Degenerative Joint Diseases: Involvement of Proteoglycan-4 (PRG4). Pharmaceuticals 2023, 16, 1425. [Google Scholar] [CrossRef]
- Heard, B.J.; Barton, K.I.; Chung, M.; Achari, Y.; Shrive, N.G.; Frank, C.B.; Hart, D.A. Single intra-articular dexamethasone injection immediately post-surgery in a rabbit model mitigates early inflammatory responses and post-traumatic osteoarthritis-like alterations. J. Orthop. Res. 2015, 33, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Heard, B.J.; Barton, K.I.; Abubacker, S.; Chung, M.; Martin, C.R.; Schmidt, T.A.; Shrive, N.G.; Hart, D.A. Synovial and cartilage responsiveness to peri-operative hyaluronic acid ± dexamethasone administration following a limited injury to the rabbit stifle joint. J. Orthop. Res. 2021, 40, 838–845. [Google Scholar] [CrossRef]
- Barton, K.I.; Heard, B.J.; Sevick, J.L.; Martin, C.R.; Shekarforoush, S.M.; Chung, M.; Achari, Y.; Frank, C.B.; Shrive, N.G.; Hart, D.A. Posttraumatic Osteoarthritis Development and Progression in an Ovine Model of Partial Anterior Cruciate Ligament Transection and Effect of Repeated Intra-articular Methylprednisolone Acetate Injections on Early Disease. Am. J. Sports Med. 2018, 46, 1596–1605. [Google Scholar] [CrossRef]
- Shumnalieva, R.; Kotov, G.; Monov, S. Obesity-Related Knee Osteoarthritis—Current Concepts. Life 2023, 13, 1650. [Google Scholar] [CrossRef] [PubMed]
- Lynskey, S.J.; Macaluso, M.J.; Gill, S.D.; McGee, S.L.; Page, R.S. Biomarkers of Osteoarthritis—A Narrative Review on Causal Links with Metabolic Syndrome. Life 2023, 13, 730. [Google Scholar] [CrossRef]
- Jiménez-Muro, M.; Soriano-Romaní, L.; Mora, G.; Ricciardelli, D.; Nieto, J.A. The microbiota-metabolic syndrome axis as a promoter of metabolic osteoarthritis. Life Sci. 2023, 329, 121944. [Google Scholar] [CrossRef]
- Ramirez, M.F.; Honigberg, M.; Wang, D.; Parekh, J.K.; Bielawski, K.; Courchesne, P.; Larson, M.D.; Levy, D.; Murabito, J.M.; Ho, J.E.; et al. Protein Biomarkers of Early Menopause and Incident Cardiovascular Disease. J. Am. Hear. Assoc. 2023, 12, e028849. [Google Scholar] [CrossRef] [PubMed]
- Isola, J.V.V.; Ko, S.; Ocañas, S.R.; Stout, M.B. Role of Estrogen Receptor α in Aging and Chronic Disease. Adv. Geriatr. Med. Res. 2023, 5, e230005. [Google Scholar] [CrossRef]
- Lee, S.-R.; Directo, D. Fish Oil Supplementation with Resistance Exercise Training Enhances Physical Function and Cardiometabolic Health in Postmenopausal Women. Nutrients 2023, 15, 4516. [Google Scholar] [CrossRef]
- Son, W.-H.; Park, H.-T.; Jeon, B.H.; Ha, M.-S. Moderate intensity walking exercises reduce the body mass index and vascular inflammatory factors in postmenopausal women with obesity: A randomized controlled trial. Sci. Rep. 2023, 13, 20172. [Google Scholar] [CrossRef] [PubMed]
- Kottilil, S.; Mathur, P. The influence of inflammation on cardiovascular disease in women. Front. Glob. Women’s Health 2022, 3, 979708. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.L. Is calcium a link between inflammatory bone resorption and heart disease? eLife 2022, 11, e83841. [Google Scholar] [CrossRef]
- Mansanguan, C.; Mansanguan, C.; Maneerat, Y.; Maneerat, Y. PPBP gene as a biomarker for coronary heart disease risk in postmenopausal Thai women. PeerJ 2022, 10, e13615. [Google Scholar] [CrossRef] [PubMed]
- Justina, V.D.; Miguez, J.S.G.; Priviero, F.; Sullivan, J.C.; Giachini, F.R.; Webb, R.C. Sex Differences in Molecular Mechanisms of Cardiovascular Aging. Front. Aging 2021, 2, 725884. [Google Scholar] [CrossRef]
- Barris, C.T.; Faulkner, J.L.; de Chantemèle, E.J.B. Salt Sensitivity of Blood Pressure in Women. Hypertension 2023, 80, 268–278. [Google Scholar] [CrossRef]
- Bedeković, D.; Bošnjak, I.; Šarić, S.; Kirner, D.; Novak, S. Role of Inflammatory Cytokines in Rheumatoid Arthritis and Development of Atherosclerosis: A Review. Medicina 2023, 59, 1550. [Google Scholar] [CrossRef]
- Mattay, S.S.; Zamani, M.; Saturno, D.; Loftus, E.V.; Ciorba, M.A.; Yarur, A.; Singh, S.; Deepak, P. Risk of Major Adverse Cardiovascular Events in Immune-Mediated Inflammatory Disorders on Biologics and Small Molecules: Network Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Schattner, A. The Cardiovascular Burden of Rheumatoid Arthritis—Implications for Treatment. Am. J. Med. 2023, 136, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Benlidayi, I.C. Exercise therapy for improving cardiovascular health in rheumatoid arthritis. Rheumatol. Int. 2023, 44, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Lahita, R.G. Sex hormones and the immune system—Part 1. Hum. Data 1990, 4, 1–12. [Google Scholar] [CrossRef]
- Mohammadisima, N.; Farshbaf-Khalili, A.; Ostadrahimi, A.; Pourmoradian, S. Positive relation between dietary inflammatory index and osteoporosis in postmenopausal women. Int. J. Vitam. Nutr. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Peng, Q.; Liu, Z.; Xie, Z.; Guo, X.; Li, Y.; Chen, C. Estrogen plays an important role by influencing the NLRP3 inflammasome. Biomed. Pharmacother. 2023, 167, 115554. [Google Scholar] [CrossRef]
- Zhang, X.; Huangfu, Z.; Wang, S. Review of mendelian randomization studies on age at natural menopause. Front. Endocrinol. 2023, 14, 1234324. [Google Scholar] [CrossRef] [PubMed]
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef]
- Mirisola, M.G. The Nutriepigenome. Genes 2023, 14, 1997. [Google Scholar] [CrossRef]
- Qiu, L.; Jing, Q.; Li, Y.; Han, J. RNA modification: Mechanisms and therapeutic targets. Mol. Biomed. 2023, 4, 25. [Google Scholar] [CrossRef]
- la Torre, A.; Vecchio, F.L.; Greco, A. Epigenetic Mechanisms of Aging and Aging-Associated Diseases. Cells 2023, 12, 1163. [Google Scholar] [CrossRef]
- De La Cruz, B.M.; Darsinou, M.; Riccio, A. From form to function: m6A methylation links mRNA structure to metabolism. Adv. Biol. Regul. 2023, 87, 100926. [Google Scholar] [CrossRef]
- Huang, M.; Guo, J.; Liu, L.; Jin, H.; Chen, X.; Zou, J. m6A demethylase FTO and osteoporosis: Potential therapeutic interventions. Front. Cell Dev. Biol. 2023, 11, 1275475. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Song, Y.; Pan, Y.; Liu, J. The essential roles of m6A modification in osteogenesis and common bone diseases. Genes Dis. 2024, 11, 335–345. [Google Scholar] [CrossRef]
- Hernández-Oliveras, A.; Zarain-Herzberg, A. The role of Ca2+-signaling in the regulation of epigenetic mechanisms. Cell Calcium 2024, 117, 102836. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhao, K.; Chen, N.; Fu, Q.; Zhou, Y.; Kong, C.; Li, P.; Yang, C. A 2-decade bibliometric analysis of epigenetics of cardiovascular disease: From past to present. Clin. Epigenetics 2023, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- Sum, H.; Brewer, A.C. Epigenetic modifications as therapeutic targets in atherosclerosis: A focus on DNA methylation and non-coding RNAs. Front. Cardiovasc. Med. 2023, 10, 1183181. [Google Scholar] [CrossRef] [PubMed]
- Kiełbowski, K.; Herian, M.; Bakinowska, E.; Banach, B.; Sroczyński, T.; Pawlik, A. The Role of Genetics and Epigenetic Regulation in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 11655. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wu, Y.; Huang, Y.; Xue, Q.; Wang, Y.; Liao, F.; Wang, X.; Miao, C. Epigenetic modification and exosome effects on autophagy in osteoarthritis. Biochem. Pharmacol. 2023, 218, 115930. [Google Scholar] [CrossRef]
- Tan, D.; Huang, Z.; Zhao, Z.; Chen, X.; Liu, J.; Wang, D.; Deng, Z.; Li, W. Single-cell sequencing, genetics, and epigenetics reveal mesenchymal stem cell senescence in osteoarthritis (Review). Int. J. Mol. Med. 2023, 53, 2. [Google Scholar] [CrossRef]
- Qureshi, I.A.; Mehler, M.F. Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility. Prog. Brain Res. 2010, 186, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Machado-Vieira, R.; Ibrahim, L.; Zarate, C.A., Jr. Histone deacetylases and mood disorders: Epigenetic programming in gene-environment interactions. CNS Neurosci. Ther. 2011, 17, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Ye, T.; Xuan, W.; Zhang, M.; Chen, Q.; Liu, J.; Zhou, P.; Song, H.; Cai, B. The effects of N6-methyladenosine RNA methylation on the nervous system. Mol. Cell. Biochem. 2023, 478, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, B.; Guo, X. Histone demethylases in neurodevelopmet and neurodegenerative diseases. Int. J. Neurosci. 2023, 1–11. [Google Scholar] [CrossRef]
- Smith, E.N.L.; Chandanathil, M.; Millis, R.M. Epigenetic Mechanisms in Obesity: Broadening Our Understanding of the Disease. Cureus 2023, 15, e47875. [Google Scholar] [CrossRef] [PubMed]
- Trang, K.; Grant, S.F. Genetics and epigenetics in the obesity phenotyping scenario. Rev. Endocr. Metab. Disord. 2023, 24, 775–793. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Carluccio, C.; Verni, T.; Carluccio, M.A. Epigenetic mechanisms in vascular in-flammation: Modulation of endothelial adhesion molecules and endothelium-leukocyte adhesion. Front. Biosci. (Landmark Ed.) 2023, 28, 194. [Google Scholar] [CrossRef]
- Bacon, E.R.; Brinton, R.D. Epigenetics of the developing and aging brain: Mechanisms that regulate onset and outcomes of brain reorganization. Neurosci. Biobehav. Rev. 2021, 125, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Bacon, E.R.; Mishra, A.; Wang, Y.; Desai, M.K.; Yin, F.; Brinton, R.D. Neuroendocrine aging precedes perimenopause and is regulated by DNA methylation. Neurobiol. Aging 2018, 74, 213–224. [Google Scholar] [CrossRef]
- Knight, A.K.; Spencer, J.B.; Smith, A.K. DNA methylation as a window into female reproductive aging. Epigenomics 2024, 16, 175–188. [Google Scholar] [CrossRef]
- Pérez-Cremades, D.; Mompeón, A.; Vidal-Gómez, X.; Hermenegildo, C.; Novella, S. miRNA as a New Regulatory Mechanism of Estrogen Vascular Action. Int. J. Mol. Sci. 2018, 19, 473. [Google Scholar] [CrossRef] [PubMed]
- Bjune, J.-I.; Strømland, P.P.; Jersin, R.; Mellgren, G.; Dankel, S.N. Metabolic and Epigenetic Regulation by Estrogen in Adipocytes. Front. Endocrinol. 2022, 13, 828780. [Google Scholar] [CrossRef]
- Shepherd, R.; Bretherton, I.; Pang, K.; Mansell, T.; Czajko, A.; Kim, B.; Vlahos, A.; Zajac, J.D.; Saffery, R.; Cheung, A.; et al. Gender-affirming hormone therapy induces specific DNA methylation changes in blood. Clin. Epigenetics 2022, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gratton, D.R.; Ladyman, S.R. Neurophysicological and cognitive changes in pregnancy. Handb. Clin. Neurol. 2020, 17, 25–55. [Google Scholar] [CrossRef]
- Gutaj, P.; Sibiak, R.; Jankowski, M.; Awdi, K.; Bryl, R.; Mozdziak, P.; Kempisty, B.; Wender-Ozegowska, E. The Role of the Adipokines in the Most Common Gestational Complications. Int. J. Mol. Sci. 2020, 21, 9408. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.R.L.; Motta-Teixeira, L.C.; Gallo, C.C.; Buonfiglio, D.D.C.; de Camargo, L.S.; Quintela, T.; Reiter, R.J.; Amaral, F.G.D.; Cipolla-Neto, J. Maternal pineal melatonin in gestation and lactation physiology, and in fetal development and programming. Gen. Comp. Endocrinol. 2020, 300, 113633. [Google Scholar] [CrossRef]
- Carvalho, D.P.; Dias, A.F.; Sferruzzi-Perri, A.N.; Ortiga-Carvalho, T.M. Gaps in the knowledge of thyroid hormones and placental biology. Biol. Reprod. 2022, 106, 1033–1048. [Google Scholar] [CrossRef]
- Rassie, K.; Giri, R.; Joham, A.E.; Teede, H.; Mousa, A. Prolactin in Pregnancies Affected by Pre-Existing Maternal Metabolic Conditions: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 2840. [Google Scholar] [CrossRef] [PubMed]
- Iosim, S.; MacKay, M.; Westover, C.; E Mason, C. Translating current biomedical therapies for long duration, deep space missions. Precis. Clin. Med. 2019, 2, 259–269. [Google Scholar] [CrossRef]
- Park, S.-K.; Stefanyshyn, D.J.; Ramage, B.; A Hart, D.; Ronsky, J.L. Relationship between knee joint laxity and knee joint mechanics during the menstrual cycle. Br. J. Sports Med. 2009, 43, 174–179. [Google Scholar] [CrossRef]
- Park, S.-K.; Stefanyshyn, D.J.; Loitz-Ramage, B.; Hart, D.A.; Ronsky, J.L. Changing Hormone Levels during the Menstrual Cycle Affect Knee Laxity and Stiffness in Healthy Female Subjects. Am. J. Sports Med. 2009, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 2017, 357, eaal2379. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A. Are secondary effects of bisphosphonates on the vascular system of bone contributing to increased risk for atypical femoral fractures in osteoporosis? BioEssays 2023, 45, e2200206. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A. Towards understanding how bisphosphonate-dependent alterations to nutrient canal integrity can contribute to risk for atypical femoral fractures: Biomechanical considerations and potential relationship to a real-world analogy. BioEssays 2023, 46, e2300117. [Google Scholar] [CrossRef] [PubMed]
- Kanherkar, R.R.; Bhatia-Dey, N.; Csoka, A.B. Epigenetics across the human lifespan. Front. Cell Dev. Biol. 2014, 2, 49. [Google Scholar] [CrossRef]
- Bar-Sadeh, B.; Rudnizky, S.; Pnueli, L.; Bentley, G.R.; Stöger, R.; Kaplan, A.; Melamed, P. Unravelling the role of epigenetics in reproductive adaptations to early-life environment. Nat. Rev. Endocrinol. 2020, 16, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Paramanik, V. Neuromodulating roles of estrogen and phytoestrogens in cognitive therapeutics through epi-genetic modifications during aging. Front. Aging Neurosci. 2022, 14, 945076. [Google Scholar] [CrossRef]
- Wilson, M.E.; Sengoku, T. Developmental regulation of neuronal genes by DNA methylation: Environment influences. Int. J. Dev. Neurosci. 2013, 31, 448–451. [Google Scholar] [CrossRef]
- Lavebratt, C.; Almgren, M.; Ekström, T.J. Epigenetic regulation in obesity. Int. J. Obes. 2011, 36, 757–765. [Google Scholar] [CrossRef]
- Bove, R.M.; Patrick, E.; Aubin, C.M.; Srivastava, G.; Schneider, J.A.; Bennett, D.A.; De Jager, P.L.; Chibnik, L.B. Reproductive period and epigenetic modifications of the oxidative phosphorylation pathway in the human prefrontal cortex. PLoS ONE 2018, 13, e0199073. [Google Scholar] [CrossRef]
- Toro, C.A.; Aylwin, C.F.; Lomniczi, A. Hypothalamic epigenetics driving female puberty. J. Neuroendocr. 2018, 30, e12589. [Google Scholar] [CrossRef] [PubMed]
- Lomniczi, A.; Loche, A.; Castellano, J.M.; Ronnekleiv, O.K.; Bosch, M.; Kaidar, G.; Knoll, J.G.; Wright, H.; Pfeifer, G.P.; Ojeda, S.R. Epigenetic control of female puberty. Nat. Neurosci. 2013, 16, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Lomniczi, A.; Wright, H.; Ojeda, S.R. Epigenetic regulation of female puberty. Front. Neuroendocr. 2015, 36, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Peterson, K.E.; Sánchez, B.N.; Dolinoy, D.C.; Mercado-Garcia, A.; Téllez-Rojo, M.M.; Goodrich, J.M. Association of blood leukocyte DNA methylation at LINE-1 and growth-related candidate genes with pubertal onset and progression. Epigenetics 2018, 13, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Le Guillou, S.; Hue-Beauvais, C.; Le Provost, F. Epigenetics: New Insights into Mammary Gland Biology. Genes 2021, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Stueve, T.R.; Wolff, M.S.; Pajak, A.; Teitelbaum, S.L.; Chen, J. CYP19A1 promoter methylation in saliva associated with milestones of pubertal timing in urban girls. BMC Pediatr. 2014, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Leka-Emiri, S.; Chrousos, G.P.; Kanaka-Gantenbein, C. The mystery of puberty initiation: Genetics and epigenetics of idiopathic central precocious puberty (ICPP). J. Endocrinol. Investig. 2017, 40, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.S.; Lee, H.S.; Hwang, J.S. Genetic factors in precocious puberty. Clin. Exp. Pediatr. 2022, 65, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.R.; Voisin, S.; Nolan, B.J.; Landen, S.; Jacques, M.; Newell, B.; Zwickl, S.; Cook, T.; Wong, A.; Ginger, A.; et al. Uncovering the effects of gender affirming hormone therapy on skeletal muscle and epigenetics: Protocol for a prospective matched cohort study in transgender individuals (the GAME study). BMJ Open 2022, 12, e060869. [Google Scholar] [CrossRef]
- Gao, F.; Das, S.K. Epigenetic regulations through DNA methylation and hydroxymethylation: Clues for early pregnancy in decidualization. Biomol. Concepts 2014, 5, 95–107. [Google Scholar] [CrossRef]
- A Michalczyk, A.; Janus, E.D.; Judge, A.; Ebeling, P.R.; Best, J.D.; Ackland, M.J.; Asproloupos, D.; A Dunbar, J. Transient epigenomic changes during pregnancy and early postpartum in women with and without type 2 diabetes. Epigenomics 2018, 10, 419–431. [Google Scholar] [CrossRef]
- Andrawus, M.; Sharvit, L.; Atzmon, G. Epigenetics and Pregnancy: Conditional Snapshot or Rolling Event. Int. J. Mol. Sci. 2022, 23, 12698. [Google Scholar] [CrossRef] [PubMed]
- Zuccarello, D.; Sorrentino, U.; Brasson, V.; Marin, L.; Piccolo, C.; Capalbo, A.; Andrisani, A.; Cassina, M. Epigenetics of pregnancy: Looking beyond the DNA code. J. Assist. Reprod. Genet. 2022, 39, 801–816. [Google Scholar] [CrossRef]
- Das, J.; Maitra, A. Maternal DNA Methylation During Pregnancy: A Review. Reprod. Sci. 2021, 28, 2758–2769. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.K.; Balakrishnan, B.; Lissaman, A.C.; Gujral, P.; Ponnampalam, A.P. Cytokines and pregnancy: Potential regulation by histone deacetylases. Mol. Reprod. Dev. 2021, 88, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Chen, L. RNA N6-methyladenosine modification in female reproductive biology and pathophysiology. Cell Commun. Signal. 2023, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Yan, J.; Yang, H. Exploring the role of m6A modification in the great obstetrical syndromes. J. Matern. Neonatal Med. 2023, 36, 2234541. [Google Scholar] [CrossRef]
- Rijnkels, M.; Freeman-Zadrowski, C.; Hernandez, J.; Potluri, V.; Wang, L.; Li, W.; Lemay, D.G. Epigenetic Modifications Unlock the Milk Protein Gene Loci during Mouse Mammary Gland Development and Differentiation. PLoS ONE 2013, 8, e53270. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.J.; Clement, K.; Jee, D.; Merlini, A.; Choudhury, S.; Maruyama, R.; Yoo, R.; Chytil, A.; Boyle, P.; Ran, F.A.; et al. Age- and Pregnancy-Associated DNA Methylation Changes in Mammary Epithelial Cells. Stem Cell Rep. 2015, 4, 297–311. [Google Scholar] [CrossRef]
- Xue, Q.; Huang, Y.; Cheng, C.; Wang, Y.; Liao, F.; Duan, Q.; Wang, X.; Miao, C. Progress in epigenetic regulation of milk synthesis, with particular emphasis on mRNA regulation and DNA methylation. Cell Cycle 2023, 22, 1675–1693. [Google Scholar] [CrossRef]
- VanHouten, J.N.; Wysolmerski, J.J. Low Estrogen and High Parathyroid Hormone-Related Peptide Levels Contribute to Accelerated Bone Resorption and Bone Loss in Lactating Mice. Endocrinology 2003, 144, 5521–5529. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.A.; Freitas, M.B.; Gonçalves, R.V. Changes in bone turnover and calcium homeostasis during pregnancy and lactation in mammals: A meta-analysis. Reprod. Fertil. Dev. 2018, 30, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Carsote, M.; Turturea, M.R.; Valea, A.; Buescu, C.; Nistor, C.; Turturea, I.F. Bridging the Gap: Pregnancy—And Lactation—Associated Osteoporosis. Diagnostics 2023, 13, 1615. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hanevik, H.I. Early menopause and epigenetic biomarkers of ageing. Reprod. Biomed. Online 2022, 45, 1313. [Google Scholar] [CrossRef]
- Jintaridth, P.; Tungtrongchitr, R.; Preutthipan, S.; Mutirangura, A. Hypomethylation of Alu Elements in Post-Menopausal Women with Osteoporosis. PLoS ONE 2013, 8, e70386. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.M.; Toriola, A.T.; Koepl, L.M.; Sandifer, T.; Poole, E.M.; Duggan, C.; McTiernan, A.; Issa, J.-P.J. Metabolic, hormonal and immunological associations with global DNA methylation among postmenopausal women. Epigenetics 2012, 7, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Song, Z.; Gao, P.; Li, M.; Ning, Z.; Song, X. Identification of an Epigenetic Signature for Coronary Heart Disease in Postmenopausal Women’s PBMC DNA. Mediat. Inflamm. 2022, 2022, 2185198. [Google Scholar] [CrossRef] [PubMed]
- Calaf Alsina, J.C. Benefits of hormone replacement therapy—Overview and update. Int. J. Fertil. Women’s Med. 1997, 42, 329–346. [Google Scholar]
- Kreatsoulas, C.; Anand, S.S. Menopausal hormone therapy for the primary prevention of chronic conditions. U.S. Preventive Services Task Force recommendation statement. Pol. Arch. Intern. Med. 2013, 123, 112–117. [Google Scholar] [CrossRef]
- Lobo, R.A.; Pickar, J.H.; Stevenson, J.C.; Mack, W.J.; Hodis, H.N. Back to the future: Hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis 2016, 254, 282–290. [Google Scholar] [CrossRef]
- Cagnacci, A.; Venier, M. The Controversial History of Hormone Replacement Therapy. Medicina 2019, 55, 602. [Google Scholar] [CrossRef] [PubMed]
- Beral, V.; Banks, E.; Reeves, G.; Appleby, P. Use of HRT and the subsequent risk of cancer. J. Epidemiol. Biostat. 1999, 4, 191. [Google Scholar]
- Tavani, A.; La Vecchia, C. The Adverse Effects of Hormone Replacement Therapy. Drugs Aging 1999, 14, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.S. Menopause and Osteoporosis: The Role of HRT. J. Am. Pharm. Assoc. 1996, 36, 235–242. [Google Scholar] [CrossRef]
- McClung, M.R. Prevention and management of osteoporosis. Best Pr. Res. Clin. Endocrinol. Metab. 2003, 17, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Salpeter, S.R.; Walsh, J.M.E.; Ormiston, T.M.; Greyber, E.; Buckley, N.S.; Salpeter, E.E. Meta-analysis: Effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab. 2005, 8, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, T.E.J.; Brincat, M.P.; Pollacco, J.; Stevenson, J.C. Effect of hormone replacement therapy on intervertebral disc height. Climacteric 2023, 26, 110–113. [Google Scholar] [CrossRef]
- Sourouni, M.; Sourouni, M.; Kiesel, L.; Kiesel, L. Menopausal Hormone Therapy and the Breast: A Review of Clinical Studies. Breast Care 2023, 18, 164–171. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, B.M.; Schiphof, D.; Groeneveld, F.P.M.J.; Koes, B.W.; van Osch, G.J.V.M.; van Meurs, J.B.J.; Bierma-Zeinstra, S.M.A. Limited evidence for a protective effect of unopposed oestrogen therapy for osteoarthritis of the hip: A systematic review. Rheumatology 2008, 48, 104–112. [Google Scholar] [CrossRef]
- Bendale, D.S.; Karpe, P.A.; Chhabra, R.; Shete, S.P.; Shah, H.; Tikoo, K. 17-β Oestradiol prevents cardiovascular dysfunction in post-menopausal metabolic syndrome by affecting SIRT1/AMPK/H3 acetylation. Br. J. Pharmacol. 2013, 170, 779–795. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Romo, R.; Arreguin, J.M.; Movahed, M.R. The effects of estrogen and hormone replacement therapy on cardiovascular systems. Futur. Cardiol. 2021, 17, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Mills, Z.B.; Faull, R.L.M.; Kwakowsky, A. Is Hormone Replacement Therapy a Risk Factor or a Therapeutic Option for Alzheimer’s Disease? Int. J. Mol. Sci. 2023, 24, 3205. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Pöllänen, E.; Ismail, K.; Sipilä, S.; Mikkola, T.M.; Berglund, E.; Lindqvist, C.M.; Syvänen, A.-C.; Rantanen, T.; Kaprio, J.; et al. Hormone Replacement Therapy Associated White Blood Cell DNA Methylation and Gene Expression are Associated with within-Pair Differences of Body Adiposity and Bone Mass. Twin Res. Hum. Genet. 2015, 18, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Hilser, J.R.; Hartiala, J.A.; Sriprasert, I.; Kono, N.; Cai, Z.; Karim, R.; DeYoung, J.; Mack, W.J.; Hodis, H.N.; Allayee, H. Effect of menopausal hormone therapy on methylation levels in early and late postmenopausal women. Clin. Epigenetics 2022, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Noronha, N.Y.; Rodrigues, G.d.S.; Noma, I.H.Y.; Brandao, C.F.C.; Rodrigues, K.P.; Bruno, A.C.; Sae-Lee, C.; Watanabe, L.M.; Pinhel, M.A.d.S.; Schineider, I.M.; et al. 14-weeks combined exercise epigenetically modulated 118 genes of menopausal women with prediabetes. Front. Endocrinol. 2022, 13, 895489. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.d.S.; Noronha, N.Y.; Noma, I.H.Y.; de Lima, J.G.R.; Sobrinho, A.C.d.S.; Pinhel, M.A.d.S.; de Almeida, M.L.; Watanabe, L.M.; Nonino, C.B.; Júnior, C.R.B. 14-Week exercise training modifies the DNA methylation levels at gene sites in non-Alzheimer’s disease women aged 50 to 70 years. Exp. Gerontol. 2024, 186, 112362. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.P.; Ogunmoroti, O.; Minhas, A.S.; Vaidya, D.; Kazzi, B.; Osibogun, O.; Whelton, S.; Kovell, L.C.; Harrington, C.M.; Honigberg, M.C.; et al. Female-specific risk factors of parity and menopause age and risk of carotid plaque: The multi-ethnic study of atherosclerosis. Am. J. Cardiovasc. Dis. 2023, 13, 222–234. [Google Scholar]
- Lai, P.M.R.; Jimenez, M.; Du, R.; Rexrode, K. Association of Reproductive Life Span and Age at Menopause with the Risk of Aneurysmal Subarachnoid Hemorrhage. Neurology 2022, 98, e2005–e2012. [Google Scholar] [CrossRef]
- Hwang, S.; Kang, S.W.; Choi, K.J.; Son, K.Y.; Lim, D.H.; Shin, D.W.; Choi, D.; Kim, S.J. Early menopause is associated with increased risk of retinal vascular occlusions: A nationwide cohort study. Sci. Rep. 2022, 12, 6068. [Google Scholar] [CrossRef]
- Han, S.-L.; Liu, D.-C.; Tan, C.-C.; Tan, L.; Xu, W. Male- and female-specific reproductive risk factors across the lifespan for dementia or cognitive decline: A systematic review and meta-analysis. BMC Med. 2023, 21, 457. [Google Scholar] [CrossRef]
- Xie, Q.; Xu, H.; Wan, Q. Correlation between parity and metabolic syndrome in Chinese women aged 40 years and older: The Reaction study. BMC Endocr. Disord. 2021, 21, 236. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Cicuttini, F.M.; Alyousef, B.; Wang, Y. Female hormonal factors and osteoarthritis of the knee, hip and hand: A narrative review. Climacteric 2018, 21, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Balkwill, A.; Cooper, C.; Roddam, A.; Brown, A.; Beral, V.; on behalf of the Million Women Study Collaborators. Reproductive history, hormonal factors and the incidence of hip and knee replacement for osteoarthritis in middle-aged women. Ann. Rheum. Dis. 2008, 68, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Muramatsu, M.; Kume, T. Organ/Tissue-Specific Vascular Endothelial Cell Heterogeneity in Health and Disease. Biol. Pharm. Bull. 2019, 42, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Parab, S.; Setten, E.; Astanina, E.; Bussolino, F.; Doronzo, G. The tissue-specific transcriptional landscape underlines the involvement of endothelial cells in health and disease. Pharmacol. Ther. 2023, 246, 108418. [Google Scholar] [CrossRef]
- Peretz, A.; Loyfer, N.; Piyanzin, S.; Ochana, B.L.; Neiman, D.; Magenheim, J.; Klochendler, A.; Drawshy, Z.; Fox-Fisher, I.; Fridlich, O.; et al. The DNA methylome of human vascular endothelium and its use in liquid biopsies. Med 2023, 4, 263–281.e4. [Google Scholar] [CrossRef] [PubMed]
- Palikuqi, B.; Nguyen, D.-H.T.; Li, G.; Schreiner, R.; Pellegata, A.F.; Liu, Y.; Redmond, D.; Geng, F.; Lin, Y.; Gómez-Salinero, J.M.; et al. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 2020, 585, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Bei, Y.; Lin, H.; Wei, T.; Dai, Y.; Hu, Y.; Zhang, C.; Dai, H. The role of class IIa histone deacetylases in regulating endothelial function. Front. Physiol. 2023, 14, 1091794. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.J.; Giles, R.W.; Bray, R.C.; Hart, D.A. Pregnancy-induced changes in rabbit medial collateral ligament vas-oregulation. Am. J. Physiol. 1998, 275, R1380–R1385. [Google Scholar] [CrossRef]
- McDougall, J.J.; Bray, R.C.; Hart, D.A. Late gestational changes in sympathomimetic sensitivity in primigravid rabbit ligaments. Can. J. Physiol Pharmacol. 2000, 78, 528–534. [Google Scholar] [CrossRef]
- Chelly, A.; Bouzid, A.; Neifar, F.; Kammoun, I.; Tekari, A.; Masmoudi, S.; Chtourou, H.; Rebai, A. Effect of Aerobic/Strength Training on RANKL Gene DNA Methylation Levels. J. Phys. Act. Health 2023, 20, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Proia, P.; Rossi, C.; Alioto, A.; Amato, A.; Polizzotto, C.; Pagliaro, A.; Kuliś, S.; Baldassano, S. MiRNAs Expression Modulates Osteogenesis in Response to Exercise and Nutrition. Genes 2023, 14, 1667. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, X.; Wei, A.; Chen, F.; Gao, Q.; Lu, K.; Jiang, Q.; Cao, W. Nrf2 epigenetic derepression induced by running exercise protects against osteoporosis. Bone Res. 2021, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Elsner, V.R.; Fraga, I.; Weber, C.; Galiano, W.B.; Iraci, L.; Wohlgemuth, M.; Morales, G.; Cercato, C.; Rodriguez, J.; Pochmann, D.; et al. Effects of a multimodal exercise protocol on functional outcomes, epigenetic modulation and brain-derived neurotrophic factor levels in institutionalized older adults: A quasi-experimental pilot study. Neural Regen. Res. 2021, 16, 2479–2485. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Bloch, W. Physical exercise and epigenetic adaptations of the cardiovascular system. Herz 2015, 40, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, F. Exercise improves vascular health: Role of mitochondria. Free Radic. Biol. Med. 2021, 177, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Mitsiou, G.; Tokmakidis, S.P.; Dinas, P.C.; Smilios, I.; Nanas, S. Endothelial progenitor cell mobilization based on exercise volume in patients with cardiovascular disease and healthy individuals: A systematic review and meta-analysis. Eur. Hear. J. Open 2022, 2, oeac078. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, S.; Wang, X.; Yang, X.; Chen, L.; Huang, T.; Zheng, Y.; Zheng, X.; Wu, X.; Sun, Y.; et al. Exercise Mitigates Endothelial Pyroptosis and Atherosclerosis by Downregulating NEAT1 Through N6-Methyladenosine Modifications. Arter. Thromb. Vasc. Biol. 2023, 43, 910–926. [Google Scholar] [CrossRef]
- Espin-Garcia, O.; Baghel, M.; Brar, N.; Whittaker, J.L.; Ali, S.A. Can genetics guide exercise prescriptions in osteoarthritis? Front. Rehabil. Sci. 2022, 3, 930421. [Google Scholar] [CrossRef]
- Barros, L.; Eichwald, T.; Solano, A.F.; Scheffer, D.d.L.; da Silva, R.A.; Gaspar, J.M.; Latini, A. Epigenetic modifications induced by exercise: Drug-free intervention to improve cognitive deficits associated with obesity. Physiol. Behav. 2019, 204, 309–323. [Google Scholar] [CrossRef]
- Barha, C.K.; Falck, R.S.; Skou, S.T.; Liu-Ambrose, T. Personalising exercise recommendations for healthy cognition and mobility in aging: Time to address sex and gender (Part 1). Br. J. Sports Med. 2020, 55, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Barha, C.K.; Falck, R.S.; Skou, S.T.; Liu-Ambrose, T. Personalizing exercise recommendations for healthy cognition and mobility in aging: Time to consider ones’ pre-existing function and genotype (Part 2). Br. J. Sports Med. 2020, 55, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Balbim, G.M.; Falck, R.S.; Barha, C.K.; Starkey, S.Y.; Bullock, A.; Davis, J.C.; Liu-Ambrose, T. Effects of exercise training on the cognitive function of older adults with different types of dementia: A systematic review and meta-analysis. Br. J. Sports Med. 2022, 56, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; Kaplon, R.E.; Hill, S.D.; McNamara, M.N.; Santos-Parker, J.R.; Pierce, G.L.; Seals, U.R.; Donato, A.J. Endothelial cell senescence with aging in healthy humans: Prevention by habitual exercise and relation to vascular endothelial function. Am. J. Physiol. Circ. Physiol. 2017, 313, H890–H895. [Google Scholar] [CrossRef] [PubMed]
- Sujkowski, A.L.; Hong, L.; Wessells, R.; Todi, S.V. The protective role of exercise against age-related neurodegeneration. Ageing Res. Rev. 2021, 74, 101543. [Google Scholar] [CrossRef] [PubMed]
- Königstein, K.; Dipla, K.; Zafeiridis, A. Training the Vessels: Molecular and Clinical Effects of Exercise on Vascular Health—A Narrative Review. Cells 2023, 12, 2544. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Geng, Q.; Jin, S.; Teng, X.; Xiao, L.; Wu, Y.; Tian, D. Exercise protects vascular function by countering senescent cells in older adults. Front. Physiol. 2023, 14, 1138162. [Google Scholar] [CrossRef] [PubMed]
- Spartano, N.L.; Wang, R.; Yang, Q.; Chernofsky, A.; Murabito, J.M.; Levy, D.; Vasan, R.S.; DeCarli, C.; Maillard, P.; Seshadri, S.; et al. Association of Physical Inactivity with MRI Markers of Brain Aging: Assessing Mediation by Cardiometabolic and Epigenetic Factors. J. Alzheimer’s Dis. 2023, 95, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-L.; Ma, X.-T.; Wang, J.-J.; Liu, H.; Chen, Y.-F.; Yang, Y. Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav. Brain Res. 2017, 317, 332–339. [Google Scholar] [CrossRef]
- Kim-Ha, J.; Kim, Y.-J. Age-related epigenetic regulation in the brain and its role in neuronal diseases. BMB Rep. 2016, 49, 671–680. [Google Scholar] [CrossRef]
- Recchioni, R.; Marcheselli, F.; Antonicelli, R.; Lazzarini, R.; Mensà, E.; Testa, R.; Procopio, A.D.; Olivieri, F. Physical activity and progenitor cell-mediated endothelial repair in chronic heart failure: Is there a role for epigenetics? Mech. Ageing Dev. 2016, 159, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, I.; Tchekalarova, J.; Iliev, D.; Tzoneva, R. Endothelial Senescence and Its Impact on Angiogenesis in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 11344. [Google Scholar] [CrossRef] [PubMed]
- Barha, C.K.; Starkey, S.Y.; Hsiung, G.Y.R.; Tam, R.; Liu-Ambrose, T. Aerobic exercise improves executive functions in females, but not males, without the BDNF Val66Met polymorphism. Biol. Sex Differ. 2023, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Barha, C.K.; Best, J.R.; Rosano, C.; Yaffe, K.; Catov, J.M.; Liu-Ambrose, T. Walking for Cognitive Health: Previous Parity Moderates the Relationship between Self-Reported Walking and Cognition. J. Gerontol. Ser. A 2022, 78, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Rode, B.; Shi, J.; Endesh, N.; Drinkhill, M.J.; Webster, P.J.; Lotteau, S.J.; Bailey, M.A.; Yuldasheva, N.Y.; Ludlow, M.J.; Cubbon, R.M.; et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat. Commun. 2017, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Debant, M.; Chuntharpursat-Bon, E.; Evans, E.L.; Musialowski, K.E.; Parsonage, G.; Morley, L.C.; Futers, T.S.; Sukumar, P.; Bowen, T.S.; et al. Endothelial Piezo1 sustains muscle capillary density and contributes to physical activity. J. Clin. Investig. 2022, 132, 141775. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.; Yu, F.; Zhang, X.; Pang, Y.; Zhao, W.; Sun, P.; Li, L.; Zong, B.; Yu, F.; Zhang, X.; et al. Mechanosensitive Piezo1 channel in physiology and pathophysiology of the central nervous system. Ageing Res. Rev. 2023, 90, 102026. [Google Scholar] [CrossRef] [PubMed]
- Zi, H.; Peng, X.; Cao, J.; Xie, T.; Liu, T.; Li, H.; Bu, J.; Du, J.; Li, J. Piezo1-dependent regulation of pericyte proliferation by blood flow during brain vascular development. Cell Rep. 2024, 43, 113652. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.F.; Manduchi, E.; Jiménez, J.M.; Jiang, Y.-Z. Biofluids, cell mechanics and epigenetics: Flow-induced epigenetic mechanisms of endothelial gene expression. J. Biomech. 2017, 50, 3–10. [Google Scholar] [CrossRef]
- Sharma, G.; Sultana, A.; Abdullah, K.M.; Pothuraju, R.; Nasser, M.W.; Batra, S.K.; Siddiqui, J.A. Epigenetic regulation of bone remodeling and bone metastasis. Semin. Cell Dev. Biol. 2024, 154, 275–285. [Google Scholar] [CrossRef]
- Castro-Muñoz, L.J.; Ulloa, E.V.; Sahlgren, C.; Lizano, M.; De La Cruz-Hernández, E.; Contreras-Paredes, A. Modulating epigenetic modifications for cancer therapy (Review). Oncol. Rep. 2023, 49, 59. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Mo, K.; Kwon, H.; Choe, S.; Park, M.; Kwak, W.; Yoon, H. Epigenetic Regulation in Breast Cancer: Insights on Epidrugs. Epigenomes 2023, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Iglesias, O.; Naidoo, V.; Carrera, I.; Corzo, L.; Cacabelos, R. Natural Bioactive Products as Epigenetic Modulators for Treating Neurodegenerative Disorders. Pharmaceuticals 2023, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Gladkova, M.G.; Leidmaa, E.; Anderzhanova, E.A. Epidrugs in the Therapy of Central Nervous System Disorders: A Way to Drive on? Cells 2023, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Gladwell, L.R.; Ahiarah, C.; Rasheed, S.; Rahman, S.M.; Choudhury, M. Traditional therapeutics and potential epidrugs for CVD: Why not both? Life 2023, 14, 23. [Google Scholar] [CrossRef]
- Musolino, E.; Pagiatakis, C.; Serio, S.; Borgese, M.; Gamberoni, F.; Gornati, R.; Bernardini, G.; Papait, R. The Yin and Yang of epigenetics in the field of nanoparticles. Nanoscale Adv. 2022, 4, 979–994. [Google Scholar] [CrossRef]
- Sharma, S.; Bhonde, R. Epigenetic Modifiers as Game Changers for Healthy Aging. Rejuvenation Res. 2023, 26, 88–104. [Google Scholar] [CrossRef]
- Farani, M.R.; Sarlak, M.; Gholami, A.; Azaraian, M.; Binabaj, M.M.; Kakavandi, S.; Tambuwala, M.M.; Taheriazam, A.; Hashemi, M.; Ghasemi, S. Epigenetic drugs as new emerging therapeutics: What is the scale’s orientation of application and challenges? Pathol.-Res. Pr. 2023, 248, 154688. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A.; Zernicke, R.F. Optimal Human Functioning Requires Exercise across the Lifespan: Mobility in a 1g Environment Is Intrinsic to the Integrity of Multiple Biological Systems. Front. Physiol. 2020, 11, 156. [Google Scholar] [CrossRef]
- Ma, L.; Liu, G.; Ding, M.; Zong, G.; Hu, F.B.; Willett, W.C.; Rimm, E.B.; Manson, J.E.; Sun, Q. Isoflavone intake and the risk of coronary heart disease in US men and women. Circulation 2020, 141, 1127–1137. [Google Scholar] [CrossRef]
- Sanchez-Martinez, L.; Periago, M.-J.; Garcia-Alonso, J.; Garcia-Conesa, M.-T.; Gonzalez-Barrio, R. A systematic review of the cardiometabolic benefits of plant products containing mixed phenolics and polyphenols in postmenopausal women: Insufficient evidence for recommendations to this specific population. Nutrients 2021, 13, 4276. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.R. Mediterranean diet and soy isoflavones for integrate3d management of menopausal metabolic syndrome. Nutrients 2022, 14, 1550. [Google Scholar] [CrossRef]
- Calderaro, A.; Patane, G.T.; Tellone, E.; Barreca, D.; Ficarra, S.; Misiti, F.; Lagana, G. The neuroprotective potentiality of flavonoids on Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 14835. [Google Scholar] [CrossRef]
- Peng, X.; Zhu, Y.; Wu, Y.; Xiang, X.; Deng, M.; Liu, L.; Li, T.; Yang, G. Genistein, a soybean isoflavone, promotes wound healing by enhancing endothelial progenitor cell mobilization in rats with hemorrhagic shock. Adv. Biol. 2023, 7, e2200236. [Google Scholar] [CrossRef] [PubMed]
- Ganai, A.A.; Farooqi, H. Bioactivity of genistein: A review of in vitro and in vivo studies. Biomed. Pharmacother. 2015, 76, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Soy isoflavones inhibit endothelial cell dysfunction and prevent cardiovascular disease. J. Cardiovasc. Pharmacol. 2019, 74, 201–209. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, Q.; Liu, J.; Li, R.; Wang, D.; Peng, W.; Wu, C. Suppression of apoptosis in vascular endothelial cell, the promising way for natural medicines to treat atherosclerosis. Pharmacol. Res. 2021, 168, 105599. [Google Scholar] [CrossRef]
- Zhang, H.; Pang, X.; Yu, H.; Zhou, H. Genistein suppresses ox-LDL-elicited oxidative stress and senescence in HUVEs through the SIRT1-p66shc-Foxo3a pathways. J. Biochem. Mol. Toxicol. 2022, 36, e22939. [Google Scholar] [CrossRef]
- Xu, K.; Qin, Q.; Yao, L.; Du, X.; Zhou, K.; Wi, X.; Wang, W.; Liu, C. Anti-oxidation effect of genistein in vascular endothelial cell after H2O2 stress. Mol. Med. Rep. 2024, 29, 2. [Google Scholar] [CrossRef]
- Squadrito, F.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; D’Anna, R.; Arcoraci, V.; Burnett, B.P.; Minutoli, L.; et al. Genistein in the metabolic syndrome: Results of a randomized clinical trial. J. Clin. Endocrinol. Metab. 2013, 98, 3366–3374. [Google Scholar] [CrossRef]
- Saad, B.; Ghareeb, B.; Kmail, A. Metabolic and epigenetics action mechanisms of antiobesity medicinal plants and phytochemicals. Evid. Based Complement. Alternat. Med. 2021, 2021, 0005903. [Google Scholar] [CrossRef] [PubMed]
- Huminiecki, L. Evidence for multilevel chemopreventive activities of natural phenols from functional genomics studies of curcumin, resveratrol, genistein, quercetin, and luteolin. Int. J. Mol. Sci. 2022, 23, 14957. [Google Scholar] [CrossRef] [PubMed]
- Kocabas, S.; Sanlier, N. A comprehensive overview of the complex relationship between epigenetics, bioactive components, cancer, and aging. Crit. Rev. Food Sci. Nutr. 2023, 63, 3333–3345. [Google Scholar] [CrossRef] [PubMed]
- Acar, Y.; Agagunduz, D.; De Cicco, P.; Capasso, R. Flavonoids: Their putative neurologic roles, epigenetic changes, and gut microbiota alterations in Parkinson’s disease. Biomed. Pharmacother. 2023, 168, 115788. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Chen, F.; Zhau, B. Epigenetics in obesity: Mechanisms and advances in therapies based on natural products. Pharmacol. Res. Perspect. 2024, 12, e1171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, D.A. The Heterogeneity of Post-Menopausal Disease Risk: Could the Basis for Why Only Subsets of Females Are Affected Be Due to a Reversible Epigenetic Modification System Associated with Puberty, Menstrual Cycles, Pregnancy and Lactation, and, Ultimately, Menopause? Int. J. Mol. Sci. 2024, 25, 3866. https://doi.org/10.3390/ijms25073866
Hart DA. The Heterogeneity of Post-Menopausal Disease Risk: Could the Basis for Why Only Subsets of Females Are Affected Be Due to a Reversible Epigenetic Modification System Associated with Puberty, Menstrual Cycles, Pregnancy and Lactation, and, Ultimately, Menopause? International Journal of Molecular Sciences. 2024; 25(7):3866. https://doi.org/10.3390/ijms25073866
Chicago/Turabian StyleHart, David A. 2024. "The Heterogeneity of Post-Menopausal Disease Risk: Could the Basis for Why Only Subsets of Females Are Affected Be Due to a Reversible Epigenetic Modification System Associated with Puberty, Menstrual Cycles, Pregnancy and Lactation, and, Ultimately, Menopause?" International Journal of Molecular Sciences 25, no. 7: 3866. https://doi.org/10.3390/ijms25073866
APA StyleHart, D. A. (2024). The Heterogeneity of Post-Menopausal Disease Risk: Could the Basis for Why Only Subsets of Females Are Affected Be Due to a Reversible Epigenetic Modification System Associated with Puberty, Menstrual Cycles, Pregnancy and Lactation, and, Ultimately, Menopause? International Journal of Molecular Sciences, 25(7), 3866. https://doi.org/10.3390/ijms25073866





